Abstract
The canonical Wnt-β-catenin signaling pathway is initiated by induction of phosphorylation of one of the Wnt receptors, low density lipoprotein receptor-related protein (LRP) 5/6, at Thr1479 and Ser1490. We identified, by screening a human kinase siRNA library, phosphatidylinositol 4-kinase type II (PI4KII) α and phosphatidylinositol-4-phosphate 5-kinase type I (PIP5KI) as required for Wnt3a-induced LRP6 phosphorylation at Ser1490 in mammalian cells and confirmed that these kinases are important for Wnt signaling in Xenopus embryos. Wnt3a stimulates the formation of phosphatidylinositol 4,5-bisphosphates [PtdIns (4,5)P2] through frizzled (Fz) and dishevelled (Dvl), the latter of which directly interacted with and activated PIP5KI. PtdIns (4,5)P2 in turn regulated phosphorylation of LRP6 at Thr1479 and Ser1490. Therefore, our study reveals a new signaling mechanism for Wnt to regulate LRP6 phosphorylation.
Members of the Wnt family of secretory glycoproteins have important roles in various physiological and pathophysiological processes, including embryonic development, bone development, neuronogenesis, adipogenesis, myogenesis, organogenesis, lipid and glucose metabolism, and tumorigenesis (1-5). Canonical Wnt binds to two receptors, LRP5/6, and Fz proteins, leading to phosphorylation of LRP6 at Thr1479 by Casein kinase (CK) 1γ and at Ser1490 by Glycogen Synthase Kinase (GSK) 3 (6-10). Wnt appears to regulate Thr1479 phosphorylation by inducing the formation of LRP6 aggregates (9), whereas it regulates Ser1490 phosphorylation through GSK in a Axin-dependent manner (10). To determine whether there are other kinases that take part in regulation of LRP6 phosphorylation, we screened a human kinase siRNA library from Applied Biosystems for effects on Wnt-induced accumulation of cytosolic β-catenin detected by an Enzyme-Linked ImmunoSorbent Assay (ELISA) and on phosphorylation of Ser1490 of LRP6 detected by protein immunoblotting in human embryonic kidney (HEK) 293T cells. Multiple phosphatidylinositol (PtdIns) kinase siRNAs inhibited cytosolic β-catenin accumulation (Fig. S1A and Table SI) and phosphorylation of LRP6 at Ser1490 (Fig. 1, A and B) in response to purified Wnt3a protein. Among the tested PtdIns kinase siRNAs, siRNAs for PI4KIIα and PIP5KIβ had the strongest inhibitory effects (Fig. S1A, and Fig. 1, A and B). These siRNA also inhibited Wnt3a-induced reporter gene activity (Fig. S1B). Additional siRNAs for both PI4KIIα and PIP5KIβ directed against different targeting sequences were also tested (Fig. S1, B and C, and Fig. 1, A and B). To further verify that the effects of these siRNAs were specific, we restored Wnt signaling by expressing the kinases knocked down by the siRNAs (Fig. S1, D to G). PI4KIIα and PIP5KIβ siRNAs did not inhibit lithium- and Axin1/2 siRNA-induced accumulation of β-catenin (Fig. S1, C and H), but β-catenin siRNA did. Thus, these siRNAs appeared to affect Wnt signaling by affecting LRP6 phosphorylation rather than Wnt signaling downstream components.
