Abstract
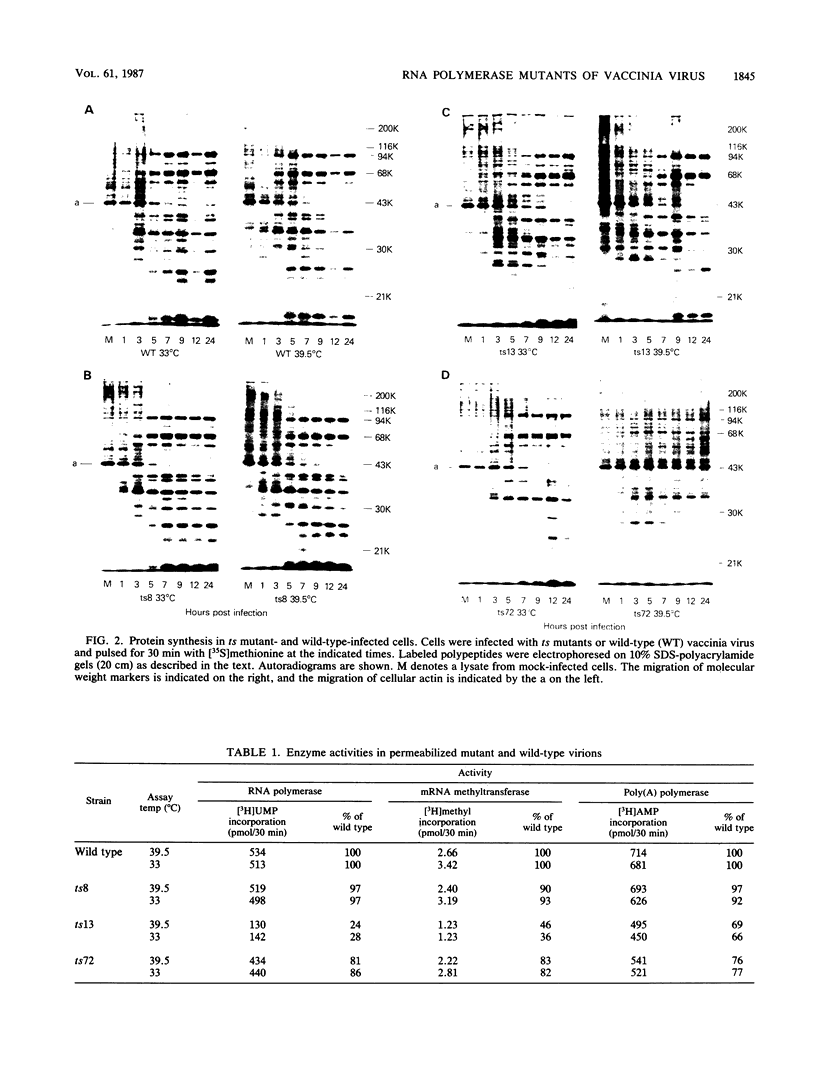
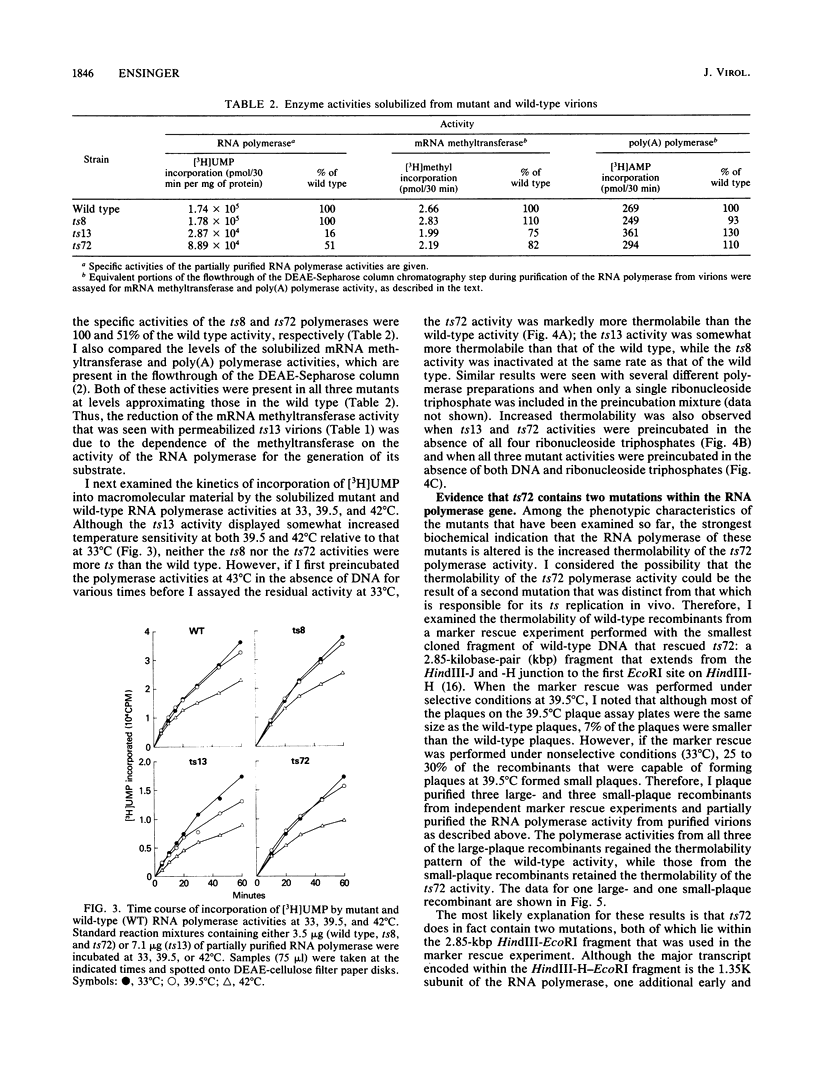
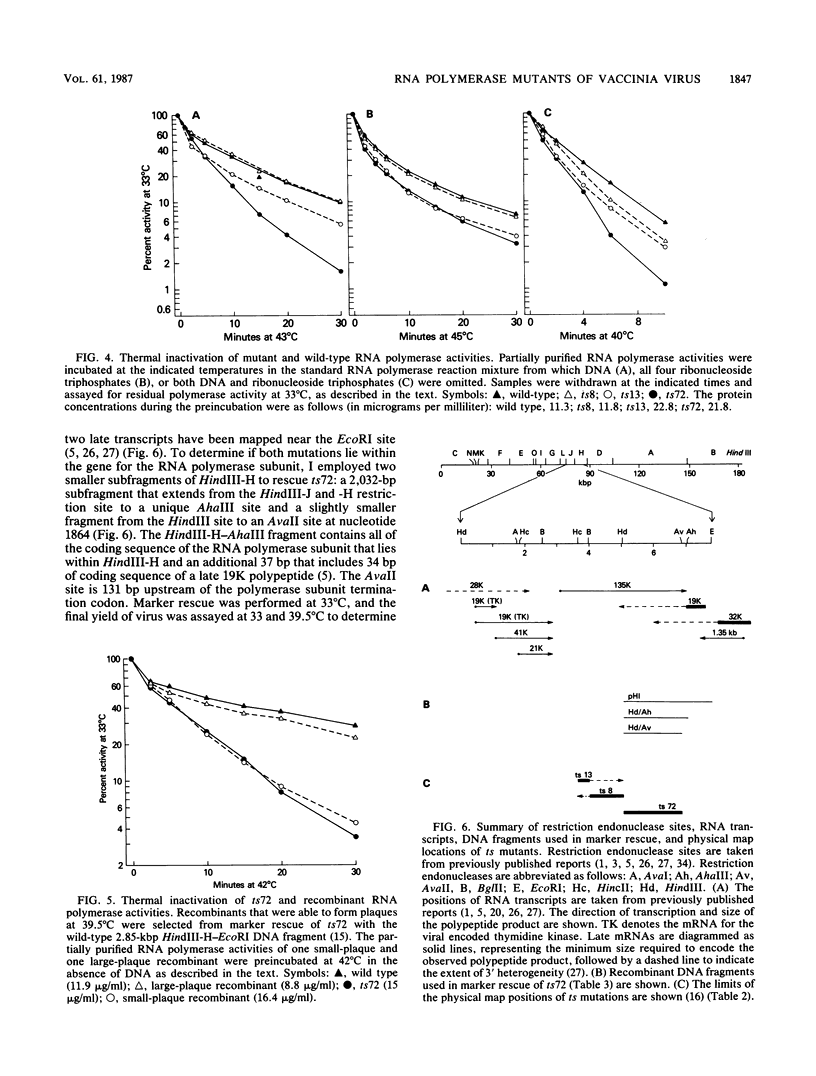
The phenotypic defects of three temperature-sensitive (ts) mutants of vaccinia virus, the ts mutations of which were mapped to the gene for one of the high-molecular-weight subunits of the virion-associated DNA-dependent RNA polymerase, were characterized. Because the virion RNA polymerase is required for the initiation of the viral replication cycle, it has been predicted that this type of mutant is defective in viral DNA replication and the synthesis of early viral proteins at the nonpermissive temperature. However, all three mutants synthesized both DNA and early proteins, and two of the three synthesized late proteins as well. RNA synthesis in vitro by permeabilized mutant virions was not more ts than that by the wild type. Furthermore, only one of three RNA polymerase activities that was partially purified from virions assembled at the permissive temperature displayed altered biochemical properties in vitro that could be correlated with its ts mutation: the ts13 activity had reduced specific activity, increased temperature sensitivity, and increased thermolability under a variety of preincubation conditions. Although the partially purified polymerase activity of a second mutant, ts72, was also more thermolabile than the wild-type activity, the thermolability was shown to be the result of a second mutation within the RNA polymerase gene. These results suggest that the defects in these mutants affect the assembly of newly synthesized polymerase subunits into active enzyme or the incorporation of RNA polymerase into maturing virions; once synthesized at the permissive temperature, the mutant polymerases are able to function in the initiation of subsequent rounds of infection at the nonpermissive temperature.