Abstract
Gram-negative bacterial infection is a major cause of sepsis and septic shock. An important inducer of inflammation underlying both syndromes is the cellular recognition of bacterial products through pattern recognition receptors (PRRs), including Toll-like receptors (TLRs). We identified a novel antagonistic mAb (named 1A6) that recognizes the extracellular portion of the TLR4–MD-2 complex. If applied to mice before infection with clinical isolates of Salmonella enterica or Escherichia coli and subsequent antibiotic therapy, 1A6 prevented otherwise fatal shock, whereas application of 1A6 after infection was ineffective. In contrast, coapplication of 1A6 and an anti-TLR2 mAb up to 4 h after infection with Gram-negative bacteria, in combination with the start of antibiotic therapy (mimicking clinical conditions), provided robust protection. Consistent with our findings in mice, dual blockade of TLR2 and TLR4 inhibited TNF-α release from human peripheral blood mononuclear cells upon Gram-negative bacterial infection/antibiotic therapy. Both murine splenocytes and human PBMCs released IFN-γ in a TLR4-dependent manner, leading to enhanced surface TLR2 expression and sensitivity for TLR2 ligands. Our results implicate TLR2 as an important, TLR4-driven sensor of Gram-negative bacterial infection and provide a rationale for blockade of both TLRs, in addition to antibiotic therapy for the treatment of Gram-negative bacterial infection.
Sepsis is a life-threatening condition that demands treatment within few hours upon clinical manifestation (1, 2). Gram-negative and -positive bacterial infections are the major causes of sepsis, which is characterized by extension of local infection to the systemic level (3–5). Typical early findings include high serum concentrations of cytokines such as TNF-α. The early phase of sepsis is followed by endocrine and cardiovascular dysregulation, often triggering fatal septic shock. Evidence of a link between the initial immune hyperactivation and a later immunoparalysis contributing to sepsis mortality may emphasize a rationale for timely and transient therapeutic immunosuppression (5).
Binding of pathogen-associated molecular patterns (PAMPs), such as envelope constituents or nucleic acids, to pattern recognition receptors (PRRs) induces inflammation upon infection. PRRs include Toll-like receptors (TLRs), which carry N-terminal leucine-rich repeat (LRR)–rich domains that interact with PAMPs. Ligand binding to the ectodomains induces TLR dimerization via the adjacent transmembrane domains. C-terminal intracellular domains recruit the cytoplasmic adaptor molecules MyD88 and/or TRIF/TICAM-1 to initiate intracellular signal transduction via specific pathways such as those involving NF-κB (6, 7). The immune-stimulatory activity of the Gram-negative bacterial outer membrane glycolipid LPS depends on binding to LPS-binding protein (LBP) and CD14. These proteins deliver LPS to the complex formed by TLR4 and MD-2 (8, 9). N-terminally oligo-acylated proteins, produced by most if not all bacteria, are PAMPs that activate TLR2–TLR1 or TLR2–TLR6 complexes (7, 10). Previous reports have shown the relative importance of TLR2 and TLR4 as sensors of Gram-negative and -positive bacteria, but have indicated involvement of additional PRRs (11, 12).
Existing strategies for the prevention of Gram-negative bacterial septic shock target inflammatory mediators or specific PRRs such as CD14. Antagonistic anti–rabbit CD14 antibody-dependent blockade of CD14 has been shown to prevent pathology such as organ injury by repetitive LPS challenge when applied, even after the initial LPS administration (13). Efforts to inhibit LPS-induced TLR4 activation include application of LBP, antagonistic lipid A, or antagonistic anti–murine (m)TLR4 mAbs (14–16).
In this study, we examined the host response to infection with clinical isolates of Escherichia coli or Salmonella enterica. Specifically, we investigated whether blockade of TLR4 and/or TLR2 on murine or human immune cells inhibits cytokine release. In addition, we studied the effect of antibiotic therapy paired with such blockade during Gram-negative bacterial infection of mice to protect against the Jarisch-Herxheimer reaction, which is induced in vivo when PAMPs are released rapidly from bacteria exposed to antibiotics (17). Results of either single or dual TLR blockade before or upon acute Gram-negative bacterial infection showed a central role of both TLR4 and TLR2 in sensing of Gram-negative bacterial challenge in vivo, a TLR4–TLR2 interrelation, and the capacity to protect from shock upon subsequent or synchronous antibiotic therapy.
