Abstract
We have compiled the p73-mediated cell cycle arrest and apoptosis pathways. p73 is a member of the p53 family, consisting of p53, p63 and p73. p73 exists in several isoforms, presenting different domain structures. p73 functions not only as a tumor suppressor in apoptosis but also as differentiator in embryo development. p53 mutations are responsible for half of the human cancers; p73 can partially substitute mutant p53 as tumor suppressor. The pathways we assembled create a p73-centered network consisting of 53 proteins and 176 interactions. We clustered our network into five functional categories: Upregulation, Activation, Suppression, Transcriptional Activity and Degradation. Our literature searches led to discovering proteins (c-Jun and pRb) with apparent opposing functional effects; these indicate either currently missing proteins and interactions or experimental misidentification or functional annotation. For convenience, here we present the p73 network using the molecular interaction map (MIM) notation. The p73 MIM is unique amongst MIMs, since it further implements detailed domain features. We highlight shared pathways between p53 and p73. We expect that the compiled and organized network would be useful to p53 family-based studies.
INTRODUCTION
p73 is a tumor suppressor protein. It is a member of the p53 family that is composed of p53, p63 and p73 (1,2). p53 is the most well-known tumor suppressor and has been suggested to be mutated in over half of the human cancers (3). It was thought to be the only member of the family until p73 was discovered in 1997 (4). In addition to the p73's tumor suppression task, p63 and p73 play roles in the development at the embryonic stage, particularly of the limb, epithelial, craniofacial, neuronal and pheromonal, respectively (5–7). The availability of data relating to the interactions with p73 should be useful in drug discovery (8,9).
All p53 family members display similar domain structures, having an N-terminal transactivation (TA) domain, a DNA-binding domain (DBD) and an oligomerization domain (OD). p73 and p63 share a sterile alpha motif domain (SAM) and an inhibitory domain (ID) at their C-termini. In addition to the similarity in the domain structures, p73 and p63 have a higher sequence homology as compared with p53. It has been further suggested that all family members have evolved from a p63/p73-like ancestral gene, which can be supported by the existence of SAM domain in squid p53 (1,4,10). The hierarchical evolution tree of the family also places p53 at the bottom, suggesting p53 as the newly evolved gene among the family members (2). p53, p63 and p73 share a high sequence similarity, especially in their DBD's. The homology between p73 and p53 is 29% in the TA domain, 63% in the DBD and 38% in the OD (11). Consequently, family members have common target genes, e.g. p21 (4,12) as well as their unique targets such as CaN19 for p73 (13). Both p53 and p73 are tumor suppressors, while p63 and p73 play a role in tissue differentiation. All three proteins form a complex network, which can be of significance in cancer formation, and p53 requires both p73 and p63 for proper function (14,15). However, how this complex mechanism is regulated is still unknown. Given that p53 is the most widely studied, we focus on p73 and its relationship with p53.
Despite high functional and sequential similarity, the p73 promoter does not share homology with the p53 promoter (16) and again unlike p53, p73 is rarely mutated in human cancers (17). p73 knockout mutants do not show rapid tumor formation; instead, some defects in normal development are detected (7). Fewer stimuli were identified for p73 as compared with p53; γ-IR and some chemotherapeutic drugs, such as cisplatin lead to p73-induced cell death (1). Several p73 isoforms are encoded with C- and/or N-terminus truncations. Dimers and tetramers can be formed not only among isoforms of p73, but also among the family members, p73 and p53. The affinities for the formation of hetero-oligomers are lower than of homo-oligomers in p73 (18). Both wild-type and some of the mutant p53 forms can be involved in hetero-tetramer formation (19,20).
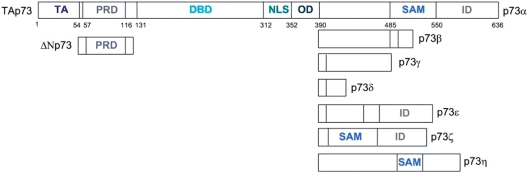
There are seven C-terminus truncation types in p73, some of which are tissue specific (20,21). N-terminus truncated isoforms are formed by an alternate promoter in intron 3 (7) and they can have C-terminus truncations regardless of the N-terminus status (Figure 1). If a truncation occurs at the N terminus, the protein is labeled as ΔNp73; otherwise as TAp73. ΔN isoforms lack the TA domain and their TA activity is significantly reduced. Having the same domain structure as TAp73 apart from the absence of the TA domain, ΔNp73 can oligomerize with p53 and p73. ΔNp73 can also compete for binding to target the DNA sites of these proteins. Thus, ΔNp73 has an inhibitory effect on p53 and p73, at the oligomerization and the DNA-binding levels (7,20). Interestingly, it was also observed that p53 and TAp73 induced TA of ΔNp73, forming a negative feedback loop (22). It is also known that ΔNp73 is overexpressed in human cancers (23). The ratio between the ΔN and TA isoforms, rather than the p73 mutations, is the key factor in the determination of cell fate (17,22).
Figure 1.
Isoforms of p73. The longest p73 isoform is TAP73α. Partial or full domains may be missing in the isoforms with N- and/or C-terminus truncations.
To further clarify the tumor suppression activity mediated by p73, we compiled its interactions which govern p73 regulation and function. We assembled and analyzed the network that centers on p73. To better organize and disseminate the data, we represented the network in MIM format. A brief comparison with the interaction network of p53 (24), is also made.
MATERIALS AND METHODS
Data compilation
Several databases (DIP, STRING, Biocarta, e-MIM, DOQCS, Biopax, Bind, Patika, Puma2, Biocyc, Mpact, Transpath, Predictome, Indigo, MINT, HPRD, BioGRID) of protein–protein interactions (PPI) and protein interaction networks are scanned for interactions of p73. In addition, a detailed literature search on article databases has been carried out, independent of the results obtained at the first step. This data compilation process is until August 2007. The interactions are verified by more than one independent source, where available, before being incorporated into the map. The independent sources could either be two articles that do not take the information from the same reference or one interaction database and one article. In those cases where the interaction presented in the article is validated through a small-scale experiment such as yeast two-hybrid or affinity purification, one reference was taken as sufficient. However, if the interaction obtained from a single reference is produced as a result of a large-scale experiment, the information was not incorporated into the map, since high-throughput data does not necessarily point out to a real physical association. We also looked for additional support before incorporating the single cited interactions into the network. Based on the supportive information, single referenced nodes can be classified into three categories: (i) proteins with available information related to specific binding sites; this could indicate an assurance of the existence of binding interaction (e.g. Itch in D6); (ii) proteins that are cited as part of the p53 network (e.g. c-Jun in A53), which may suggest that they also have a function in p73 network and (iii) proteins whose interaction sites are known and are also cited as existing in the p53 network (e.g. Chk1 in A31).
When contradictory information was encountered, the publication years and sources were compared. Under such circumstances, a more detailed search on these proteins has been performed by investigating their properties and interactions apart from the pathways related to p73 function. The decision as to the incorporation of the interactions has been reached only after this detailed analysis was made.
PPI network diagrams
Network diagrams can be classified into three main categories: explicit, heuristic and combinatorially complex. Heuristic and combinatorial diagrams can display large networks in a compact form, covering all possible interactions, without space and time restrictions. On the other hand, explicit diagrams include also the directed and sequential relationships of the pathways (25). Thus, heuristic and combinatorial diagrams can present the logic of the network in a human-readable format, while explicit diagrams are more suitable for computer simulations (26).
Several notations have been proposed for the representation of processes in interaction networks. Two well-known notations are the molecular interaction maps (MIM), proposed by Kohn and coworkers (24,27) and process diagrams, proposed by Kitano et al. (28,29). While being able to represent explicit diagrams, the MIM notation is prepared mainly for construction of heuristic and combinatorially complex diagrams (26). On the other hand, process diagrams are generally used for the construction of explicit diagrams (25). Detailed comparisons of notations can be found in the literature (25,30). Considering the number and characteristics of p73 interactions, we have decided to use the MIM notations for the representation of the p73 interaction network.
MIM notation and symbols
In a MIM each species is represented at one place only. The interactions, complexes and effects of the proteins or interactions are represented using defined symbols. In the MIM notation, the symbols representing the interactions are mainly grouped into two types, reaction symbols and contingency symbols (Figure 2b). By classifying the relations among nodes (proteins or promoters), the reaction symbols define physical interactions, protein complexes, covalent binding and other such events. Contingencies represent effects like inhibition and stimulation (25), which may emerge from and be directed to nodes or edges (reactions or contingencies of the MIM). The notation of MIM can be found in the literature together with examples (27).
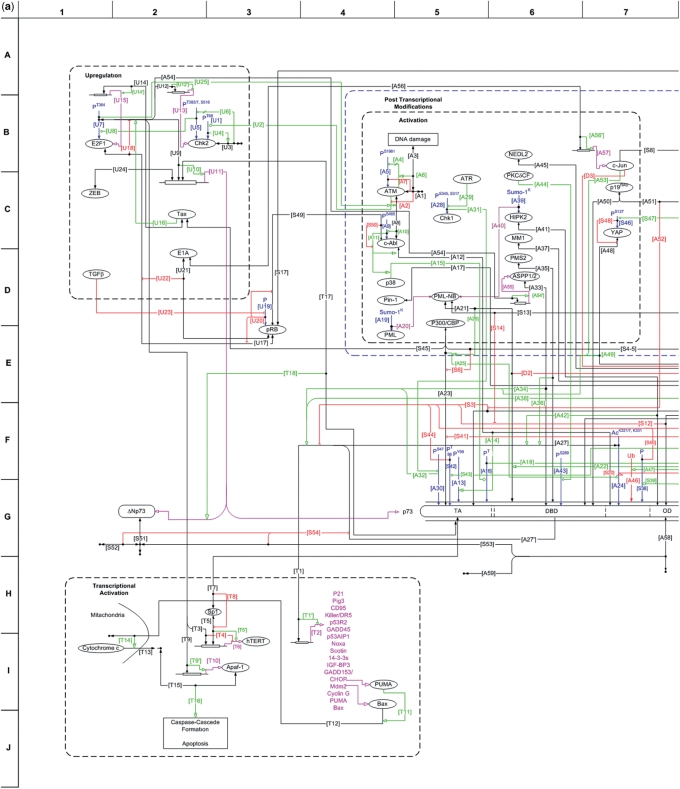
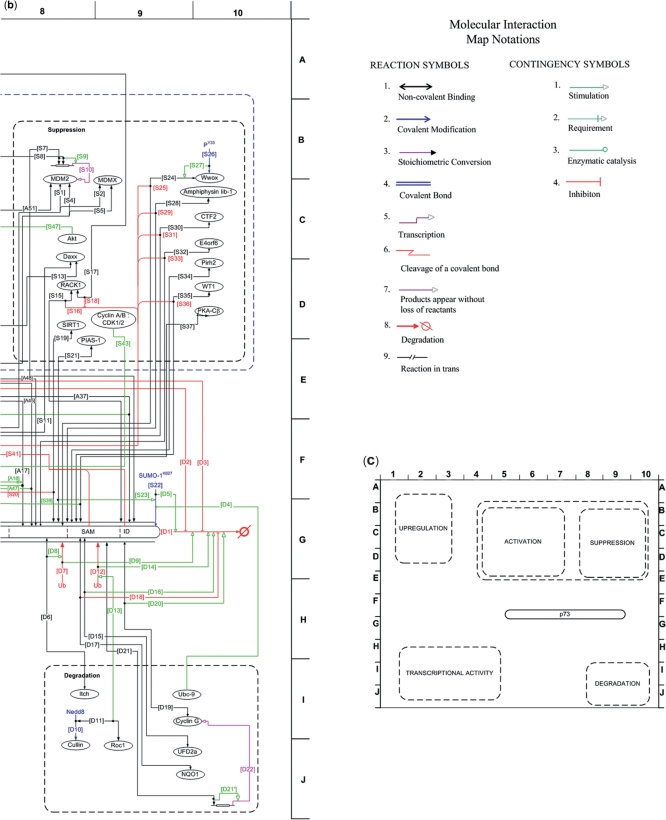
Figure 2.
