Abstract
Small noncoding RNAs, microRNAs (miRNAs), bind to messenger RNAs through base pairing to suppress gene expression. Despite accumulating evidence that miRNAs play critical roles in various biological processes across diverse organisms, their roles in mammalian skeletal development have not been demonstrated. Here, we show that Dicer, an essential component for biogenesis of miRNAs, is essential for normal skeletal development. Dicer-null growth plates show a progressive reduction in the proliferating pool of chondrocytes, leading to severe skeletal growth defects and premature death of mice. The reduction of proliferating chondrocytes in Dicer-null growth plates is caused by two distinct mechanisms: decreased chondrocyte proliferation and accelerated differentiation into postmitotic hypertrophic chondrocytes. These defects appear to be caused by mechanisms downstream or independent of the Ihh-PTHrP signaling pathway, a pivotal signaling system that regulates chondrocyte proliferation and differentiation. Microarray analysis of Dicer-null chondrocytes showed limited expression changes in miRNA-target genes, suggesting that, in the majority of cases, chondrocytic miRNAs do not directly regulate target RNA abundance. Our results demonstrate the critical role of the Dicer-dependent pathway in the regulation of chondrocyte proliferation and differentiation during skeletal development.
Keywords: microRNA, skeletal development
Endochondral bone development is composed of the initial formation of a cartilage template and its subsequent replacement by mineralized bone. Longitudinal bone growth is driven by regulated proliferation and differentiation of chondrocytes in the growth plate cartilage. In developing growth plates, periarticular chondrocytes proliferate and differentiate into flat columnar chondrocytes that proliferate further to form orderly columns. Columnar chondrocytes stop proliferating and then differentiate into postmitotic hypertrophic chondrocytes. This process is tightly controlled by multiple layers of regulatory mechanisms, thus allowing persistent longitudinal bone growth.
Regulation of gene expression is the major mechanism to control a variety of cellular functions, including proliferation and differentiation. Small noncoding microRNAs (miRNAs) encoded in the genome regulate gene expression at the posttranscriptional level. Genetic ablation of miRNA genes has demonstrated that loss of single miRNAs can results in significant physiological consequences in mice (1–4). In addition, germ-line ablation of genes encoding components for miRNA biogenesis results in embryonic or perinatal lethality in mice (5–7). These examples suggest that posttranscriptional gene regulation by miRNAs plays a critical role in regulating fundamental cellular functions in mice. miRNAs are generated from long primary transcripts (primiRNAs) through multiple processing steps (8). primiRNAs are cleaved into small-hairpin premiRNAs by the microprocessor complex containing Drosha and DGCR8. premiRNAs are exported into the cytoplasm, where the RNase III, Dicer, removes the loop region of the hairpin. This step is essential for generation of mature miRNAs. To investigate the role of Dicer-dependent small RNAs in skeletal development, we conditionally disrupted the Dicer gene in this study. We show that Dicer plays a critical role in maintaining the proliferating pool of chondrocytes through regulation of chondrocyte proliferation and inhibition of premature differentiation to postmitotic hypertrophic chondrocytes.
Results
Dicer Deficiency in Chondrocytes Causes Defects in Skeletal Development.
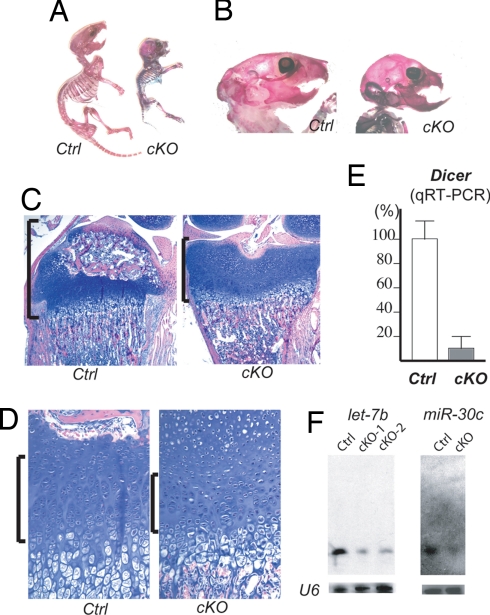
To investigate the physiological role of Dicer-dependent small RNAs in skeletal development, we deleted the Dicer gene in cartilage by crossing mice containing a floxed Dicer allele (Dicerfl/fl) (9) with transgenic mice expressing Cre recombinase under the control of a Col2a1 promoter (Col2-Cre). This Cre allele exhibited efficient Cre-recombinase activity in chondrocytes (10). Col2-Cre:Dicerfl/fl mice showed a significant growth defect and mostly die by the time of weaning. Skeletal preparation demonstrated relatively proportional reduction in skeletal size (Fig. 1A). However, the growth defect of the skull and maxilla in Col2-Cre:Dicerfl/fl mice caused relative overgrowth of the mandible and lower incisors (Fig. 1B). This is likely due to growth defects in the nasal cartilage, because we observed histological changes similar to those of limb growth plates (data not shown). Histological examination of postnatal 12-day-old (P12) mice shows a delay in formation of the secondary ossification center and flattening of the tibial epiphysis of Col2-Cre:Dicerfl/fl mice (Fig. 1C). The chondrocyte density in Col2-Cre:Dicerfl/fl growth plates was reduced because of the reduction in the number of columnar proliferating chondrocytes (1,656 ± 49/mm3 vs. control 2,266 ± 108/mm3; P < 0.05 by ANOVA, n = 3 per each group) (Fig. 1D). To assess the efficiency of Dicer removal, we quantified the mRNA level of Dicer mRNA by quantitative RT-PCR (qRT-PCR) using RNA isolated form microdissected hindlimb cartilage of 3-day-old mice. The Dicer mRNA level of Col2-Cre:Dicerfl/fl cartilage was reduced by 87% of that of control cartilage (Fig. 1E). As expected, the expression levels of ubiquitous miRNAs, let-7a, and miR-30c were significantly reduced in Col2-Cre:Dicerfl/fl mice (Fig. 1F).