RESULTS AND DISCUSSION
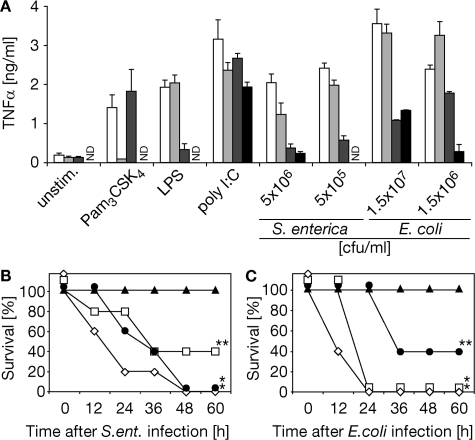
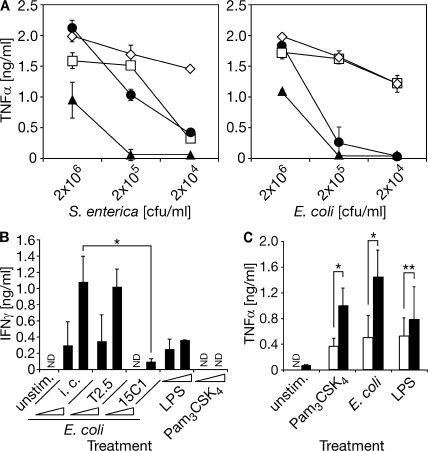
Investigating the involvement of TLR2 in addition to TLR4, we applied experimental models of Gram-negative bacterial infection and subsequent antibiotic therapy both in vitro and in vivo (18). We observed TLR2/4-independent activity of primary macrophages upon high-dose infection and subsequent antibiotic therapy affirming the involvement of further PRRs (such as TLR9 binding bacterial DNA) (6) in the recognition of Gram-negative bacteria. However, at lower infection doses, TLR2−/−/TLR4−/− macrophages were barely responsive. Substantial TLR4−/− macrophage activity upon Gram-negative bacterial challenge thus implied a contributing TLR2 activity (Fig. 1 A). Next, we infected mice with clinical isolates of Gram-negative bacteria. After 1 h, infection was terminated by antibiotic therapy, which was effective as indicated not only by the sterility of blood cultures 18 h after infection (not depicted) but also by the survival of all TLR2−/−/TLR4−/− mice (Fig. 1, B and C). As opposed to resistance of TLR2−/−/TLR4−/− mice, >50% of mice lacking expression of TLR2 or TLR4 and all WT mice succumbed to fatal shock upon S. enterica and E. coli infection (followed by antibiotic therapy; Fig. 1, B and C).
Figure 1.
Impaired responsiveness to Gram-negative bacterial infection followed by antibiotic therapy in the absence of TLR2/TLR4 expression. (A–C) Wild-type (white bars, ⋄), TLR2−/− (light gray bars, □), TLR4−/− (dark gray bars, •), and TLR2−/−/TLR4−/− (black bars, ▴; A) macrophages or mice (B and C) were infected with S. enterica (A and B) or E. coli (A and C) and subjected to antibiotic therapy after 1 h (A–C). (A) Cell culture supernatants were analyzed for TNF-α content by ELISA 16 h after challenge or infection as indicated (Pam3CSK4, tripalmitoylated hexapeptide; ND, not detected). (B and C) Mice were infected with 109 CFU S. enterica (ent.) or 5 × 109 CFU E. coli. *, P < 0.003; **, P < 0.052; P values result from comparison to results of respective TLR2−/−/TLR4−/− group analysis; n = 5 for each experimental group.
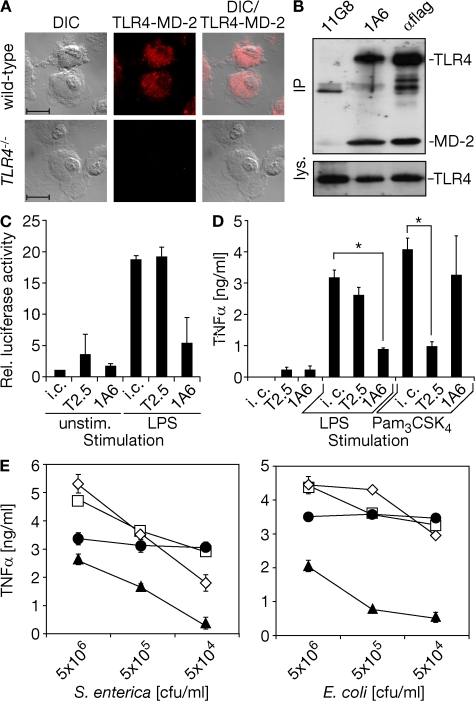
In the face of predominant intracellular TLR2 localization in primary immune cells, the effectiveness of anti-TLR2 mAb (T2.5) application to inhibit surface TLR2-driven fatal shock upon challenge with the Gram-positive bacterium Bacillus subtilis has been surprising (12, 19). To extend the concept of sole TLR2 blockade and to translate it to an experimental setting mimicking sepsis upon bacterial infection, we first identified a novel mAb (1A6). 1A6 bound the murine TLR4–MD-2 complex in a dose-dependent fashion, as well as a human (h)TLR4–mMD-2 complex (Fig. S1, A–C, available at http://www.jem.org/cgi/content/full/jem.20071990/DC1). Fluorescent staining of WT, but not TLR4−/−, macrophages using 1A6 and subsequent flow cytometry or microscopy confirmed primarily intracellular TLR4 localization (Fig. S1 D and Fig. 2 A). Co-precipitation of mTLR4 and mMD-2 from cells overexpressing both proteins (Fig. 2 B) indicated the specificity of 1A6 for an epitope formed by both chains of the murine TLR4–MD-2 complex together. Binding of 1A6 to TLR4–MD-2, but not to TLR2, resulted in TLR4 neutralization and contrasted with the inverse features of T2.5 (Fig. 2, C and D; Fig. S2 A). Furthermore, preapplication of 1A6 reduced phosphorylation of the mitogen-activated protein kinase p38 upon E. coli infection highly effectively, whereas blockade of TLR2 on RAW264.7 macrophages that constitutively express TLR2 at a high level was less effective (Fig. S2 B and not depicted). Co-application of 1A6 and T2.5, but not single mAb application, inhibited TNF-α release from murine macrophages upon S. enterica or E. coli infection followed by antibiotic therapy (Fig. 2 E).