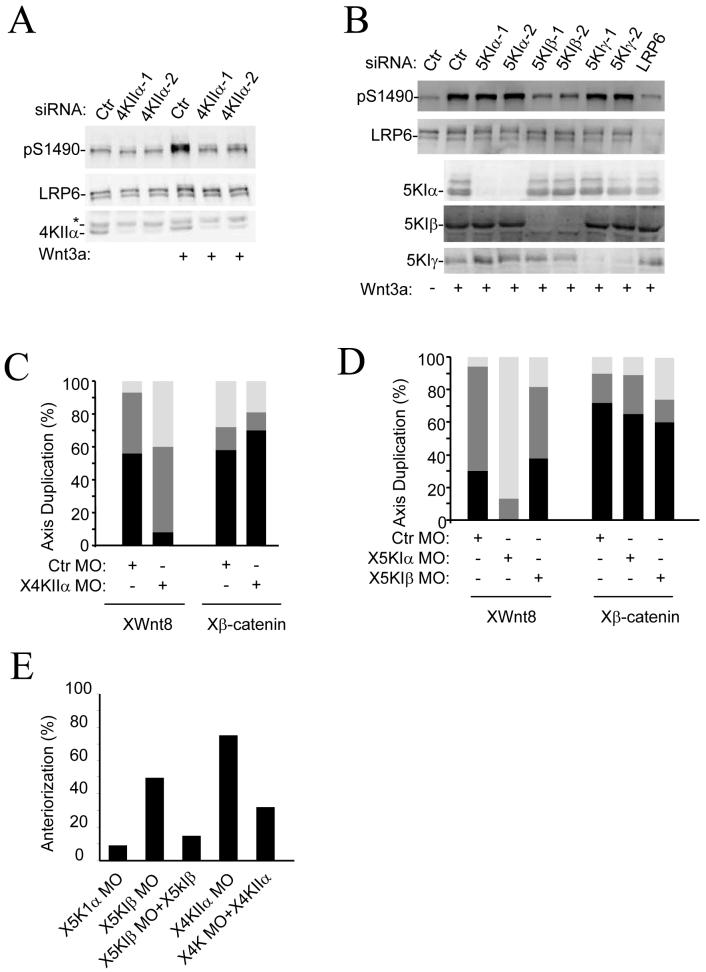
Figure 1. Effect of depletion of PtdIns kinases on Wnt3a signaling.
A,B) Effects of PtdIns kinase siRNAs on Wnt3a-induced phosphorylation of LRP6 at Ser1490. HEK293T cells were transfected with siRNAs as indicated for 48 hours and then treated with Wnt3a (50ng/ml) for 30mins. Phosphorylated proteins were assayed by Western blotting. The experiments were repeated at least three times. Representative images are shown.
C,D) A control morpholino oligos (Ctr MO, 10 nM) or MO (10 nM) targeting Xenopus PI4KIIα (C), PIP5KIα, or PIP5KIβ (D) was injected with XWnt8 (2 pg) or Xβ-catenin (10 pg) mRNA into four-cell stage embryos. n>40 for all of the Xenopus embryo studies. Open bar, no double axis; shaded, incomplete double axis; closed, complete double axis.
E) Four-cell stage embryos were injected with XPIP5KIβ MO (40ng) or XPI4KIIα MO (40ng), or XPIP5KIα MO (40ng) with or without XPIP5KIβ (10pg) or XPI4KIIα (5pg) RNA in the dorsal region and cultured to tailbud stages. XPIP5KIα MO (n=30), XPIP5KIβ MO (n=45), XPIP5KIβ MO+XPIP5KIβ (n=29), XPI4KIIα MO (n=55), and XPI4KIIα MO+XPI4KIIα (n=30).
PIP5KIγ siRNAs showed a weak effect, whereas PIP5KIα siRNAs showed little effect in HEK293T cells (Fig. 1B). However, treatment of the cells with combinations of the PIP5KI siRNAs showed that the combination targeting all three PIP5KI isoforms reduced Wnt3a-indcued accumulation of β-catenin and phosphorylation of LRP6 almost to basal levels (Fig. S1, C and I), suggesting that PIP5KIα and 1γ may also contribute to Wnt signaling in these cells.
