Abstract
The polymeric Ig receptor (pIgR) transcytoses its ligand, dimeric IgA (dIgA), from the basolateral to the apical surface of epithelial cells. Although the pIgR is constitutively transcytosed in the absence of ligand, binding of dIgA stimulates transcytosis of the pIgR. We recently reported that dIgA binding to the pIgR induces translocation of protein kinase C, production of inositol triphosphate, and elevation of intracellular free calcium. We now report that dIgA binding causes rapid, transient tyrosine phosphorylation of several proteins, including phosphatidyl inositol-specific phospholipase C-γl. Protein tyrosine kinase inhibitors or deletion of the last 30 amino acids of pIgR cytoplasmic tail prevents IgA-stimulated protein tyrosine kinase activation, tyrosine phosphorylation of phospholipase C-γl, production of inositol triphosphate, and the stimulation of transcytosis by dIgA. Analysis of pIgR deletion mutants reveals that the same discrete portion of the cytoplasmic domain, residues 727–736 (but not the Tyr734), controls both the ability of pIgR to cause dIgA-induced tyrosine phosphorylation of the phospholipase C-γl and to undergo dIgA-stimulated transcytosis. In addition, dIgA transcytosis can be strongly stimulated by mimicking phospholipase C-γl activation. In combination with our previous results, we conclude that the protein tyrosine kinase(s) and phospholipase C-γl that are activated upon dIgA binding to the pIgR control dIgA-stimulated pIgR transcytosis.
INTRODUCTION
In recent years, major findings have led to a good understanding of the mechanisms by which protein-sorting signals and vesicular coat proteins control membrane traffic (Rothman, 1994; Schekman and Orci, 1996). Similarly, most of the major pathways for intracellular signaling have been elucidated (Fantl et al., 1993; Cano and Mahadevan, 1995; Ihle et al., 1995; Guan and Chen, 1996; Alberola-Ila et al., 1997; Gudermann et al., 1997). Although these fields are largely distinct, one area of overlap has come from the realization that most steps in membrane and protein traffic are regulated by signaling pathways superimposed on the sorting machinery. The regulation of membrane traffic was first appreciated in neurons and specialized secretory cells, where exocytosis of synaptic vesicles or secretory granules occurs in response to extracellular signals, which act to raise the intracellular free calcium concentration, leading to fusion with the plasma membrane (Blondel et al., 1995; Südhof, 1995; Corvera and Czech, 1996). Regulation of protein traffic has been also documented in the endocytotic pathway and often occurs in response to ligand binding to a receptor. The best understood example of this is the epidermal growth factor receptor (EGFR).1 In the absence of EGF the wild-type EGFR recycles constitutively, but in the presence of ligand the EGFR is more rapidly internalized and degraded (Felder et al., 1990; Lund et al., 1990; Wiley et al., 1991; Opresko et al., 1995; Futter et al., 1996). In contrast, the kinase-deficient mutant is recycled to the plasma membrane even in the presence of EGF. Recently, a protein (SNX1) that binds to the EGFR in the yeast two-hybrid system has been cloned (Kurten et al., 1996). Cotransfection of this protein with the EGFR in fibroblastic CV-1 cells leads to the degradation of the EGFR, which is increased in the presence of EGF. In addition, the SNX1 protein does not lead to the degradation of the kinase-deficient EGFR (Kurten et al., 1996). These results are strong evidence that the intrinsic tyrosine kinase of the EGFR stimulated by the ligand, in addition to other portions of the cytoplasmic domain, might control its targeting to a degradative pathway.
We have been investigating the regulation of transcytosis of the polymeric Ig receptor (pIgR), which transcytoses dimeric IgA (dIgA) from the basolateral to the apical surface of polarized epithelial cells, such as Madin-Darby canine kidney (MDCK) cells. Transcytosis can be divided biochemically and morphologically into three steps. Step 1 is internalization from the basolateral plasma membrane and delivery into basolateral early endosomes. Step 2 is the microtubule-dependent translocation from basolateral early endosomes to apical recycling endosomes (ARE), which are mainly localized underneath the apical plasma membrane. Step 3 is the delivery from the ARE to the apical plasma membrane. At the apical plasma membrane the extracellular, ligand-binding domain of the pIgR is cleaved off and released into the apical medium. This cleaved fragment is called secretory component (SC) (Casanova et al., 1990, 1991; Hunziker et al., 1990; Okamoto et al., 1992; Hirt et al., 1993; Apodaca et al., 1994; Mostov, 1994; Song et al., 1994a).
Each of these steps is regulated. Step 1 depends on two signals centered around Tyr668 and Tyr734 in the cytoplasmic domain of the pIgR (Okamoto et al., 1992). These signals resemble many other signals for rapid internalization. However, rapid internalization of the pIgR also requires Ser726, a major site of phosphorylation of the pIgR (Okamoto et al., 1994). Step 2 is promoted by phosphorylation of Ser664, the other major site of phosphorylation of the pIgR (Casanova et al., 1990). Ordinarily, targeting of the pIgR from the trans-Golgi network (TGN) to the basolateral surface, as well as recycling of pIgR from the endocytotic pathway back to the basolateral surface, are promoted by a basolateral signal localized to residues 653–670, which comprise the most membrane-proximal region of pIgR’s C-terminal cytoplasmic domain (Casanova et al., 1991; Aroeti et al., 1993). Phosphorylation of Ser664 in the middle of this signal inactivates the signal, thereby reducing basolateral recycling and promoting transcytosis to the apical surface. Step 3 is the most highly regulated step in transcytosis. In the absence of external stimuli, this step is rate limiting, so that a large fraction of pIgR and dIgA internalized from the basolateral surface can accumulate in the ARE (Apodaca et al., 1994). Binding of the ligand, dIgA, to the pIgR stimulates step 3 of pIgR transcytosis (Song et al., 1994a, 1994b). The effect is especially pronounced (and was first discovered) in a pIgR where Ser664 has been mutated to a nonphosphorylatable Ala (pIgR-664A). Due to the absence of phosphorylation of Ser664, this mutant exhibits a lower level of constitutive transcytosis, i.e., transcytosis in the absence of bound dIgA is lower than that of the wild-type receptor (Hirt et al., 1993). Stimulation of pIgR transcytosis by dIgA binding has recently been shown to be a principal regulator of transcytosis in liver of the intact rat, suggesting that this phenomenon is significant in vivo (Giffroy et al., 1998). Both the production and transcytosis of pIgR are coordinated and regulated to accommodate variations in the amount of dIgA that must be transcytosed to achieve an efficient immune response.
The ability of the pIgR to increase its transcytosis in response to ligand binding suggests that the pIgR is capable of transducing a signal to the intracellular sorting machinery. We recently reported that dIgA binding to the pIgR leads to activation of protein kinase C (PKC), release of inositol tri-phosphate (IP3), and elevation of intracellular free calcium. These intracellular events stimulate step 3 of transcytosis (Cardone et al., 1994, 1996). A likely explanation for these results is that binding of dIgA to the pIgR leads to activation of a phosphatidyl inositol-specific phospholipase C (PLC). There are two well known mechanisms by which a plasma membrane receptor can activate PLC: PLC-β is activated via a heterotrimeric G protein, while PLC-γ is activated by tyrosine phosphorylation (Majerus, 1992). The cytoplasmic domain of the pIgR contains two short segments of amino acids that are homologous to regions of other proteins (e.g., the insulin-like growth factor-2 receptor) that have been shown to interact with heterotrimeric G proteins. Therefore, Hirt et al. (1993) and we (Bomsel and Mostov, 1992; Bomsel and Mostov, 1993) had proposed that the pIgR would activate PLC-β via an interaction with a G protein. However, so far we have been unable to find any evidence for the involvement of a heterotrimeric G protein and activation of PLC-β in ligand- induced stimulation of pIgR transcytosis.
Here we report the surprising result that dIgA binding to the pIgR leads to rapid activation of PTK and tyrosine phosphorylation of PLC-γ1. Blocking this PTK activity by specific PTK inhibitors or by deletion of a short domain (726–736) in the pIgR cytoplasmic tail also selectively prevents IgA-stimulated transcytosis of pIgR, but not its constitutive transcytosis. We additionally showed that IgA-stimulated transcytosis of pIgR utilizes activation of phospholipase C-γ1.
MATERIALS AND METHODS
Cells
The MDCK strain II cell line and its transfectants were maintained as previously described (Breitfeld et al., 1989). All the mutants, except pIgR-747t and pIgR-737t, have been reported elsewhere (Breitfeld et al., 1990; Casanova et al., 1991; Okamoto et al., 1992, 1994). The pIgR-747t and pIgR-737t mutants have been created by PCR reaction introducing a stop codon after the codon encoding for the Ala746 or Ala736. Cells were grown on 0.4-μm pore Transwell filters (Corning-Costar, Cambridge, MA) and the medium changed every day. Cells were used on days 3 or 4 after plating. All experiments have been reproduced with at least two different clones of each mutant.
Reagents
Wortmannin, trypsin, leupeptin, and soybean trypsin inhibitor were from Sigma Chemical (St. Louis, MO). N-Hydroxy-succinimide-long chain-biotin was obtained from Pierce Chemical (Rockford, IL). NP40, ionomycin, and phorbol 12-myristate 13-acetate (PMA) were from Calbiochem (San Diego, CA). The anti-phosphotyrosine antibody 4G10 and the mixed monoclonal antibodies against PLC-γ1 were from Upstate Biotechnology (Lake Placid, NY). The anti-mouse IgG horseradish peroxidase secondary antibody was purchased from Bio-Rad (Richmond, CA). The avidin-HRP and the ECL system were obtained from Amersham (Arlington Heights, IL). The dIgA was kindly provided by Professor J.-P. Vaerman (Catholic University of Louvain, Brussels, Belgium).
Protein Tyrosine Kinase (PTK) Inhibitors
Genistein and daidzein were purchased from Calbiochem and herbimycin A was purchased from BIOMOL Research Labs (Plymouth Meeting, PA). PP1 was a generous gift form Dr. Kevan Shokat. All the drugs were dissolved and kept as stock solution in DMSO. Cells were pretreated with genistein (200 μM) or daidzein (200 μM) 45 min before the experiment, with PP1 (10 μM) 15 min before the experiment, and for 18 h with herbimycin A (5 μg/ml). The drugs were present throughout the different assays and the control cells were treated with DMSO. At the concentration used none of the drugs had any effect on polarity as measured by the integrity of the tight junctions by transepithelial resistance or the restricted basolateral localization of E-cadherin, as confirmed by cell surface biotinylation (our unpublished data).