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajszár G., Wittek R., Weir J. P., Moss B. Vaccinia virus thymidine kinase and neighboring genes: mRNAs and polypeptides of wild-type virus and putative nonsense mutants. J Virol. 1983 Jan;45(1):62–72. doi: 10.1128/jvi.45.1.62-72.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroudy B. M., Moss B. Purification and characterization of a DNA-dependent RNA polymerase from vaccinia virions. J Biol Chem. 1980 May 10;255(9):4372–4380. [PubMed] [Google Scholar]
- Broyles S. S., Moss B. Homology between RNA polymerases of poxviruses, prokaryotes, and eukaryotes: nucleotide sequence and transcriptional analysis of vaccinia virus genes encoding 147-kDa and 22-kDa subunits. Proc Natl Acad Sci U S A. 1986 May;83(10):3141–3145. doi: 10.1073/pnas.83.10.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAIRNS J. The initiation of vaccinia infection. Virology. 1960 Jul;11:603–623. doi: 10.1016/0042-6822(60)90103-3. [DOI] [PubMed] [Google Scholar]
- Chernos V. I., Belanov E. F., Vasilieva N. N. Temperature-sensitive mutants of vaccinia virus. I. Isolation and preliminary characterization. Acta Virol. 1978 Mar;22(2):81–90. [PubMed] [Google Scholar]
- Condit R. C., Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981 Aug;113(1):224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A., Spizz G. Isolation, characterization, and physical mapping of temperature-sensitive mutants of vaccinia virus. Virology. 1983 Jul 30;128(2):429–443. doi: 10.1016/0042-6822(83)90268-4. [DOI] [PubMed] [Google Scholar]
- Dales S., Milovanovitch V., Pogo B. G., Weintraub S. B., Huima T., Wilton S., McFadden G. Biogenesis of vaccinia: isolation of conditional lethal mutants and electron microscopic characterization of their phenotypically expressed defects. Virology. 1978 Feb;84(2):403–428. doi: 10.1016/0042-6822(78)90258-1. [DOI] [PubMed] [Google Scholar]