To further investigate the involvement of PtdIns kinases in Wnt signaling, we examined whether these PtdIns kinases also functioned in Wnt signaling in Xenopus. A morpholino (MO) targeting Xenopus PI4KIIα inhibited XWnt8-induced, but not β-catenin-induced, axis duplication in Xenopus embryos (Fig. 1C and S2A). Although PIP5KIβ-MO showed little effect, a MO targeting its close homolog PIP5KIα inhibited XWnt8-induced, but not β-catenin-induced, axis duplication (Fig. 1D). Consistent with the phenotypes, PIP5KIα and PI4KIIα MOs reduced phosphorylation of LRP6 (Fig. S2B). In addition, expression of catalytically inactive PIP5KIα and PI4KIIα mutants suppressed axial duplication induced by XWnt8, but not β-catenin (Fig. S2C), further confirming the importance of these kinases in Wnt signaling.
Inhibition of zygotic Wnt-β-catenin signaling induces anteriorized phenotypes that include enlarged cement glands and head structures (11). PI4KIIα-MO that injected into the dorsal regions of Xenopus embryos induced strong anteriorized phenotypes in over 70% embryos. This effect could be partially reversed by coinjection of Xenopus PI4KIIα mRNA (Fig. 1E and S2D). Although PIP5KIα-MO had little effect on the phenotype, PIP5KIβ-MO induced anteriorized phenotypes in around 50% of treated embryos. The PIP5KIβ MO effect could be almost completely reversed by injection of XPIP5KIβ mRNA (Fig. 1E and S2D). These results together indicate that PtdIns kinases regulate endogenous Wnt signaling in Xenopus embryos.
Because sequential phosphorylation of PtdIns lipids by PI4KII and PIP5KI constitutes the major pathway for PtdIns (4,5)P2 production in most cells (12, 13), we suspected that PtdIns (4,5)P2 might regulate phosphorylation of Ser1490. To test this hypothesis, we delivered PtdIns plus all of the 7 possible isoforms of PtdIns Phosphates at equal molar concentrations into HEK 293T cells in a lipid carrier. PtdIns (4,5)P2 showed the strongest stimulatory effect on Wnt3a-induced phosphorylation of Ser1490 (Fig. 2A). In addition, delivery of PtdIns (4)P, but not PtdIns, rescued the effect of PI4K siRNA, whereas delivery of PtdIns (4,5)P2, but not PtdIns (4)P, rescued the effect of PIP5K siRNA on Wnt3a-induced phosphorylation of LRP6 (Fig. 2, B and C) and β-catenin accumulation (Fig. S3). These results suggest that PtdIns (4,5)P2 may be the primary PtdIns lipid involved in the regulation of Ser1490 phosphorylation.
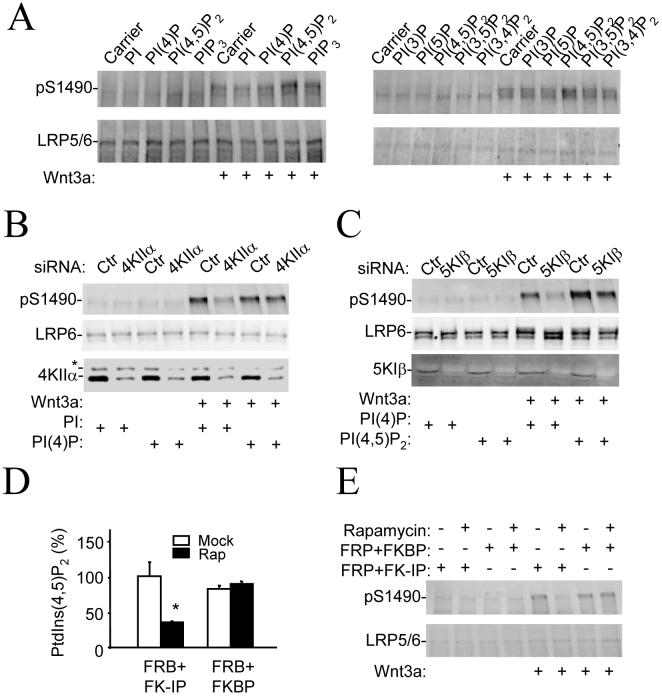
Figure 2. Effect of PtdIns (4,5)P2 on Wnt3a signaling.