IgA Stimulation, Immunoprecipitation, and Anti-phosphotyrosine Western Blot
MDCK cells were grown on 75-mm filters for 3–4 d. The filters were washed three times in MEM BSA (MEM, 6 mg/ml BSA, 0.35 g/l NaHCO3, 20 mM HEPES, pH 7.4, and antibiotics) at 37°C. MEM BSA (5 ml) was added into the apical chamber and the filter was placed onto a 300 μl drop of MEM BSA with or without 0.3 mg/ml of dIgA for different periods of time. At the indicated time point the filter was immediately plunged into 500 ml of ice-cold PBS. The filter was rapidly placed onto an ice-cold metal plate covered with parafilm and 1 ml of fresh lysis buffer (1% NP40, 125 mM NaCl, 20 mM HEPES, pH 7.4, 10 mM NaF, 2 mM NaVanadate, and a cocktail of proteases inhibitors) was added into the apical chamber. All the following steps were done at 4°C. The filters were gently shaken for 15 min and the cells were scraped with a plastic rubber policeman. The lysates were transferred into an Eppendorf tube, vigorously vortexed for 30 s, and placed on a rotator for 15 min. The lysates were spun at high speed for 20 min in an Eppendorf microfuge, and the supernatants were precleared twice for 30 min and immunoprecipitated for 4–5 h. The protein concentration in each sample was quantitated using a Bradford assay (Pierce) and standardized before immunoprecipitation. The immunoprecipitates were resolved by SDS-PAGE, transferred onto a polyvinyl difluoride (PVDF) membrane (Millipore) in 3-(cyclohexylamino)propane sulfonic acid buffer (2.2 g/l, pH 11). The membrane was blocked with PBS with 5% BSA, probed with the anti-phosphotyrosine antibody 4G10, washed extensively, and revealed by an anti-mouse HRP antibody and ECL.
Measurement of IP3 Production
The assay to measure the IP3 production in response to dIgA stimulation was performed as previously described (Cardone et al., 1996). Briefly, MDCK cells grown on filters were transferred into MEM BSA 24 h before the assay and then treated with 10 mM LiCl in MEM BSA for 10 min. The basolateral surface of the cells was exposed to dIgA at 0.3 mg/ml in MEM BSA and 10 mM LiCl for 5 min. After trichloroacetic acid extraction the IP3 was determined with a competitive binding assay kit (Dupont-NEN, Boston, MA). When indicated, cells were pretreated for 45 min with 200 μM genistein. Measurements were adjusted to protein concentration in each sample.
Ligand Transcytosis and Endocytosis
The dIgA was iodinated by the ICl method and stored at a concentration of 75 μg/ml, 4 × 105 to 1 × 106 cpm/μl. Cells cultured on 12-mm Transwell filters for 3–4 d were washed three times in MEM BSA. The filter units were placed on a 10-μl drop of MEM BSA containing 125I-dIgA and the ligand was internalized for 10 min at 37°C. The filters were rapidly washed four to five times with MEM BSA and transferred into a 12-well culture plate, and fresh medium was added to both apical (300 μl) and basolateral chambers (500 μl). The medium was collected and the filters transferred into new wells at 7.5, 15, 30, 60, and 120 min. At the end of the chase the filters were cut out from the holders, and all the fractions (cells, apical and basolateral media) were counted in a Packard γ-counter (Packard Instrument, Downers Grove, IL). In some experiments the internalization of the ligand was performed for 30 min at 17°C to load the basolateral endosomes as previously shown. The cells were then trypsinized (25 μg/ml) for 90 min at 4°C to remove all the 125I-IgA bound at the basolateral membrane. Cells were then washed three times with cold MEM BSA and the ligand transcytosis was assayed as described above.
For endocytosis assay, cells grown 3–4 d on 12-mm filters were incubated at 4°C for 1 h on a 30-μl drop of MEM BSA containing radioiodinated dIgA. The unbound dIgA was washed away, and the cells were incubated at 37°C for 5 min. After cooling down the cells at 4°C, the basolateral surface of the cells was trypsinized to remove noninternalized dIgA and washed. The trypsin washes and the intracellular counts per min were counted in a Packard γ-counter. The amount of internalized dIgA is plotted as percentage of total initial binding of the iodinated dIgA.
Transcytosis Assay after Metabolic Labeling
Cells grown 3–4 d on 12-mm filters were labeled on 10 μl of cysteine-free MEM containing [35S]cysteine at 1 mCi/ml for 15 min. Filters were rinsed and chased in MEM BSA at 37°C for various periods of time. In some cases dIgA (0.3 mg/ml) was included in the basolateral chase medium. The SC fragment was immunoprecipitated from the apical and the basolateral medium as well as from the cells at the end of the chase. Immunoprecipitates were analyzed on SDS-PAGE, and radioactivity was determined with a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). Cumulative radioactivity in apical SC is plotted as percentage of total initial radioactivity in pIgR.
Transcytosis Assay after Cell Surface Biotinylation
Cells grown 3–4 d on 12-mm filters were washed three times in HBSS containing 25 mM HEPES pH 7.4 (HBSS), and the basolateral cell surface was biotinylated for 30 min at 17°C with 500 μl of a solution containing 0.2 mg/ml sulfo-NHS-biotin dissolved in HBSS. During the biotinylation 300 μl of MEM BSA were present in the apical chamber. Cells were then washed three times with MEM BSA at 17°C to quench the excess biotin. MEM BSA (200 μl) was added to the apical chambers, and the filter units were placed onto a drop of MEM BSA containing or lacking 0.3 mg/ml of dIgA and incubated at 37°C for various periods of time. SC fragment was immunoprecipitated as described above, but the SDS-polyacrylamide gel was transferred on a PVDF membrane and the SC fragment was detected in a Western blot using biotin-HRP. The amount of SC fragment was quantitated using a Molecular Dynamics scanner. Cumulative signal in apical SC is plotted as percentage of total initial biotinylated pIgR.
Third-Step Assay
This assay quantitatively measured the transport of a preloaded biotinylated pIgR from the ARE to the apical plasma membrane and has been described in detail elsewhere (Song et al., 1994b). Cells grown 3–4 d on 12-mm filters were used. After three quick washes with HBSS containing 25 mM HEPES pH 7.4 (HBSS), the basolateral cell surface was biotinylated for 30 min at 17°C with 500 μl of a solution containing 0.2 mg/ml sulfo-NHS-biotin dissolved in HBSS. During the biotinylation, 300 μl of MEM BSA were present in the apical chamber. Cells were then washed three times with MEM BSA at 17°C to quench the excess biotin. MEM BSA (200 μl) was added to the apical chambers, and the filter units were placed onto a drop of MEM BSA containing or lacking 0.3 mg/ml of dIgA and incubated at 17°C for 10 min. Cells were then chased for 15 min at 37°C with trypsin (15 μg/ml) in the apical medium and with or without dIgA at the basolateral surface. The chase was stopped by moving the cells to cold MEM BSA and by three washes in cold MEM BSA containing 15% horse serum and further incubated for 1 h at 4°C in the presence of 33 μM nocodazole to depolymerize the microtubules. The cells went on to a second chase for 20 min at 37°C with trypsin (25 μg/ml) in the apical medium and soybean trypsin inhibitor (0.125 mg/ml) in the basolateral medium. At the end of the chase the cells were washed twice in cold MEM BSA containing 15% horse serum and incubated 5 min in MEM BSA with soybean trypsin inhibitor (0.125 mg/ml) to quench the trypsin. Cells were washed three more times in MEM BSA-15% horse serum and once in PBS before lysis. The lysates were precleared and the pIgR immunoprecipitated with a sheep anti-rabbit SC antiserum. The immunoprecipitates were analyzed on SDS-PAGE and transferred to a Millipore PVDF membrane in CAPS buffer (2.2 g/l, pH 11). The biotinylated pIgR was revealed by probing the membrane with streptavidin-HRP and ECL and quantitated with a Molecular Dynamics densitometer. A set of filters was used as a standard and lysed after the nocodazole treatment. The amount of biotinylated pIgR in these samples was considered as 100%. In the samples subjected to the second chase at 37°C, the amount of remaining pIgR in the cells was estimated as a percentage of the standard set.
Statistical Analysis
Results represent the mean ± SD where indicated. Statistical significance was calculated by Student’s t test.
RESULTS
dIgA Binding to the pIgR Induces Tyrosine Phosphorylation of Several Proteins Including PLC-γ1
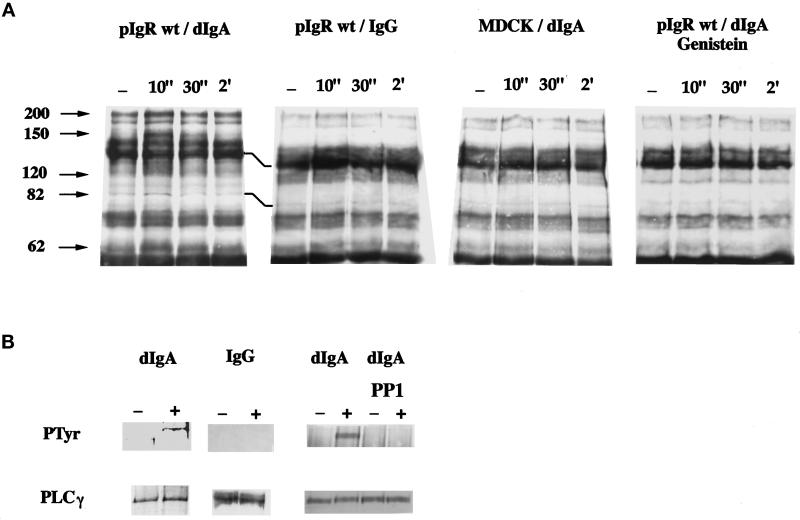
To investigate the involvement of a PTK activity, we first examined the tyrosine phosphorylation of total cell proteins after dIgA treatment of the pIgR-expressing MDCK cells by using an anti-phosphotyrosine antibody Western blot. As early as 10 s after dIgA exposure, we observed a clear increase in the level of tyrosine phosphorylation of several proteins of ∼200, 150, 120, 82, and 62 kDa (Figure 1A). MDCK cells that do not express the receptor were insensitive to dIgA. Similarly, MDCK cells expressing the receptor were insensitive to IgG. These results indicated that the signal was both pIgR and dIgA specific.