MIM of p73. A softcopy of the MIM of p73 can be downloaded from ‘http://www.prc.boun.edu.tr/PRC/software.html’. (a) The MIM of the p73 protein, including the interactions related to cell cycle arrest and apoptosis mediated by p73. The interactions are grouped into functional categories, and each interaction is labeled accordingly. The details of each interaction can be found in Table 3, which includes the Annotations. (b) The list of symbols used in the representation of the interactions, i.e. the MIM Symbols (24). (c) The schematic representation of p73 MIM, highlighting the functional categories on the map.
Extensions made on the Kohn notation
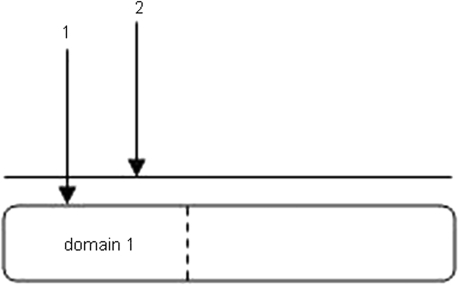
The domain representation that is already available in the Kohn notation (24) has been extended by a line, which is drawn above the node, representing the whole protein, regardless of the domains. The distinction between the interactions known to take place between specific domains of the involved protein, and interactions without such available detailed information, is incorporated into the map (Figure 3). Interaction number 1 is specific to domain 1, but interaction number 2 implies no currently known domain specificity. Therefore, an isoform lacking domain 1 will not have interaction 1, whereas the status of interaction 2 is unknown, since the place of the binding site involved in the interaction is not known.
Figure 3.
Extended domain representation. The extension made on MIM notation for interactions acting on the same protein, depending on the availability of information on domains; (1) an interaction occurring specifically through domain 1 of the protein is directed to this domain; (2) is a known interaction of the protein without available information on which domains are affected. Here the interaction line, arrow 2, is directed to the line drawn above the node, thus representing the whole protein.
As discussed in the Introduction section, the ratio among ΔN and TA isoforms is the key factor in cancer research on p73 (17,22). Thus, it is reasonable to represent the ΔN and TA isoforms separately on the p73 MIM. Almost all interactions are common for the ΔN and TA isoforms except for the ones taking part through the TA domain. These common interactions are not shown on the ΔN isoform to avoid redundancy.
Assessment of data
The nodes of p73 MIM are grouped into functional categories, and the interactions are labeled accordingly. Each label is a combination of a letter, representing the category interaction it is associated with, and a number. While there is no such restriction, in most cases the numerical sequence of interaction labels represent the occurrence of interactions on the pathways (see Table 3, Annotations).
Table 3.
Molecular interaction map annotations
| Edge | Details |
|---|---|
| Upregulation | |
| [U1] | Chk2 is phosphorylated at Thr68 (50,51). |
| [U2] | ATM induces phosphorylation of Chk2 (50,51). |
| [U3] [U4] | Phosphorylated Chk2 mediates its own dimerization (51,52). |
| [U5] [U6] | Chk2 dimer is activated further by regulating its own autophosphorylation (in trans at residues 383, 387 and 516.) (51,52). |
| [U7] | E2F1 is phosphorylated at its consensus Chk phospohorylation site; Ser364, which results in its activation (40,53). |
| [U8] | Activated Chk2 catalyzes the phosphorylation of E2F1 (40,53). |
| [U9] [U10] | p73 promoter contains E2F1 responsive elements. E2F1 can bind to these elements. But whether it can bind selectively to the promoter regions of different isoforms or it can induce both of them simultaneously is still a controversial issue (54,53). |
| [U11] | p73 promoter becomes transcriptionally active via E2F1 (54,11). |
| [U12] | E2F1 upregulates Chk2 (51,55). |
| [U13] | Since E2F1 can also stimulate the transcription of Chk2, it can be stated that there is a positive feedback loop among Chk2 and E2F1 (51,55). |
| [U14] [U14′] [U15] [U16] | Tax increases the level of E2F-1 (56). |
| [U17] | pRB directly binds to active E2F1 (57,58). |
| [U18] | Activated E2F1 protein can induce the apoptotic activity of p73. If the cell wants to stay arrested in the cell cycle, pRB mediates inhibiton of the E2F1 effect on p73 (24,57,59,60). |
| [U19] | If pRB is phosphorylated, its functioning is disturbed (57,58). |
| [U21 [U22] | E1A is a viral protein and it indirectly enhances the upregulation of p73; because it can bind to pRB and inhibit pRB mediated E2F1 repression (57,61). |
| [U23] | TGFβ prevents the phosphorylation of pRB. Thus, it indirectly stimulates the inhibition effect of pRB on E2F1 (54). |
| [U24] | On the intronic fragment, which lies between the promoters of TAp73 and ΔNp73, there are six consensus ZEB-binding sites. ZEB binding on p73 promoter is a known fact; however, how this protein regulates the amounts of the isoforms is still a controversial topic (62). |
| Activation | |
| [A1] [A2] | ATM normally exists as an inactive dimer (51,63). |
| [A3] | The configuration of ATM changes as a result of its binding to the damaged DNA (51,63). |
| [A4] | ATM can catalyze its autophosphorylation in trans at Ser1981 (51,63). |
| [A5] | ATM is phosphorylated at Ser1981 (51,63). |
| [A6] | The configurational change of ATM is required for the stimulation of its autophosphorylation (51,63). |
| [A7] | Phosphorylation of ATM inhibits its dimer formation. In this way, ATM is fully activated (51,63). |
| [A8] | ATM interacts with c-Abl (64,65). |
| [A9] | c-Abl is phosphorylated at Ser465 (64,65). |
| [A10] | The interaction of ATM and c-Abl results in phosphorylation of c-Abl (64,65). |
| [A11] | c-Abl becomes active via its phosphorylation. After its activity is established, it can start the signaling cascade, which leads to the stabilization and activation of p73. In response to DNA damage, c-abl signaling cascade requires the following events in sequential order: the phosohorylation of p73 which is mediated by p38; conformational change of p73 which is induced by Pin1; participation of p73 in PML-NB and acetylation of p73 as a result of its interaction with p300 (37,66–68). |
| [A12] | c-Abl binds via its SH3 domain to p73's PXXP motif, which is between residues 322 and 339 (44,69). |
| [A13] | p73 is phosphorylated at Tyr99 (44,69). |
| [A14] | Formation of the p73:c-Abl complex is a requirement for the phosphorylation of p73 at its Tyr99 (44,69). |
| [A15] [A16] | p38 mediates the phosphorylation of p73. The phosphorylation sites on are Thr residues adjacent to prolines (66,68). |
| [A17] | To stabilize p73, Pin1 binds to it and induces its conformational change. Pin1-binding sites on p73 are 412,442,482 (67). |
| [A18] | p73 phosphorylation is a requirement for its association with Pin1 (67). |
| [A19] | PML is sumoylated at three lysine residues (70,71). |
| [A20] | PML should be covalently modified by SUMO-1, to form PML-NB complex (70,72). |
| [A21] | PML-NB binds to p73 and rearranges its half-life (66). |
| [A22] | Pin1 and p73 interaction is a step in the c-abl signaling cascade. The consecutive step after that is the formation of the PML-NB:p73 (66,67). |
| [A23] | p300/CBP (via its CH1 Domain) interacts with p73 (at its TA Domain) (73,74). |
| [A24] | p73 is acetylated at lysine residues; 321, 327 and 331 (37,66). |
| [A25] | The binding of p300/CBP to p73, stimulates acetylation of p73 (37,66). |
| [A26] | The acetylation of p73 which is mediated by p300/CBP is PML-NB dependent (66). |
| [A27] [A27′] | The last step of the c-abl signaling cascade is accomplished by the acetylation of p73, which leads to the enhancement of p73's TA activity (37,66,74). |
| [A28] | Chk1 is phosphorylated at Ser317 and Ser345. And this phosphorylation leads to its activation (75,76). |
| [A29] | The phosphorylation of Chk1 is mediated by ATR (75,76). |
| [A30] | p73 is phosphorylated at its TA; at Serine 47 (77). |
| [A31] | Chk1 can regulate the activity of p73 by enhancing its phosphorylation at its TA (40,77). |
| [A32] | Phosphorylation of p73 by Chk1 increases its proapoptotic activity (40,77). |
| [A33] | ASPP1/2 can bind to the DBD of both p73 and p63 (78). |
| [A34] | The binding of ASPP1/2 to p73 stimulates activation of genes; Bax, PUMA, PIG3 (78). |
| [A35] | The physical interaction between PMS2 and p73 stabilizes p73 by increasing its half-life (79). |
| [A36] | The stabilization process of p73, which is mediated by PMS2 results in the induction of apoptosis. And it is claimed that this process is somehow dependent on c-abl. Basing on this claim, it would be reasonable to propose that PMS2 participates in the c-abl signaling cascade (79). |
| [A37] | MM1 can bind to the C-terminus of p73 (80). |
| [A38] | Binding of MM1 enhances the TA of p73 on Bax and Pig3 (80). |
| [A39] | HIPK2 is modified by SUMO-1 (81). |
| [A40] | Sumoylated HIPK2 can enter in to PML-NB (81). |
| [A41] | HIPK2 and p73 form complex via their 817–907 and 345–380 residue ranges, respectively (39). |
| [A42] | Formation of p73:HIPK2 increases the apoptotic activity of p73. Since HIPK2 also localized in PML-NB, it would be logical to hypothesize that the action of HIPK2 is a part of the c-abl signaling cascade (39). |
| [A43] | p73 is phosphorylated at Ser289, which is located at DBD (82). |
| [A44] | PKCdCF stimulates phosphorylation of p73 at its DBD, thus increases its activity (82). |
| [A45] | p73:NEDL2 is modulated via p73's COOH terminal region (PY motif) and WW domain of NEDL2 (83). |
| [A46] | Ubiquitination of p73 results in its stabilization (83). |
| [A47] | NEDL2 regulates the stabilization of p73 by enhancing its ubiquitination (83,84). |
| [A48] | p73 and YAP directly interact via their PPPPY motif and WW domain, respectively (85). |
| [A49] | YAP can enhance the transcriptional activity of p73 by forming p73:YAP complex (85). |
| [A50] | p19ras (via the region between 56 and 87) interacts with p73 (at DBD) (86). |
| [A51] | p19ras can also interact with MDM2 via the same region which is used in its interaction with p73 (86). |
| [A52] | When p19ras: MDM2 complex is formed, MDM2 can not inhibit the activity of p73 anymore (86). |
| [A53] | c-Jun modulates the stability of p73 to stimulate its transcriptional activity. It is proposed that c-Jun affects only TAp73 isoforms. So, this protein may be one of the selective effectors among p73 isoforms (45). |
| [A54] [A54′] [A55] | E2F1 can bind and active the promoter of ASPPs (87). |
| [A56] [A56′] [A57] | E1A stimulates the transcription of c-Jun (88). |
| [A58] [A59] | Homo-dimerization and also homo-tetramerization of TAp73 is regulated by OD (4,18). |
| Suppression | |
| [S1] [S2] | Both MDM2 and MDMX bind to p73 at its N-terminus; between the residues 1–70 (43,89). |
| [S3] | MDM2/MDMX and p300 share the same binding site on p73. If this specific site is occupied by MDM2/MDMX, p300 cannot bind to p73 and enhance its TA activity (43,95). |
| [S4] [S5] | MDM2/MDMX competes with p73 for the same binding site on p300, which is located at its N-terminus (43). |
| [S6] | If MDM2/MDMX:p300 complex is formed, p73 can no longer be activated via c-abl signaling cascade (43). |
| [S7] | p73 can activate the promoter of MDM2. So, it would be reasonable to state that the feedback loop among MDM2 and p73 is negative (41,43). |
| [S8] | MDM2 can also be transactivated by c-Jun (90). |
| [S9] [S10] | The level of MDM2 is upregulated (41,43,90). |
| [S11] | S/P/T-rich C-terminal domain of Daxx, which lies between 667 and 740, can interact with the OD of p73. The interaction specific region of p73 is composed of 345–380 residue range (91,92). |