Figure 2.
mTLR4–MD-2 specificity of 1A6 and effective TLR4 and TLR2 blockade on murine macrophages infected with Gram-negative bacteria. (A) Macrophages incubated with anti-TLR4 mAb (1A6) shown by Nomarski differential interference contrast microscopy (DIC), fluorescence recording (middle), and superimposition of both recordings (right). Bar, 10 μm. (B) Lysates of HEK293 cells that overexpressed murine TLR4 and MD-2 were subjected to immunoprecipitation (IP; α, anti) and analyzed using flag-specific antiserum (lys., total lysates). (C and D) HEK293 cells overexpressing mTLR4–MD-2 transiently or RAW264.7 macrophages, respectively, were challenged as indicated upon preincubation with anti-TLR2 mAb (T2.5), 1A6, or isotype control (i.c.) for 30 min. HEK293 cells were lysed 16 h after challenge and assayed for NF-κB–driven relative (Rel.) luciferase activity (C), whereas RAW264.7 macrophage supernatants were sampled 16 h after challenge (Pam3CSK4, tripalmitoylated hexapeptide) and analyzed by ELISA (D). *, P < 0.003. (E) RAW264.7 macrophages were infected 30 min after incubation with 1A6 (•), T2.5 (□), 1A6 and T2.5 (▴), or isotype control (⋄) mAb. Antibiotic therapy started 1 h after infection, and supernatants were analyzed by ELISA 6 h after infection. Results illustrated represent similar results (A, B, and E) or summarize the results (C, D) of at least three independent experiments.
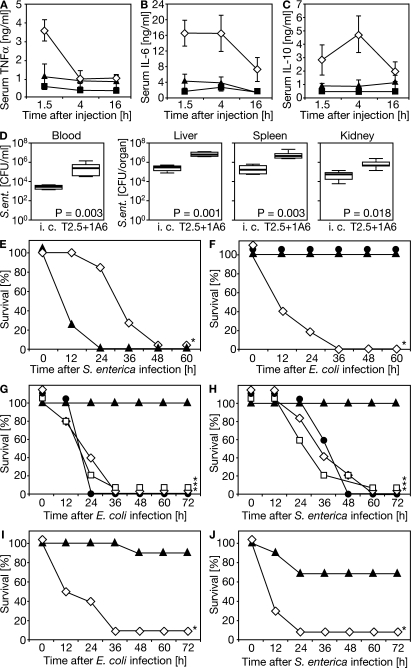
The inhibitory effect of TLR4 blockade during systemic LPS challenge depended on both the LPS dose and the 1A6 dose (Fig. S3, A and B, available at http://www.jem.org/cgi/content/full/jem.20071990/DC1). For example, if 1A6 was applied 4 h after LPS injection, a fully protective 1A6 dose was 30 mg/kg body weight (Fig. S3 C). Mice were completely protected from high-dose LPS shock if 1A6 was applied within a time window of 5 h before challenge and 4 h after challenge (Fig. S3, D and E). The different requirements in respect to 1A6 doses might depend on specific demands for celerity of TLR4 blockade (Fig. S3, B and C). The protective effect of systemic 1A6 administration, however, was not caused by induction of TLR4+ cell-specific depletory activity, and TLR4–MD-2–bound 1A6 persisted on the surface of macrophages in vitro for at least 4 h (Fig. S4, A–C), which indicated a slow TLR4–MD-2–1A6 complex uptake. Based on the results of our KO mice and murine macrophage analyses (Fig. 1), we evaluated dual TLR blockade in respect to its protective potential. To this we applied both mAbs systemically before challenge with antibiotic-treated E. coli. Amounts of serum-borne TNF-α, IL-6, and IL-10 were equally low in TLR2−/−/TLR4−/− mice and 1A6/T2.5-treated mice when compared with control mice (Fig. 3, A–C). Notably, upon low-dose S. enterica infection, dual TLR blockade in the absence of antibiotic therapy increased bacterial loads in different compartments 24 h after infection to a significant degree (Fig. 3 D). Failure to apply antibiotic therapy upon acute infection with S. enterica consequently accelerated pathogenesis within the first 12 h, as indicated by increased fatality of mice in which dual TLR blockade was performed (Fig. 3 E).
Figure 3.