Fig. 1.
Dicer is required for normal skeletal development. (A) Skeletal preparation of 9-day-old mice. Col2-Cre:Dicer fl/fl mice (cKO) show postnatal growth retardation compared with control littermates (Ctrl). (B) cKO mice at postnatal day 17 show growth defect of the maxilla, causing a relative overgrowth of the mandible. (C and D) Hematoxylin/eosin-stained 12-day-old tibiae. The epiphysis (brackets) of cKO mice is flattened, and formation of the secondary ossification center is delayed (C). The number of column-forming proliferating chondrocytes (brackets) in cKO mice is reduced (D). (E) qRT-PCR analysis using RNA isolated from microdissected hindlimb cartilage of 3-day-old mice shows a reduction in Dicer mRNA level by 87% in cKO mice. (F) Northern blot analysis on RNA of hindlimb cartilage of 3-day-old mice shows reduced expression of let-7b and miR-30c in cKO mice.
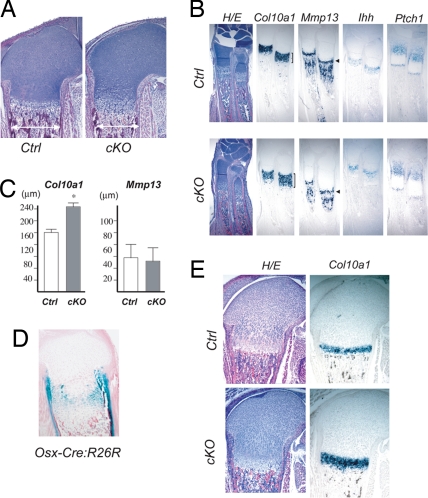
Analysis of fetal and neonatal Col2-Cre:Dicerfl/fl bones revealed a reduction in bone width and an expansion of the hypertrophic region of the growth plate (Fig. 2 A and B). The reduction in bone width was already evident at embryonic day (E)14.5, and the expansion of the hypertrophic region was present after the initiation of bone marrow formation at E15.5 (data not shown). At E18.5, the hypertrophic region, indicated by Col10a1 expression, was expanded, whereas the Mmp13 expression domain of the cartilage was not expanded in Col2-Cre:Dicerfl/fl mice (Fig. 2 B and C). Because Mmp13 is expressed only by terminally differentiated hypertrophic chondrocytes in the growth plate, these observations suggest that the expansion of the hypertrophic region was caused by an acceleration of hypertrophic differentiation of proliferating chondrocytes rather than a reduction in cartilage resorption by bone cells, which would cause an increase in terminally differentiated hypertrophic chondrocytes. Expansion of the hypertrophic region can also occur as a consequence of stimulation of chondrocyte differentiation at earlier steps (11, 12). To test whether Dicer deficiency directly stimulated hypertrophic differentiation of proliferating chondrocytes, we deleted Dicer only in late proliferating chondrocytes using Osx-Cre transgenic mice (13). Osx-Cre mice show Cre recombination activity in late-proliferating chondrocytes of the growth plate and in osteoblasts (Fig. 2D). Osx-Cre:Dicerfl/fl mice showed an expansion of hypertrophic region without affecting the size of the periarticular region (Fig. 2E). We also observed increased Col10a1 expression in primary rib chondrocytes upon Dicer deletion in vitro [supporting information (SI) Fig. 6]. These observations strongly suggest that the loss of Dicer in proliferating chondrocytes directly stimulates their hypertrophic differentiation, leading to the expansion of the hypertrophic region during fetal and neonatal stages.
Fig. 2.