| [S12] | The interaction between Daxx and p73 leads the repression of the p73's transcriptional activity (91,92). |
| [S13] | Daxx is recruited to PML complex (91–93). |
| [S14] | Via forming a complex with PML, the repression activity of Daxx (on p73) can be relived (91,92). |
| [S15] | RACK1 regulates the activity of p73 by binding to its C-terminus (94). |
| [S16] | RACK1 regulates the activity of p73 negatively (94). |
| [S17] | pRB and RACK1 interacts physically (94). |
| [S18] | RACK1 imposes a transcriptional repression on p73. And this repression can be overcome by the formation of pRB:RACK1 complex (94). |
| [S19] | SIRT1 and p73 are interacting proteins (95). |
| [S20] | SIRT1 is a NAD-dependent deacetylase. After it binds to p73, it cleaves the acetylations, which have been modulated by p300. So, if the interaction between SIRT1 and p73 is established, the transcriptional activity of p73 is repressed; since c-abl mediated signaling cascade cannot survive without having the acetylated p73 residues (95). |
| [S21] | PIAS-1 binds to a, b and g types of p73 isoforms (96,97). |
| [S22] | There is a specific residue at the extreme C-terminus of p73 (Lys627) for sumoylation. Therefore, only p73a can be covalently modified by SUMO-1. The modifications regulated by SUMO-1 can alter the subcellular localization of p73 but does not effect much its transactivity (96,98). |
| [S23] | PIAS-1 can stimulate sumoylation of p73 (96,98). |
| [S24] | p73 and Wwox interacts via their PPPPY motif and first WW domain, respectively (99). |
| [S25] | If p73 forms a complex with Wwox, nuclear p73 is transported to the cytoplasm resulting in its inactivation of p73 (99). |
| [S26] [S27] | Wwox is phosphorylated at Y33 and this phosphorylation enhances its binding to p73 (99). |
| [S28] | By use of the SH3 domain of amphiphysin IIb-1 and C-terminus of p73b, amphiphysin IIb-1: p73b complex is formed (100). |
| [S29] | Formation of amphiphysin IIb-1: p73b complex results in relocalization of nuclear p73 in to cytoplasm, which is an inhibitory effect on p73's TA function (100). |
| [S30] | CTF2 interacts with p73 at it the 228–312 residue range (78). |
| [S31] | CTF2 suppresses the induction ability of p73 on p21 promoter (78). |
| [S32] | E4orf6 is a viral protein and it can bind to the common C-terminal region of p73a and p73b (48,101). |
| [S33] | E4orf6 downregulates the apoptotic activity of p73 (48,101). |
| [S34] | Pirh2 interacts with p73 (102). |
| [S35] | p73 binds to zinc finger region of WT1 (103). |
| [S36] | WT1 can inhibit the transcription activity of p73 by direct binding. It specifically overcomes the stimulation effect of p73 on MDM2 promoter (103). |
| [S37] | PKA-Cβ interacts with both N- and C-termini of p73 (104). |
| [S38] [S39] | Bound PKA-Cβ can catalyze phosphorylation of p73 (104). |
| [S40] | One of the suppression mechanisms of p73's transcriptional activity depends on the kinase activity of PKA-Cβ (104). |
| [S41] | SAM and ID inhibit the association of p300/CBP with p73 (105,106). |
| [S42] [S43] | Cyclin A/B:Cdk1/2 complexes are essential components in cell cycle because they can induce progression in to another phase in the cycle. If this complex is present, it can phosphorylate p73 on Thr86 (38,107). |
| [S44] | If p73 is phosphorylated by Cyclin A/B:Cdk1/2 complexes, it can no longer induce cell cycle arrest or apoptosis, since the cell is forced to end mitosis (38,107). |
| [S45] | Tax associates with p300 (108,109). |
| [S46] | YAP is phosphorylated at Ser127 (110). |
| [S47] | Phosphorylation of YAP is mediated by Akt (110). |
| [S48] | YAP can no longer stimulate the TA of activity of p73 if it is phosphorylated by Akt (85,110). |
| [S49] | pRb and c-abl forms a complex (24,60). |
| [S50] | In the cells which are in G0/G1 phase of mitosis, pRb binds to c-abl to interrupt the c-abl mediated signaling cascade (24,60). |
| [S51] [S52] | Similar to p53, DNp73 will be active after it forms a homo-tetramer (17,18,22). |
| [S53] | Heterotetramers can be formed among p73 family members (17,18,22,111). |
| [S54] | The activity of TAp73 is suppressed by DNp73 via two distinct mechanisms; either DN or TA isoforms competes for the same DNA-binding sites, such as p21, MDM2 and 14-3-3σ, or they form inactive heterotetramers (11,49). |
| Degradation | |
| [D1] | This edge represents the degradation of p73. |
| [D2] | If p73 is recruited in to PML-NB complex it is going to be protected from proteosomal degradation (66). |
| [D3] | c-Jun increases the stability of p73 by increasing its half-life. And this action prevents p73 from proteosomal degradation (45). |
| [D4] | Ubc9 catalyzes the interaction between SUMO-1 and p73 (98,112). |
| [D5] | SUMO-1 mediated modification recruits p73 in to proteosome (98). |
| [D6] | Itch is a ligase and it can bind to p73's PY motif via its WW domain (113). |
| [D7] | p73 is ubiquitinated as a result of p73:Itch formation (113). |
| [D8] | Itch promotes the ubiquitination of p73 (113). |
| [D9] | Ubiquitinated p73 is rapidly degraded (113). |
| [D10] | Cullin is modified by Nedd8 covalently (49,114). |
| [D11] | Neddylated-Cullin:Roc1 ring is formed to establish the core module of E3 ubiquitin ligase (49,114). |
| [D12] [D13] | When neddylated-Cullin:Roc1 is formed, p73 is exposed to ubiquitination (49,114). |
| [D14] | The detection of ubiquitinated p73 residues leads to its degradation (49,114). |
| [D15] | SAM domain of p73 is required for its interaction with UFD2a (115). |
| [D16] | UFD2a stimulates the degradation of p73 without changing its ubiquitination levels (115). |
| [D17] | NQO1 binds to SAM domain of p73 in a NADH-mediated manner (116). |
| [D18] | The physical interaction between NQO1 and NADH hinders p73 degradation, which is controlled by 20S proteosome (116). |
| [D19] | Cyclin G and p73 are interacting proteins (117). |
| [D20] | Cyclin G enhances degradation of p73 without implying any ubiquitinations on it (117). |
| [D21] [D21′] [D22] | The transcription of Cyclin G is enhanced by p73. So, basing on this fact, it would be logical to claim that there is a negative feedback loop between p73 and Cyclin G (118). |
| Transcirptional activation | |
| [T1] [T1′] | p73 binds to the promoters of various genes. |
| [T2] | p73 induces the transcription of following genes; p21, Pig3, CD95, GADD45, p53AIP1, Noxa, Scotin, 14-3-3s, IGF-BP3, GADD153/CHOP, Mdm2, Cyclin G, PUMA, Bax (13,17,19,41). |
| [T3] [T4] | If the level of E2F1 is high, it can repress hTERT level directly (119). |
| [T5] [T5′] [T6] | hTERT core promoter can directly be activated by Sp1 (111,120). |
| [T7] | p73 and Sp1 form a complex (111,120). |
| [T8] | The association among p73 and Sp1 inhibits the formation of the Sp1:hTERT promoter complex (111,120). |
| [T9] [T9′] [T10] | E2F1 stimulates the transcription of Apaf-1 (51,121). |
| [T11] | PUMA imposes a conformational modification on Bax (122,123). |
| [T12] | As a result of the modification in its conformation, Bax is delocalized in to mitochondria (122,123). |
| [T13] | Cytochrome c is released (122,123). |
| [T14] | Release of cytochrome c is mediated by Bax translocation (122,123). |
| [T15] [T16] | As the complex between Apaf1 and cytochrome c is formed, the mechanism for caspase cascade is triggered (122–124). |
| [T17] | TAp73 can bind to DNp73 promoter (22,125). |
| [T18] | One of the most important negative feedback loops in p73 network arises as TAp73 stimulates DNp73 transcription (22,125). |
In the majority of the publications aiming to test the p73 activity level, the transcription levels of one selected protein, mostly p21, is measured experimentally and the effect is stated as transcriptional activation or inhibition of p73. Unless stated otherwise, these effects are accepted as related to transcriptional activation of all target promoters (combined promoter). Mapping it on the p73 MIM, the stimulation or inhibition effect is directed to the interaction labeled ‘A29’.
Clustering
In agglomerative hierarchical clustering, the clustering starts at the binary level and attempts to get higher degree clusters until a united cluster is obtained (31). The main criterion of the algorithm is the distance between the members. The nearer the members are, the higher the probability that they will get under the same tree branch. Keeping the distance measure constant, groups are constructed according to three norms: single-link (farthest neighbor), complete-link (nearest neighbor) and average-link. In single-link clustering, the distance is designated as the smallest; in complete-link as the largest; and in average-link as the mean distance between the clustered elements of each iteration (31).
The input for the clustering algorithm was a connectivity matrix (CM). CM is binary, square and its dimension is n × n, where n is the number of the nodes in the network. Each element (i,j) of the CM corresponds to the availability of an interaction between any proteins in the network. The interacting nodes are recorded as CM (i,j) = 1 and noninteracting nodes as CM (i,j)= 0.
For clustering purposes, the tool EXpression Analyzer and DisplayER (EXPANDER) is used (32). Average-link agglomerative hierarchical clustering is employed with a distance expression inversely related to the Pearson correlation coefficient.
RESULTS
MIM of p73 is a heuristic MIM constructed for p73 mediated cell cycle arrest and apoptosis
Using the MIM notations proposed by Kohn et al. (27) with the additional notations including the extended domain representation, distinct representation of isoforms (see Materials and methods section), and organizing the information related to the interactions, the p73 MIM is constructed. At the data gathering stage only the interactions which are directly or indirectly related to the function of p73 in cell cycle arrest and apoptosis are taken into account. The interactions of p73 related to its role in differentiation are excluded from the map. For ease of use in future studies, the interaction information is stored in both computer readable (using Systems Biology Markup Language) and human readable formats.
In the p73 MIM, p73 is placed at the center of the map. The interactions are organized around the p73 node, without time and space constraints. The diverse roles of the p73 domains in the p73 functions are presented in detail. All interactions are directed to the relevant domain when information is available. The resulting p73 MIM consists of 53 nodes (proteins) and 176 edges (physical interactions or functional relations) (Figure 2a). In addition to the nodes and edges, the p73 MIM also contains several promoters and definition boxes. The boxes represent states or events, such as DNA damage or degradation. The p73 MIM includes only end points of some pathways, where the leading path is not directly related to p73 function, such as cleaved PKCδ, PKCδCF. We excluded the details of each box and such pathways, since they are beyond the scope of this work. The proteins represented as nodes of the p73 MIM may have interactions other than those displayed in the map, related to their individual functions which are apart from p73 mediated apoptosis.
A list of proteins represented in the map with their accession codes is also provided (Supplementary Table 1). A softcopy of the MIM of p73 can be downloaded from http://www.prc.boun.edu.tr/PRC/software.html.