Systemic blockade of both TLR4 and TLR2 upon S. enterica or E. coli challenge inhibits cytokine release, enhances pathology if an infection is not followed by antibiotic therapy, and protects from septic shock if synchronized with the start of antibiotic therapy. (A–C) Mice received 1A6 and T2.5 mAb (⋄; n = 6) or isotype control mAb (▴; n = 6) 1 h before i.p. challenge with 5 × 107 CFU E. coli that had been pretreated with antibiotics in vitro immediately before injection (▪, TLR2−/−/TLR4−/− mice, used as positive control; n = 3). Serum samples drawn as indicated were subjected to ELISA. Each experimental group was analyzed in the course of three individual experiments. (D) Wild-type mice received mAbs (i.c., isotype control; T2.5, anti-TLR2; 1A6, anti-TLR4) 1 h before infection with 106 CFU S. enterica (ent.) by i.p. injection. 24 h later, mice were killed and bacterial loads of compartments indicated were determined (n = 6 for each experimental group). (E–J) Wild-type mice received mAb (⋄, isotype control; •, 1A6; ▿, T2.5; ▴, 1A6 and T2.5; *, P < 0.004 for comparison to specific dual TLR blockade groups): 1 h before infection with 108 CFU S. enterica to be left untreated thereafter (E; n = 7 per group split for three independent experiments), 1 h before infection with 5 × 109 CFU E. coli and 2 h before antibiotic therapy (F; n = 5 per group split for two independent experiments), at the start of antibiotic therapy 1 h after infection with 5 × 109 CFU E. coli or 109 CFU S. enterica (G and H; two individual experiments with n = 5 per group), or (results of three individual experiments) at the start of antibiotic therapy 4 h after infection with 5 × 108 CFU E. coli (n = 9 for ▴, n = 10 for ⋄) or 108 CFU S. enterica (n = 10 for both experimental groups; I and J).
Surprisingly, prophylactic pretreatment with 1A6 alone, but not T2.5 alone, protected WT mice from otherwise lethal E. coli infection/antibiotic therapy (Fig. 3 F and not depicted). Accordingly, early TLR4 blockade in otherwise untreated experimental peritonitis has been protective (16). Our findings on the necessity of concordant dual TLR blockade (Fig. 2 E), however, somehow opposed exclusiveness of TLR4 involvement; possibly caused by the high TLR2 expression level in RAW264.7 macrophages used in our initial experiments here, which is not apparent in primary macrophages (19). That TLR4−/− mice lacked significant resistance to Gram-negative bacterial challenge (Fig. 1 C) could not be explained by a compensatory hyperactivity of TLR2 in TLR4−/− mice, because average serum TNF-α concentrations in WT and TLR4−/− mice upon challenge with bacterial lipopeptide analogue for 90 min (n = 14 for each of the two genotypes) did not differ significantly (not depicted). Instead, a systemically operative TLR4–TLR2 interrelation, which is outlined in the following paragraphs, provides a possible explanation for the effectiveness of TLR4 preblockade.
In modeling treatment of an established Gram-negative bacterial sepsis, we first infected mice with E. coli or S. enterica. After 1 h, an initial antibiotics administration was performed that was accompanied by application of either one of the two mAbs or both mAbs together. In accordance with the results of our initial experiments, neither of the two mAbs alone conferred protection (Fig. 3, G and H). Notably, dual mAb application resulted in complete protection against infection/antibiotics treatment–induced shock (Fig. 3, G and H). Dual TLR blockade was protective, even if performed in synchrony with the start of antibiotic therapy 4 h after infection (Fig. 3, I and J), even though mice already displayed symptoms of severe illness 3 h after infection. The 4-h time window of effective treatment is consistent with specific mAb-mediated protection upon single TLR-specific challenge (Fig. S3 E) (19). Our findings suggest effectiveness of TLR2/TLR4 blockade in the advanced phase of sepsis pathogenesis in which infection becomes clinically manifest, and therefore antibiotics are applied.
Infection with E. coli is among the most important causes of sepsis (4), which might depend on E. coli access to the bloodstream by mechanisms such as trauma. In contrast, salmonellae cause enteric disease because of their capacity to traverse epithelial cells lining the intestine or upon breaching tight junctions between them (20). However, only upon infection with S. enterica alone for longer time periods (Fig. 3, D and E), or if antibiotic therapy was delayed for 4 h (Fig. 3 J), might epithelial breakage or intracellular inhabitation have contributed to evasion from host surveillance, and thus to the increase of bacterial load. Indeed, infection with S. enterica was more pathogenic than infection with E. coli as judged from the necessity for application of S. enterica doses that were reduced by 80% as compared with E. coli doses to induce similar hyperinflammation, despite antibiotic therapy.
