Abstract
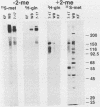
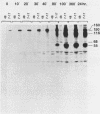
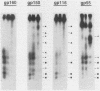
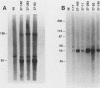
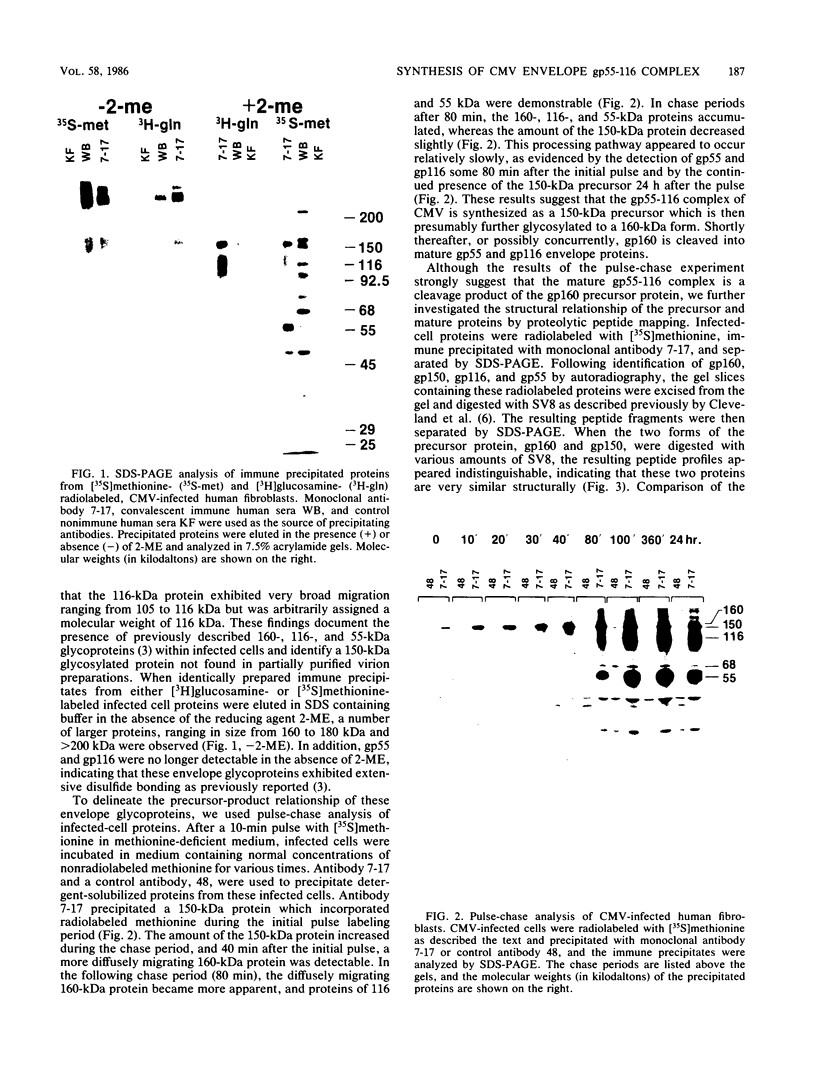
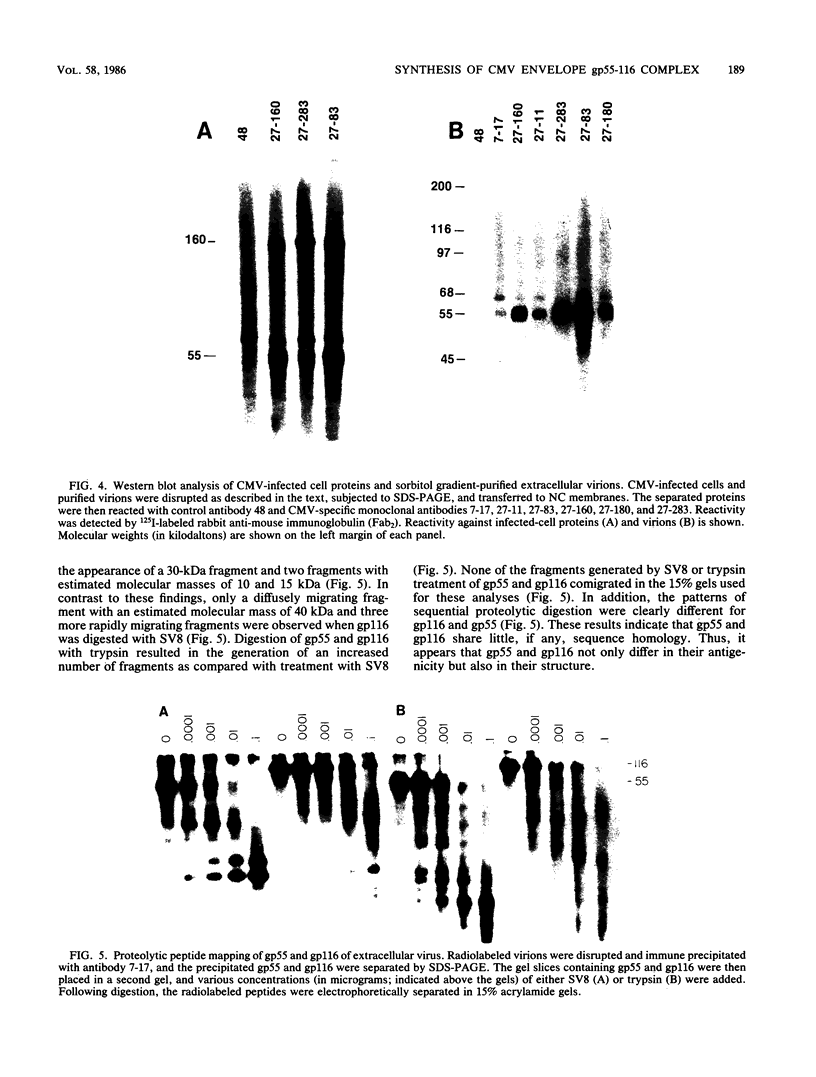
The envelope of human cytomegalovirus has been reported to contain between three and eight glycoproteins. Major constituents of the envelope include two abundant glycoproteins with estimated molecular weights of 55,000 (gp55) and 116,000 (gp116). These two glycoproteins have been shown to exist as a disulfide-linked complex (gp55-116) within the envelope of mature virions. Utilizing a panel of monoclonal antibodies reactive with the gp55-116 complex, we characterized the synthesis and processing of these two virion proteins. Infected cells were shown to contain two glycosylated proteins of 160,000 and 150,000 daltons as well as the mature gp55 and gp116. Pulse-chase analysis indicated that gp150 was a precursor protein of gp160. The mature gp55 and gp116 were generated, in turn, by cleavage of gp160. Antigenic and structural analysis revealed that gp55 and gp116 shared little structural homology and no detectable antigenic cross-reactivity. The results of this study are discussed in relation to the synthesis of envelope proteins of other herpesviruses.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen H. K. Complement-fixing and virus-neutralizing antibodies in cytomegalovirus infection as measured against homologous and heterologous antigen. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(4):504–508. doi: 10.1111/j.1699-0463.1970.tb04334.x. [DOI] [PubMed] [Google Scholar]
- Britt W. J. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology. 1984 Jun;135(2):369–378. doi: 10.1016/0042-6822(84)90193-4. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Britt W., Evans L., Wehrly K., Nishio J., Cloyd M. Characterization of monoclonal antibodies reactive with murine leukemia viruses: use in analysis of strains of friend MCF and Friend ecotropic murine leukemia virus. Virology. 1983 May;127(1):134–148. doi: 10.1016/0042-6822(83)90378-1. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Farrar G. H., Oram J. D. Characterization of the human cytomegalovirus envelope glycoproteins. J Gen Virol. 1984 Nov;65(Pt 11):1991–2001. doi: 10.1099/0022-1317-65-11-1991. [DOI] [PubMed] [Google Scholar]