- Drillien R., Spehner D., Kirn A. Complementation and genetic linkage between vaccinia virus temperature-sensitive mutants. Virology. 1982 Jun;119(2):372–381. doi: 10.1016/0042-6822(82)90096-4. [DOI] [PubMed] [Google Scholar]
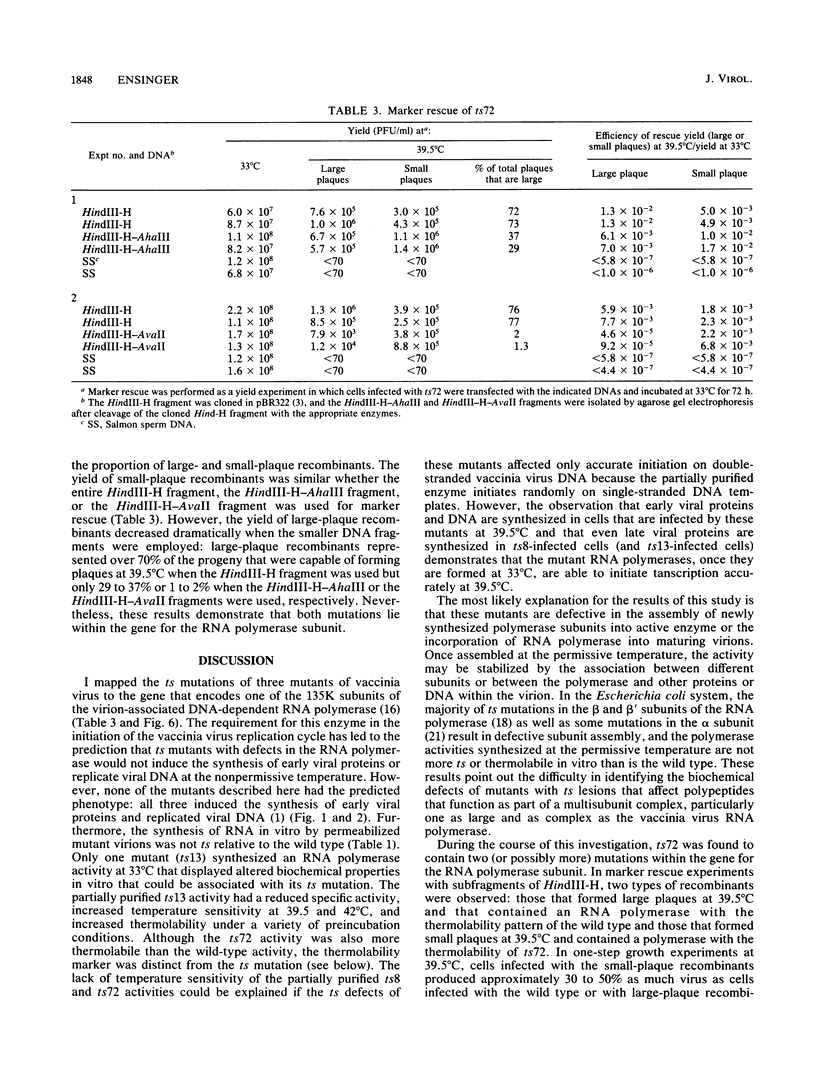
- Ensinger M. J. Isolation and genetic characterization of temperature-sensitive mutants of vaccinia virus WR. J Virol. 1982 Sep;43(3):778–790. doi: 10.1128/jvi.43.3.778-790.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger M. J., Rovinsky M. Marker rescue of temperature-sensitive mutations of vaccinia virus WR: correlation of genetic and physical maps. J Virol. 1983 Nov;48(2):419–428. doi: 10.1128/jvi.48.2.419-428.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger M. J., Weir J. P., Moss B. Fine structure marker rescue of temperature-sensitive mutations of vaccinia virus within a central conserved region of the genome. J Virol. 1985 Dec;56(3):1027–1029. doi: 10.1128/jvi.56.3.1027-1029.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilead Z., Jeng Y. H., Wold W. S., Sugawara K., Rho H. M., Harter M. L., Green M. Immunological identification of two adenovirus 2-induced early proteins possibly involved in cell transformation. Nature. 1976 Nov 18;264(5583):263–266. doi: 10.1038/264263a0. [DOI] [PubMed] [Google Scholar]
- Gross G. C., Fields D. A., Bautz E. K. Temperature-sensitive mutants of Escherichia coli with defects in the assembly of RNA polymerase in vitro. Eur J Biochem. 1977 Dec 1;81(2):333–338. doi: 10.1111/j.1432-1033.1977.tb11956.x. [DOI] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Hruby D. E., Maki R. A., Miller D. B., Ball L. A. Fine structure analysis and nucleotide sequence of the vaccinia virus thymidine kinase gene. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3411–3415. doi: 10.1073/pnas.80.11.