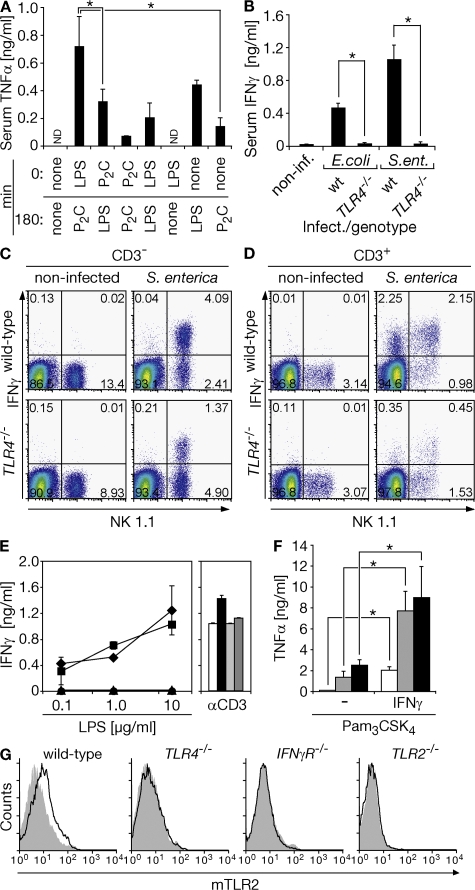
We speculated that the startling protection by TLR4 blockade before infection, but not after infection (Fig. 3, F and G), might indicate a TLR2 trigger function of TLR4 (21). To evaluate this hypothesis, we challenged mice with TLR2 and TLR4 ligands consecutively, at a low dose. Serum TNF-α concentrations peaked at 90 min and were reduced to background levels 180 min after single challenge of each TLR (Fig. 4 A and not depicted). Consistently, sequential (3-h interval) TLR4–TLR2 activation caused the strongest serum TNF-α accumulation after 4.5 h, as compared with single TLR2, TLR2–TLR4, TLR2–TLR2, or even single TLR4 or TLR4–TLR4 activation (Fig. 4 A). This finding was paralleled by the persistence of an increased TNF-α level 180 min after the second challenge (6 h upon first challenge) and a fatal outcome upon consecutive TLR4–TLR2 challenge specifically (not depicted). Thus, enhanced TLR2 sensitivity rather than tolerance was operative upon TLR4 activation.
Figure 4.
Timely graduated TLR4–TLR2 activation induces maximal cell activation and correlates with TLR4- and MyD88-dependent release of IFN-γ by NK and NKT cells to enhance TLR2-specific sensitivity and surface TLR2 expression upon Gram-negative bacterial infection. (A) Mice were challenged by i.p. injection of 50 μg LPS or 50 μg dipalmitoylated hexapeptide (P2C), or they were left untreated (none; n = 6 per experimental group). After a second challenge (or none) at 180 min, serum was drawn at 270 min and analyzed by ELISA. *, P < 0.004. ND, not detected. (B) Mice were infected with 5 × 108 CFU E. coli or 108 CFU S. enterica (ent.) for 3 h, after which serum was sampled and analyzed by ELISA. *, P < 0.004 (inf., infected; WT, n = 4; n = 3 for each TLR4−/− group). (C and D) Murine splenocytes were prepared 2 h after systemic infection with 108 CFU S. enterica. Viable CD3− and CD3+ fractions of splenocytes were analyzed for NK 1.1 (NK and NKT, respectively) and intracellular IFN-γ expression. (E) Splenocytes from wild-type (♦, white bar), TRIF−/− (▪, black bar), MyD88−/− (•, light gray bar), and TRIF−/−/MyD88−/− (▴, dark gray bar) mice were challenged for 24 h ex vivo upon which supernatants were analyzed by ELISA (3.5 μg/ml αCD3 as control, represents two independent experiments). (F) Primary macrophages were IFN-γ primed for 3 h or left untreated (−). Cells were washed twice and challenged with 1 ng/ml lipopeptide (Pam3CSK4, white bars), 10 ng/ml (gray bars), or 100 ng/ml (black bars) for an additional 6 h and analyzed by ELISA. *, P < 0.006 (represents three independent experiments). (G) Splenocytes were isolated 3 h after S. enterica infection of mice (108 CFU) with indicated genotypes and the CD11b+ fraction was analyzed for surface TLR2 expression (representative of at least three independent experiments).
We reasoned that IFN-γ might mediate the TLR4-dependent effect described above, because impairment of IFN-γ function has been reported to attenuate Gram-negative bacterial challenge–induced pathology (22), and because of the protective effect of prophylactic TLR4 blockade (Fig. 3 F). Accordingly, Gram-negative bacterial infection caused accumulation of substantial amounts of IFN-γ within a 3-h time frame in the sera of WT mice in a TLR4-dependent manner (Fig. 4 B). Splenic NK cells (CD3−NK1.1+), NKT cells (CD3+NK1.1+), and a low but substantial frequency of T cells from WT mice were found to already express IFN-γ 2 h after systemic infection with S. enterica, whereas the number of IFN-γ producers was substantially lower in spleens from TLR4−/− mice (Fig. 4, C and D, and Fig. S5, A and B, available at http://www.jem.org/cgi/content/full/jem.20071990/DC1). Activated TLR4 induces proinflammatory cytokine production by recruitment of MyD88, whereas it mediates late NF-κB activation and type I IFN- synthesis through TRIF/TICAM-1 (7). Notably, TLR4-driven IFN-γ release from splenocytes was MyD88-dependent, but did not depend on TRIF/TICAM-1 (Fig. 4 E). In addition, IFN-γ priming for 3 h increased cellular responsiveness to TLR2 ligand challenge (Fig. 4 F) and cell surface TLR2 expression on C57BL/6 or 129Sv WT CD11b+ cells was increased 3 h after S. enterica infection, whereas an up-regulation of TLR2 was undetectable in infected TLR4−/− and IFN-γR–deficient (IFN-γR−/−) mice (Fig. 4 G and not depicted).