Proteins of p73 MIM are grouped into functional categories
The proteins involved in the p73 MIM are grouped into functional categories, according to their effects on the network (Table 1). The defined categories are Upregulation, Activation, Suppression, Degradation, and Transcriptional Activation. A protein may have roles in more than one pathway, belonging to different functional categories. In such cases, the protein is located in a category according to its major function based on current literature. However p73, its ΔN isoform and pRB could not be assigned to a single functional category and are represented as separate nodes, since their roles are critical in several paths. The edges are labeled according to functional categories so that the letters used in the labels of the edges are the initials of their related categories (see Materials and methods section). The details of all interactions and their corresponding references can be found at the end of the article, in the Annotations (Table 3). The Annotations are organized with respect to the defined categories and the labels of the edges.
Table 1.
Proteins of the p73 MIM grouped into functional categories
| Functional categories | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Upregulation | Activation | Suppression | Degradation | Transcriptional activation | |||||
| E2F1 | (B:1) | Pin-1 | (D:4) | RACK1 | (D:8) | Cyclin G | (I:10) | hTERT | (I:3) |
| Chk2 | (B:2) | c-Jun | (B:7) | MDM2 | (B:8) | Ubc-9 | (I:10) | Apaf-1 | (I:3) |
| TGFβ | (D:1) | ASPP1/2 | (D:6) | MDMX | (B:9) | Cullin | (J:8) | PUMA | (I:4) |
| ZEB | (C:1) | p38 | (D:4) | CTF2 | (C:10) | Itch | (I:8) | Bax | (I:4) |
| Tax | (C:2) | PML | (E:4) | WT1 | (D:10) | NQO1 | (J:10) | Scotin | (I:4) |
| E1A | (D:2) | MM1 | (C:6) | SIRT1 | (D:8) | UFD2a | (J:10) | p21 | (H:4) |
| c-Abl | (C:4) | E4orf6 | (C:10) | Roc1 | (J:9) | GADD45 | (H:4) | ||
| YAP | (C:7) | Akt | (C:8) | 14-3-3σ | (I:4) | ||||
| p300/CBP | (D:5) | PIAS-1 | (E:8) | IGF-BP3 | (I:4) | ||||
| Chk1 | (C:5) | Daxx | (C:8) | GADD 153/CHOP | (I:4) | ||||
| Ungrouped | p19ras | (C:7) | Amphiphysin IIb-1 | (C:10) | Noxa | (I:4) | |||
| pRB | (D:3) | PKCδCF | (C:6) | Wwox | (B:10) | Killer/DR5 | (H:4) | ||
| p73 | (G:5-9) | ATM | (C:4) | Cyclin A/B:CDK1/2 | (D:9) | PIG3 | (H:4) | ||
| ΔNp73 | (G:2) | ATR | (C:5) | PKA-Cβ | (D:10) | CD95 | (H:4) | ||
| PMS2 | (D:6) | Pirh2 | (D:10) | p53AIP1 | (I:4) | ||||
| NEDL2 | (B:6) | p53R2 | (H:4) | ||||||
| HIPK2 | (C:6) | ||||||||
| PML-NB | (D:5) | ||||||||
In the related Results section, we investigated the following characteristics of each functional category: (i) the number of members of each category; (ii) important nodes which are assigned to each category and (iii) interrelationships between members of different categories.
The Upregulation functional category
The Upregulation category contains the nodes and edges that are related to the transcription of the p73. In this category, there are 6 nodes, 24 edges and 3 promoters. This group is directly related to the Activation category, since both categories are essential for p73 function. The key node of this unit is E2F1 which has the highest number of interactions and is of crucial biological importance in the activation of the p73 promoter. E2F1 is a transcription factor which controls the progression of the cell-cycle from the G1 to the S phase. E2F1 is activated via phosphorylation (U7; see label U7 in the Map and its details in the Annotations, Table 3). Active E2F1 can stimulate either cell cycle arrest or apoptosis, as in the case of the DNA damage. By enhancing the activity of ATM on Chk2 (labels U2, U11), E2F1 forms a positive feedback loop, where ATM activates Chk2 and Chk2 further increases the activity of E2F1 by phosphorylation. Additionally, E2F1 can bind to either p73 (U9) or the Apaf-1 promoter (T6) to stimulate the transcription of p73 and Apaf-1 (U10, T8). Furthermore, the activity of E2F1 is inhibited when it forms a complex with pRB. Under such circumstances, the concentration of E2F1 is regulated by pRB (U17–18).
There are controversial data in the literature regarding the viral oncogene E1A. According to some sources, E1A hinders the activation of p73 (33). In contrast, other studies (34) point out that E1A can activate p73, by binding to pRB and thus releasing E2F1. Following a comprehensive literature search, examining the sources of the arguments, and the dates of the relevant publications, it appears to us that E1A plays a role in the activation of p73 (U21–22). Consequently, only this aspect of the behavior of E1A is mapped on the p73 MIM.
The Activation and Suppression functional categories
The Activation and Suppression categories can be grouped under the same heading, since the nodes (proteins) belonging to those categories function by modifying p73 at the posttranscriptional level.
The nodes included in the Activation functional category are related to the posttranscriptional activation of the p73 protein. This category consists of 18 nodes, 2 promoters and 59 edges. Within this category DNA damage is represented by a box. p73 is activated when DNA damage results as an outcome of γ-IR or some chemotherapeutic drug (1). The main activation pathway of p73 is the signaling cascade initiated by the recognition of the damaged DNA by ATM (A3). After the activation of ATM, it activates c-Abl by phosphorylation (A8–10). c-Abl binds to the PXXP motif of p73 (A12) and causes its phosphorylation at the tyrosine (Y99) (A14) and threonine (A16) residues, directly and by activating p38, respectively. Phosphorylated p73 is recognized by Pin-1 isomerase with a consequent conformational change of p73. In the next step, p73 is recruited to PML-NBs where it is acetylated by p300/CBP and becomes fully active (A21, A25–27). In addition to the main pathway, there are several proteins which can lead to an increase in the activity of p73 either by direct binding or via posttranscriptional modifications.
The Suppression functional category contains 15 nodes, one promoter and 54 edges, which are related to the suppression of p73 function. The most important node in this category is MDM2, which is also a crucial node in the p53 network. MDM2 leads to the inactivation of p73, but does not degrade it. In addition, the MDM2 promoter is activated by p73, forming a negative feedback loop. Most of the proteins involved in p73 suppression are p73's first-degree interactions. The inhibitory effects of p73 SAM and ID domains, on the p73 protein itself are also assigned to the Suppression functional category (S41).
The Activation and Suppression functional categories are interrelated through few direct interactions, as can be seen from the p73 MIM. (Figure 2a) The functional relationship between these categories occurs largely at two successive steps, such that a member of one category blocks the effect of a member of the other, through p73. The Activation functional category is interrelated to the Upregulation by first-degree interactions, with a larger number of direct interactions as compared with the Suppression category.
The Degradation functional category
This category contains the nodes that are related to the degradation of p73. In this category, there are 7 nodes, 1 promoter and 22 edges. Among these seven, three regulate the ‘standard’ degradation mechanism via ubiquitination (D6–9, 11–14); one by sumoylation (D4–5, S22); three by forming a complex with p73 (D15–20).
Neddylated Cullin:Roc1 complex (D10–11) constitutes the core module of E3 ubiquitin ligase. The formation of the module stimulates the ubiquitination of p73 resulting in the proteasomal degradation of p73 (D12–14). In a similar manner, another ligase Itch, promotes an increase in the ubiquitination level of p73 (D6–9). Other than these, the SUMO conjugating enzyme, Ubc9 stimulates the sumoylation of p73 at the C-terminus (D4–5, S22). This is another way for p73 to be flagged for rapid degradation. Additionally, there is a negative feedback loop between p73 and Cyclin G. p73 stimulates the transcription of Cyclin G, while Cyclin G enhances its degradation (D19–22).
The nodes involved in this category exert their effects on p73 directly, without forming complex paths. There are no direct interrelationships with other categories.
The Transcriptional Activation functional category
The Transcriptional Activation category contains 6 nodes, 3 promoters, 17 edges and an Apoptosis box. In this functional category, the promoters which are activated by p73 are represented by one combined promoter (T1, T2) (see Materials and methods section). Currently available data on the interactions between p73 and specific promoters are provided in the Annotations (Table 3). If the edges directed to T1 are traced in the map, specific information about each promoter can be reached through the Annotations. Following TA of the promoters by p73, the translated proteins can promote cell cycle arrest and apoptosis.
The targets of p73 that are involved in the differentiation are not included in the list, since in principle the map is constructed for apoptosis. If a promoter is significant in the p73 MIM, other than being a target of p73, such as MDM2 promoter, then it is also represented in other relevant categories (e.g. S10). Other than the activated promoters, the apoptosis pathway that is induced by Bax and PUMA proteins (T9–10) and the inhibition of hTert transcription (T3–5) are of importance in this category. In the apoptosis pathway, PUMA and Bax are transcribed by p73 (T2). After being transcribed, PUMA causes conformational change of Bax, and induces its translocation inside the mitochondria (T9). Once Bax is located in the mitochondria, it causes cytochrome c release. Binding of Apaf-1 to the released cytochrome c leads to the formation of the caspase cascade and eventually apoptosis (T12–15). The hTert upregulation is a well-known cause of the maintenance of the telomerase length in most of human cancers. The binding of p73–Sp1 (T6), leads to inhibition of the hTERT transcription; thus is also important in the p73 mediated tumor suppression.
The interrelation of this category with other categories is limited to the interaction of p73 with promoters (T1) and activation of specific promoters by E2F1 (T3/6).
The MIM of p73 includes anticoherent interactions
To be preferred by evolution, the function of a protein should provide robustness to the cell. And mechanisms involved in a robust biological network should be regulated in a coherent manner to maintain homeostasis at any level of an organism (28). Therefore, if a specific form of a protein is a positive regulator of a particular event in one path, then its effect via other paths toward that outcome should also be positive. Similarly, a negative regulator should have negative effects on the same node through different interactions. In the literature, interactions of a protein may be stated to lead to opposing end points by using different paths. In such cases, the node is defined as anticoherent. When related edges of each node are tracked on the p73 MIM, two important proteins can be determined as anticoherent nodes of the system. These proteins are pRB, which is not assigned to any functional category, and c-Jun in The Activation and Suppression functional categories.
The activity of E2F1, which is discussed in detail under The Upregulation functional category section, is suppressed when E2F1 forms a complex with pRB (U17–18). With this interaction, pRB has a negative effect on the p73 activity. However, binding of pRB to RACK1 (S17) appears to have a positive effect on p73. RACK1 is a repressor of p73; when there is a physical interaction between p73 and RACK1, the p73 transcriptional activity is inhibited (S15–16). The pRB interaction with RACK1 frees the p73 from repression (S18). These two opposing actions suggest that pRB is an anticoherent node in the p73 tumor suppression network.
c-Jun appears to be a positive regulator of p73. It inhibits p73 degradation (D3) and stimulates p73 transcriptional activity (A53). On the other hand, c-Jun also binds to the MDM2 promoter and enhances its transcription (S8, S10). As mentioned in The Activation and Suppression functional categories section, MDM2 suppresses the p73 activity. These two opposing actions indicate that c-Jun is an anticoherent node in the p73 network. Interestingly, there is a direct relationship between those two anticoherent proteins. pRB binds to the c-Jun promoter and stimulates its transcription (A57–58).
The p73 MIM and the network of p53 share proteins with similar and opposing roles
A good starting point to understand the logic of the p73 tumor suppression mechanism would be a comparison between the p53 and p73 networks. The common and distinct features of the p53 and p73 networks may describe parallel or diverse functions of these tumor suppressors. Expression of the compared features of both p53 and p73 networks in the p73 MIM would require implementation of the p53 protein and p53 related pathways into the MIM. Such an implementation would cause divergence from the focus on p73 interactions in cell cycle and apoptosis in the MIM. Therefore, a brief comparison among the nodes in the p73 MIM and the members of the p53 network is made. For this purpose, a preexisting p53 MIM (24) and the data obtained from the literature are used. Among the compared nodes, 12 proteins (ATM, p300, Cyclin A/Cdk2, HIPK2, Chk1, Chk2, p21, 14–3–3σ, Gadd45, Bax, PUMA, Cyclin G) function in the same manner or lead to similar effects using different mechanisms, 2 (MDM2, c-Abl) have different roles and two nodes (c-Jun, CTF2) have diverse end effects on p53 and p73. A summary of the comparison is given in Table 2.
