Abstract
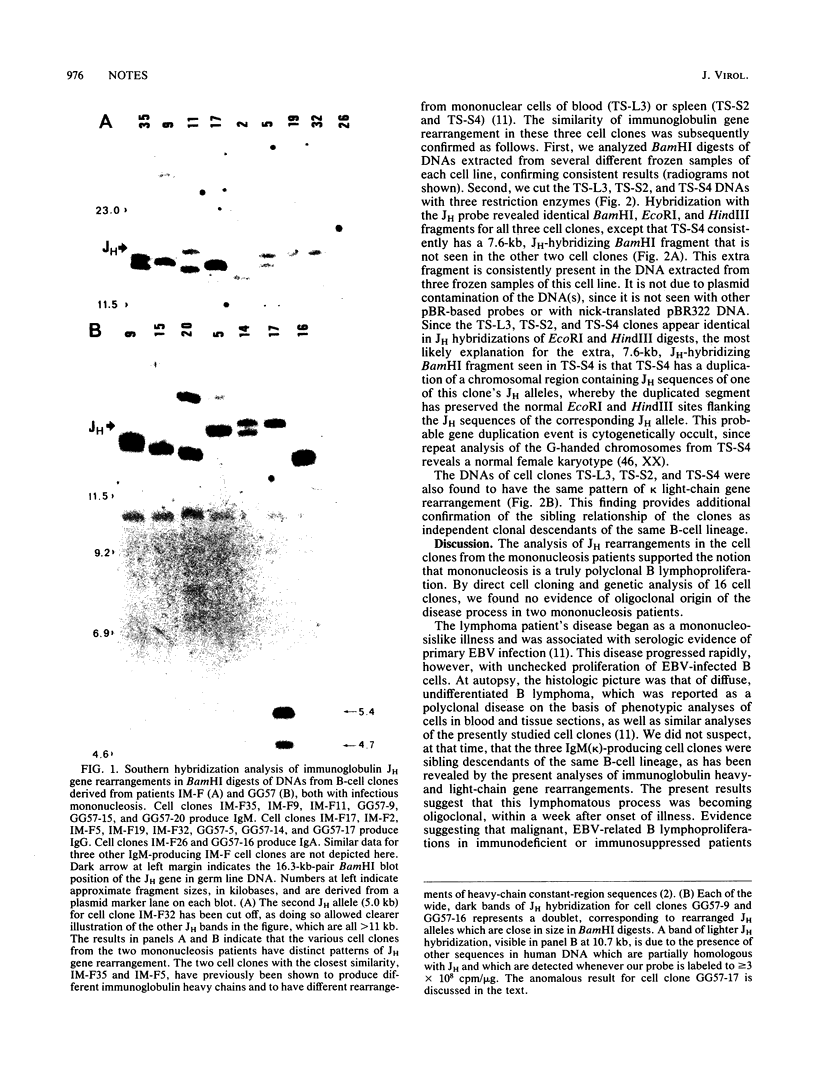
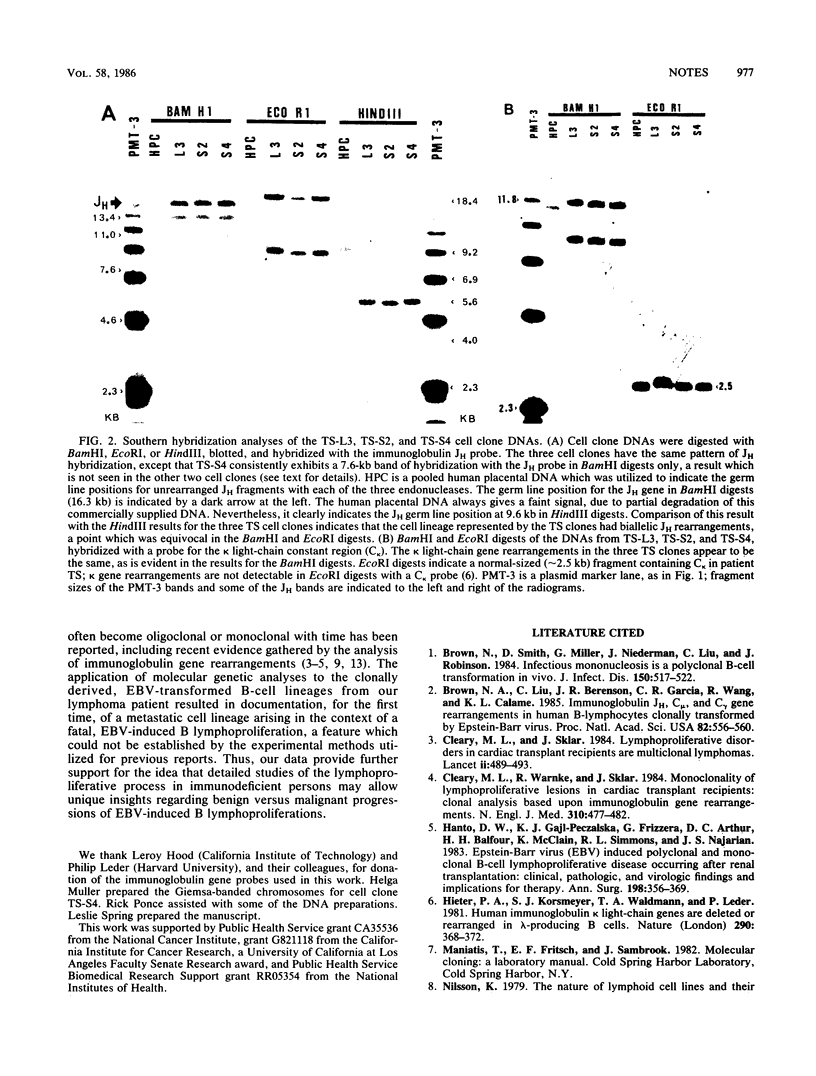
Analysis of immunoglobulin gene rearrangements in 16 B-cell lineages clonally propagated from two mononucleosis patients supported the notion that mononucleosis is a polyclonal B-lymphoproliferative disorder. Three of seven cell clones from a patient with a fatal B lymphoma revealed the same pattern of immunoglobulin gene rearrangement, indicating that this patient's disease was oligoclonal. The three similar clones were propagated from two sites (blood and spleen), indicating that they represent a metastatic cell lineage which arose during the patient's fatal B lymphoproliferation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown N. A., Liu C., Berenson J. R., Garcia C. R., Wang R., Calame K. L. Immunoglobulin JH, C mu, and C gamma gene rearrangements in human B lymphocytes clonally transformed by Epstein-Barr virus. Proc Natl Acad Sci U S A. 1985 Jan;82(2):556–560. doi: 10.1073/pnas.82.2.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N., Smith D., Miller G., Niederman J., Liu C., Robinson J. Infectious mononucleosis: a polyclonal B cell transformation in vivo. J Infect Dis. 1984 Oct;150(4):517–522. doi: 10.1093/infdis/150.4.517. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Sklar J. Lymphoproliferative disorders in cardiac transplant recipients are multiclonal lymphomas. Lancet. 1984 Sep 1;2(8401):489–493. doi: 10.1016/s0140-6736(84)92566-2. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Warnke R., Sklar J. Monoclonality of lymphoproliferative lesions in cardiac-transplant recipients. Clonal analysis based on immunoglobulin-gene rearrangements. N Engl J Med. 1984 Feb 23;310(8):477–482. doi: 10.1056/NEJM198402233100801. [DOI] [PubMed] [Google Scholar]