Figure 1.
dIgA binding to the pIgR induces tyrosine phosphorylation of several proteins, including PLC-γ1. Cells were exposed to dIgA or IgG (0.3 mg/ml) at the basolateral surface for the indicated period of time. In panel A the cells were lysed and the lysates immunoprecipitated with a specific anti-phosphotyrosine monoclonal antibody (4G10). The arrowheads indicate bands that exhibit an increase in tyrosine phosphorylation. Where indicated, the cells were pretreated for 1 h with 200 μM genistein. In panel B, MDCK cells expressing wild-type pIgR after a 30-s exposure to dIgA or IgG (0.3 mg/ml) in the absence or the presence of the PTK inhibitor Pp 1 were lysed, and the lysates were immunoprecipitated with a mixture of monoclonal anti-PLC-γ1 antibodies. The immunoprecipitates were resolved on SDS-PAGE, the gel transferred to a PVDF membrane, and the membrane first probed with the anti-phosphotyrosine antibody (top gels). After stripping, the membrane was reprobed with the same anti-PLC-γ1 monoclonal antibodies (bottom gels).
To confirm that we were looking at a PTK activation, we analyzed the effect of the PTK inhibitor genistein. Genistein has been shown to inhibit PTK by acting as a competitive inhibitor of ATP (Akiyama et al., 1987). Pretreatment of the cells for 1 h with 200 μM genistein completely blocked the increase in protein tyrosine phosphorylation induced by dIgA (Figure 1A).
As the tyrosine phosphorylation of the PLC-γ isoform is an indication of its activation (Rhee, 1991; Weiss et al., 1991), we next specifically examined PLC-γ1 tyrosine phosphorylation by using an anti-phosphotyrosine Western blot. Upon dIgA binding for 15 s, there is a significant increase of the PLC-γ1 tyrosine phosphorylation (Figure 1B). This signal decreased at 30 s and was gone by 2 min (our unpublished results). Again, as a control IgG did not affect tyrosine phosphorylation of the PLC-γ1. Also, PP1, a inhibitor that is specific for the src family of PTKs, totally blocked dIgA-induced tyrosine phosphorylation of PLC-γ1 (Figure 1B). After stripping of the blot, we reprobed the membrane with the anti-PLC-γ1 antibody to verify that comparable amounts of this protein had been immunoprecipitated in the activated and nonactivated samples. Note that PLC-γ1 is not one of the proteins detected in Figure 1A; the PLC-γ1 signal is too weak to detect if this enzyme has not been first specifically immunoprecipitated.
The dIgA-stimulated Production of IP3 Is Blocked by the PTK Inhibitor Genistein
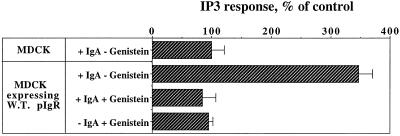
We have shown that dIgA binding causes an increase in the phosphorylation on tyrosine of several proteins, including PLC-γ1, which can be inhibited by pretreating the cells with the PTK inhibitor genistein. This PLC-γ1 activation is in agreement with our previous observation that dIgA induced the production of IP3 (Cardone et al., 1996). To confirm that we were looking at the same signaling pathway, we analyzed the effect of genistein on IP3 production. As expected, pretreatment of the cells with the same concentration of genistein (200 μM) that blocks protein tyrosine phosphorylation completely inhibited the strong dIgA-stimulated production of IP3 (Figure 2).
Figure 2.
The IgA-stimulated production of IP3 is inhibited by genistein. The intracellular concentration of IP3 was measured as in MATERIALS AND METHODS in MDCK cells and MDCK expressing wild-type pIgR in response to 0.3 mg/ml of basolaterally added dIgA for 5 min. Where indicated, cells were pretreated for 45 min with genistein (200 μM) before dIgA exposure. Data are mean ± SD of quadruplicate filters and are representative of four separate experiments.
Mimicking PLC-γ1 Activation Stimulates dIgA Transcytosis
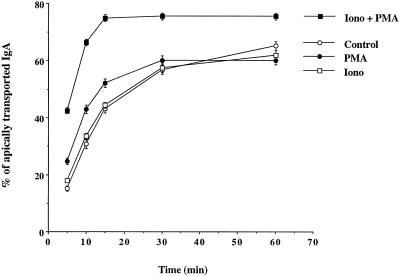
By hydrolyzing phosphatidylinositol, PLC-γ produces IP3 and diacylglycerol. IP3 releases calcium from intracellular stores, which in turn induces a massive calcium entry from the extracellular medium, thereby increasing the intracellular calcium concentration. On the other hand, the diacylglycerol stimulates the calcium- and phospholipid-dependent PKC. The synergistic role of PKC and calcium has been clearly demonstrated to be an important mechanism of signaling in many systems. It is possible to mimic both events and stimulate downstream effects of PLC-γ by using ionomycin, which induces calcium entry from the extracellular medium, in conjunction with PMA, which acts as an analog of diacylglycerol to stimulate PKC (Kaibuchi et al., 1983; Katakami et al., 1983; Yamanishi et al., 1983). To mimic a specific effect of PLC-γ that involves both down-stream effectors, PKC and calcium, it is important to use concentrations of both drugs that when used separately do not have any effect but when combined have a synergistic effect. We used PMA at 20 nM and showed that it has very little effect on dIgA transcytosis. Ionomycin used at 500 nM does not have any effect on dIgA transcytosis. However, when both drugs are used together, there is a dramatic effect on dIgA transcytosis (Figure 3). After only 5 min, dIgA transcytosis is increased by 200–300%, as compared with control or with cells treated singly with either ionomycin or PMA. The plateau of transcytosis is reached after only 15 min as compared with 60 min for the other samples.
Figure 3.
dIgA transcytosis is stimulated by mimicking PLC-γ activation. Cells expressing wild-type pIgR were allowed to internalize 125I-labeled dIgA from the basolateral surface for 10 min at 37°C. The cells were then rapidly washed and the ligand transcytosis was assayed as described in MATERIALS AND METHODS. The chase was performed in the absence (open circle) or the presence of 500 nM ionomycin (open square), 20 nM PMA (closed circle), or ionomycin + PMA (closed square).
dIgA-stimulated Transcytosis of pIgR Is Blocked by PTK Inhibitors
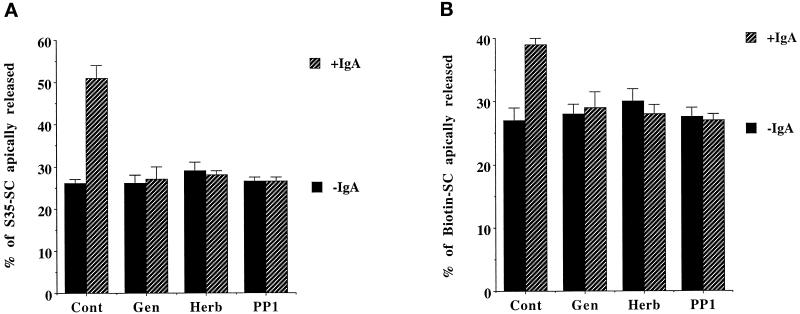
Next we analyzed the effect of three PTK inhibitors, genistein, herbimycin A, and PP1, on both the constitutive and ligand-stimulated transcytosis of the pIgR. Herbimycin A and PP1 are more specific for the p60src PTK family members; herbimycin A works by inducing their degradation as well as reducing their specific activity (Uehara et al., 1989a, 1989b; Hanke et al., 1996). We used two independent methods to detect transcytosis of the receptor in the presence or absence of ligand and with or without pretreatment of the cells with PTK inhibitors. In the first assay, cells were metabolically labeled, and pIgR transcytosis was measured by quantifying the amount of radiolabeled SC fragment released into the apical media. For constitutive transcytosis, after 60 min 25% of radiolabeled SC fragment was found in the apical media. In the presence of dIgA in the basolateral media during the chase the amount of SC fragment found in the apical media had approximately doubled to 51% (Figure 4A). The dIgA also gave a large stimulation of transcytosis at later time points (our unpublished results). This dIgA-stimulated transport of pIgR was blocked by all three PTK inhibitors, genistein, herbimycin A, and PP1; however, none of the drugs had any effect on the constitutive rate of receptor transcytosis.
Figure 4.
dIgA-stimulated transcytosis of pIgR is blocked by PTK inhibitors. Cells grown 3–4 d on 12-mm filters were metabolically labeled (A) or basolaterally biotinylated (B). After washes, cells were incubated at 37°C for 60 min or 30 min, respectively, in the presence or absence of 0.3 mg/ml dIgA. The indicated drugs (Gen, genistein; Herb, herbimycin A; PP1) were present throughout the assay. The amount of SC released in the apical media was quantitated as a percentage of total labeled pIgR as indicated in MATERIALS AND METHODS. The experiments were performed at least three times, with each experiment containing triplicate points, and the data from all experiments were combined. Values are mean ± SD.
The assay described in the preceding paragraph measures the entire pathway of the pIgR, from its synthesis in the endoplasmic reticulum (ER) through basolateral delivery, transcytosis, and apical cleavage to SC. Previous work has shown that the delivery of newly made pIgR from the ER and Golgi to the basolateral plasma membrane is not affected by the presence of dIgA, so that the stimulation by dIgA must be at a later step in the pathway of the pIgR. We therefore used a second assay to follow specifically the transport of pIgR from the basolateral to the apical surface. A pool of receptor was biotinylated by exposing the basolateral surface of the cells to a membrane impermeant biotinylation reagent at 17°C for 30 min. Under these conditions, it is known that the receptor mostly recycles between the basolateral membrane and the basolateral endosomes but is not significantly transcytosed (Song et al., 1994a, 1994b). After this labeling period, in the absence or presence of dIgA, the cells were chased for 30 min at 37°C. The biotinylated SC fragment released into the apical media was collected and quantitated as described in MATERIALS AND METHODS. Again, in the presence of dIgA, the amount of biotinylated SC released in the apical media was increased from 27% to 39% at the 30-min time point, and this stimulation was blocked by both PTK inhibitors (Figure 4B). The stimulation of transcytosis caused by dIgA measured by this assay is less than in the metabolic labeling assay described in the preceding paragraph (Song et al., 1994a, 1994b). As we have previously shown, there are two reasons why the biotinylation assay gives a smaller stimulation. First, the dIgA ligand is fragile, and its ability to bind the pIgR is destroyed by the high concentration of biotinylation reagent. Therefore, we find it necessary to first label the pIgR with biotin and then only subsequently to expose the cells to dIgA. With such a sequential protocol, it is likely that many biotinylated pIgR molecules will not have the opportunity to actually bind dIgA. Second, biotinylation also damages the pIgR, which in consequence may attenuate binding for dIgA, and so the pIgR molecules that are biotinylated will tend not to be the ones that bind dIgA. The labeling conditions chosen (17°C, 30 min, 0.2 mg/ml biotinylation reagent) were a compromise chosen to give a signal that could be reliably quantitated but that would otherwise minimize damage. The time of chase after metabolic labeling was 30 min longer compared with the biotinylation experiment. This delay corresponds to the time necessary for the receptor to be transported through the Golgi apparatus and reach the basolateral surface before internalization and transcytosis. This allows us to compare the two different experiments in a comparable time frame.