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihama A., Shimamoto N., Aiba H., Kawakami K., Nashimoto H., Tsugawa A., Uchida H. Temperature-sensitive mutations in the alpha subunit gene of Escherichia coli RNA polymerase. J Mol Biol. 1980 Feb 25;137(2):137–150. doi: 10.1016/0022-2836(80)90321-6. [DOI] [PubMed] [Google Scholar]
- Isle H. B., Venkatesan S., Moss B. Cell-free translation of early and late mRNAs selected by hybridization to cloned DNA fragments derived from the left 14 million to 72 million daltons of the vaccinia virus genome. Virology. 1981 Jul 15;112(1):306–317. doi: 10.1016/0042-6822(81)90636-x. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- Kates J., Dahl R., Mielke M. Synthesis and intracellular localization of vaccinia virus deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Virol. 1968 Sep;2(9):894–900. doi: 10.1128/jvi.2.9.894-900.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lake J. R., Cooper P. D. Synthesis of virus DNA and polypeptides by temperature-sensitive mutants of rabbitpox virus. J Gen Virol. 1980 Apr;47(2):243–259. doi: 10.1099/0022-1317-47-2-243. [DOI] [PubMed] [Google Scholar]
- Mahr A., Roberts B. E. Arrangement of late RNAs transcribed from a 7.1-kilobase EcoRI vaccinia virus DNA fragment. J Virol. 1984 Feb;49(2):510–520. doi: 10.1128/jvi.49.2.510-520.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahr A., Roberts B. E. Organization of six early transcripts synthesized from a vaccinia virus EcoRI DNA fragment. J Virol. 1984 Feb;49(2):497–509. doi: 10.1128/jvi.49.2.497-509.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. K., Carter J. K., Moyer R. W. Isolation and characterization of monoclonal antibodies directed against two subunits of rabbit poxvirus-associated, DNA-directed RNA polymerase. J Virol. 1985 Sep;55(3):670–680. doi: 10.1128/jvi.55.3.670-680.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Paoletti E. Polyadenylate polymerase from vaccinia virions. Nat New Biol. 1973 Sep 12;245(141):59–63. doi: 10.1038/newbio245059a0. [DOI] [PubMed] [Google Scholar]