A) Effect of exogenous PtdIns (4,5)P2 on Wnt3a-induced phosphorylation of LRP6 at Ser1490. HEK293T cells were treated with various PtdIns lipids in a lipid carrier for 10mins and incubated with Wnt3a (20ng/ml) for additional 20 mins before assayed by immunoblotting.
B, C) Rescuing the effects of PI kinase siRNAs by direct delivery of PtdIns lipids.
D,E) Reduction in PtdIns (4,5)P2 levels decreases LRP6 Ser1490 phosphorylation. HEK293T cells transfected with FRB (PM-FRB-CFP), FKBP (mRFP-FKBP12) or FK-IP (mRFP-FKBP12-5-ptase-dom) were treated with Wnt3a (20ng/ml) in the presence or absence of rapamycin (100nM) for 30 mins before they were collected for the lipid assay (D) and Immunoblotting analysis (E). * stands for P<0.01 compared to the absence of rapamycin (Student’s t-Test).
To further investigate the involvement of PtdIns (4,5)P2 in regulating phosphorylation of LRP6, we used a rapidly inducible PtdIns (4,5)P2 hydrolysis system, in which rapamycin induces the heterodimerization of membrane-targeted FRP (fragment of mammalian target of rapamycin that binds FKBP) and FKBP12 (FK560-binding protein 12) fused with a truncated form of type IV phosphoinositide 5-phosphatase, leading to activation of the phosphatase (14, 15). In this system, rapamycin reduced the amount of PtdIns (4,5)P2 in cells expressing both FRP and phosphatase-fused FKBP12, but not in those expressing FRP and FKBP12 alone (Fig. 2D). Rapamycin also attenuated phosphorylation of LRP6 only in cells expressing both FRP and phosphatase-fused FKBP12 (Fig. 2E).
We next tested whether Wnt3a can stimulate PtdIns (4,5)P2 production by establishing an ELISA to determine the PtdIns (4,5)P2 contents and detected significant Wnt3a-induced stimulation of PtdIns (4,5)P2 production (more than 2-fold increases) in HEK293T, Hela, and NIH3T3 cells (Fig. S4A). We confirmed these results by High Performance Liquid Chromatography (HPLC) and Thin Layer Chromatography (TLC) (Fig. 3A, and S4, B and C). Therefore, these results demonstrate that Wnt3a can induce PtdIns (4,5)P2 formation, which may regulate phosphorylation of LRP6. The findings that the PI4KIIα siRNA abolished, and the PIP5KIβ siRNA reduced, Wnt3a-induced accumulation of PtdIns (4,5)P2 (Fig. 3B) confirm the involvement of these PtdIns kinases in Wnt3a-induced formation of PtdIns (4,5)P2.
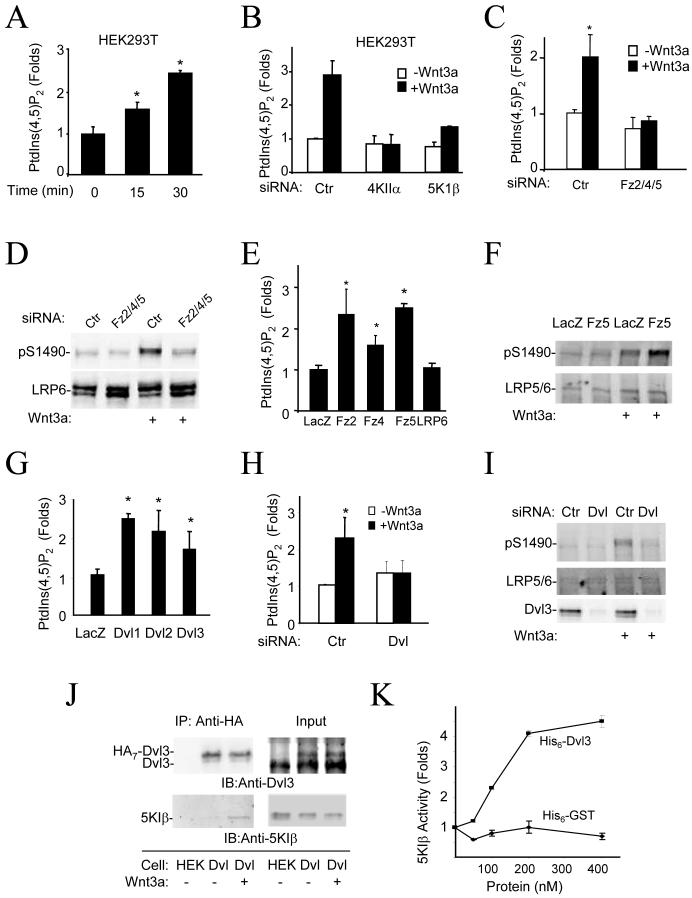
Figure 3. Stimulation of PtdIns (4,5)P2 formation by Wnt3a through Fz and Dvl.