Importantly, in both assays the constitutive transport of the receptor was unaffected by the PTK inhibitors. This suggests that transcytosis of the receptor has two components: a constitutive portion that is independent of PTK and a dIgA-stimulated PTK-dependent portion.
Altogether, these results suggest that dIgA stimulation of pIgR transcytosis is regulated by dIgA-stimulated PTK and may involve PLC-γ1.
pIgR Endocytosis Is Unaffected by PTK Inhibitors
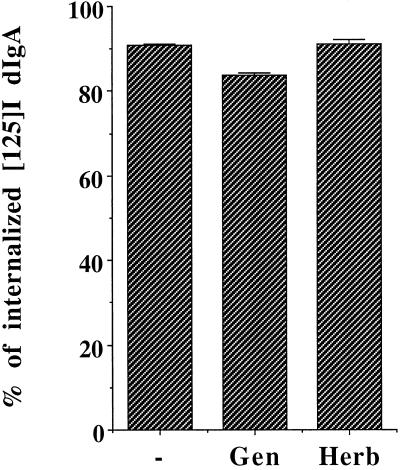
We have previously reported that of the three steps of transcytosis, only the third step is stimulated by dIgA. The first step of transcytosis, internalization, is not increased in the presence of the ligand. One possible complication in our assays was that the PTK inhibitors may have affected internalization and consequently decreased transcytosis. However, neither of the PTK inhibitors, genistein nor herbimycin A, significantly inhibited the internalization of basolaterally prebound radioiodinated dIgA, and so this cannot account for the block of the dIgA-stimulated transcytosis (Figure 5).
Figure 5.
pIgR endocytosis is unaffected by PTK inhibitors. Endocytosis of radioiodinated dIgA was performed as described in MATERIALS AND METHODS. The amount of internalized dIgA is plotted as percentage of total initial binding of the iodinated dIgA. Cells were pretreated for 1 h with 200 μM genistein or 16 h with herbimycin A (5 μg/ml), and the drugs were present throughout the assay. The experiment was performed at least three times, with each experiment containing triplicate points, and the data from all experiments were combined. Values are mean ± SD.
The dIgA-stimulated Third Step of pIgR Transcytosis Is Blocked by PTK Inhibitors
We have recently described an assay that allowed us to show that dIgA binding to the pIgR stimulates primarily the third step of transcytosis, i.e., the transport from the ARE to the apical plasma membrane (Song et al., 1994a). We therefore used this assay to directly address the role of the dIgA-stimulated PTK in dIgA-stimulated transcytosis. This method presents numerous advantages: it measures the last step independently of the first two steps (internalization and translocation from basolateral endosome to the ARE), it is independent of entry to and recycling from the ARE, it discriminates constitutive from dIgA-stimulated transport, and finally it analyzes directly the pIgR and not its ligand. However, as demonstrated in the previous assay, biotinylation of pIgR reduces the effect of the dIgA stimulation; hence, the stimulation of receptor transcytosis is underestimated, and the effects observed are smaller but still highly reproducible and statistically significant. In this assay, pIgR and other proteins at the basolateral cell surface are biotinylated at 17°C. At this temperature the pIgR recycles between the basolateral membrane and the basolateral endosome but does not translocate to the ARE (Apodaca et al., 1994, 1996). After biotinylation, the cells are exposed or not to dIgA at 17°C for 10 min. Then the pIgR (±dIgA) is chased from the basolateral side to the ARE and the apical membrane by a 15-min incubation at 37°C. During this first chase, trypsin is present in the apical media to cleave any pIgR that reaches the apical membrane and therefore prevent apical recycling. Then the cells are rapidly cooled down and treated for 1 h with nocodazole to depolymerize the microtubules and block any further transport from the basolateral endosomes or basolateral surface of the cell to the ARE. The cells are then reheated for 20 min for a second chase at 37°C to allow transport from the ARE to the apical plasma membrane. It has previously been shown that nocodazole does not affect movement from the ARE to the apical surface (Hunziker et al., 1990; Apodaca et al., 1994). Again, trypsin is present in the apical media to prevent apical recycling. At the end of the second chase, cells are cooled down, washed, and lysed. The amount of biotinylated pIgR remaining inside the cells is quantitated by Western blot after immunoprecipitation. Although this assay involves several manipulations, it gives reproducible results, which have been thoroughly documented to primarily measure the third step of transcytosis (Apodaca et al., 1994). This assay was originally used to show that dIgA binding stimulates the third step of transcytosis, while phosphorylation of Ser664 affects both the second and third steps (Song et al., 1994a).
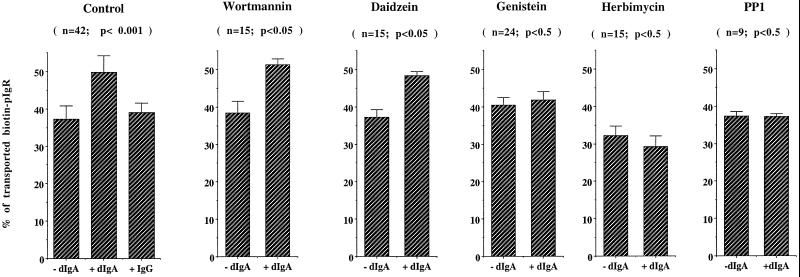
Figure 6 demonstrates that with the wild-type pIgR and no drug, addition of dIgA (but not IgG) decreases the amount of biotinylated pIgR remaining inside the cell; this is plotted as an increase in the percent of biotinylated pIgR that is apically transported. We previously showed that the decrease in the amount of pIgR was due to transcytosis and cleavage at the apical surface and not due to an increase in its degradation in lysosomes (Song et al., 1994b). We performed an additional control to confirm that this experiment measures only the third step of transcytosis independently of the two first steps. We used the PI3 kinase inhibitor wortmannin, which has recently been shown to inhibit dIgA transcytosis (Cardone and Mostov, 1995; Hansen et al., 1995) and specifically affects only the second step of transcytosis, namely the transport between the basolateral endosomes and apical recycling endosomes (Hansen et al., 1995). Wortmannin did not affect the transport of pIgR as measured in our assay, confirming that this assay analyzes a step on the pIgR transcytotic pathway that is after the wortmannin-sensitive second step, i.e., apical exocytosis of a pool of biotinylated pIgR localized in the apical recycling compartment (Figure 6).
Figure 6.
dIgA-stimulated third step of pIgR transcytosis is blocked by PTK inhibitors. The assay for the third step of transcytosis was performed as described in MATERIALS AND METHODS. Where indicated, the cells were pretreated for 45 min with 1 μM wortmannin, 200 μM daidzein, 200 μM genistein or 15 min with 10 μM PP1 or 16 h with herbimycin A (5 μg/ml). The drugs were present throughout the assay. Values are mean ± SD. This experiment was performed at least five times, with each experiment containing triplicate or quadruplicate points, and the data from all experiments were combined. The total number of points (n) and the statistical significance (Student’s t test) are indicated.
We next tested the effect of the PTK inhibitors, genistein, herbimycin A, and PP1, in this assay as described in MATERIALS AND METHODS. All three inhibitors, but not the inactive analog daidzein, completely blocked the dIgA-induced stimulation of transcytosis. Genistein increased constitutive transport only slightly, and herbimycin A slightly decreased it (Figure 6); these differences were not statistically significant. In contrast, PP1 did not have any effect on constitutive transport. These results strongly suggest that the stimulation of the third step of pIgR transcytosis by dIgA is dependent on PTK activation.
dIgA-induced PTK Activation, PLC-γ1 Tyrosine Phosphorylation, and IP3 Production Require the Same Short Sequence within pIgR’s Cytoplasmic Tail
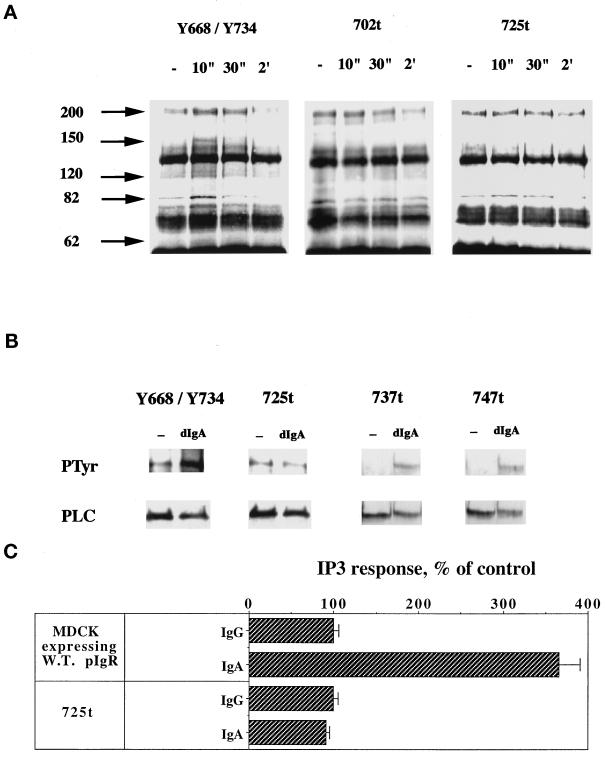
In addition to the biochemical data, we wanted to provide genetic evidence of the involvement of PTK and PLC-γ1 in ligand-stimulated transcytosis. To determine the region within the cytoplasmic tail of the pIgR required for signaling, we took advantage of our large collection of pIgRs with different mutations and truncations in the cytoplasmic domain. These mutants were exposed to dIgA, and we analyzed the content of ligand-induced tyrosine-phosphorylated proteins by anti-phosphotyrosine Western blot. The C-terminal, cytoplasmic domain of the pIgR extends from residue 653 to residue 755. A tail-minus construct (truncated after residue 655) was unable to stimulate protein tyrosine phosphorylation, indicating that the signaling machinery interacts with the cytoplasmic domain (our unpublished results). C-Terminal truncations after amino acids 702 or 725 largely abolished the signal of tyrosine phosphorylation, suggesting that the domain containing the last 30 amino acids was responsible for signaling protein tyrosine phosphorylation upon dIgA binding (Figure 7A). Some residual stimulation of tyrosine phosphorylation of a band of 82 kDa is seen in Figure 7A, for pIgR-725t, but this was highly variable among experiments. In contrast to deletions in the distal C-terminal region, deletion of only the basolateral targeting domain (residues 655–670) did not affect dIgA-induced protein tyrosine phosphorylation, indicating that this membrane-proximal region is not required for signaling (our unpublished results).