Table 2.
The role in the p73 MIM and the p53 network for the proteins with available information
| Protein | Effect | Mechanism | References |
|---|---|---|---|
| ATM | Same | Different | (35,36,64,65) |
| c-Abl | Different | – | (24,44,69) |
| Chk1 | Same | Same | (40) |
| Chk2 | Same | Different | (40) |
| c-jun | Opposing | – | (45) |
| CTF2 | Opposing | – | (46,78) |
| CyclinA/Cdk2 | Same | Same | (17,38,102) |
| Cyclin G | Same | Different | (117) |
| E4orf6 | No int. w/p53 | – | (48,96,98) |
| HIPK2 | Same | Same | (39) |
| Itch | No int. w/p53 | – | (47,113) |
| MDM2 | Different | – | (42,43,89) |
| MM1 | No int. w/p53 | – | (45,80) |
| NEDL2 | No int. w/p53 | – | (47,83) |
| p300 | Same | Same | (24,37,73,74) |
| Pin1 | Same | Same | (17,67) |
Following the interactions of ATM in the p53 network and the p73 MIM, it can be seen that ATM directly activates p53 by phosphorylation at Ser15 (35) and leads to activation of p73 indirectly (36), (A8). Pin1 imposes a conformational change on p73 and p53 (A17) (17). p300 binds to the TA domains of p53 and p73, to enhance their functional activities (24,37), (A23). Cyclin A/Cdk2 can phosphorylate p53 and p73 (38,17), (S43). Serine/threonine kinase HIPK2 physically interacts with the ODs of both p73 and p53, resulting in their colocalization into the nuclear bodies (A41). Chk1 targets the TA domains of p53 and p73 to improve the TA of p53 and p73. Chk2 mediates the posttranslational modification of p53 and p73, by direct and indirect means, respectively (40). Cyclin G interacts both with p53 and p73, causing downregulation of these proteins. (D19) The mechanisms differ such that the negative effect of Cyclin G on p53 is MDM2-dependent. In addition, some promoters e.g. p21, 14–3–3σ, Gadd45, Bax, PUMA (41), (T2) are also transactivated by both p73 and p53.
MDM2 is a significant protein in the p53 network, since it mediates ubiquitination and degradation of p53 (42). It is also associated with p73 by direct binding and instead of its degradation, binding of MDM2 results in stabilization of p73 (42), (S1). This binding also leads to the inactivation of p73, by disrupting the interaction between p73 and p300/CBP (43). The tyrosine kinase, c-Abl stimulates DNA binding by p53 through direct interaction, but it stabilizes p73 by posttranslational modification (24,44), (A12). c-Jun stabilizes p73, increasing its pro-apoptotic activity (A53), while it negatively regulates p53 (45). Another protein having opposing affects on p73 and p53 is CTF2. It enhances p53 activity but inhibits DNA binding to p73 (46), (S30).
There are also proteins that affect p73 without having any interaction with p53, such as NEDL2 (47), (A45); MM1 (45), (A37); adenovirus protein E4orf6 (48), (S23) and Itch, (D6). Among these proteins, Itch stands out as being the protein having a role in the degradation of p73. Itch functions in a manner similar to that of the MDM2 in p53 network (47).
Hierarchical clustering can define paths with small number of interactions, but not large functional categories
The interactions of the p73 MIM are defined at a binary level by two different approaches. In the first approach, only direct (first degree) physical interactions between proteins are taken into consideration. The results were unable to define meaningful clusters. They also excluded many proteins, which are related to the network via contingencies only. In the second approach, first-degree contingencies where the two proteins having an interaction defined by a contingency are also counted as binary interactions. However, contingencies affecting other contingencies (higher degree) are not included.
As a result of the second approach, the clustering program was able to define paths with small number of interactions in the network, although large clusters corresponding to functional categories could not be obtained (See Supplementary material). For relatively small categories (Figure 2a), namely Transcriptional Activity and Degradation, the proteins involved could mostly be distinguished in the hierarchical tree. The main pathway leading to apoptosis in the Transcriptional Activation functional category, starting from the PUMA and Bax transcription (T2), and ending at the apoptosis box (T15) is defined as a branch, where Bax and PUMA have a closer relation, merging with cytochrome c, Apaf-1 and the Apoptosis box. In the case of the Degradation functional category, the proteins influencing degradation of p73 by direct interactions are also defined as a branch including Cyclin G, Cyclin G promoter, NQO1, UFD2a and the degradation of p73. However, other members of this category which lead to the degradation of p73 by covalent modifications could not be mined from the given input information. In the Suppression and Activation functional categories, theÿ 11 nodes—including theΔNp73 isoform and its promoter—that affect the p73 activity in the same path, acting on T1, are well clustered. In addition to those mentioned clusters, there are also small groups (3–4 nodes) of interrelated proteins placed in close relation in the hierarchical tree, such as hTERT, hTERT promoter and Sp1.
DISCUSSION
Biological information on the interactions of p73 are organized in the p73 MIM with special emphasis on domain specificity
p73 has major roles in the embryonic differentiation and in the tumor suppression networks. Due to the scarcity of data, p73 interactions related to differentiation are not considered in this work. Here, we focus on the apoptotic function of p73. MIM notation (Figure 2b) is selected for the network representation. We added some extensions for the representation of domains (Figure 3) and isoforms. The extensions which emphasize the domain specificity made it possible to demonstrate whether an interaction is valid for a specific p73 isoform of interest. The data are stored in both human and computer readable formats for ease of use in future studies.
The constructed p73 MIM organizes the information related to the biology of the p73. While constructing the map, special attention was paid to the domain specificity of the p73 interactions. This feature makes the p73 MIM distinguishable as compared with other PPI maps. The organization of the 53 nodes and 176 edges of the p73 MIM into five functional categories provide a clear and compact representation of the biological mechanisms related to p73 function.
Functional categories demonstrate biologically significant interrelations
The functional categories identified from the nodes and edges of the p73 MIM provide a compact structure of the network, yielding five functional groups, Upregulation, Activation, Suppression, Degradation, Transcriptional Activation and their interrelationships.
The Upregulation and Activation categories are related via the node E2F1 of the Upregulation category, and the major pathway for p73 activation (A54/U11). When ATM (A1–7) is activated after DNA damage, it activates the stimulators of p73 (A8) and E2F1 (U2). In turn, E2F1 increases the activity of ATM (U11), forming a positive feedback loop. This loop is biologically significant and it can be followed from the p73 MIM. The PML-NB:p73 complex and c-Jun can inhibit the degradation of p73 (D2-3), relating the Activation and Degradation functional categories. Furthermore, recruitment of p73 into PML-NBs complements paths, which are stimulated by ATM upon recognition of DNA damage (A3). The existence of the interactions in different paths initiated by the same signal, DNA damage in this case, demonstrates the robust structure of this biological network.
In the Suppression functional category, instead of forming complex pathways for p73 suppression, the proteins mostly bind directly to p73 or inhibit the interactions that activate p73. When p73 is no longer required for the cell activity, it is degraded via sumoylation (D4–5, S22), ubiquitination (D6–9, D11–14) or direct association with proteins such as UFD2a or Cyclin G (D16–20) which may not need proteasomal activity. The Transcriptional Activation and the Upregulation functional categories are related via the activation of the Apaf-1 promoter by E2F1 (T3).
Through the map, we observe numerous paths for p73 degradation, and a rather simple composition for interactions relating to p73 suppression. p73 is kept at very low concentrations when there is no DNA damage (49), which demonstrates the biological priority of the degradation invoked by the suppression mechanism. Here, we illustrate that the structure of the Suppression and Degradation functional categories in the p73 network correlates with this biological preference.
Anticoherent interactions of the p73 MIM suggest further experimental investigation
Construction of the interaction networks allows identification of the regions where there are inconsistencies in the functions of proteins. By tracing the interaction paths, we point out two nodes, pRB and c-Jun, which are anticoherent in their tasks in the p73 MIM. pRB is considered as anticoherent, since it regulates p73 both negatively and positively, by suppressing the activities of E2F1 and RACK1 (U17–18, S17), respectively. c-Jun has strong effects on the positive regulation of p73, by repressing its degradation and stimulating its transcriptional activity (D3). In contrast to these positive effects, transcription of MDM2, a p73 suppressor, is also stimulated by c-Jun (S8). Furthermore, those two anticoherent nodes are interrelated: pRB binds to the promoter of c-Jun and enhances its transcription (A56–57).
The observed apparent inconsistency in the p73 network for pRB and c-Jun may indicate the existence of interactions at different spatial or temporal cell states, an experimental misidentification or missing data regarding these proteins. Either way, based on our analysis of the p73 interaction network, we propose that the pathways involving pRB and c-Jun, should be further explored experimentally to clarify the regulatory mechanisms in p73 network.
Comparison of the p73 MIM and the p53 network presents similarities and differences in the interactions
The compared nodes of the p73 MIM and of the p53 network can be classified into four categories: proteins (i) with the same effects in both networks; (ii) with similar effects on p73 and p53 via different mechanisms; (iii) with different, or in some cases, opposing roles in the networks and (iv) existing in the p73 network which have no effect on p53, as explained in the Results section. This classification indicates that p73 and p53 can use parallel or alternate interactions leading to apoptosis.
Eleven nodes are shared by the networks of p73 and p53. This fact emphasizes the crucial importance of p73 in cancer research. The common pathways also indicate that tumor formation caused by malfunctioning of the shared proteins could affect both p53 and p73, and that p73 could be an alternative tumor suppressor in cases of cancer formation related to p53 mutations. Finally, they indicate a competition between p73 and p53 in cases where they target the same promoters. Furthermore, proteins that do not exist in the p53 network and are related to the transcription mechanism of p73 can be good candidates for further research, since proteins related to the transcription of p73 have important roles in both activation and inhibition of apoptosis pathways.
Pathways with fewer interactions are extracted from the network by hierarchical clustering with introduction of first-degree contingencies
For cross-validating the manually organized functional categories, the p73 MIM is analyzed by a clustering algorithm, using two different input matrices. The first approach, which excluded the contingencies, was unable to define the paths of the network. In the second approach, which included both the first-degree physical interactions and the first-degree contingencies, the large categories, e.g. Activation cannot be thoroughly extracted from the network. These categories include many high-order contingencies; more importantly, common ending points of the pathways are mostly contingencies. However, via the additional information present in the second approach, nodes which construct pathways with small number of interactions can be clustered together and most members of small functional categories can be extracted. This improvement suggests that by using a proper representation of the MIM notation in a computer readable format, computer-based analyses can supply information. Such knowledge can be used for improvement of the maps, assisting in the construction of functional categories.
Tracing the edges of the map and considering the results of the network analysis will provide an organized way to review and analyze the underlying apoptosis machinery of p73. Such a study of p73 is essential both for experimentalists and theoreticians working with p73 or proteins in its network. Clearly, pointing to specific regions in the network may help in avoiding some misleading effects which are the outcome of the sparse data, an evident fact in cancer research.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
ACKNOWLEDGEMENTS
We are grateful to Dr Kurt Kohn for suggesting the project to us. Moreover, Dr Kohn and Dr Mirit Aladjem followed the project making many useful comments. We would like to acknowledge financial support from the following sources: EU FP6-2004-ACC-SSA-2 (517991); Turkish Academy of Sciences (TUBA); The Scientific and Technological Research Council of Turkey (TUBITAK, 107T382). This project has been funded in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health (N01-CO-12400). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported (in part) by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. Funding to pay the Open Access publication charges for this article was provided by NCI contract N01-CO-12400.
Conflict of interest statement. None declared.