Acceleration of hypertrophic differentiation of Dicer-null chondrocytes. (A) The bone width (double arrows) is reduced, and the hypertrophic region of the growth plate is expanded in neonatal Col2-Cre:Dicer fl/fl (cKO) mice. (B) In situ hybridization analysis demonstrates the expansion of the hypertrophic region in E18.5 cKO growth plates of the radius, as indicated by the enlargement of the Col10a1 domain, whereas there is no expansion of the Mmp13 domain that marks terminally differentiated hypertrophic chondrocytes in the growth plate (arrowheads), suggesting that acceleration of hypertrophic differentiation, rather than reduction of cartilage resorption, is responsible for the expansion of the hypertrophic region. Mmp13 is also expressed in osteoblasts in the bone marrow. Expression of mRNAs for Indian hedgehog (Ihh) and its transcriptional target, Ptch1 is preserved. (C) The longitudinal length of the Col10a1 domain was significantly increased in cKO mice by 35% (ANOVA; n = 3 P < 0.05), whereas the Mmp13 domain was not expanded in the cKO growth plate. (D and E) Deletion of Dicer in late-proliferating chondrocytes using Osx-Cre mice results in expansion of the hypertrophic region. Cre activity of Osx-Cre transgenic mice, assessed by R26R reporter assay (54), is observed in late proliferating chondrocytes in addition to cells in the osteoblast lineage (C). Neonatal Osx-Cre:Dicer fl/fl mice (cKO) show expansion of hypertrophic region marked with Col10a1 (D).
Indian hedgehog (Ihh) regulates chondrocyte differentiation and proliferation through PTHrP-dependent and -independent pathways (12, 14). The expression of Ihh and the transcriptional target of Ihh signaling, Patched (Ptch1), was preserved in Col2-Cre:Dicerfl/fl mice, suggesting that the acceleration of hypertrophic differentiation was caused by defects either downstream or independent of Ihh signaling. Another critical signaling system downstream of Ihh that negatively regulates hypertrophic differentiation is the PTHrP signaling pathway. Because the basal expression level of PTHrP in cartilage was too low to reliably detect its possible down-regulation in Col2-Cre:Dicerfl/fl growth plates, we took advantage of transgenic mice expressing a constitutively active PTHrP receptor (Col2-caPPR) to test whether possible impairment of PTHrP signaling was involved in the accelerated hypertrophic differentiation in Col2-Cre:Dicerfl/fl mice. Col2-caPPR transgenic mice were able to successfully rescue growth plate abnormalities caused by loss or impairment of PTHrP signaling in vivo (11, 15, 16). The expansion of the hypertrophic region in Col2-Cre:Dicerfl/fl mice was not reversed in compound mutant mice, Col2-caPPR:Col2-Cre:Dicerfl/fl mice, suggesting that the acceleration of hypertrophic differentiation in Col2-Cre:Dicerfl/fl chondrocytes was caused by a defect either independent or downstream of PTHrP receptor signaling (SI Fig. 7A). Because Dicer deficiency would affect multiple pathways, it is also possible that Dicer deficiency indeed affected PTHrP signaling, but because defects in other pathway played a dominant role, caPPR overexpression could not rescue the phenotype.
Dicer Is Required for Maintenance of Proliferating Chondrocytes in the Growth Plate.
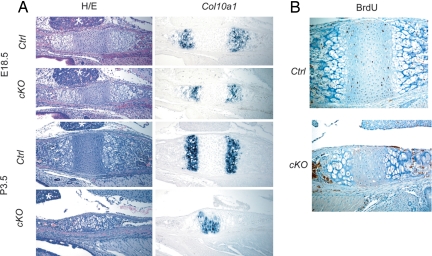
The skeletal growth defect and reduced width of the growth plate suggested a decrease in number of chondrocytes in Col2-Cre:Dicerfl/fl mice. Indeed, the reduction in proliferating chondrocytes in Col2-Cre:Dicerfl/fl mice was particularly well demonstrated in the growth plate between the basisphenoidal and basioccipital bones in the skull base (Fig. 3). This bidirectional growth plate had two Col10a1 domains flanking the region containing proliferating chondrocytes. The basisphenoidal–basioccipital growth plate of Col2-Cre:Dicerfl/fl mice was thinner in width, and the domain of proliferating chondrocytes progressively became smaller (Fig. 3A). The diminished number of proliferating chondrocytes at P3.5 in Col2-Cre:Dicerfl/fl mice was shown by the fused Col10a1 domains and the reduction in the BrdU-positive domain (Fig. 3). Overexpression of caPPR failed to restore the mass of proliferating chondrocytes, again supporting that defective PTHrP receptor signaling is not the major cause for the phenotype (SI Fig. 7B). Loss of Dicer has been reported to increase cell death in multiple types of cells (9, 17–21). We did not find an overt increase in cell death assessed by the TUNEL assay (SI Fig. 8C), and therefore it was unlikely that cell death contributed to the decrease in proliferating chondrocytes. In addition, we found a dramatic decrease in chondrocyte proliferation in Col2-Cre:Dicerfl/fl mice (Fig. 4). These findings suggest that loss of Dicer results in a reduction in the number of proliferating chondrocytes through two distinct mechanisms: acceleration of differentiation into postmitotic hypertrophic chondrocytes and reduction in chondrocyte proliferation.
Fig. 3.