Figure 7.
dIgA-induced PTK activation requires the C-terminal extremity of the pIgR cytoplasmic tail. In panel A, cells were exposed to dIgA (0.3 mg/ml) at the basolateral surface for the indicated period of time. The cells were lysed and the lysates immunoprecipitated with a specific anti-phosphotyrosine monoclonal antibody (4G10) as in Figure 1. Y668/Y734 cells express the double tyrosine pIgR mutant. The pIgR-702t and pIgR-725t cells express truncated constructs of pIgR immediately after residue 702 or 725, respectively. The arrowheads indicate bands with increased tyrosine phosphorylation. In panel B, pIgR-Y668/Y734, pIgR-725t, pIgR-737t, and pIgR-747t mutant cells were lysed after a 30-s exposure to dIgA or IgG (0.3 mg/ml), and the lysates were immunoprecipitated with a mixture of monoclonal anti-PLC-γ1 antibodies. The immunoprecipitates were resolved on SDS-PAGE, the gel was transferred to a PVDF membrane, and the membrane was first probed with the anti-phosphotyrosine antibody (top gels). After stripping, the membrane was reprobed with the same anti-PLC-γ1 monoclonal antibodies (bottom gels). In panel C, the intracellular concentration of IP3 was measured as in MATERIALS AND METHODS in MDCK expressing wild-type pIgR or pIgR-725t mutant in response to 0.3 mg/ml of basolaterally added dIgA for 5 min. Data are mean ± SD of quadruplicate filters and are representative of four separate experiments.
To further define the region within the cytoplasmic tail of pIgR involved in receptor signaling, we analyzed the effect of a series of truncations and point mutations on PLC-γ1 tyrosine phosphorylation and activation. The increase in tyrosine phosphorylation of the PLC-γ1 upon dIgA stimulation was completely blocked in the pIgR-725t but not in the truncated pIgR-737t or pIgR-747t cell lines, suggesting that the 726–736 segment is necessary for PLC-γ1 tyrosine phosphorylation (Figure 7B). Note that we deliberately overexposed the Western blot for phosphotyrosine (Figure 7B, WB PTyr) to show the PLC-γ1 band in the non-dIgA-treated cells and that dIgA treatment did not increase tyrosine phosphorylation. In addition, we analyzed IP3 production by the pIgR-725t mutant. As expected, deletion of the last 30 amino acids of pIgR’s cytoplasmic tail completely blocked IP3 production (Figure 7C). The pIgR-737t and pIgR-747t mutants showed normal IP3 production (our unpublished results).
Receptor PTK or receptors associated with nonreceptor PTK are usually tyrosine phosphorylated, thereby allowing SH2 domain-containing proteins to bind to the activated receptors and transduce the stimulatory input to the signaling machinery (Cohen et al., 1995). The pIgR has two tyrosines in its cytoplasmic domain, one in the basolateral targeting signal at position 668 and one in the last 30 amino acids at position 734. The tyrosine residue 668 is conserved among all sequenced species (human, rabbit, rat, mouse, cow) while the tyrosine 734 is a phenylalanine in the bovine pIgR. Dimeric IgA exposure of the double tyrosine mutant (Y668/Y734) (Figure 7A), as well as the single tyrosine mutants (our unpublished results) led to an increase in overall cellular protein tyrosine phosphorylation comparable to that of the wild-type pIgR. In addition, the double tyrosine mutant is still capable of transducing the specific increase in tyrosine phosphorylation of the PLC-γ1 upon dIgA binding (Figure 7B). Note again that the Western blot for phosphotyrosine (Figure 7B, WB PTyr) is deliberately overexposed to show the PLC-γ1 band in the non-dIgA–treated cells, but in this case tyrosine phosphorylation is stimulated by dIgA binding. Taken together, these results indicated that dIgA-stimulated protein tyrosine phosphorylation required a segment of 11 amino acids (726–736) within the pIgR cytoplasmic tail but, interestingly, not the tyrosine 734.
The Cytoplasmic Segment Required for dIgA-stimulated PTK Activation and PLC-γ1 Tyrosine Phosphorylation Controls dIgA Stimulation of Transcytosis of pIgR
The data in the preceding paragraphs indicated that the segment from 726 to 736 is necessary to transduce the signal leading to tyrosine phosphorylation of several proteins, including PLC-γ1, upon dIgA binding. Further we propose that phosphorylation of PLC-γ1 is upstream and might control dIgA-stimulated transcytosis. To test this we examined dIgA-stimulated transcytosis by several of the mutants. We would predict that the pIgR-725t mutant defective in PLC-γ1 phosphorylation should be insensitive to stimulation of transcytosis by dIgA treatment. However, mutations that did not alter PLC-γ1 phosphorylation (pIgR-737t and pIgR-747t truncations) would not affect dIgA-stimulated transcytosis.
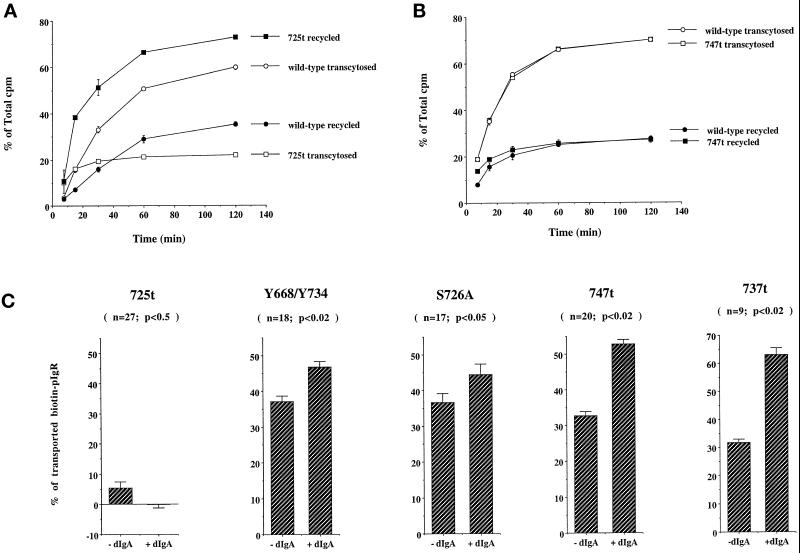
We first attempted to study transport of radioiodinated dIgA, but as pIgR internalization is known to be dramatically decreased in the pIgR-725t mutant (Breitfeld et al., 1990), we analyzed dIgA transport after preinternalization for 30 min at 17°C and subsequent stripping of the basolateral surface. Transcytosis of dIgA was dramatically decreased (Figure 8A). In a further experiment we incubated the cells with radioiodinated dIgA at the basolateral surface at 37°C for 30 min and, after washing, chased for 2 additional hours. Again we did not observe more than 5% transcytosis (Okamoto and Mostov, unpublished observations). The fate of this preinternalized iodinated dIgA is therefore independent of its rate of internalization. The extent of the decrease in transcytosis suggested that not only the stimulated but also the constitutive pathway was altered by the truncation. In contrast, transport of iodinated dIgA by the pIgR-747t–truncated receptor was indistinguishable from that of the wild-type receptor (Figure 8B).
Figure 8.
The cytoplasmic domain required for PTK activation controls pIgR transcytosis. (A) Cells expressing wild-type pIgR (open and closed circles) or pIgR-725t (open and closed squares) were allowed to internalize 125I-labeled dIgA from the basolateral surface for 30 min at 17°C to load the basolateral endosomes. The cells were then trypsinized (25 μg/ml) for 90 min at 4°C to remove all the 125I-labeled IgA bound at the basolateral membrane. Cells were then washed and the ligand transcytosis was assayed as described in MATERIALS AND METHODS. The transcytosed (open symbols) and recycled (closed symbols) ligands were expressed as a percentage of total collected ligand in the apical and basolateral media and cells. (B) Cells expressing either wild-type pIgR or pIgR-747t were allowed to internalize 125I-labeled dIgA from the basolateral surface for 10 min at 37°C. The cells were then washed and chased up to 2 h. Apical and basal media were collected at different time points and the radioactive ligand was counted. The transcytosed (open symbols) and recycled (closed symbols) ligands were expressed as a percentage of total ligand in the apical and basolateral media and cells. (C) The third step assay was performed as described in MATERIALS AND METHODS. The controls are shown in Figure 3. Values are mean ± SD. This experiment was performed at least five times, with each experiment containing triplicate or quadruplicate points, and the data from all experiments were combined. The total number of points (n) and the statistical significance (Student’s t test) are indicated.
We additionally analyzed the behavior of these truncated receptors in our third-step assay, as this assay is also not influenced by the reduced rate of internalization. For the pIgR-725t mutant the constitutive pathway was dramatically reduced and, consistent with our previous results, the dIgA treatment did not rescue this defect (Figure 8C and Figure 6 for the controls), indicating that dIgA-stimulated transcytosis was affected. Indeed, it has been previously reported that although the constitutive pathway is dramatically reduced with the pIgR-664A mutant, addition of dIgA almost completely rescues transcytosis of the pIgR-664A mutant (Hirt et al., 1993; Song et al., 1994b). To further pinpoint the region in the cytoplasmic tail of pIgR required for dIgA-stimulated transcytosis, we analyzed the pIgR-737t and pIgR-747t mutant cell lines. Both truncated mutants were fully responsive to dIgA stimulation in our third-step assay and, in fact, pIgR-737t gave even greater responses than the wild-type receptor. (The reason for the exaggerated response is not clear but raises the possibility that although the C-terminal-most residues of the pIgR tail are not required for stimulation of transcytosis, they may play a secondary modulatory role.)