- Nagaya A., Pogo B. G., Dales S. Biogenesis of vaccinia: separation of early stages from maturation by means of rifampicin. Virology. 1970 Apr;40(4):1039–1051. doi: 10.1016/0042-6822(70)90150-9. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Joklik W. K. Isolation and properties of the vaccinia virus DNA-dependent RNA polymerase. J Biol Chem. 1977 Oct 10;252(19):6930–6938. [PubMed] [Google Scholar]
- Pitkanen A., McAuslan B., Hedgpeth J., Woodson B. Induction of poxvirus ribonucleic acid polymerases. J Virol. 1968 Dec;2(12):1363–1367. doi: 10.1128/jvi.2.12.1363-1367.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plucienniczak A., Schroeder E., Zettlmeissl G., Streeck R. E. Nucleotide sequence of a cluster of early and late genes in a conserved segment of the vaccinia virus genome. Nucleic Acids Res. 1985 Feb 11;13(3):985–998. doi: 10.1093/nar/13.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Spencer E., Shuman S., Hurwitz J. Purification and properties of vaccinia virus DNA-dependent RNA polymerase. J Biol Chem. 1980 Jun 10;255(11):5388–5395. [PubMed] [Google Scholar]
- Sridhar P., Condit R. C. Selection for temperature-sensitive mutations in specific vaccinia virus genes: isolation and characterization of a virus mutant which encodes a phosphonoacetic acid-resistant, temperature-sensitive DNA polymerase. Virology. 1983 Jul 30;128(2):444–457. doi: 10.1016/0042-6822(83)90269-6. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C. M., Moss B. Methylation of newly synthesized viral messenger RNA by an enzyme in vaccinia virus. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3014–3018. doi: 10.1073/pnas.71.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]