Reduced number of proliferating chondrocytes in the basisphenoidal-baisoccipital growth plate. (A) Hematoxylin/eosin (H/E) staining and Col10a1 expression in the basiosphenoidal/basioccipital growth plate at E18.5 and P3.5. Col2-Cre:Dicer fl/fl mice (cKO) show a progressive reduction in the width of the growth plate and the proliferating chondrocytes flanked by Col10a1-positive hypertrophic chondrocytes. Asterisks indicate the pituitary gland. (B) Reduced BrdU-positive chondrocytes in the cKO growth plate at P3.5.
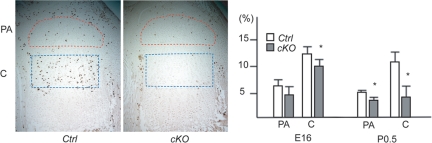
Fig. 4.
Reduced chondrocyte proliferation in Col2-Cre:Dicer fl/fl mice (cKO). The BrdU-labeling assay demonstrates a dramatic reduction in chondrocyte proliferation in cKO growth plates. The BrdU-labeling index was calculated in the periarticular (PA) and columnar proliferating (C) region of the proximal growth plate of the tibia at the indicated ages. Representative BrdU-staining pictures of P0.5 tibiae are shown. The BrdU-labeling index in cKO mice was significantly reduced in the columnar region at E16.5, and in both periarticular and columnar regions at P0.5. Asterisks indicate statistical significance with the P < 0.05.
miRNA and Gene Expression Profiling Suggests Limited Direct Regulation of mRNA Expression by Chondrocytic miRNAs.
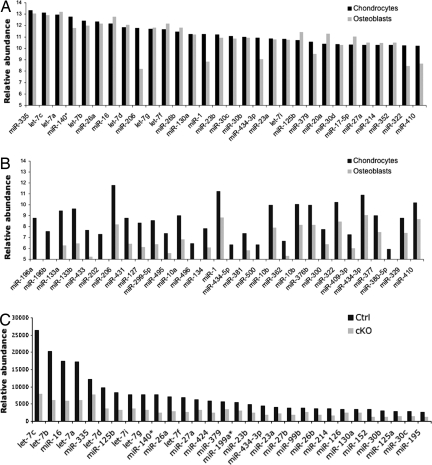
Although Dicer has been shown to process RNA species besides premiRNAs in mammalian cells (22), it is believed that the primary role of Dicer in mice is to generate mature miRNAs. Therefore, we hypothesized that the reduction in levels of chondrocytic miRNAs caused the physiologic changes in Dicer-deficient chondrocytes. To understand the roles of miRNAs in chondrocytes, first we performed miRNA profiling using a bead-based array (23). miRNA profiles from different chondrocyte sources showed modestly different patterns, whereas the miRNA profile of calvarial cells showed greater dissimilarity from those of chondrocytes (SI Fig. 9 and SI Table 1). The majority of miRNAs abundantly expressed in hindlimb chondrocytes and calvarial osteoblasts were evolutionary conserved and relatively ubiquitous ones such as members of the let-7 family, miR-16, and miR-26 (Fig. 5A). The “cartilage-specific” miRNAs, miR-140 and miR-140*, are expressed at high levels, a result consistent with previous reports (24, 25) (Fig. 5A). We also found that several miRNAs were preferentially expressed in chondrocytes compared with calvarial cells (Fig. 5B and SI Fig. 9B). miRNA profiling comparing control and Col2-Cre:Dicerfl/fl mice showed that most miRNAs were reduced by ≈60–70% in neonatal Col2-Cre:Dicerfl/fl hindlimb growth plates (Fig. 5C and SI Table 2). We noticed that some miRNAs, such as miR-133 and miR-1, showed no reduction. This is probably due to contaminating muscle tissues into microdissected cartilage specimens, because these miRNAs are expressed at very high levels in muscle (26, 27). The observation that certain amounts of miRNAs were still detectable in the Col2-Cre:Dicerfl/fl sample was unexpected. Considering the relatively proportional reduction in the majority of miRNAs and the efficient elimination of Dicer mRNA, it is unlikely that contamination of noncartilaginous tissues or inefficient Dicer deletion is the reason for the remaining miRNAs. Because mature miRNAs appear quite stable (28), these miRNAs in mutant growth plates are probably a carryover of preexisting miRNAs.
Fig. 5.
miRNA expression in chondrocytes. (A) Relative expression levels of the 30 most-abundant miRNAs in neonatal hindlimb cartilage (chondrocytes) and calvarial cells (osteoblasts). Many abundant miRNAs are similarly expressed in chondrocytes and osteoblasts. (B) Thirty miRNAs preferentially expressed in chondrocytes. The expression of muscle-specific miRNAs, mir-206, -133, and -1 could be overestimated because of possible contamination of muscle tissues to cartilage specimens. (C) Expression of 30 miRNAs in control and Dicer-deficient chondrocytes. RNA isolated from neonatal hindlimb cartilage was subjected to miRNA profiling. Expression levels of most of miRNAs were reduced by 60–70% in Dicer-deficient chondrocytes. Complete data are shown in SI Table 2.