REFERENCES
- 1.Levrero M, De Laurenzi V, Costanzo A, Gong J, Wang JY, Melino G. The p53/p63/p73 family of transcription factors: overlapping and distinct functions. J. Cell Sci. 2000;113:1661–1670. doi: 10.1242/jcs.113.10.1661. [DOI] [PubMed] [Google Scholar]
- 2.Yang A, Kaghad M, Caput D, McKeon F. On the shoulders of giants: p63, p73 and the rise of p53. Trends Genet. 2002;18:90–95. doi: 10.1016/s0168-9525(02)02595-7. [DOI] [PubMed] [Google Scholar]
- 3.Bullock AN, Fersht AR. Rescuing the function of mutant p53. Nat. Rev. Cancer. 2001;1:68–76. doi: 10.1038/35094077. [DOI] [PubMed] [Google Scholar]
- 4.Kaghad M, Bonnet H, Yang A, Creancier L, Biscan JC, Valent A, Minty A, Chalon P, Lelias JM, Dumont X, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 5.Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 6.Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 7.Yang A, Walker N, Bronson R, Kaghad M, Oosterwegel M, Bonnin J, Vagner C, Bonnet H, Dikkes P, Sharpe A, et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature. 2000;404:99–103. doi: 10.1038/35003607. [DOI] [PubMed] [Google Scholar]
- 8.Irwin MS, Kondo K, Marin MC, Cheng LS, Hahn WC, Kaelin WG., Jr Chemosensitivity linked to p73 function. Cancer Cell. 2003;3:403–410. doi: 10.1016/s1535-6108(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 9.Vayssade M, Haddada H, Faridoni-Laurens L, Tourpin S, Valent A, Bénard J, Ahomadegbe JC. P73 functionally replaces p53 in Adriamycin-treated, p53-deficient breast cancer cells. Int. J. Cancer. 2005;116:860–869. doi: 10.1002/ijc.21033. [DOI] [PubMed] [Google Scholar]
- 10.Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dostch V, Andrews NC, Caput D, McKeon F. p63, a p53 Homolog at 3q27–29, Encodes Multiple Products with Transactivating, Death-Inducing, and Dominant-Negative Activities. Moll. Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 11.Bénard J, Douc-Rasy S, Ahomadegbe JC. TP53 family members and human cancers. Hum. Mutat. 2003;21:182–191. doi: 10.1002/humu.10172. [DOI] [PubMed] [Google Scholar]
- 12.Dohn M, Zhang S, Chen X. p63a and DNp63a can induce cell cycle arrest and apoptosis and differentially regulate p53 target genes. Oncogene. 2001;20:3193–3205. doi: 10.1038/sj.onc.1204427. [DOI] [PubMed] [Google Scholar]
- 13.Fontemaggi G, Kela I, Amariglio N, Rechavi G, Krishnamurthy J, Strano S, Sacchi A, Givol D, Blandino G. Identification of direct p73 target genes combining DNA microarray and chromatin immunoprecipitation analyses. J. Biol. Chem. 2002;277:43359–43368. doi: 10.1074/jbc.M205573200. [DOI] [PubMed] [Google Scholar]
- 14.Flores ER, Tsai KY, Crowley D, Sengupta S, Yang A, McKeon F, Jacks T. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature. 2002;416:560–564. doi: 10.1038/416560a. [DOI] [PubMed] [Google Scholar]
- 15.Zmijewski FM, Lane DP, Bourdon JC. p53/p63/p73 isoforms: an orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ. 2006;13:962–972. doi: 10.1038/sj.cdd.4401914. [DOI] [PubMed] [Google Scholar]
- 16.Ding Y, Inoue T, Kamiyama J, Tamura Y, Ohtani-Fujita N, Igata E, Sakai T. Molecular cloning and functional characterization of the upstream promoter region of the human p73 gene. DNA Res. 1999;6:347–351. doi: 10.1093/dnares/6.5.347. [DOI] [PubMed] [Google Scholar]
- 17.Scoumanne A, Harms KL, Chen X. Structural basis for gene activation by p53 family members. Cancer Biol. Ther. 2005;4:1178–1185. doi: 10.4161/cbt.4.11.2254. [DOI] [PubMed] [Google Scholar]
- 18.Davidson TS, Vagner C, Kaghad M, Ayed A, Caput D, Arrowsmith CH. p73 and p63 are homotetramers capable of weak heterotypic interactions with each other but not with p53. J. Biol. Chem. 1999;274:18709–18714. doi: 10.1074/jbc.274.26.18709. [DOI] [PubMed] [Google Scholar]
- 19.Di Como CJ, Gaiddon C, Prives C. p73 function is inhibited by tumor-derived p53 mutants in mammalian cells. Mol. Cell Biol. 1999;19:1438–1449. doi: 10.1128/mcb.19.2.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melino G, De Laurenzi V, Vousden KH. p73: friend or foe in tumorigenesis. Nat. Rev. Cancer. 2002;2:605–615. doi: 10.1038/nrc861. [DOI] [PubMed] [Google Scholar]
- 21.Loiseau H, Arsaut J, Demotes MJ. p73 gene transcripts in human brain tumors: overexpression and altered splicing in ependymomas. Neurosci. Lett. 1999;263:173–176. doi: 10.1016/s0304-3940(99)00130-5. [DOI] [PubMed] [Google Scholar]
- 22.Rossi M, Sayan AE, Terrinoni A, Melino G, Knight RA. Mechanism of induction of apoptosis by p73 and its relevance to neuroblastoma biology. Ann. N Y Acad. Sci. 2004;1028:143–149. doi: 10.1196/annals.1322.015. [DOI] [PubMed] [Google Scholar]
- 23.Zaika AI, Slade N, Erster SH, Sansome C, Joseph TW, Pearl M, Chalas E, Moll UM. ΔNp73, A dominant-negative inhibitor of wild-type p53 and TAp73, is up-regulated in human tumors. J. Exp. Med. 2002;196:765–780. doi: 10.1084/jem.20020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohn KW. Molecular interaction map of the mammalian cell cycle control and DNA repair systems. Mol. Biol. Cell. 1999;10:2703–2734. doi: 10.1091/mbc.10.8.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohn KW, Aladjem MI, Kim S, Weinstein JN, Pommier Y. Depicting combinatorial complexity with the molecular interaction map notation. Mol. Syst. Biol. 2006;2:51. doi: 10.1038/msb4100088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohn KW. Molecular interaction maps as information organizers and simulation guides. Chaos. 2001;11:84–97. doi: 10.1063/1.1338126. [DOI] [PubMed] [Google Scholar]
- 27.Kohn KW, Aladjem MI, Weinstein JN, Pommier Y. Molecular interaction maps of bioregulatory networks: a general rubric for systems biology. Mol. Biol. Cell. 2006;17:1–13. doi: 10.1091/mbc.E05-09-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oda K, Matsuoka Y, Funahashi A, Kitano H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol. Syst. Biol. 2005;1:e1–e17. doi: 10.1038/msb4100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitano H, Funahashi A, Matsuoka Y, Oda K. Using process diagrams for the graphical representation of biological networks. Nat. Biotechnol. 2005;23:961–966. doi: 10.1038/nbt1111. [DOI] [PubMed] [Google Scholar]
- 30.Kohn KW, Aladjem MI. Circuit diagrams for biological networks. Mol. Syst. Biol. 2006;2:2006.0002. doi: 10.1038/msb4100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alpaydın E. Introduction to Machine Learning. The MIT Press: London, pp.; 2004. Clustering; pp. 133–153. [Google Scholar]
- 32.Sharan R, Maron-Katz A, Shamir R. CLICK and EXPANDER: a system for clustering and visualizing gene expression data. Bioinformatics. 2003;19:1787–1799. doi: 10.1093/bioinformatics/btg232. [DOI] [PubMed] [Google Scholar]
- 33.Das S, El-Deiry WS, Somasundaram K. Regulation of the p53 homolog p73 by adenoviral Oncogene E1A. J. Biol. Chem. 2003;278:18313–18320. doi: 10.1074/jbc.M211704200. [DOI] [PubMed] [Google Scholar]
- 34.Zaika A, Irwin M, Sansome C, Moll UM. Oncogenes induce and activate endogenous p73 protein. J. Biol. Chem. 2001;276:11310–11316. doi: 10.1074/jbc.M005737200. [DOI] [PubMed] [Google Scholar]
- 35.Keramaris E, Hirao A, Slack RS, Mak TW, Park DS. ATM can regulate p53 and neuronal death independent of Chk2 in response to DNA damage. J. Biol. Chem. 2003;278:37782–37789. doi: 10.1074/jbc.M304049200. [DOI] [PubMed] [Google Scholar]
- 36.Cuddihy AR, Bristow RG. The p53 protein family and radiation sensitivity: Yes or no? Cancer Metastasis Rev. 2004;23:237–257. doi: 10.1023/B:CANC.0000031764.81141.e4. [DOI] [PubMed] [Google Scholar]
- 37.Costanzo A, Merlo P, Pediconi N, Fulco M, Sartorelli V, Cole PA, Fontemaggi G, Fanciulli M, Schiltz L, Blandino G, et al. DNA damage-dependent acetylation of p73 dictates the selective activation of apoptotic target genes. Moll. Cell. 2002;9:175–186. doi: 10.1016/s1097-2765(02)00431-8. [DOI] [PubMed] [Google Scholar]
- 38.Gaiddon C, Lokshin M, Gross I, Levasseur D, Taya Y, Loeffler J, Prives C. Cyclin-dependent Kinases Phosphorylate p73 at Threonine 86 in a Cell Cycle-dependent Manner and Negatively Regulate p73. J. Biol. Chem. 2003;278:27421–27431. doi: 10.1074/jbc.M300251200. [DOI] [PubMed] [Google Scholar]
- 39.Kim EJ, Park JS, Um SJ. Identification and characterization of HIPK2 interacting with p73 and modulating functions of the p53 family in vivo. J. Biol. Chem. 2002;277:32020–32028. doi: 10.1074/jbc.M200153200. [DOI] [PubMed] [Google Scholar]
- 40.Urist M, Tanaka T, Poyurovsky MV, Prives C. p73 induction after DNA damage is regulated by checkpoint kinases Chk1 and Chk2. Genes. Dev. 2004;18:3041–3054. doi: 10.1101/gad.1221004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramadan S, Terrinoni SA, Catani MV, Sayan AE, Knight RA, Mueller M, Krammer PH, Melino G, Candi E. p73 induces apoptosis by different mechanisms. Biochem. Biophys. Res. Commun. 2005;331:713–717. doi: 10.1016/j.bbrc.2005.03.156. [DOI] [PubMed] [Google Scholar]
- 42.Ozaki T, Nakagawara A. p73, a sophisticated p53 family member in the cancer world. Cancer. Sci. 2005;96:729–737. doi: 10.1111/j.1349-7006.2005.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng X, Che L, Jost CA, Maya R, Keller D, Wang X, Jr, Kaelin WG, Oren M, Chen J, Lu H. MDM2 suppresses p73 function without promoting p73 degradation. Mol. Cell Biol. 1999;19:3257–3266. doi: 10.1128/mcb.19.5.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan Z, Shioya H, Ishiko T, Sun X, Gu J, Huang Y, Lu H, Kharbanda S, Weichselbaum R, Kufe D. p73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damage. Nature. 1999;399:814–817. doi: 10.1038/21704. [DOI] [PubMed] [Google Scholar]
- 45.Toh WH, Siddique MM, Boominathan L, Lin KW, Sabapathy K. c-Jun regulates the stability and activity of the p53 homologue, p73. J. Biol. Chem. 2004;279:44713–44722. doi: 10.1074/jbc.M407672200. [DOI] [PubMed] [Google Scholar]