- Fiala M., Honess R. W., Heiner D. C., Heine J. W., Jr, Murnane J., Wallace R., Guze L. B. Cytomegalovirus proteins. I. Polypeptides of virions and dense bodies. J Virol. 1976 Jul;19(1):243–254. doi: 10.1128/jvi.19.1.243-254.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W. Protein counterparts of human and simian cytomegaloviruses. Virology. 1983 Jul 30;128(2):391–406. doi: 10.1016/0042-6822(83)90265-9. [DOI] [PubMed] [Google Scholar]
- Grose C., Edwards D. P., Weigle K. A., Friedrichs W. E., McGuire W. L. Varicella-zoster virus-specific gp140: a highly immunogenic and disulfide-linked structural glycoprotein. Virology. 1984 Jan 15;132(1):138–146. doi: 10.1016/0042-6822(84)90098-9. [DOI] [PubMed] [Google Scholar]
- Hampl H., Ben-Porat T., Ehrlicher L., Habermehl K. O., Kaplan A. S. Characterization of the envelope proteins of pseudorabies virus. J Virol. 1984 Nov;52(2):583–590. doi: 10.1128/jvi.52.2.583-590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Sapienza V. J., Carp R. I., Moon H. M. Analysis of structural polypeptides of purified human cytomegalovirus. J Virol. 1976 Dec;20(3):604–611. doi: 10.1128/jvi.20.3.604-611.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Diggelmann H. Extracellular cleavage of the glycoprotein precursor of Rous sarcoma virus. J Virol. 1979 Jan;29(1):285–292. doi: 10.1128/jvi.29.1.285-292.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak B., Sullivan C., Sarnow P., Thomas R., Bricout F., Nicolas J. C., Fleckenstein B., Levine A. J. Characterization of monoclonal antibodies and polyclonal immune sera directed against human cytomegalovirus virion proteins. Virology. 1984 Jan 30;132(2):325–338. doi: 10.1016/0042-6822(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Pass R. F., Stagno S., Britt W. J., Alford C. A. Specific cell-mediated immunity and the natural history of congenital infection with cytomegalovirus. J Infect Dis. 1983 Dec;148(6):953–961. doi: 10.1093/infdis/148.6.953. [DOI] [PubMed] [Google Scholar]
- Pereira L., Dondero D., Roizman B. Herpes simplex virus glycoprotein gA/B: evidence that the infected Vero cell products comap and arise by proteolysis. J Virol. 1982 Oct;44(1):88–97. doi: 10.1128/jvi.44.1.88-97.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Hoffman M., Gallo D., Cremer N. Monoclonal antibodies to human cytomegalovirus: three surface membrane proteins with unique immunological and electrophoretic properties specify cross-reactive determinants. Infect Immun. 1982 Jun;36(3):924–932. doi: 10.1128/iai.36.3.924-932.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Hoffman M., Tatsuno M., Dondero D. Polymorphism of human cytomegalovirus glycoproteins characterized by monoclonal antibodies. Virology. 1984 Nov;139(1):73–86. doi: 10.1016/0042-6822(84)90331-3. [DOI] [PubMed] [Google Scholar]