Next, we performed microarray analysis to identify genes whose expression was altered in Dicer-deficient chondrocytes. RNA isolated from whole growth plates of P3.5 control and Col2-Cre:Dicerfl/fl hindlimbs was subjected to microarray analysis. Of the 45,036 probes, 2,658 showed >1.5-fold expression changes (SI Table 3). To test whether the reduction of miRNAs directly influenced RNA levels of miRNA-target genes in Dicer-deficient chondrocytes, we examined expression levels of predicted miRNA-target genes that had binding sites for five or more different seed sequences of the 30 most-abundant chondrocytic miRNAs using the microarray data. These miRNAs had 17 different seed sequences (seven-nucleotide sequences in positions two to eight of mature miRNAs) that were used to predict target genes by the computer program, TargetScanS (29). A total 4,310 genes were predicted as potential target genes of these miRNAs abundantly expressed in chondrocytes (SI Table 4). Two hundred and fourteen genes (5.0%) had binding sites for five or more different seed sequences. Among these genes, analysis of 71 genes whose expression data were available showed that most were either not expressed or had unchanged expression (SI Fig. 10A). Only Hmga2 was found up-regulated in the Col2-Cre:Dicerfl/fl growth plate, a result subsequently confirmed by quantitative RT-PCR and in situ hybridization (SI Fig. 10B). This finding is consistent with the reports that Hmga2 transcripts were destabilized by miRNAs, let-7 and miR-98 (30–32). We also examined the 3′-UTR sequences of the 1,437 genes up-regulated in the Col2-Cre:Dicerfl/fl growth plate for potential binding sites of chondrocytic miRNAs. The frequencies of sequences complementary to the 17 miRNA seed sequences in 3′ UTRs were calculated by using the MotifADE (33). We did not find significant enrichment in miRNA-binding sites in genes up-regulated in Col2-Cre:Dicerfl/fl chondrocytes (data not shown). This finding may suggest that Dicer deficiency in chondrocytes has limited effects in miRNA-target gene expression at the RNA level.
Discussion
In this article, we demonstrate that Dicer is essential for normal skeletal growth. Dicer deficiency in chondrocytes results in a reduction in the number of proliferating chondrocytes through two distinct mechanisms: decreased proliferation and accelerated differentiation into postmitotic hypertrophic chondrocytes. Studies investigating the role of Dicer in mammalian cells and tissues have demonstrated variable consequences of Dicer deficiency (2, 5, 9, 17–21, 28, 34–39). Whereas proliferation defects have been reported in several Dicer-deficient cell types as in our model, we did not find an overt increase in cell death or derepression of heterochromatin-derived transcripts (SI Fig. 8B), as observed in other types of cells (9, 17–21, 36, 37). Defects or alterations in cell differentiation have been reported in other types of cells missing Dicer (9, 20, 34, 35, 39). However, stimulation rather than blockage of cell differentiation like that observed in our model appears unique. These observations suggest that consequences of Dicer deficiency depend on cell type. It is also possible that differences in efficiency of Dicer or miRNA elimination are responsible for the different outcomes, because we observed small amounts of miRNAs in Col2-Cre:Dicerfl/fl growth plates.
We observed a dramatic reduction in cell proliferation in Dicer-deficient chondrocytes. Whereas Dicer-null ES cells show proliferation defects (36, 37), loss of Dicer in thymocytes (9) or limb bud (18) does not cause detectable proliferation defects. In contrast, Dicer deficiency stimulates proliferation of lung cancer cells and Ras-induced tumorigenesis (40). These examples show that effects of loss of Dicer on cell proliferation differ in a cell-specific manner. The cause of the reduced proliferation of Dicer-deficient chondrocytes is not clear. In microarray analysis, we did not find clear changes in expression of genes involved in cell cycle progression except a mild decrease in cyclin E2 expression. We found no indication of activation of the p53, stress response, or senescence pathways (data not shown).
miRNA profiling and gene expression profiling data showed limited changes in RNA abundance of predicted miRNA-target genes in Dicer-deficient chondrocytes (SI Fig. 10A). These observations may support the notion that miRNAs primarily regulate gene expression at the translational level, although recent reports have shown that many miRNAs reduce target RNA levels by facilitating deadenylation and decapping (41–47). Alternatively, the direct effects of miRNAs may be modified by subsequent cellular events in the steady state in vivo. In maturing oocytes, whereas maternal transcript degradation depends on Dicer, there is no or only limited enrichment of predicted target genes of miRNAs among genes up-regulated in Dicer-deficient oocytes (38, 48). These findings along with ours may suggest that RNA levels of most genes are not directly regulated by miRNA-mRNA interaction. We did find up-regulation of Hmga2 mRNA in Dicer-deficient chondrocytes. This RNA has 13 binding sites for chondrocytic miRNAs in the 3′ untranslated region (data not shown). This result is consistent with recent reports that let-7 and miR-98 suppress Hmga2 expression (30–32) by destabilizing its RNA (31).
miRNA expression analysis confirmed abundant expression of “cartilage-specific” miR-140 that was previously suggested to regulate histone deacetylase 4 (HDAC4) (24). Because HDAC4 inhibits hypertrophic differentiation of chondrocytes (49), this miRNA is particularly interesting from the point of view of chondrocyte biology. However, the finding that chondrocyte hypertrophy is stimulated rather than inhibited in Dicer-deficient chondrocytes suggests that the reduction of possible suppression of HDAC4 by miR-140 in Dicer-deficient chondrocytes is an unlikely mechanism for the phenotype.