- Hanto D. W., Gajl-Peczalska K. J., Frizzera G., Arthur D. C., Balfour H. H., Jr, McClain K., Simmons R. L., Najarian J. S. Epstein-Barr virus (EBV) induced polyclonal and monoclonal B-cell lymphoproliferative diseases occurring after renal transplantation. Clinical, pathologic, and virologic findings and implications for therapy. Ann Surg. 1983 Sep;198(3):356–369. doi: 10.1097/00000658-198309000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieter P. A., Korsmeyer S. J., Waldmann T. A., Leder P. Human immunoglobulin kappa light-chain genes are deleted or rearranged in lambda-producing B cells. Nature. 1981 Apr 2;290(5805):368–372. doi: 10.1038/290368a0. [DOI] [PubMed] [Google Scholar]
- Purtilo D. T., Tatsumi E., Manolov G., Manolova Y., Harada S., Lipscomb H., Krueger G. Epstein-Barr virus as an etiological agent in the pathogenesis of lymphoproliferative and aproliferative diseases in immune deficient patients. Int Rev Exp Pathol. 1985;27:113–183. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robinson J. E., Brown N., Andiman W., Halliday K., Francke U., Robert M. F., Andersson-Anvret M., Horstmann D., Miller G. Diffuse polyclonal B-cell lymphoma during primary infection with Epstein-Barr virus. N Engl J Med. 1980 Jun 5;302(23):1293–1297. doi: 10.1056/NEJM198006053022306. [DOI] [PubMed] [Google Scholar]
- Robinson J. E., Smith D., Niederman J. Plasmacytic differentiation of circulating Epstein-Barr virus-infected B lymphocytes during acute infectious mononucleosis. J Exp Med. 1981 Feb 1;153(2):235–244. doi: 10.1084/jem.153.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer W. T., Ritz J., Finegold M. J., Guerra I. C., Rosenblatt H. M., Lewis D. E., Pollack M. S., Taber L. H., Sumaya C. V., Grumet F. C. Epstein-Barr virus-associated B-cell proliferations of diverse clonal origins after bone marrow transplantation in a 12-year-old patient with severe combined immunodeficiency. N Engl J Med. 1985 May 2;312(18):1151–1159. doi: 10.1056/NEJM198505023121804. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]