Two amino acids in the 726–736 segment have been previously implicated in pIgR endocytosis: Tyr734 and Ser726 (Okamoto et al., 1992, 1994). To determine whether the phenotype we observed with the pIgR-725t mutant was due to the loss of one of these amino acids, we analyzed two additional mutants: the double tyrosine mutant pIgR-668A/734A and pIgR-726A. In the third-step assay both behaved the same as the wild-type pIgR (Figure 8C). This result indicated that neither of the two tyrosine residues nor serine 726 is involved in stimulation of transcytosis. This result confirms that even a large reduction in the rate of internalization alone (as is known to occur with the Tyr mutants or pIgR-726A) cannot account for the defect in dIgA-stimulated or constitutive transcytosis, which was observed only with the pIgR-725t mutant.
Taken together, our genetic analysis strongly argues that the signal(s) required for dIgA-stimulated transport of pIgR and PLC-γ1 tyrosine phosphorylation are both contained within the segment 726–736, but do not require residues Ser726 or Tyr734.
DISCUSSION
dIgA Binding Leads to Activation of Tyrosine Kinase Activity
We previously showed that binding of dIgA to the pIgR stimulated translocation of the ε isoform of PKC, IP3 production, and a calcium signal (Cardone et al., 1996). These events are known to be downstream of the PLC-β or PLC-γ activation that follows either stimulation of heterotrimeric G protein or activation of a PTK, respectively (Majerus, 1992). Although it was originally predicted that PLC-β would be involved, we found, surprisingly, that it was the PTK-regulated PLC-γ1. Indeed, anti-phosphotyrosine Western blots revealed a clear increase in the phosphotyrosine content of several proteins after dIgA treatment. This signal is very rapid and transient, making it difficult to observe. From one experiment to another, the extent of increased tyrosine phosphorylation of the substrates was variable, but the same proteins were always detected. The level of phosphorylation of the proteins at 150 kDa and 62 kDa was the most constant, while the one at 82 kDa was more variable, probably due to the rapid kinetics. This suggested that protein tyrosine phosphatases rapidly countered PTK activation by differentially dephosphorylating the substrates. Finally, this signal was completely inhibited by pretreatment of the cells with the PTK inhibitor genistein.
The cytoplasmic domain of the pIgR contains tyrosine residues at positions 668 and 734 that could be potential sites of phosphorylation and therefore docking sites for signaling molecules displaying SH2 or PTB domains (Kavanaugh and Williams, 1994; Cohen et al., 1995). However, several groups have reported that pIgR is not phosphorylated on tyrosine. We also did not detect any signal after immunoprecipitation of the receptor and Western blot with the anti-phosphotyrosine antibody after dIgA treatment or even after exposure of the cells to the protein tyrosine phosphatase inhibitor pervanadate (Luton, unpublished observations). In addition, tyrosine 734 is replaced by a phenylalanine residue in the bovine pIgR, suggesting that it is its aromatic feature that is important, rather than its potential for phosphorylation. Finally, mutation of one or both of these tyrosines to alanine did not alter dIgA-induced tyrosine phosphorylation (including of PLC-γ1) or dIgA-induced stimulation of transcytosis. These data strongly suggest that the tyrosine residues in the cytoplasmic domain of pIgR are not involved in signaling. Moreover, the pIgR cytoplasmic region does not contain any classic signaling domain, such as the SH2, SH3, PH, PTB, or ITAM domains (Reth, 1989; Musacchio et al., 1993; Kavanaugh and Williams, 1994; Bork and Margolis, 1995; Cohen et al., 1995; Cambier, 1996), or sequences known to interact with these domains, which will allow interaction with a PTK or other signaling proteins. Therefore, the pIgR may use a novel mechanism for coupling to the kinase. The pIgR is, in one view, a member of the Fc receptor family among which the FcεRI and the FcγRIII, which interact indirectly with a PTK of the src-family through the γ subunit, which is part of the CD3ζ family (Ravetch, 1994). We have not been able to detect the presence of the Fcγ subunit in MDCK cells. It might be that the antibody we used does not cross-react with the canine form or, more likely, that the pIgR has a specific subunit. Such a subunit may be widely expressed in epithelial cells. The Fcγ and CD3ζ chains interact within multisubunit receptors by their transmembrane domains. A similar mechanism might associate a transducing subunit to pIgR since it has a very highly conserved transmembrane domain for which no function has been assigned.
We have not yet characterized the PTK involved in pIgR transcytosis. Nevertheless, we have been able to coimmunoprecipitate, with a specific anti-pIgR antibody, a tyrosine kinase activity that is increased by dIgA treatment of the cells (Luton and Mostov, unpublished). However, this activity is extremely weak, and we are currently trying to improve our signal by purifying this protein kinase from rat liver. Both PTK inhibitors, herbimycin A and especially PP1, are selective for src family members (Uehara et al., 1989a, 1989b; Hanke et al., 1996), and it is known in many different systems that src PTK activity is directly responsible or upstream of PLCγ activation (Weber et al., 1992; Nakanishi et al., 1993; Marrero et al., 1995; Linnekin et al., 1997; Melford et al., 1997). For instance, PP1 has been used to explore the role of p60src in PLCγ activation in polarized intestinal cells (Khare et al., 1997). We are currently investigating the possibility of a role for p60src or p62yes, both of which are expressed in MDCK cells.
Consequences of dIgA-induced Tyrosine Phosphorylation
These results suggested that the previously observed dIgA-induced IP3 production and calcium signal were the result of the activation of PLC-γ1, which is known to be stimulated as a consequence of its tyrosine phosphorylation. In support of this idea, we have observed that genistein also inhibits the production of IP3 in response to dIgA binding, and that after only 15 s of dIgA exposure, a greatly decreased fraction of the immunoprecipitated PLC-γ1 was tyrosine phosphorylated. In addition, the kinetic analysis of all these signaling events is in agreement with a signaling cascade. Indeed, the PLC-γ1 tyrosine phosphorylation is clearly seen after 30 s of dIgA treatment (Figure 1B) and maintained up to 2 min (data not shown). The IP3 signal can be detected after 60 s of dIgA treatment and lasts for about 10 min with a peak at 5 min (Figure 2 and Cardone et al., 1996). The calcium signal is detected as soon as 90 s after dIgA exposure of the cells and reaches a plateau after another 2 min (Cardone et al., 1996). Hence, we conclude that the pIgR is associated with a signaling pathway involving PTK activation, leading to the tyrosine phosphorylation of the PLC-γ1 and subsequently to IP3 production and a calcium signal.
If the PTK-signaling pathway controls dIgA-stimulated pIgR transcytosis, one can expect that selective PTK inhibitors will block this dIgA-stimulated pIgR transcytosis. We used three PTK inhibitors, genistein, herbimycin A, and PP1, that have different mechanisms of inhibition (Akiyama et al., 1987; Uehara et al., 1989a, 1989b), and we tested them in three different transport assays. One of these transport assays was designed to analyze solely the third step of pIgR transcytosis — transport from the ARE to the apical surface — which is the only step stimulated by dIgA (Song et al., 1994a). Strikingly, all three inhibitors completely blocked the stimulation of pIgR transport by dIgA in all three assays. It is also noteworthy that in the presence of the inhibitors, the transport of pIgR was brought down to the constitutive level, suggesting that the PTK inhibitors were blocking only the stimulated part of pIgR transcytosis and that the constitutive portion of the transport is not controlled by PTK.
Although very helpful and widely used, PTK inhibitors have nonspecific effects, which can make the interpretation of results difficult. For this reason we have performed the following controls: 1) Four different very well-known PTK inhibitors (genistein, herbimycin A, PP1, and tyrphostin 25 (Luton, unpublished data) that have different mechanisms of inhibition always gave very similar results. 2) An inactive analog of genistein, daidzein, had no effect (Figure 6). 3) The specificity of the effect of the PTK inhibitors on the dIgA-stimulated transport of pIgR was checked by analysis of the internalization and polarized sorting of iodinated transferrin endocytosed by its receptor at the basolateral surface. Internalization, recycling to the basolateral surface, and transcytosis were all virtually unaffected by the PTK inhibitors (our unpublished results). Similarly, pIgR internalization was also unaffected by the PTK inhibitors (Figure 5). 4) The range of concentrations that inhibit PTK activation by dIgA is the same as that which blocks pIgR transport stimulated by dIgA. 5) The well-known inhibition of protein synthesis by genistein and herbimycin A had no effect on pIgR transport since inhibition of protein synthesis with cycloheximide had no effect on pIgR transcytosis.
A great advantage of working with the pIgR system is that we have previously created and analyzed a large number of mutations within the cytoplasmic tail of the pIgR. By using our collection of point mutations and truncations, we determined that 11 amino acids (726–736) within the cytoplasmic tail of pIgR were absolutely required for PTK activation, as well as for PLC-γ1 tyrosine phosphorylation and dIgA-stimulated pIgR transcytosis. By analyzing point mutants, we ruled out that the pIgR-725t phenotype was due to the loss of the Ser726 phosphorylation or Tyr734.
Taken together, our data support the hypothesis that dIgA-induced tyrosine phosphorylation is necessary for the dIgA-induced stimulation of transcytosis. Furthermore, the results illustrate a close tie between the activation of PLC-γ1 activation by tyrosine phosphorylation and dIgA-induced transcytosis. 1) dIgA specifically stimulates PLC-γl tyrosine phosphorylation and IP3 production. 2) PTK inhibitors block PLC-γl tyrosine phosphorylation, production of IP3, and dIgA-stimulated transcytosis. 3) Residues 726–736 of the pIgR’s cytoplasmic tail are necessary for both tyrosine phosphorylation of PLC-γl and dIgA-stimulated transcytosis. However, neither Tyr734 nor Ser726 within this segment are required for these events. 4) dIgA transcytosis can be strongly stimulated by mimicking PLC-γl activation, indicating that PLC-γ1 activation is sufficient for stimulation of transcytosis. It should be kept in mind that signaling generally occurs through complex networks, rather than simple linear pathways. This is likely to be true for the pIgR signaling, as well. Indeed, we and others have shown that PI3 kinase is also involved in regulation of pIgR transcytosis (Cardone and Mostov, 1995; Hansen et al., 1995).