Materials and Methods
Mice.
Col2-Cre transgenic mice (10), floxed Dicer mice (9), Osx-Cre transgenic mice (13), and Col2-caPPR transgenic mice (15) were described. Genotyping of Cre transgenic mice was performed by PCR using primers detecting the Cre sequence (11). The floxed and wild-type Dicer alleles were detected by using primers, P1: 5′-AGTGTAGCCTTAGCCATTTGC-3′ and P2: 5′-CTGGTGGCTTGAGGACAAGAC-3′. These primers amplify the region spanning the downstream loxP sequence. Littermates were used as control. The Col2-caPPR allele was genotyped by PCR by using primers specific to the transgene, P3: 5′-TAGTTGGCCCAGGTCCTGT-3′ and P4: 5′-TAACCATGTTCATGCCTTCTTC-3′.
Because Col2-Cre:Dicer+/+, Col2-Cre:Dicerfl/+, and Dicerfl/fl mice were indistinguishable from wild-type mice in growth, growth plate morphology, and chondrocyte marker expression (data not shown), either Col2-Cre:Dicerfl/+ or Dicerfl/fl littermates were used as control in this study.
Skeletal Preparation, Histology, and Rosa-26R Cre Reporter Assay.
Alizarin red and alcian blue staining was performed by using a modified McLeod's method (50). Carcasses were fixed in 95% ethanol, stained with alcian blue and alizarin red, cleared in 1% KOH, and kept in 50% glycerol. For histological analysis, mice were dissected, fixed in 10% formalin, decalcified in 10% EDTA, paraffin-processed, cut, and subjected to hematoxylin/eosin staining, in situ hybridization, and BrdU staining. X-gal staining for the Rosa 26-R Cre reporter assay was performed as described (51).
In Situ Hybridization.
In situ hybridization was carried out as described (52). Probes for Col10a1, MMP13, Ihh, and Ptch1 mRNAs were described (11).
BrdU Labeling and Detection.
For BrdU labeling, 50 μg of BrdU per gram of body weight was given to mice i.p. 2 h before death. Tissues were fixed in 10% formalin solution, processed, and sectioned by using standard procedures. BrdU was detected by using the BrdU-staining kit (Zymed). The BrdU-labeling index was calculated as the ratio of BrdU-positive nuclei over total nuclei in each region of the growth plate.
miRNA Expression Profiling.
Total RNA was extracted from growth plates and calvariae of neonatal C57BL/6 mice. Limbs were freshly frozen in OCT media, cryosectioned with 60-μm thickness, and dissected under a dissecting microscope using 28-gauge needle tips to obtain the desired populations of chondrocytes. Chondrocytes from the periarticular, columnar, and hypertrophic regions were separately isolated from the distal femur by using TRIzol (Invitrogen). RNA was purified also from whole growth plates of the femur and tibia (hindlimb chondrocytes), the humerus (forelimb chondrocytes), and calvarial cells. miRNA profiling was performed by using the Luminex bead-based array (23).
Northern Blot Analysis.
Small RNA was isolated from whole growth plate samples of the femur and tibia of 3-day-old mice by using the mirVana miRNA isolation kit (Ambion). RNA was separated on a denaturing 15% polyacrylamide gel containing 8M urea and 1× TBE and electroblotted onto a nylon membrane. Synthesized antisense DNA oligo probes were labeled with digoxigenin by using the DIG oligonucleotide tailing kit, second generation (Roche Applied Science). Hybridization and washing were performed by using the ULTAHyb-Oligo Hybridization Buffer (Ambion) according to the manufacturer's protocol. Signal detection was performed by using the DIG Luminescent Detection Kit (Roche Applied Science).
Quantitative RT-PCR.
Total RNA was extracted from growth-plate cartilage of the distal femur and proximal tibia of P3.5-old mice by using RNeasy mini kit (Qiagen). cDNA synthesis was performed by using random hexamers with the Protoscript First Strand cDNA Synthesis Kit (New England Biolabs), and quantitative PCR was performed by using the DNA Engine Opticon 2 Continuous Fluorescence Detection System (Biolab) and the Sybr green mix (Applied Biosystems). Signals were normalized to β-actin. Each reaction was performed in quadruplicate and repeated on different sample sets to confirm the results. Primer sequences were: Dicer1-F, 5′-AATTGGCTTCCTCCTGGTTAT-3′ and Dicer1-R, GTCAGGTCCTCCTCCTCCTC-3′; β-actin-F, 5′-GCACTGTGTTGGCATAGAGG-3′ and β-actin-R, 5′-GTTCCGATGCCCTGAGGCTC-3′.