Our findings are consistent with both TLR2 mRNA augmentation and cell surface TLR2 increase upon LPS challenge in humans (23, 24). They also correspond with the enhanced cell surface TLR2 expression in farmers' children as compared with controls, a finding that has been linked to exposure to higher amounts of LPS (25). The translation potential of our preclinical data is further supported by the effective inhibition of TNF-α release from hPBMCs upon infection with each of the two Gram-negative bacteria through mAb-mediated TLR2/TLR4 blockade (Fig. 5 A). TLR4, but not TLR2, blockade on hPBMCs inhibited rapid IFN-γ release upon E. coli infection. Accordingly, although LPS challenge induced IFN-γ release from hPBMCs, acylated hexapeptide did not (Fig. 5 B). Furthermore, IFN-γ challenge enhanced TLR2-specific hPBMC activation if applied 3 h before TLR2 challenge (Fig. 5 C).
Figure 5.
Dual TLR blockade inhibits TNF-α release, whereas TLR4-specific blockade impedes IFN-γ release from hPBMCs upon Gram-negative bacterial infection and IFN-γ enhances TLR2-specific, but not TLR4-specific responsiveness of hPBMCs. (A) hPBMCs were preincubated with mAbs for 30 min (•, 15C1; □, T2.5; ▴, 15C1 and T2.5; ⋄, isotype control), infected with the indicated doses of S. enterica or E. coli, and treated by application of antibiotics after 1 h. Supernatant was analyzed 6 h after infection by ELISA. Illustration represents one out of three equivalent results of three independent experiments. (B) 30 min before infection with 105 CFU/ml or 106 CFU/ml E. coli and subsequent antibiotic therapy, hPBMCs were pretreated with mAbs (i.c., isotype control). Nonpretreated cells were challenged with 100 ng/ml or 1 μg/ml LPS or tripalmitoylated hexapeptide (Pam3CSK4). Triangles indicate smaller and larger doses. IFN-γ in the supernatants was analyzed 16 h after challenge by ELISA (ND, not detected; illustration represents summarized results of three independent experiments; *, P = 0.027). (C) hPBMCs were primed with IFN-γ for 3 h (shaded bars) or left untreated (open bars). Subsequently, cells were washed twice by centrifugation and challenged with TLR agonists (Pam3CSK4, lipopeptide) or infected with 106 CFU/ml E. coli and subjected to antibiotic therapy after 1 h. Supernatants sampled 5 h after challenge were analyzed by ELISA (summarized result of five independent experiments; *, P < 0.02; **, P = 0.26).
Antagonism of primary inflammatory mediators, such as of TNF-α, IL-6, or IL-1β, is being evaluated. Blockades of different cytokines are currently being used as therapies of chronic inflammatory diseases. Yet, they have proved less successful for the treatment of acute infection, possibly caused by redundant activities via untargeted cytokines. Targeting late mediators of sepsis has proved successful in experimental models of sepsis, as demonstrated by antagonism of macrophage migration inhibitory factor or high-mobility group box 1 protein (26, 27). Using an experimental model of hyperinflammation induced by Gram-negative bacterial infection coupled to antibiotic therapy, we show a 4-h window of opportunity for protective TLR2/TLR4 blockade, contrasting the hypothesis of immediate early TLR activation as a point of no return. Our data also imply a time-dependent accumulation of inflammatory TLR signals encompassing one signal that “switches on” second line TLR2-specific sensitivity, which might depend on first line TLR4 activation upon a Gram-negative bacterial insult. Therefore, effective interference with pattern recognition concomitant with initiation of antibiotic therapy might be possible even in an advanced phase of sepsis pathology after infection. It is conceivable that dual TLR antagonism (as demonstrated in this study), as well as late mediator blockade and other concepts of sepsis pathology inhibition might have to match with each other or complement one another to define the most effective therapy.
It remains to be shown whether, in addition to averting a “storm” of cytokines, transient TLR blockade upon infection also reduces sepsis-related apoptosis and/or immunoparalysis (5), as deduced from TLR2/TLR4 blockade–dependent IL-10 reduction (Fig. 3 C). In conclusion (Fig. S6, available at http://www.jem.org/cgi/content/full/jem.20071990/DC1), our data implicate IFN-γ as a TLR4–MyD88–driven inducer of up-regulation of surface TLR2 expression and toxemia-related TLR2 sensitivity. Our preclinical results suggest that blockade of both TLR2 and the TLR4–MD-2 complex is a therapeutic approach to effectively inhibit Gram-negative bacterial infection–induced immunopathology during antibiotic therapy.
MATERIALS AND METHODS
Reagents, bacteria, cells, cell lines, mice, and TLR2/TLR4 blockade.