A) Effect of Wnt3a treatment on PtdIns (4,5)P2 content. HEK293T Cells were stimulated with Wnt3a protein (50ng/ml) before lipid extraction. PtdIns (4,5)P2 content was determined by HPLC. * P<0.01 compared to Time 0 (Student’s t-Test).
B) Requirement of PI4KIIα and PIP5KIβ for Wnt3a-induced formation of PtdIns (4,5)P2. Cells were transfected with siRNAs as indicated for 48 hours and then treated with Wnt3a (50ng/ml) for 30mins. PtdIns (4,5)P2 were detected by ELISA.
C) Effect of Fz siRNAs on Wnt3a-induced formation of PtdIns (4,5)P2. Cells were transfected with control siRNA or a combination of Fz2, Fz4, and Fz5 siRNAs for 48 hours and then treated with Wnt3a (50ng/ml) for 30mins before assays. * P<0.01 compared to control siRNA transfection in the absence of Wnt3a (Student’s t-Test).
D) Effect of Fz siRNAs on Wnt3a-induced phosphorylation of LRP6 at Ser1490. Cells were transfected as in C for 48 hours and then treated with Wnt3a (50ng/ml) for 30mins.
E) Effect of Fz overexpression on accumulation of PtdIns (4,5)P2. HEK293T cells were transfected with the Lac Z, Fz5, or LRP6 expression plasmids for 18 hours, and PtdIns (4,5)P2 levels were determined by ELISA.
F) Effect of Fz5 expression on phosphorylation of LRP6 at Ser1490. Cells were transfected with Fz5 expression plasmid for 18 hrs and then treated with Wnt3a (20ng/ml) for 20mins.
G) Effect of Dvl expression on the PtdIns (4,5)P2 levels. HEK293T cells were transfected with the mouse Dvl1, 2, or 3 expression plasmid for 18 hours before the PtdIns (4,5)P2 ELISA assay. * P<0.01 compared to the sample expressing LacZ (Student’s t-Test).
H,I) Effect of Dvl siRNAs on formation of PtdIns (4,5)P2 and phosphorylation of LRP6 at Ser1490. HEK293T cells were transfected with control siRNA or Dvl siRNA mixture targeting Dvl1, 2 and 3 for 48 hours and then treated with Wnt3a (50ng/ml) for 30mins. * P<0.01 compared to control siRNA transfection in the absence of Wnt3a (Student’s t-Test).
J) Interaction of Dvl3 with endogenous PIP5KIβ. HEK293T cells (Dvl) stably expressing Dvl3-HA7 whose expression level is similar to that of the endogenous Dvl3 were used in immunoprecipitation by an anti-HA antibody. The parent HEK293T cells (HEK) were used as a control. Immunocomplexes were detected by the anti-Dvl3 and anti-PIP5KIβ antibodies.
K) Effect of purified recombinant Dvl3 protein on kinase activity of purified recombinant PIP5KIβ protein. PIP5KIβ (50 nM) was incubated with GST or Dvl3 proteins at concentrations indicated in the figure for 2 hours at room temperature. One-tenth of the samples was taken for Western blotting and the rest was subjected to in vitro kinase assay with PtdIns (4)P as a substrate. The product PtdIns (4,5)P2 is separated by TLC, detected, and quantified by a phosphoimager.