- Pinter A., Lieman-Hurwitz J., Fleissner E. The nature of the association between the murine leukemia virus envelope proteins. Virology. 1978 Dec;91(2):345–351. doi: 10.1016/0042-6822(78)90382-3. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Esber E., Saral R., Manischewitz J. F., Rogers J. L., Rook A. H., Santos G. W., Burns W. H. HLA-restricted cytotoxic T lymphocyte and nonthymic cytotoxic lymphocyte responses to cytomegalovirus infection of bone marrow transplant recipients. J Immunol. 1981 May;126(5):2036–2041. [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Rook A. H., Manischewitz J. F., Jackson L., Moreschi G., Santos G. W., Saral R., Burns W. H. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982 Jul 1;307(1):7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- Rasmussen L., Kelsall D., Nelson R., Carney W., Hirsch M., Winston D., Preiksaitis J., Merigan T. C. Virus-specific IgG and IgM antibodies in normal and immunocompromised subjects infected with cytomegalovirus. J Infect Dis. 1982 Feb;145(2):191–199. doi: 10.1093/infdis/145.2.191. [DOI] [PubMed] [Google Scholar]
- Sarov I., Abady I. The morphogenesis of human cytomegalovirus. Isolation and polypeptide characterization of cytomegalovirions and dense bodies. Virology. 1975 Aug;66(2):464–473. doi: 10.1016/0042-6822(75)90218-4. [DOI] [PubMed] [Google Scholar]
- Stinski M. F. Human cytomegalovirus: glycoproteins associated with virions and dense bodies. J Virol. 1976 Aug;19(2):594–609. doi: 10.1128/jvi.19.2.594-609.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zezulak K. M., Spear P. G. Limited proteolysis of herpes simplex virus glycoproteins that occurs during their extraction from vero cells. J Virol. 1984 Apr;50(1):258–262. doi: 10.1128/jvi.50.1.258-262.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., van den Hurk J. V., Gilchrist J. E., Misra V., Babiuk L. A. Interactions of monoclonal antibodies and bovine herpesvirus type 1 (BHV-1) glycoproteins: characterization of their biochemical and immunological properties. Virology. 1984 Jun;135(2):466–479. doi: 10.1016/0042-6822(84)90201-0. [DOI] [PubMed] [Google Scholar]