- 46.Uramoto H, Izumi H, Nagatani G, Ohmori H, Nagasue N, Ise T, Yoshida T, Yasumoto K, Kohno K. Physical interaction of tumour suppressor p53/p73 with CCAAT-binding transcription factor 2 (CTF2) and differential regulation of human high-mobility group 1 (HMG1) gene expression. Biochem. J. 2003;371:301–310. doi: 10.1042/BJ20021646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dulloo I, Sabapathy K. Transactivation-dependent and -independent Regulation of p73 Stability. J. Biol. Chem. 2005;280:28203–28214. doi: 10.1074/jbc.M501702200. [DOI] [PubMed] [Google Scholar]
- 48.Steegenga WT, Shvarts A, Riteco N, Bos JL, Jochemsen AG. Distinct Regulation of p53 and p73 Activity by Adenovirus E1A, E1B, and E4orf6 Proteins. Mol. Cell Biol. 1999;19:3885–3894. doi: 10.1128/mcb.19.5.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oberst A, Rossi M, Salomoni P, Pandolfi PP, Oren M, Melino G, Bernassola F. Regulation of the p73 protein stability and degradation. Biochem. Biophys. Res. Commun. 2005;331:707–712. doi: 10.1016/j.bbrc.2005.03.158. [DOI] [PubMed] [Google Scholar]
- 50.Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc. Natl Acad. Sci. USA. 2000;97:10389–10394. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pommier Y, Weinstein JN, Aladjem MI, Kohn KW. Chk2 molecular interaction map and rationale for Chk2 inhibitors. Clin. Cancer. Res. 2006;12:2657–2661. doi: 10.1158/1078-0432.CCR-06-0743. [DOI] [PubMed] [Google Scholar]
- 52.Buscemi G, Carlessi L, Zannini L, Lisanti S, Fontanella E, Canevari S, Delia D. DNA damage-induced cell cycle regulation and function of novel Chk2 phosphoresidues. Mol. Cell Biol. 2006;26:7832–7845. doi: 10.1128/MCB.00534-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsantoulis PK, Gorgoulis VG. Involvement of E2F transcription factor family in cancer. Eur. J. Cancer. 2005;41:2403–2414. doi: 10.1016/j.ejca.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 54.Irwin M, Marin MC, Phillips AC, Seelan RS, Smith DI, Liu W, Flores ER, Tsai KY, Jacks T, Vousden KH, et al. Role for the p53 homologue p73 in E2F-1-induced apoptosis. Nature. 2000;407:645–648. doi: 10.1038/35036614. [DOI] [PubMed] [Google Scholar]
- 55.Rogoff HA, Pickering MT, Frame FM, Debatis ME, Sanchez Y, Jones S, Kowalik TF. Apoptosis associated with deregulated E2F activity is dependent on E2F1 and Atm/Nbs1/Chk2. Mol. Cell Biol. 2004;24:2968–2977. doi: 10.1128/MCB.24.7.2968-2977.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ohtani K, Iwanaga R, Arai M, Huang Y, Matsumura Y, Nakamura M. Cell type-specific E2F activation and cell cycle progression induced by the oncogene product tax of human T-cell leukemia virus Type I. J. Biol. Chem. 2000;275:11154–11163. doi: 10.1074/jbc.275.15.11154. [DOI] [PubMed] [Google Scholar]
- 57.Camperano MR, Flemington EK. Regulation of E2F through ubiquitin–proteasome-dependent degradation: Stabilization by the pRB tumor suppressor protein. Proc. Natl Acad. Sci. USA. 1997;94:2221–2226. doi: 10.1073/pnas.94.6.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frederick AD, Dyson N. pRB Contains an E2F1-specific binding domain that allows E2F1-Induced apoptosis to be regulated separately from other E2F activities. Mol. Cell. 2003;12:639–649. doi: 10.1016/s1097-2765(03)00344-7. [DOI] [PubMed] [Google Scholar]
- 59.Braithwaite AW, Del Sal G, Lu X. Some p53-binding proteins that can function as arbiters of life and death. Cell Death Differ. 2006;13:984–93. doi: 10.1038/sj.cdd.4401924. [DOI] [PubMed] [Google Scholar]
- 60.Wang JYJ. Regulation of cell death by the Abl tyrosine kinase. Oncogene. 2000;19:5643–5650. doi: 10.1038/sj.onc.1203878. [DOI] [PubMed] [Google Scholar]
- 61.Flinterman M, Guelen L, Ezzati-Nik S, Killick R, Melino G, Tominaga K, Mymryk JS, Gaken J, Tavassoli M. E1A activates transcription of p73 and Noxa to induce apoptosis. J. Biol. Chem. 2005;280:5945–5959. doi: 10.1074/jbc.M406661200. [DOI] [PubMed] [Google Scholar]
- 62.Fontemaggi G, Gurtner A, Strano S, Higashi Y, Sacchi A, Piaggio G, Blandino G. The transcriptional repressor ZEB regulates p73 expression at the crossroad between proliferation and differentiation. Mol. Cell Biol. 2001;21:8461–8470. doi: 10.1128/MCB.21.24.8461-8470.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 64.Baskaran R, Wood LD, Whitaker LL, Canman CE, Morgan SE, Xu Y, Barlow C, Baltimore D, Wynshaw-Boris A, Kastan MB, et al. Ataxia telangiectasia mutant protein activates c-Abl tyrosine kinase in response to ionizing radiation. Nature. 1997;387:516–519. doi: 10.1038/387516a0. [DOI] [PubMed] [Google Scholar]
- 65.Shafman T, Khanna KK, Kedar P, Spring K, Kozlov S, Yen T, Hobson K, Gatei M, Zhang N, Watters D, et al. Interaction between ATM protein and c-Abl in response to DNA damage. Nature. 1997;387:520–523. doi: 10.1038/387520a0. [DOI] [PubMed] [Google Scholar]
- 66.Bernassola F, Salomoni P, Oberst A, Como CJD, Pagano M, Melino G, Pandolfi PP. Ubiquitin-dependent Degradation of p73 Is Inhibited by PML. J. Exp. Med. 2004;199:1545–1557. doi: 10.1084/jem.20031943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mantovani F, Piazza S, Gostissa M, Strano S, Zacchi P, Mantovani R, Blandino G, Del Sal G. Pin1 links the activities of c-Abl and p300 in regulating p73 function. Mol. Cell. 2004;14:625–636. doi: 10.1016/j.molcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 68.Sanchez-Prieto R, Sanchez-Arevalo VJ, Servitja JM, Gutkind JS. Regulation of p73 by c-Abl through the p38 MAP kinase pathway. Oncogene. 2002;21:974–979. doi: 10.1038/sj.onc.1205134. [DOI] [PubMed] [Google Scholar]
- 69.Agami R, Blandino G, Oren M, Shaul Y. Interaction of c-Abl and p73alpha and their collaboration to induce apoptosis. Nature. 1999;399:809–813. doi: 10.1038/21697. [DOI] [PubMed] [Google Scholar]
- 70.Müller S, Matunis MJ, Dejean A. Conjugation with the ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus. EMBO J. 1998;17:61–70. doi: 10.1093/emboj/17.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kamitani T, Kito K, Nguyen HP, Wada H, Fukuda Kamitani T, Yeh ET. Identification of three major sentrinization sites in PML. J. Biol. Chem. 1998;283:26675–26682. doi: 10.1074/jbc.273.41.26675. [DOI] [PubMed] [Google Scholar]
- 72.Zhong S, Müller S, Ronchetti S, Freemont PS, Dejan A, Pandolfi PP. Role of SUMO-1-modified PML in nuclear body formation. Blood. 2000;95:2748–2752. [PubMed] [Google Scholar]
- 73.Lee CW, La Thangue NB. Promoter specificity and stability control of the p53-related protein p73. Oncogene. 1999;18:4171–4181. doi: 10.1038/sj.onc.1202793. [DOI] [PubMed] [Google Scholar]
- 74.Zeng X, Li X, Miller A, Yuan Z, Yuan W, Kwok RP, Goodman R, Lu H. The N-terminal domain of p73 interacts with the CH1 domain of p300/CREB binding protein and mediates transcriptional activation and apoptosis. Mol. Cell Biol. 2000;20:1299–1310. doi: 10.1128/mcb.20.4.1299-1310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Q, Guntuku S, Cui WS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes. Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao H, Piwnica-Worms H. ATR-Mediated Checkpoint Pathways Regulate Phosphorylation and Activation of Human Chk1. Mol. Cell Biol. 2001;21:4129–4139. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gonzalez S, Prives C, Cordon-Cardo C. p73α regulation by Chk1 in response to DNA damage. Mol. Cell Biol. 2003;23:8161–8171. doi: 10.1128/MCB.23.22.8161-8171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Bergamaschi D, Samuels Y, Jin B, Duraisingham S, Crook T, Lu X. ASPP1 and ASPP2, Common activators of p53 family members. Mol. Cell Biol. 2004;24:1341–1350. doi: 10.1128/MCB.24.3.1341-1350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shimodaira H, Yamashita AY, Kolodner RD, Wang JYJ. Interaction of mismatch repair protein PMS2 and the p53-related transcription factor p73 in apoptosis response to cisplatin. Proc. Natl Acad. Sci. USA. 2003;100:2420–2425. doi: 10.1073/pnas.0438031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Watanabe K, Ozaki T, Nakagawa T, Miyazaki K, Takahashi M, Hosoda M, Hayashi S, Todo S, Nakagawara A. Physical interaction of p73 with c-Myc and MM1, a c-Myc-binding protein, and modulation of the p73 function. J. Biol. Chem. 2002;277:15113–15123. doi: 10.1074/jbc.M111281200. [DOI] [PubMed] [Google Scholar]
- 81.Kim YH, Choi CY, Kim Y. Covalent modification of the homeodomain-interacting protein kinase 2 (HIPK2) by the ubiquitin-like protein SUMO-1. Proc. Natl Acad. Sci. USA. 1999;96:12350–12355. doi: 10.1073/pnas.96.22.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ren J, Datta R, Shioya H, Li Y, Oki E, Biedermann V, Bharti A, Kufe D. p73β is regulated by protein kinase Cδ catalytic fragment generated in the apoptotic response to DNA damage. J. Biol. Chem. 2002;277:33758–33765. doi: 10.1074/jbc.M110667200. [DOI] [PubMed] [Google Scholar]
- 83.Miyazaki K, Ozaki T, Kato C, Hanamoto T, Fujita T, Irino S, Watanabe K, Nakagawa T, Nakagawara A. A novel HECT-type E3 ubiquitin ligase, NEDL2, stabilizes p73 and enhances its transcriptional activity. Biochem. Biophys. Res. Commun. 2003;308:106–113. doi: 10.1016/s0006-291x(03)01347-0. [DOI] [PubMed] [Google Scholar]
- 84.Wu L, Zhu H, Nie L, Maki CG. A link between p73 transcriptional activity and p73 degradation. Oncogene. 2004;23:4032–4036. doi: 10.1038/sj.onc.1207538. [DOI] [PubMed] [Google Scholar]
- 85.Strano S, Munarriz E, Rossi M, Castagnoli L, Shaul Y, Sacchi A, Oren M, Sudol M, Cesareni M, Blandino G. Physical Interaction with Yes-associated Protein Enhances p73 Transcriptional Activity. J. Biol. Chem. 2001;276:15164–15173. doi: 10.1074/jbc.M010484200. [DOI] [PubMed] [Google Scholar]
- 86.Jeong MH, Bae J, Kim WH, Yoo SM, Kim JW, Song PI, Choi KH. p19ras interacts with and activates p73 by involving the MDM2 protein. J. Biol. Chem. 2006;281:8707–8715. doi: 10.1074/jbc.M513853200. [DOI] [PubMed] [Google Scholar]