Many effectors can be found downstream of PLC-γ activation. Nevertheless it is tempting to speculate that the calcium signal may have an important role in pIgR transcytosis, as it does in many other transport events, such as in neuronal cells (Mayorga et al., 1994; Burgoyne and Morgan, 1995; Littleton and Bellen, 1995). We showed previously that drugs decreasing or increasing the intracellular concentration of calcium affect dIgA transcytosis accordingly (Cardone et al., 1996). It has been shown that calcium release, which poorly diffuses through the cell in contrast to IP3, can be restricted to local sites within a cell (Thomas et al., 1996). For instance, in polarized pancreatic acinar cells the calcium signal can be limited to the apical part of the cell. This has been ascribed to the preferential apical localization of the IP3 receptors (Kasai et al., 1993; Thorn et al., 1993). Interestingly, in MDCK cells there exist distinct membrane localizations and multiple isoforms of IP3 receptors, providing an explanation for the complex spatio-temporal patterns of calcium release from IP3-sensitive calcium pools in epithelial cells (Bush et al., 1994). In MDCK cells, IP3 receptors have been found not only in the ER but also at the basolateral membrane and throughout the cells in the lysosomes and possibly other internal compartments (Bush et al., 1994; Haller et al., 1996). One can imagine that the IP3 production at the basolateral surface following the rapid activation of the PLC-γ1 by dIgA diffuses across the cell to release calcium from apical stores, where calcium will act to stimulate pIgR transcytosis. Finally, MDCK cells possess a “capacitative calcium entry” system, which can sustain prolonged calcium entry into the cells independently of IP3. Initially, there is release of calcium from intracellular stores induced by IP3, and subsequently this small calcium signal triggers the entry of calcium from other intracellular stores or the extracellular medium independently of IP3 production (Delles et al., 1995).
A Possible Cytoplasmic Signal for Constitutive Transcytosis?
The requirement of residues 726–736 for protein tyrosine phosphorylation is congruent with the lack of dIgA stimulation of transcytosis of the pIgR-725t mutant. However, the reduced constitutive level of transcytosis of this mutant in the absence of dIgA was surprising. PTK inhibitors did not affect the constitutive pathway, suggesting that PTK may not play a role in the absence of dIgA. Our previous view of constitutive transcytosis was that phosphorylation of Ser664 in the basolateral targeting signal of the pIgR inactivates this signal and thereby facilitates transcytosis of the pIgR. Transcytosis of the pIgR after inactivation of the basolateral signal is either by default or due to an apical targeting signal, which is normally overridden by the fully active basolateral targeting signal. The apical signal was thought not to reside in the cytoplasmic domain of the pIgR (or other receptors), as receptors lacking the entire cytoplasmic domain are targeted apically, at least from the TGN (Matter and Mellman, 1994). However, our finding that the constitutive transcytosis of pIgR-725t is greatly reduced suggests that the deleted region of the pIgR plays a previously unsuspected role in promoting apical delivery in the transcytotic pathway. Perhaps this region contains an apical targeting signal. We have previously found that the delivery of the pIgR-725t from the TGN to the basolateral surface is faster than the delivery of the wild-type pIgR (Breitfeld et al., 1990). This would be consistent with the removal of an apical signal or basolateral inhibitory sequences from the pIgR-725t mutant.
In conclusion, we have provided a consistent body of evidence from several types of experiments indicating that a PTK-signaling pathway activated upon dIgA binding to the pIgR controls ligand-stimulated transcytosis of pIgR. Compared with many other PTK signal transduction events, this system presents several unusual and interesting features, which are likely to yield novel insights of general importance to cell biology. Although the ligand binds to the pIgR at the basolateral surface, the response of increased transcytosis takes place across the cell, i.e., in delivery from the apical recycling endosome to the apical plasma membrane. This system is therefore ideal for examining compartmentalization and spatial organization of signaling in polarized cells, which is a largely unexplored and potentially quite fruitful area. Moreover, as mentioned above, the pIgR contains none of the well known domains involved in signaling, suggesting that it may utilize a novel mechanism for coupling to a tyrosine kinase.
ACKNOWLEDGMENTS
We thank Dr. Anthony DeFranco, Dr. Arthur Weiss, and Dr. David Morgan for valuable discussions, and Professor Jean-Pierre Vaerman for dIgA. A special thanks to Dr. Kevan Shokat for his valuable advice and for generously providing us with the PTK inhibitor PP1. This work was supported by National Institutes of Health grants AI-25144 and AI-36953 and an American Heart Association Established Investigator Award to K.M. F.L. was supported by the Fondation pour la Recherche Médicale and the Association pour la Recherche contre le Cancer.
Footnotes
Abbreviations: ARE, apical recycling endosome; dIgA, dimeric Ig A; EGFR, epidermal growth factor receptor; IP3, inositol tri-phosphate; pIgR, polymeric Ig receptor; PKC, protein kinase C; PLC, phosphatidyl inositol-specific phospholipase C; PTK, protein tyrosine kinase; SC, secretory component.
REFERENCES
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Alberola-Ila J, Takaki S, Kerner JD, Perlmutter RM. Differential signaling by lymphocytes antigen receptors. Annu Rev Immunol. 1997;15:125–154. doi: 10.1146/annurev.immunol.15.1.125. [DOI] [PubMed] [Google Scholar]
- Apodaca G, Cardone MH, Whiteheart SW, DasGupta BR, Mostov KE. Reconstitution of transcytosis in SLO-permeabilized MDCK cells: existence of an NSF-dependent fusion mechanism with the apical surface of MDCK cells. EMBO J. 1996;15:1471–1481. [PMC free article] [PubMed] [Google Scholar]
- Apodaca G, Katz LA, Mostov KE. Receptor-mediated transcytosis of IgA in MDCK cells via apical recycling endosomes. J Cell Biol. 1994;125:67–86. doi: 10.1083/jcb.125.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroeti B, Kosen PA, Kuntz ID, Cohen FE, Mostov KE. Mutational and secondary structural analysis of the basolateral sorting signal of the polymeric immunoglobulin receptor. J Cell Biol. 1993;123:1149–1160. doi: 10.1083/jcb.123.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel O, Bell GI, Seino S. Inositol 1,4,5-triphosphate receptors, secretory granules and secretion in endocrine and neuroendocrine cells. Trends Neurosci. 1995;18:157–161. doi: 10.1016/0166-2236(95)93894-4. [DOI] [PubMed] [Google Scholar]
- Bomsel M, Mostov K. Role of heterotrimeric G proteins in membrane traffic. Mol Biol Cell. 1992;3:1317–1328. doi: 10.1091/mbc.3.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel M, Mostov KE. Possible role of both the α and βγ subunits of the heterotrimeric G protein, Gs, in transcytosis of the polymeric immunoglobulin receptor. J Biol Chem. 1993;268:25824–25835. [PubMed] [Google Scholar]
- Bork P, Margolis B. A phosphotyrosine interaction domain. Cell. 1995;80:693–394. doi: 10.1016/0092-8674(95)90347-x. [DOI] [PubMed] [Google Scholar]
- Breitfeld P, Casanova JE, Harris JM, Simister NE, Mostov KE. Expression and analysis of the polymeric immunoglobulin receptor. Methods Cell Biol. 1989;32:329–337. doi: 10.1016/s0091-679x(08)61178-4. [DOI] [PubMed] [Google Scholar]
- Breitfeld PP, Casanova JE, McKinnon WC, Mostov KE. Deletions in the cytoplasmic domain of the polymeric immunoglobulin receptor differentially affect endocytotic rate and postendocytotic traffic. J Biol Chem. 1990;265:13750–13757. [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A. Ca2+ and secretory-vesicle dynamics. Trends Neurosci. 1995;18:191–196. doi: 10.1016/0166-2236(95)93900-i. [DOI] [PubMed] [Google Scholar]
- Bush KT, Stuart RO, Li S-H, Moura LA, Sharp AH, Ross CA, Nigam SK. Epithelial inositol 1,4,5-triphosphate receptors. J Biol Chem. 1994;269:23694–23699. [PubMed] [Google Scholar]
- Cambier JC. New nomenclature for the Reth motif. Immunol Today. 1996;16:110. doi: 10.1016/0167-5699(95)80105-7. [DOI] [PubMed] [Google Scholar]
- Cano E, Mahadevan LC. Parallel signal processing among mammalian MAPKs. Trends Biochem Sci. 1995;20:117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- Cardone M, Mostov K. Wortmannin inhibits transcytosis of dimeric IgA by the polymeric immunoglobulin receptor. FEBS Lett. 1995;376:74–76. doi: 10.1016/0014-5793(95)01251-8. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Smith BL, Mennit PA, Mochly-Rosen D, Silver RB, Mostov KE. Signal transduction by the polymeric immunoglobulin receptor suggests a role in regulation of receptor transcytosis. J Cell Biol. 1996;133:997–1005. doi: 10.1083/jcb.133.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone MH, Smith BL, Song W, Mochley-Rosen D, Mostov KE. Phorbol myristate acetate-mediated stimulation of transcytosis and apical recycling in MDCK cells. J Cell Biol. 1994;124:717–727. doi: 10.1083/jcb.124.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova JE, Apodaca G, Mostov KE. An autonomous signal for basolateral sorting in the cytoplasmic domain of the polymeric immunoglobulin receptor. Cell. 1991;66:65–75. doi: 10.1016/0092-8674(91)90139-p. [DOI] [PubMed] [Google Scholar]
- Casanova JE, Breitfeld PP, Ross SA, Mostov KE. Phosphorylation of the polymeric immunoglobulin receptor required for its efficient transcytosis. Science. 1990;248:742–745. doi: 10.1126/science.2110383. [DOI] [PubMed] [Google Scholar]
- Cohen GB, Ren R, Baltimore D. Modular binding domains in signal transduction proteins. Cell. 1995;80:237–248. doi: 10.1016/0092-8674(95)90406-9. [DOI] [PubMed] [Google Scholar]
- Corvera S, Czech MP. Intracellular trafficking of the GLUT4 glucose transporter. Semin Dev Biol. 1996;7:249–257. [Google Scholar]