Microarray Analysis.
RNA was isolated from microdissected whole growth-plate cartilage of the distal femur of neonatal mice by using the RNeasy mini Kit (Qiagen). Up to 10 limbs were pooled for a group. Two samples per each group were separately prepared. The biotynylated cRNA was hybridized to the Affymetrix MOE430 v2 chip. Normalization and comparison between groups were performed by using the dChip (53). Genes considered to be absent in more than two sample sets based on the Affymetrix detection (present/absent) call were removed from the comparison analysis.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Greg Nachtrab for technical assistance, Dr. Changzhong Chen and Dr. Edward Fox at the Dana Farber Cancer Institute for help in Affymetrix microarray analysis, and Dr. Vamsi Mootha and David Keller at the Broad Institute for MotifADE analysis. T.K. is supported by National Institutes of Health (NIH) Grant 1R21AR054500. Part of this work is supported by NIH Grant DK56246 (to A.P.M. and H.M.K.). S.J.R. was supported by postdoctoral fellowships from the National Health and Medical Research Council of Australia (Grant 301299) and the Arthritis Foundation (Grant 401683).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707900105/DC1.
References
- 1.Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, et al. Science. 2007;316:608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, et al. Science. 2007;316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 3.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D. Cell. 2007;129:303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda T, Yamagata K, Fujiyama S, Matsumoto T, Koshida I, Yoshimura K, Mihara M, Naitou M, Endoh H, Nakamura T, et al. Nat Cell Biol. 2007;9:604–611. doi: 10.1038/ncb1577. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R. Nat Genet. 2007;39:380–385. doi: 10.1038/ng1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YS, Kim HK, Chung S, Kim KS, Dutta A. J Biol Chem. 2005;280:16635–16641. doi: 10.1074/jbc.M412247200. [DOI] [PubMed] [Google Scholar]
- 9.Cobb BS, Nesterova TB, Thompson E, Hertweck A, O'Connor E, Godwin J, Wilson CB, Brockdorff N, Fisher AG, Smale ST, Merkenschlager M. J Exp Med. 2005;201:1367–1373. doi: 10.1084/jem.20050572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Genesis. 2000;26:145–146. [PubMed] [Google Scholar]
- 11.Kobayashi T, Chung UI, Schipani E, Starbuck M, Karsenty G, Katagiri T, Goad DL, Lanske B, Kronenberg HM. Development. 2002;129:2977–2986. doi: 10.1242/dev.129.12.2977. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi T, Soegiarto DW, Yang Y, Lanske B, Schipani E, McMahon AP, Kronenberg HM. J Clin Invest. 2005;115:1734–1742. doi: 10.1172/JCI24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodda SJ, McMahon AP. Development. 2006;133:3231–3244. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 14.Karp SJ, Schipani E, St-Jacques B, Hunzelman J, Kronenberg H, McMahon AP. Development. 2000;127:543–548. doi: 10.1242/dev.127.3.543. [DOI] [PubMed] [Google Scholar]
- 15.Schipani E, Lanske B, Hunzelman J, Luz A, Kovacs CS, Lee K, Pirro A, Kronenberg HM, Juppner H. Proc Natl Acad Sci USA. 1997;94:13689–13694. doi: 10.1073/pnas.94.25.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soegiarto DW, Kiachopoulos S, Schipani E, Juppner H, Erben RG, Lanske B. Endocrinology. 2001;142:5303–5310. doi: 10.1210/endo.142.12.8553. [DOI] [PubMed] [Google Scholar]
- 17.Fukagawa T, Nogami M, Yoshikawa M, Ikeno M, Okazaki T, Takami Y, Nakayama T, Oshimura M. Nat Cell Biol. 2004;6:784–791. doi: 10.1038/ncb1155. [DOI] [PubMed] [Google Scholar]
- 18.Harfe BD, McManus MT, Mansfield JH, Hornstein E, Tabin CJ. Proc Natl Acad Sci USA. 2005;102:10898–10903. doi: 10.1073/pnas.0504834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris KS, Zhang Z, McManus MT, Harfe BD, Sun X. Proc Natl Acad Sci USA. 2006;103:2208–2213. doi: 10.1073/pnas.0510839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muljo SA, Ansel KM, Kanellopoulou C, Livingston DM, Rao A, Rajewsky K. J Exp Med. 2005;202:261–269. doi: 10.1084/jem.20050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaefer A, O'Carroll D, Tan CL, Hillman D, Sugimori M, Llinas R, Greengard P. J Exp Med. 2007;204:1553–1558. doi: 10.1084/jem.20070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krol J, Fiszer A, Mykowska A, Sobczak K, de Mezer M, Krzyzosiak WJ. Mol Cell. 2007;25:575–586. doi: 10.1016/j.molcel.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 23.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, et al. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 24.Tuddenham L, Wheeler G, Ntounia-Fousara S, Waters J, Hajihosseini MK, Clark I, Dalmay T. FEBS Lett. 2006;580:4214–4217. doi: 10.1016/j.febslet.2006.06.080. [DOI] [PubMed] [Google Scholar]