We detected expression of Fz2, 3, 4, 5, and 6 in HEK293T cells by RT-PCR and made and validated two sets of siRNAs for each of these Fz genes (Table SII). Fz5 siRNA showed the strongest inhibition of Wnt3a-induced accumulation of β-catenin, whereas Fz2 and Fz4 siRNAs also had some inhibitory effect (Fig. S5A and Table SII). Combination of Fz2, 4, and 5 siRNAs virtually abolished Wnt3a-induced accumulation of β-catenin (Table SII). The combination also abrogated Wnt3a-induced formation of PtdIns (4,5)P2 (Fig. 3C) and phosphorylation of LRP6 at Ser1490 (Fig. 3D, Table SII, and Fig. S5, B and C). Expression of Fz5, Fz2 and Fz4 stimulated formation of PtdIns (4,5)P2 (Fig. 3E) and Wnt3a-induced phosphorylation of Ser1490 (Fig. 3F and S5, D and E). These results together indicate that Wnt3a acts through Fz to stimulate PtdIns (4,5)P2 formation in HEK293T cells and regulates LRP6 phosphorylation.
As Dvl is required for phosphorylation of LRP6 (9, 10), we questioned whether Dvl might have a role in formation of PtdIns (4,5)P2. Expression of Dvl1-3 increased the amount of PtdIns (4,5)P2 in HEK293T cells (Fig. 3G and S6A). When HEK293T cells were transfected with a mixture of three Dvl siRNAs targeting each of the three Dvl isoforms (16), both Wnt3a-induced formation of PtdIns (4,5)P2 and phosphorylation of Ser1490 were inhibited (Fig. 3, H and I and S6B). Dvl is a scaffold protein with no known enzymatic domains. Dvl and PIP5KIβ coimmunoprecipitated when they were overexpressed in HEK293T cells (Fig. S6C). We mapped Dvl interaction site to the N-terminal half of PIP5KIβ kinase domain (Fig. S6D) and PIP5KI-binding sites to two fragments of Dvl1 that contain the DIX and PDZ domain, respectively (Fig. S6E). The interaction of Dvl3 and PIP5KIβ was also examined in a HEK293T cell line that stably expressed Dvl3 carrying seven Hemagglutinin (HA) tags at its C-terminus at a level lower than that of endogenous Dvl3 (the right upper panel of Fig. 3J). Although we did not observe coimmunoprecipitation in the absence of Wnt3a, an interaction of Dvl3-HA with endogenous PIP5KIβ was detected in the presence of Wnt3a (Fig. 3J), suggesting that Wnt3a may regulate the interaction. In a pull-down assay with recombinant proteins prepared in E coli., Dvl1 interacted with PIP5KIβ and Iα in vitro (Fig. S6F). We then tested whether Dvl could directly regulate PIP5KI kinase activity. Using the recombinant Dvl3 and PIP5KIβ prepared from E coli., we found that, in an in vitro kinase assay, Dvl directly stimulated PIP5KIβ in a dose-dependent manner (Fig. 3K and S6G). All of the above data, together with the knowledge that Fz can interact with Dvl and recruit it to the membrane (9, 17-22), suggest that Wnt3a may induce, through Fz, Dvl to bind and activate PIP5KI.