LPS from S. enterica serovar Minnesota strain R595, polyinosinic-polycytidylic acid (poly-I:C; both from Sigma-Aldrich) and di- or tripalmitoyl-cysteinyl-seryl-(lysyl)3-lysine (EMC microcollections) were applied at 100 ng/ml unless otherwise indicated. Thiolated DNA (#1668) was applied at 2 μM (TIB MOLBIOL) and anti–mouse-CD3ε was applied at 1 μg/ml (145-2C11; BD Biosciences), or IFN-γ (PeproTech) at 20 ng/ml. As the isotype-matched control for 1A6 (rat IgG2b) and T2.5 (mouse IgG1, mTLR2, and hTLR2-specific; HBT), equal amounts of unspecific 11G8 (rat IgG2b) and mTLR2-specific mouse mT2.13 (neutral, mouse IgG1), respectively, were blended (12, 19). Anti-hTLR4 mAb (15C1; isotype control mT2.13) has been previously described (12). mAbs were applied at 25 μg/ml in vitro or 30 mg/kg in vivo. Clinical isolate clones of S. enterica subspecies enterica serovar enteritidis and E. coli were cultured (16 h, 37°C) in standard media. Bacteria were used for infections both in vivo and in vitro. The bacterial dosage applied in vivo corresponded to a minimal dose that was lethal, despite antibiotic therapy. For antibiotic therapy in vitro, antibiotics (100 μg/ml ampicillin, 10 μg/ml ofloxacin; Sigma-Aldrich) were applied once 1 h after infection. Upon systemic infection, 68 mg/kg ampicillin and 2.8 mg/kg ofloxacin were applied i.p. at the antibiotic therapy starting time points indicated, and an additional 3 times (hourly) without mAbs. For determination of bacterial loads upon infection of mice and subsequent cervical dislocation, aliquots of serial dilutions of blood and organ suspensions were plated.
Immunization and mAb identification.
Male Wistar rats were subcutaneously immunized 3 times within 6 wk with 106 CHO/mTLR4–MD-2 cells suspended in monophosphoryl-lipid A/trehalose dicorynomycolate adjuvant (RIBI; Sigma-Aldrich). Immunized rats were challenged subcutaneously with 10 μg recombinant mTLR4-mMD-2 (mTLR4 ectodomain aa 1–629 fused to mMD-2 aa 19–170 via a peptide linker in RIBI). Lymph node cells were fused with Sp2/0 myeloma cells after 3 d (12). Hybridoma supernatants were screened for binding to mTLR4–MD-2 by flow cytometry.
Mice.
TLR2−/− (provided by Amgen, South San Francisco, CA) and TLR4−/− (provided by K. Hoshino and S. Akira, Osaka University, Osaka, Japan) mice were backcrossed toward the C57BL/6 background (WT) nine times and intercrossed (TLR2−/−/TLR4−/−) (27, 28). MyD88−/− and TRIF−/− mice were backcrossed toward the C57BL/6 background (WT) six times and intercrossed (MyD88−/−/TRIF −/−; provided by T. Kawaii, K. Hoshino, and S. Akira, Osaka University, Osaka, Japan) (29). IFN-γR−/− mice were on 129Sv background (30). All animal experiments were approved by the Government of Upper Bavaria, Germany.
Flow cytometry.
CD3 (FITC), IFN-γ (APC), CD11b (APC), NK1.1 (PE), CD8 (Alexa405), CD4 (PE; all from BD Bioscience), flag-tag (M2; Sigma-Aldrich), MTS510 (rat anti–mouse TLR4; Abcam), and/or TLR2 (FITC, mT2.7, or T2.5; HBT) for analysis by flow cytometry. For detection of unlabeled rat 1A6 or mouse T2.5, mouse anti–rat Fcγ or rat anti–mouse Fcγ mAb coupled with FMAT Blue (Applied Biosystems) or FITC (Jackson ImmunoResearch Laboratories), respectively, were used as secondary mAbs. Intracellular IFN-γ was analyzed using Cytofix/Cytoperm plus fixation/permeabilization and GolgiPlug solutions for incubation of cultured cells 4 h before staining (BD Biosciences). Primary cells were analyzed on a CyAn ADP LX9 analyzer (Dako) using FlowJo software (Tree Star, Inc.). Transfected CHO and HEK293 cells were analyzed using a FACSCalibur (BD Biosciences).
Analysis of supernatants and sera by ELISA.
Supernatant or mouse serum cytokine concentrations were determined by species-specific TNF-α, IL-6, IL-10, and IFN-γ ELISA (R&D Systems).
Immunocytochemistry, immunoprecipitation, and immunoblot analysis.
Immunofluorescence analysis of 2% aldehyde-fixed macrophages was performed after TLR4-specific staining using 1A6 and goat anti–rat–Alexa546 (Invitrogen) in 0.2% saponin/0.5% bovine serum albumin with a laser-scanning microscope using LSM Image software (Carl Zeiss, Inc.). Lysates of 4 × 106 cells of a HEK293 line stably overexpressing flag-tagged mTLR4–MD-2 were immunoprecipitated as described for lysates of 5 × 105 RAW264.7 cells applied to immunoblot analysis (19).
NF-κB–driven reporter gene assay.
Cell lysates of HEK293 cells transfected with plasmids for expression of PRRs and reporter proteins and challenged specifically were analyzed for NF-κB–dependent firefly luciferase activity (19).
Statistical analysis.
Student's t test for unconnected samples was applied for P value calculations. Mortality was analyzed by the Kaplan-Meier and log-rank methods. Differences were considered significant for P < 0.05. All P values are two tailed.