- 25.Wienholds E, Kloosterman WP, Miska E, Alvarez-Saavedra E, Berezikov E, de Bruijn E, Horvitz HR, Kauppinen S, Plasterk RH. Science. 2005;309:310–311. doi: 10.1126/science.1114519. [DOI] [PubMed] [Google Scholar]
- 26.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HK, Lee YS, Sivaprasad U, Malhotra A, Dutta A. J Cell Biol. 2006;174:677–687. doi: 10.1083/jcb.200603008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Rourke JR, Georges SA, Seay HR, Tapscott SJ, McManus MT, Goldhamer DJ, Swanson MS, Harfe BD. Dev Biol. 2007;311:359–368. doi: 10.1016/j.ydbio.2007.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 30.Hebert C, Norris K, Scheper MA, Nikitakis N, Sauk JJ. Mol Cancer. 2007;6:5. doi: 10.1186/1476-4598-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee YS, Dutta A. Genes Dev. 2007;21:1025–1030. doi: 10.1101/gad.1540407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayr C, Hemann MT, Bartel DP. Science. 2007;315:1576–1579. doi: 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mootha VK, Handschin C, Arlow D, Xie X, St Pierre J, Sihag S, Yang W, Altshuler D, Puigserver P, Patterson N, et al. Proc Natl Acad Sci USA. 2004;101:6570–6575. doi: 10.1073/pnas.0401401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andl T, Murchison EP, Liu F, Zhang Y, Yunta-Gonzalez M, Tobias JW, Andl CD, Seykora JT, Hannon GJ, Millar SE. Curr Biol. 2006;16:1041–1049. doi: 10.1016/j.cub.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cobb BS, Hertweck A, Smith J, O'Connor E, Graf D, Cook T, Smale ST, Sakaguchi S, Livesey FJ, Fisher AG, Merkenschlager M. J Exp Med. 2006;203:2519–2527. doi: 10.1084/jem.20061692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K. Genes Dev. 2005;19:489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murchison EP, Partridge JF, Tam OH, Cheloufi S, Hannon GJ. Proc Natl Acad Sci USA. 2005;102:12135–12140. doi: 10.1073/pnas.0505479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murchison EP, Stein P, Xuan Z, Pan H, Zhang MQ, Schultz RM, Hannon GJ. Genes Dev. 2007;21:682–693. doi: 10.1101/gad.1521307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yi R, O'Carroll D, Pasolli HA, Zhang Z, Dietrich FS, Tarakhovsky A, Fuchs E. Nat Genet. 2006;38:356–362. doi: 10.1038/ng1744. [DOI] [PubMed] [Google Scholar]
- 40.Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Nat Genet. 2007;39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 41.Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli AE. Cell. 2005;122:553–563. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 42.Behm-Ansmant I, Rehwinkel J, Doerks T, Stark A, Bork P, Izaurralde E. Genes Dev. 2006;20:1885–1898. doi: 10.1101/gad.1424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giraldez AJ, Mishima Y, Rihel J, Grocock RJ, Van Dongen S, Inoue K, Enright AJ, Schier AF. Science. 2006;312:75–79. doi: 10.1126/science.1122689. [DOI] [PubMed] [Google Scholar]
- 44.Jing Q, Huang S, Guth S, Zarubin T, Motoyama A, Chen J, Di Padova F, Lin SC, Gram H, Han J. Cell. 2005;120:623–634. doi: 10.1016/j.cell.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 45.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 46.Schmitter D, Filkowski J, Sewer A, Pillai RS, Oakeley EJ, Zavolan M, Svoboda P, Filipowicz W. Nucleic Acids Res. 2006;34:4801–4815. doi: 10.1093/nar/gkl646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu L, Fan J, Belasco JG. Proc Natl Acad Sci USA. 2006;103:4034–4039. doi: 10.1073/pnas.0510928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang F, Kaneda M, O'Carroll D, Hajkova P, Barton SC, Sun YA, Lee C, Tarakhovsky A, Lao K, Surani MA. Genes Dev. 2007;21:644–648. doi: 10.1101/gad.418707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X, Meadows E, McAnally J, Pomajzl C, Shelton JM, Richardson JA, et al. Cell. 2004;119:555–566. doi: 10.1016/j.cell.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 50.McLeod MJ. Teratology. 1980;22:299–301. doi: 10.1002/tera.1420220306. [DOI] [PubMed] [Google Scholar]
- 51.Chung UI, Lanske B, Lee K, Li E, Kronenberg H. Proc Natl Acad Sci USA. 1998;95:13030–13035. doi: 10.1073/pnas.95.22.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murtaugh LC, Zeng L, Chyung JH, Lassar AB. Dev Cell. 2001;1:411–422. doi: 10.1016/s1534-5807(01)00039-9. [DOI] [PubMed] [Google Scholar]
- 53.Li C, Wong WH. Proc Natl Acad Sci USA. 2001;98:31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soriano P. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.