- 87.Fogal V, Kartasheva NN, Trigiante G, Llanos S, Yap D, Vousden KH, Lu X. ASPP1 and ASPP2 are new transcriptional targets of E2F. Cell Death. Differ. 2005;12:369–376. doi: 10.1038/sj.cdd.4401562. [DOI] [PubMed] [Google Scholar]
- 88.van Dam H, Offringa R, Meijer I, Stein B, Smits AM, Herrlich P, Bos JL, van der Eb AJ. Differential effects of the adenovirus E1A oncogene on members of the AP-1 transcription factor family. Mol. Cell Biol. 1990;10:5857–5864. doi: 10.1128/mcb.10.11.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wanga X, Arooza T, Siua WY, Chiua CHS, Laua A, Yamashitab K, Poona RYC. MDM2 and MDMX can interact differently with ARF and members of the p53 family. FEBS Lett. 2001;490:202–208. doi: 10.1016/s0014-5793(01)02124-x. [DOI] [PubMed] [Google Scholar]
- 90.Ries S, Biederer C, Woods D, Shifman O, Shirasawa S, Sasazuki T, McMahon M, Oren M, McCormick F. Opposing Effects of Ras on p53: Transcriptional Activation of mdm2 and Induction of p19ARF. Cell. 2000;103:321–330. doi: 10.1016/s0092-8674(00)00123-9. [DOI] [PubMed] [Google Scholar]
- 91.Kim EJ, Park JS, Um SJ. Identifcation of Daxx interacting with p73, one of the p53 family, and its regulation of p53 activity by competitive interaction with PML. Nucleic Acids Res. 2003;31:5356–5367. doi: 10.1093/nar/gkg741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Müller S, Hoege C, Pyrowolakis G, Jentsch S. SUMO, ubiquitin's mysterious cousin. Nat. Rev. Mol. Cell Biol. 2001;2:202–210. doi: 10.1038/35056591. [DOI] [PubMed] [Google Scholar]
- 93.Salomoni P, Pandolfi PP. The Role of PML in tumor suppression. Cell. 2002;108:165–170. doi: 10.1016/s0092-8674(02)00626-8. [DOI] [PubMed] [Google Scholar]
- 94.Ozaki T, Watanabe K, Nakagawa T, Miyazaki K, Takahashi M, Nakagawara A. Function of p73, but not of p53, is inhibited by the physical interaction with RACK1 and its inhibitory effect is counteracted by pRB. Oncogene. 2003;22:3231–3242. doi: 10.1038/sj.onc.1206382. [DOI] [PubMed] [Google Scholar]
- 95.Dai JM, Wang ZY, Sun DS, Lin RX, Wang SQ. SIRT1 Interacts With p73 and Suppresses p73-Dependent Transcriptional Activity. J. Cell Physiol. 2007;210:161–166. doi: 10.1002/jcp.20831. [DOI] [PubMed] [Google Scholar]
- 96.Kotaja N, Karvonen U, Jänne OA, Palvimo JJ. PIAS Proteins Modulate Transcription Factors by Functioning as SUMO-1 Ligases. Mol. Cell Biol. 2002;22:5222–5234. doi: 10.1128/MCB.22.14.5222-5234.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Munarriz E, Barcaroli D, Stephanou A, Townsend PA, Maisse C, Terrinoni A, Neale MH, Martin SJ, Latchman DS, Knight AS, et al. PIAS-1 Is a Checkpoint Regulator Which Affects Exit from G1 and G2 by Sumoylation of p73. Mol. Cell Biol. 2004;24:10593–10610. doi: 10.1128/MCB.24.24.10593-10610.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Minty A, Dumont X, Kaghad M, Caput D. Covalent modification of p73alpha by SUMO-1. Two-hybrid screening with p73 identifies novel SUMO-1-interacting proteins and a SUMO-1 interaction motif. J. Biol. Chem. 2000;275:36316–36323. doi: 10.1074/jbc.M004293200. [DOI] [PubMed] [Google Scholar]
- 99.Aqeilan RI, Pekarsky Y, Herrero JJ, Palamurchuk A, Letofsky J, Druck T, Trapasso F, Han SH, Melina G, Huebner K, et al. Functional association between Wwox tumor suppressor protein and p73, a p53 homolog. Proc. Natl Acad. Sci. USA. 2004;101:4401–4406. doi: 10.1073/pnas.0400805101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim KC, Kim TS, Kang KH, Choi KH. Amphiphysin IIb-1, a novel splicing variant of amphiphysin II, regulates p73b function through protein-protein interactions. Oncogene. 2001;20:6689–6699. doi: 10.1038/sj.onc.1204839. [DOI] [PubMed] [Google Scholar]
- 101.Higashino F, Pipas JM, Shenk T. Adenovirus E4orf6 oncoprotein modulates the function of the p53-related protein, p73. Proc. Natl Acad. Sci. USA. 1998;95:15683–15687. doi: 10.1073/pnas.95.26.15683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leng RP, Lin Y, Ma W, Wu H, Lemmers B, Chung S, Parant JM, Lozano G, Hakem R, Benchimol S. Pirh2, a p53-Induced Ubiquitin-Protein Ligase, Promotes p53 Degradation. Cell. 2003;112:779–791. doi: 10.1016/s0092-8674(03)00193-4. [DOI] [PubMed] [Google Scholar]
- 103.Scharnhorst V, Dekker P, Eb AJ, Jochemsen AG. Physical interaction between Wilms tumor 1 and p73 protein modulates their functions. J. Biol. Chem. 2000;275:10202–10211. doi: 10.1074/jbc.275.14.10202. [DOI] [PubMed] [Google Scholar]
- 104.Hanamoto T, Ozaki T, Furuya K, Hosoda M, Hayashi S, Nakanishi M, Yamamoto H, Kikuchi H, Todo S, Nakagawara A. Identification of Protein Kinase A Catalytic Subunit as a Novel Binding Partner of p73 and Regulation of p73 Function. J. Biol. Chem. 2005;280:16665–16675. doi: 10.1074/jbc.M414323200. [DOI] [PubMed] [Google Scholar]
- 105.Liu G, Chen X. The C-terminal Sterile {alpha} Motif and the Extreme C Terminus Regulate the Transcriptional Activity of the {alpha} Isoform of p73. J. Biol. Chem. 2005;280:20111–20119. doi: 10.1074/jbc.M413889200. [DOI] [PubMed] [Google Scholar]
- 106.Serber Z, Lai HC, Yang A, Ou HD, Sigal MS, Kelly AE, Darimont BD, Duijf PH, Van Bokhoven H, McKeon F, et al. A C-Terminal Inhibitory Domain Controls the Activity of p63 by an Intramolecular Mechanism. Mol. Cell Biol. 2002;22:8601–8611. doi: 10.1128/MCB.22.24.8601-8611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fulco M, Costanzo A, Merlo P, Mangiacasale R, Strano S, Blandino G, Balsano C, Lavia P, Levrero M. p73 Is Regulated by Phosphorylation at the G2/M Transition. J. Biol. Chem. 2003;278:49196–49202. doi: 10.1074/jbc.M304921200. [DOI] [PubMed] [Google Scholar]
- 108.Kaida A, Ariumi Y, Ueda Y, Lin JY, Hijikata M, Ikawa S, Shimotohno K. Functional impairment of p73 and p51, the p53-related proteins, by the human T-cell leukemia virus type 1 Tax oncoprotein. Oncogene. 2000;19:827–830. doi: 10.1038/sj.onc.1203387. [DOI] [PubMed] [Google Scholar]
- 109.Lemasson I, Nyborg JK. Human T-cell Leukemia Virus Type I Tax Repression of p73b Is Mediated through Competition for the C/H1 Domain of CBP. J. Biol. Chem. 2001;276:15720–15727. doi: 10.1074/jbc.M100131200. [DOI] [PubMed] [Google Scholar]
- 110.Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt Phosphorylates the Yes-Associated Protein, YAP, to Induce Interaction with 14-3-3 and Attenuation of p73-Mediated Apoptosis. Mol. Cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 111.Beitzinger M, Oswald C, Beinoraviciute-Kellner R, Stiewe T. Regulation of telomerase activity by the p53 family member p73. Oncogene. 2006;25:813–826. doi: 10.1038/sj.onc.1209125. [DOI] [PubMed] [Google Scholar]
- 112.Moschos SJ, Mo YY. Role of SUMO/Ubc9 in DNA damage repair and tumorigenesis. J. Mol. Hist. 2006;37:309–319. doi: 10.1007/s10735-006-9030-0. [DOI] [PubMed] [Google Scholar]
- 113.Rossi M, De Laurenzi V, Munarriz E, Green DR, Liu YC, Vousden KH, Cesareni G, Melino G. The ubiquitin-protein ligase Itch regulates p73 stability. EMBO J. 2005;24:836–848. doi: 10.1038/sj.emboj.7600444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Horil T, Osaka F, Chiba T, Miyamoto C, Okabayashi K, Shimbara N, Katoi S, Tanaka K. Covalent modifcation of all members of human cullin family proteins by NEDD8. Oncogene. 1999;18:6829–6834. doi: 10.1038/sj.onc.1203093. [DOI] [PubMed] [Google Scholar]
- 115.Hosoda M, Ozaki T, Miyazaki K, Hayashi S, Furuya K, Watanabe K, Nakagawa T, Hanamoto T, Todo S, Nakagawara A. UFD2a mediates the proteasomal turnover without promoting p73 ubiquitination. Oncogene. 2005;24:7156–7169. doi: 10.1038/sj.onc.1208872. [DOI] [PubMed] [Google Scholar]
- 116.Asher G, Tsvetkov P, Kahana C, Shaul Y. A mechanism of ubiquitin-independent proteasomal degradation of the tumor suppressors p53 and p73. Genes Dev. 2005;19:316–321. doi: 10.1101/gad.319905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ohtsuka T, Ryu H, Minamishima YA, Ryo A, Lee SW. Modulation of p53 and p73 levels by cyclin G: implication of a negative feedback regulation. Oncogene. 2003;22:1678–1687. doi: 10.1038/sj.onc.1206306. [DOI] [PubMed] [Google Scholar]
- 118.Gaiddon C, Lokshin M, Ahn J, Zhang T, Prives C. A Subset of Tumor-Derived Mutant Forms of p53 Down-Regulate p63 and p73 through a Direct Interaction with the p53 Core Domain. Mol. Cell Biol. 2001;21:1874–1887. doi: 10.1128/MCB.21.5.1874-1887.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Crowe DL, Nguyen DC, Tsang KJ, Kyo S. E2F-1 represses transcription of the human telomerase reverse transcriptase gene. Nucleic Acids Res. 2001;29:2789–2794. doi: 10.1093/nar/29.13.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Racek T, Mise N, Li Z, Stoll A, Putzer BM. C-terminal p73 Isoforms Repress Transcriptional Activity of the Human Telomerase Reverse Transcriptase (hTERT) Promoter. J. Biol. Chem. 2005;280:40402–40405. doi: 10.1074/jbc.C500193200. [DOI] [PubMed] [Google Scholar]
- 121.Furukawa Y, Nishimura N, Matsuda M, Kano Y, Nakamura M. Apaf-1 Is a Mediator of E2F-1-induced Apoptosis. J. Biol. Chem. 2002;277:39760–39768. doi: 10.1074/jbc.M200805200. [DOI] [PubMed] [Google Scholar]
- 122.Melino G, Bernassola F, Ranalli M, Yee K, Zong WX, Corazzari M, Knight RA, Green DR, Thompson C, Vousden KH. p73 Induces Apoptosis via PUMA Transactivation and Bax Mitochondrial Translocation. J. Biol. Chem. 2004;279:8076–8083. doi: 10.1074/jbc.M307469200. [DOI] [PubMed] [Google Scholar]
- 123.Webster KA. Puma joins the battery of BH3-only proteins that promote death and infarction during myocardial ischemia. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H20–H22. doi: 10.1152/ajpheart.00111.2006. [DOI] [PubMed] [Google Scholar]
- 124.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. Garland Science: New York, pp.; 2008. Apoptosis; pp. 1115–1129. [Google Scholar]
- 125.Coates PJ. Regulating p73 isoforms in human tumours. J. Pathol. 2006;210:385–389. doi: 10.1002/path.2080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.