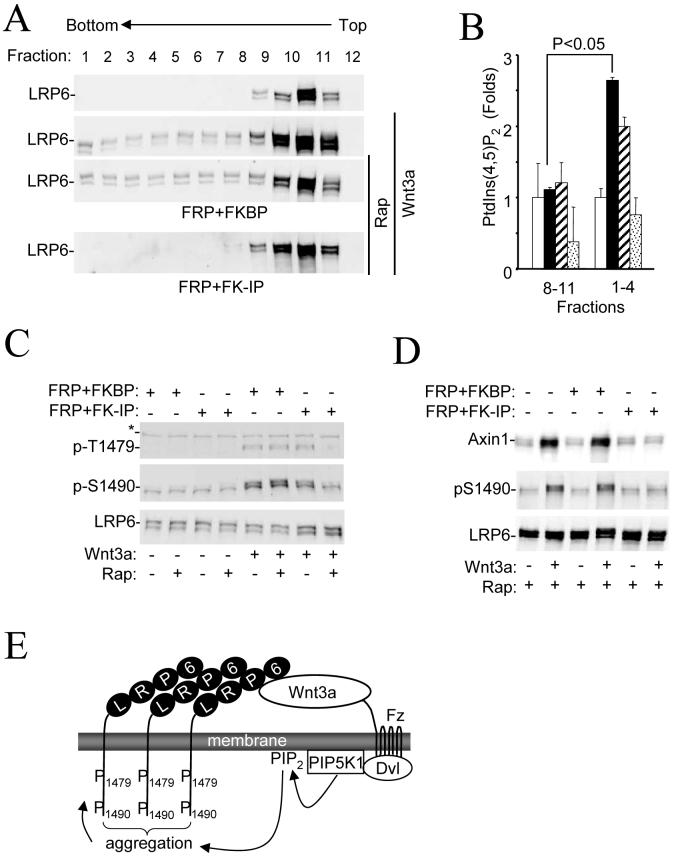
We next tested whether PtdIns (4,5)P2 is required for Wnt3a-induced formation of LRP6 aggregates, referred to as “signalosomes”, which precedes phosphorylation of LRP6 at Thr1479 (9). We used sucrose density gradient centrifugation to detect LRP6 aggregates in cells treated with Wnt3a (Fig. 4A). Fractions that contained LRP6 aggregates also had a higher PtdIns (4,5)P2 content than those containing non-aggregated LRP6 (Fig. 4B). Importantly, the aggregation was sensitive to the elimination of PtdIns (4,5)P2 through rapamycin-induced PtdIns (4,5)P2 hydrolysis (Fig. 4A). We also examined LRP6 aggregation using confocal microscopy in Hela cells expressing LRP6-YFP (9). We observed the aggregates in control cells, but not in cells transfected with the PIP5KI siRNAs (Fig. S7). Elimination of PtdIns (4,5)P2 also led to decreased phosphorylation of Thr1479 (Fig. 4C) (9). Therefore, we conclude that PtdIns (4,5)P2 is required for Wnt-induced LRP6 aggregation and Thr1479 phosphorylation.
Figure 4. Requirement of PtdIns (4,5)P2 for formation of LRP6 aggregate and membrane translocation of Axin and GSK3.
A) Requirement of PtdIns (4,5)P2 for Wnt3a-induced LRP6 aggregation. HEK293T cells were transfected and treated with Wnt3a and rapamycin as indicated. Cell lysates were subjected to sucrose density gradient ultracentrifugation, and fractions were analyzed by Western analysis.
B) PtdIns (4,5)P2 amounts in two sucrose density gradient ultracentrifugation fraction pools. Fractions 8-11 and 1-4 from A were pooled, and PtdIns (4,5)P2 amounts were measured by ELISA. Open bars correspond to the samples from the top panel of A; black bars to the second panel; shaded bars to the third panel; and dotted bars to the last panel. The PtdIns (4,5)P2 amounts are presented relative to those in untreated cells.
C) Requirement of PtdIns (4,5)P2 for phosphorylation of LRP6 at Thr1479. HEK293T cells were transfected with plasmids as indicated for 20 hrs and then treated with Wnt3a (20ng/ml) for 30mins in the presence or absence of rapamycin (100nM) before they were collected for immunoblotting analysis.
D) Requirement of PtdIns (4,5)P2 for Wnt3a-induced membrane recruitment of Axin1. HEK293T cells were transfected and treated as indicated. The membrane fractions were prepared and analyzed by Western analysis.
E) A model for Wnt3a cross-membrane signaling.
