Abstract
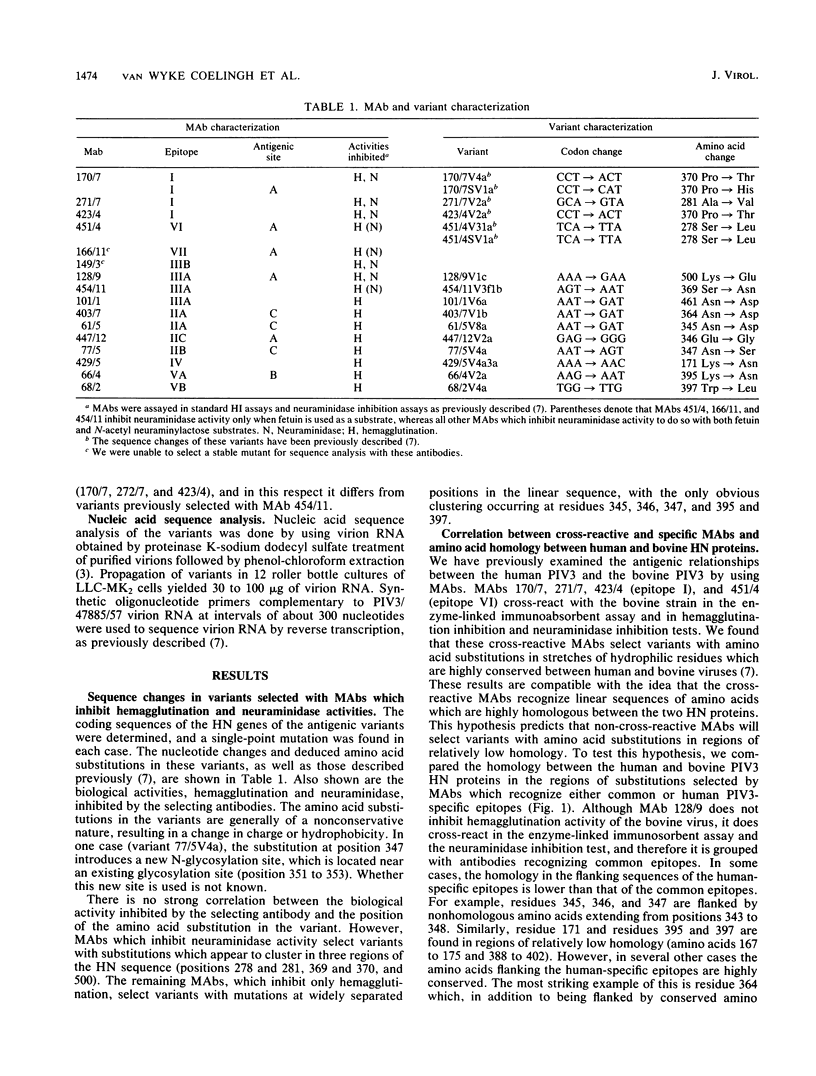
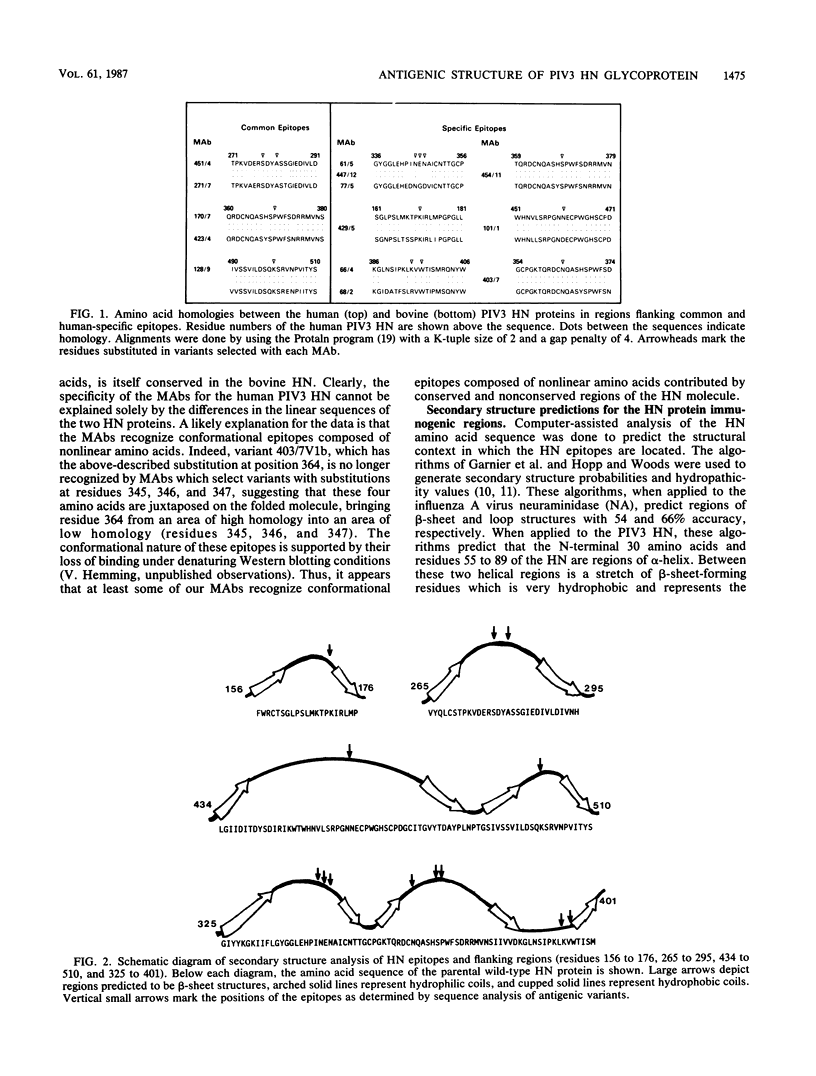
The hemagglutinin-neuraminidase (HN) gene sequence was determined for 16 antigenic variants of human parainfluenza virus type 3 (PIV3). The variants were selected by using monoclonal antibodies (MAbs) to the HN protein which inhibit neuraminidase, hemagglutination, or both activities. Each variant had a single-point mutation in the HN gene, coding for a single amino acid substitution in the HN protein. Operational and topographic maps of the HN protein correlated well with the relative positions of the substitutions. There was little correlation between the cross-reactivity of a MAb with the bovine PIV3 HN and the amount of amino acid homology between the human and bovine PIV3 HN proteins in the regions of the epitopes, suggesting that many of the epitopes are conformational in nature. Computer-assisted analysis of the HN protein predicted a secondary structure composed primarily of hydrophobic beta sheets interconnected by random hydrophilic coil structures. The HN epitopes were located in predicted coil regions. Epitopes recognized by MAbs which inhibit neuraminidase activity of the virus were located in a region which appears to be structurally conserved among several paramyxovirus HN proteins and which may represent the sialic cid-binding site of the HN molecule.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Air G. M., Els M. C., Brown L. E., Laver W. G., Webster R. G. Location of antigenic sites on the three-dimensional structure of the influenza N2 virus neuraminidase. Virology. 1985 Sep;145(2):237–248. doi: 10.1016/0042-6822(85)90157-6. [DOI] [PubMed] [Google Scholar]
- Bean W. J., Jr, Sriram G., Webster R. G. Electrophoretic analysis of iodine-labeled influenza virus RNA segments. Anal Biochem. 1980 Feb;102(1):228–232. doi: 10.1016/0003-2697(80)90343-7. [DOI] [PubMed] [Google Scholar]
- Blumberg B., Giorgi C., Roux L., Raju R., Dowling P., Chollet A., Kolakofsky D. Sequence determination of the Sendai virus HN gene and its comparison to the influenza virus glycoproteins. Cell. 1985 May;41(1):269–278. doi: 10.1016/0092-8674(85)90080-7. [DOI] [PubMed] [Google Scholar]
- CHANOCK R. M., PARROTT R. H., COOK K., ANDREWS B. E., BELL J. A., REICHELDERFER T., KAPIKIAN A. Z., MASTROTA F. M., HUEBNER R. J. Newly recognized myxoviruses from children with respiratory disease. N Engl J Med. 1958 Jan 30;258(5):207–213. doi: 10.1056/NEJM195801302580502. [DOI] [PubMed] [Google Scholar]