- Delles C, Haller T, Dietl P. A highly calcium-selective cation current activated by intracellular calcium release in MDCK cells. J Physiol. 1995;486.3:557–569. doi: 10.1113/jphysiol.1995.sp020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantl WJ, Johnson DE, Williams LT. Signalling by receptor tyrosine kinases. Annu Rev Biochem. 1993;62:453–481. doi: 10.1146/annurev.bi.62.070193.002321. [DOI] [PubMed] [Google Scholar]
- Felder S, Miller K, Moehren G, Ullrich A, Schlessinger J, Hopkins CR. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990;61:623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffroy D, Langendries A, Maurice M, Daniel F, Lardeux B, Courtoy PJ, Vaerman J-P. In vivo stimulation of polymeric Ig receptor-transcytosis by circulating polymeric IgA in rat liver. Int Immunol. 1998;10:347–354. doi: 10.1093/intimm/10.3.347. [DOI] [PubMed] [Google Scholar]
- Guan JL, Chen HC. Signal transduction in cell-matrix interactions. Int Rev Cytol. 1996;168:81–121. [PubMed] [Google Scholar]
- Gudermann T, Schoneberg T, Schultz G. Functional and structural complexity of signal transduction via G-protein-coupled receptors. Annu Rev Neurosci. 1997;20:399–427. doi: 10.1146/annurev.neuro.20.1.399. [DOI] [PubMed] [Google Scholar]
- Haller T, Volkl H, Deetjen P, Dietl P. The lysosomal Ca2+ pool in MDCK cells can be released by Ins(1, 4, 5)P3-dependent hormones or thapsigargin but does not activate store-operated Ca2+ entry. Biochem J. 1996;319:909–912. doi: 10.1042/bj3190909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and src family-selective tyrosine kinase inhibitor. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- Hansen SH, Olsson A, Casanova JE. Wortmannin, an inhibitor of phosphoinositide 3-kinase, inhibits transcytosis in polarized epithelial cells. J Biol Chem. 1995;270:28425–28432. doi: 10.1074/jbc.270.47.28425. [DOI] [PubMed] [Google Scholar]
- Hirt RP, Hughes GJ, Frutiger S, Michetti P, Perregaux C, Poulain-Godefroy O, Jeanguenat N, Neutra MR, Kraehenbuhl J-P. Transcytosis of the polymeric Ig receptor requires phosphorylation of Serine 664 in the absence but not the presence of dimeric IgA. Cell. 1993;74:245–255. doi: 10.1016/0092-8674(93)90416-n. [DOI] [PubMed] [Google Scholar]
- Hunziker W, Mâle P, Mellman I. Differential microtubule requirements for transcytosis in MDCK cells. EMBO J. 1990;9:3515–3525. doi: 10.1002/j.1460-2075.1990.tb07560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle JN, Witthuhn BA, Quelle FW, Yamamoto K, Silvennoinen O. Signaling through the hematopoietic cytokine receptors. Annu Rev Immunol. 1995;13:369–398. doi: 10.1146/annurev.iy.13.040195.002101. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K, Takai Y, Sawamura M, Hoshijima M, Fujikura T, Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983;258:6701–6704. [PubMed] [Google Scholar]
- Kasai H, Li YX, Miyashita Y. Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell. 1993;74:669–677. doi: 10.1016/0092-8674(93)90514-q. [DOI] [PubMed] [Google Scholar]
- Katakami Y, Kaibuchi K, Sawamura M, Takai Y, Nishizuka Y. Synergistic action of protein kinase C and calcium for histamine release from rat peritoneal mast cells. Biochem Biophys Res Commun. 1983;121:573–578. doi: 10.1016/0006-291x(84)90220-1. [DOI] [PubMed] [Google Scholar]
- Kavanaugh WM, Williams LT. A novel alternative to SH2 domains for binding tyrosine-phosphorylated proteins. Science. 1994;266:1862–1865. doi: 10.1126/science.7527937. [DOI] [PubMed] [Google Scholar]
- Khare S, et al. 1,25 dihydroxyvitamin D3 stimulates phospholipase C-γ in rat colonocytes: role of c-src in PLC-γ activation. J Clin Invest. 1997;99:1831–1841. doi: 10.1172/JCI119350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurten RC, Cadena DL, Gill GN. Enhanced degradation of EGF receptors by a sorting nexin, SNX1. Science. 1996;272:1008–1010. doi: 10.1126/science.272.5264.1008. [DOI] [PubMed] [Google Scholar]
- Linnekin D, DeBerry CS, Mou S. Lyn associates with the juxtamembrane region of c-kit and is activated by stem cell factor in hematopoietic cell lines and normal progenitor cells. J Biol Chem. 1997;272:27450–27455. doi: 10.1074/jbc.272.43.27450. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Bellen HJ. Synaptotagmin controls and modulates synaptic-vesicle fusion in a Ca(2+)-dependent manner. Trends Neurosci. 1995;18:177–183. doi: 10.1016/0166-2236(95)93898-8. [DOI] [PubMed] [Google Scholar]
- Lund KA, Lazar CS, Chen WS, Walsh BJ, Welsh JB, Herbst JJ, Walton GM, Rosenfeld MG, Gill GN, Wiley HS. Phosphorylation of the epidermal growth factor receptor at threonine 654 inhibits ligand-induced internalization and down-regulation. J Biol Chem. 1990;265:20517–20523. [PubMed] [Google Scholar]
- Majerus PW. Inositol phosphate biochemistry. Annu Rev Biochem. 1992;61:225–250. doi: 10.1146/annurev.bi.61.070192.001301. [DOI] [PubMed] [Google Scholar]
- Marrero MB, Schieffer B, Paxton WG, Schieffer E, Bernstein KE. Electroporation of Pp 60c-src antibodies inhibits the angiotensin II activation of phospholipase C-γ1 in rat aortic smooth muscle cells. J Biol Chem. 1995;270:15734–15738. doi: 10.1074/jbc.270.26.15734. [DOI] [PubMed] [Google Scholar]
- Matter K, Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr Opin Cell Biol. 1994;6:545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Mayorga LS, Berón W, Sarrouf MN, Colombo MI, Creutz C, Stahl PD. Calcium-dependent fusion among endosomes. J Biol Chem. 1994;269:30927–30934. [PubMed] [Google Scholar]
- Melford SK, Turner M, Briddon SJ, Tybulewicz VLJ, Watson SP. Syk and Fyn are required by mouse megakaryocytes for the rise in intracellular calcium induced by a collagen-related peptide. J Biol Chem. 1997;272:27539–27542. doi: 10.1074/jbc.272.44.27539. [DOI] [PubMed] [Google Scholar]
- Mostov KE. Transepithelial transport of immunoglobulins. Annu Rev Immunol. 1994;12:63–84. doi: 10.1146/annurev.iy.12.040194.000431. [DOI] [PubMed] [Google Scholar]
- Musacchio A, Gibson T, Rice P, Thompson J, Saraste M. The PH domain: a common piece in the structural patchwork of signalling proteins. Trends Biochem Sci. 1993;18:343–348. doi: 10.1016/0968-0004(93)90071-t. [DOI] [PubMed] [Google Scholar]
- Nakanishi O, Shibasaki F, Hidaka M, Homma Y, Takenawa T. Phospholipase C-γ1 associates with viral and cellular src kinases. J Biol Chem. 1993;268:10754–10759. [PubMed] [Google Scholar]
- Okamoto CT, Shia S-P, Bird C, Mostov KE, Roth MG. The cytoplasmic domain of the polymeric immunoglobulin receptor contains two internalization signals that are distinct from its basolateral sorting signal. J Biol Chem. 1992;267:9925–9932. [PubMed] [Google Scholar]
- Okamoto CT, Song W, Bomsel M, Mostov KE. Rapid internalization of the polymeric immunoglobulin receptor requires phosphorylated serine 726. J Biol Chem. 1994;269:15676–15682. [PubMed] [Google Scholar]
- Opresko LK, Chang CP, Will BH, Burke PM, Gill GN, Willey HS. Endocytosis and lysosomal targeting of epidermal growth factor receptors are mediated by distinct sequences independent of the tyrosine kinase domain. J Biol Chem. 1995;270:43325–43333. doi: 10.1074/jbc.270.9.4325. [DOI] [PubMed] [Google Scholar]
- Ravetch JV. Fc receptors: rubor redux. Cell. 1994;78:553–560. doi: 10.1016/0092-8674(94)90521-5. [DOI] [PubMed] [Google Scholar]
- Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. [PubMed] [Google Scholar]
- Rhee SG. Inositol phospholipid-specific phospholipase C: interaction of the γ1 isoform with tyrosine kinase. Trends Biochem Sci. 1991;16:297–301. doi: 10.1016/0968-0004(91)90122-c. [DOI] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Song W, Apodaca G, Mostov K. Transcytosis of the polymeric immunoglobulin receptor is regulated in multiple intracellular compartments. J Biol Chem. 1994a;269:29474–29480. [PubMed] [Google Scholar]
- Song W, Bomsel M, Casanova J, Vaerman J-P, Mostov KE. Stimulation of transcytosis of the polymeric immunoglobulin receptor by dimeric IgA. Proc Natl Acad Sci USA. 1994b;91:163–166. doi: 10.1073/pnas.91.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Thomas AP, Bird GSJ, Hajnoczky G, Robb-Gaspers LD, Putney JJW. Spatial and temporal aspects of cellular calcium signaling. FASEB J. 1996;10:1505–1517. [PubMed] [Google Scholar]
- Thorn P, Lawrie AM, Smith PM, Gallacher DV, Petersen OH. Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol triphosphate. Cell. 1993;74:661–668. doi: 10.1016/0092-8674(93)90513-p. [DOI] [PubMed] [Google Scholar]
- Uehara Y, Fukazawa H, Murakami Y, Mizuno S. Irreversible inhibition of v-src tyrosine kinase activity by herbimycin A and its abrogation by sulfhydryl compounds. Biochem Biophys Res Commun. 1989a;163:803–809. doi: 10.1016/0006-291x(89)92293-6. [DOI] [PubMed] [Google Scholar]
- Uehara Y, Murakami Y, Sugimoto Y, Mizuno S. Mechanism of reversion of rous sarcoma virus transformation by herbimycin A: reduction of total phosphotyrosine levels due to reduced activity and increased turnover of p60v-src1. Cancer Res. 1989b;49:780–785. [PubMed] [Google Scholar]
- Weber JR, Bell GM, Han MY, Pawson T, Imboden JB. Association of the tyrosine kinase LCK with phospholipase C-γ1 after stimulation of the T cell antigen receptor. J Exp Med. 1992;176:373–379. doi: 10.1084/jem.176.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Koretzky G, Schatzman RC, Kadlecek T. Functional activation of the T-cell antigen receptor induces tyrosine phosphorylation of phospholipase C-γ1. Proc Natl Acad Sci USA. 1991;88:5484–5488. doi: 10.1073/pnas.88.13.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley HS, Herbst JJ, Walsh BJ, Lauffenburger DA, Rosenfeld MG, Gill GN. The role of tyrosine kinase activity in endocytosis, compartmentation, and down-regulation of the epidermal growth factor receptor. J Biol Chem. 1991;266:11083–11094. [PubMed] [Google Scholar]
- Yamanishi J, Takai Y, Kaibuchi K, Sano K, Castagna M, Nishizuka Y. Synergistic functions of phorbol ester and calcium in serotonin release from human platelets. Biochem Biophys Res Commun. 1983;112:778–786. doi: 10.1016/0006-291x(83)91529-2. [DOI] [PubMed] [Google Scholar]