Because LRP6 aggregates appear to have a high affinity for Axin (9) and Axin membrane translocation is required for GSK3-mediated phosphorylation of Ser1490 (10), we examined if PtdIns (4,5)P2 is involved in Wnt-induced Axin membrane translocation. Elimination of PtdIns (4,5)P2 using the rapamycin-inducible system abrogated Wnt3a-idncued Axin translocation (Fig. 4D). Putting all these results together, we propose a model (Fig. 4E) that Wnt3a regulates the activity of PIP5KI via Fz and Dvl and induces the formation of PtdIns (4,5)P2. PtdIns (4,5)P2 is required, but not sufficient, for LRP6 aggregation and phosphorylation at Thr1479 and Ser1490 as well as Axin translocation. Precise mechanisms by which PtdIns (4,5)P2 regulates these events need to be further studied.
Supplementary Material
Acknowledgments
We thank Dr. De Camilli for making available resources from his lab in connection to this work, for discussion and for critical reading of the manuscript. We thank Zhong Li, Michelle Orsulak, Yazhou Zhang, Linling Tang, Wenzhong Liu, Ying Xi, Yizhong Wu, and Xiaoqing Gan for technical assistance, D. Sussman, C. Carpenter, R. Grosschedl, T. Balla, B. Williams, M.F. Roussel, X He, C. Niehrs, R. Nusse, and J. Nathans for providing experimental materials. This work is supported by grants from NIH (AR051476, CA132317, NS36251 and DA018343), and from NIDA to Yale Neuro-proteomics center.
REFERENCES
- 1.Logan CY, Nusse R. Annu Rev Cell Dev Biol. 2004;20:781. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 2.Reya T, Clevers H. Nature. 2005 Apr 14;434:843. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 3.Clevers H. Cell. 2006 Nov 3;127:469. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 4.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. Nat Rev Genet. 2004 Sep;5:691. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 5.Mani A, et al. Science. 2007 Mar 2;315:1278. [Google Scholar]
- 6.Davidson G, et al. Nature. 2005 Dec 8;438:867. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- 7.Nusse R. Nature. 2005 Dec 8;438:747. doi: 10.1038/438747a. [DOI] [PubMed] [Google Scholar]
- 8.Zeng X, et al. Nature. 2005 Dec 8;438:873. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilic J, et al. Science. 2007 Jun 15;316:1619. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 10.Zeng X, et al. Development. 2007 [Google Scholar]
- 11.Harland R, Gerhart J. Annu Rev Cell Dev Biol. 1997;13:611. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- 12.Hsuan JJ, Minogue S, dos Santos M. Adv Cancer Res. 1998;74:167. doi: 10.1016/s0065-230x(08)60767-8. [DOI] [PubMed] [Google Scholar]
- 13.Doughman RL, Firestone AJ, Anderson RA. The Journal of membrane biology. 2003 Jul 15;194:77. doi: 10.1007/s00232-003-2027-7. [DOI] [PubMed] [Google Scholar]
- 14.Heo WD, et al. Science. 2006 Dec 1;314:1458. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varnai P, Thyagarajan B, Rohacs T, Balla T. J Cell Biol. 2006 Nov 6;175:377. doi: 10.1083/jcb.200607116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Mao J, Sun L, Liu W, Wu D. J Biol Chem. 2002;277:5977. doi: 10.1074/jbc.M111131200. [DOI] [PubMed] [Google Scholar]
- 17.Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Genes Dev. 1998;12:2610. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong HC, et al. Mol. Cell. 2003 Nov;12:1251. doi: 10.1016/s1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W, et al. Science. 2003;301:1391. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- 20.Cong F, Schweizer L, Varmus H. Development. 2004 Oct;131:5103. doi: 10.1242/dev.01318. [DOI] [PubMed] [Google Scholar]
- 21.Pan WJ, et al. Cell Res. 2004 Aug;14:324. doi: 10.1038/sj.cr.7290232. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama N, Yin D, Malbon CC. J Mol Signal. 2007;2:11. doi: 10.1186/1750-2187-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.