- Coelingh K. J., Winter C. C., Murphy B. R., Rice J. M., Kimball P. C., Olmsted R. A., Collins P. L. Conserved epitopes on the hemagglutinin-neuraminidase proteins of human and bovine parainfluenza type 3 viruses: nucleotide sequence analysis of variants selected with monoclonal antibodies. J Virol. 1986 Oct;60(1):90–96. doi: 10.1128/jvi.60.1.90-96.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman P. M., Varghese J. N., Laver W. G. Structure of the catalytic and antigenic sites in influenza virus neuraminidase. Nature. 1983 May 5;303(5912):41–44. doi: 10.1038/303041a0. [DOI] [PubMed] [Google Scholar]
- Elango N., Coligan J. E., Jambou R. C., Venkatesan S. Human parainfluenza type 3 virus hemagglutinin-neuraminidase glycoprotein: nucleotide sequence of mRNA and limited amino acid sequence of the purified protein. J Virol. 1986 Feb;57(2):481–489. doi: 10.1128/jvi.57.2.481-489.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knossow M., Daniels R. S., Douglas A. R., Skehel J. J., Wiley D. C. Three-dimensional structure of an antigenic mutant of the influenza virus haemagglutinin. Nature. 1984 Oct 18;311(5987):678–680. doi: 10.1038/311678a0. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Colman P. M., Webster R. G., Hinshaw V. S., Air G. M. Influenza virus neuraminidase with hemagglutinin activity. Virology. 1984 Sep;137(2):314–323. doi: 10.1016/0042-6822(84)90223-x. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Scheid A., Choppin P. W. Immunological studies of the functions of paramyxovirus glycoproteins. Virology. 1981 Feb;109(1):94–105. doi: 10.1016/0042-6822(81)90474-8. [DOI] [PubMed] [Google Scholar]
- Orvell C., Norrby E. Immunologic properties of purified Sendai virus glycoproteins. J Immunol. 1977 Dec;119(6):1882–1887. [PubMed] [Google Scholar]
- Portner A. The HN glycoprotein of Sendai virus: analysis of site(s) involved in hemagglutinating and neuraminidase activities. Virology. 1981 Dec;115(2):375–384. doi: 10.1016/0042-6822(81)90118-5. [DOI] [PubMed] [Google Scholar]
- Varghese J. N., Laver W. G., Colman P. M. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 A resolution. Nature. 1983 May 5;303(5912):35–40. doi: 10.1038/303035a0. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Brown L. E., Laver W. G. Antigenic and biological characterization of influenza virus neuraminidase (N2) with monoclonal antibodies. Virology. 1984 May;135(1):30–42. doi: 10.1016/0042-6822(84)90114-4. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C., Murphy B. R. Antigenic variation in the hemagglutinin-neuraminidase protein of human parainfluenza type 3 virus. Virology. 1985 Jun;143(2):569–582. doi: 10.1016/0042-6822(85)90395-2. [DOI] [PubMed] [Google Scholar]