Online supplemental material.
Fig. S1 illustrates the capacity of 1A6 to stain overexpressed and endogenous cell surface TLR4–MD-2 specifically. Fig. S2 and Fig. S3 provide evidence for effectiveness, specificity, dose-dependence, and duration of 1A6-mediated TLR4–MD-2 blocking in vitro and in vivo, respectively. Fig. S4 demonstrates absence of CD11b+ cell depletion upon systemic 1A6 administration, as well as persistence of 1A6 on the surface of murine macrophages in vitro. Fig. S5 shows TLR4-dependent IFN-γ production induced by 2 h of systemic S. enterica infection by both CD4+ and CD8+ T cells (flow cytometry), respectively, as well as infection-induced surface TLR2 up-regulation on human PBMCs. Fig. S6 abstracts procedures and findings. The online version of this article is available at http://www.jem.org/cgi/content/full/jem.20071990/DC1.
Supplementary Material
Acknowledgments
We thank S. Fichte for assistance; T. Miethke for provision of clinical isolates of bacteria and N. Wantia for drawing blood from healthy authors to obtain PBMCs; M. Schiemann, C. Stemberger, and D. H. Busch for help with flow cytometry; F. Schmitz and T. Haas for help with microscopy; S. Akira, K. Hoshino, T. Kawai, and M. Yamamoto for TLR4−/−, MyD88−/−, and TRIF/TICAM-1−/− mice; D. Goeddel for TLR2−/− mice; M. Hammer and R. Lang for help with serum IFN-γ detection; HBT for support with T2.5; and T. Calandra, R. Ulevitch, C. Galanos, U. Koedel, S. Bauer, and G. Häcker for helpful discussions.
We thank The German Research Foundation for support of this study through SFB/TR22-A5.
G. Elson and B. Daubeuf are employed by NovImmune SA, Geneva, Switzerland, whose potential product, anti–human TLR4 (15C1) mAb was studied in this work. All other authors declare no financial interests.
S. Spiller and G. Elson contributed equally to this paper.
References
- 1.Cohen, J. 2002. The immunopathogenesis of sepsis. Nature. 420:885–891. [DOI] [PubMed] [Google Scholar]
- 2.Rivers, E.P., L. McIntyre, D.C. Morro, and K.K. Rivers. 2005. Early and innovative interventions for severe sepsis and septic shock: taking advantage of a window of opportunity. CMAJ. 173:1054–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan, C. 2002. Points of control in inflammation. Nature. 420:846–852. [DOI] [PubMed] [Google Scholar]
- 4.Annane, D., E. Bellissant, and J.M. Cavaillon. 2005. Septic shock. Lancet. 365:63–78. [DOI] [PubMed] [Google Scholar]
- 5.Hotchkiss, R.S., and D.W. Nicholson. 2006. Apoptosis and caspases regulate death and inflammation in sepsis. Nat. Rev. Immunol. 6:813–822. [DOI] [PubMed] [Google Scholar]
- 6.Latz, E., A. Verma, A. Visintin, M. Gong, C.M. Sirois, D.C. Klein, B.G. Monks, C.J. McKnight, M.S. Lamphier, W.P. Duprex, T. Espevik, and D.T. Golenbock. 2007. Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat. Rev. Immunol. 4:499–511. [DOI] [PubMed] [Google Scholar]
- 7.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499–511. [DOI] [PubMed] [Google Scholar]
- 8.Ulevitch, R.J., and P.S. Tobias. 1995. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu. Rev. Immunol. 13:437–457. [DOI] [PubMed] [Google Scholar]
- 9.Beutler, B., and E.T. Rietschel. 2003. Timeline: innate immune sensing and its roots: the story of endotoxin. Nat. Immunol. 8:772–779. [DOI] [PubMed] [Google Scholar]
- 10.Liu, P.T., S.R. Krutzik, and R.L. Modlin. 2007. Therapeutic implications of the TLR and VDR partnership. Trends Mol. Med. 13:117–124. [DOI] [PubMed] [Google Scholar]
- 11.Lembo, A., C. Kalis, C.J. Kirschning, V. Mitolo, E. Jirillo, H. Wagner, C. Galanos, and M.A. Freudenberg. 2003. Differential contribution of Toll-like receptors 4 and 2 to the cytokine response to Salmonella enterica serovar Typhimurium and Staphylococcus aureus in Mice. Infect. Immun. 71:6058–6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elson, G., I. Dunn-Siegrist, B. Daubeuf, and J. Pugin. 2007. Contribution of Toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood. 109:1574–1583. [DOI] [PubMed] [Google Scholar]
- 13.Schimke, J., J. Mathison, J. Morgiewicz, and R.J. Ulevitch. 1998. Anti-CD14 mAb treatment provides therapeutic benefit after in vivo exposure to endotoxin. Proc. Natl. Acad. Sci. USA. 95:13875–13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamping, N., R. Dettmer, N.W. Schroder, D. Pfeil, W. Hallatschek, R. Burger, and R.R. Schumann. 1998. LPS-binding protein protects mice from septic shock caused by LPS or gram-negative bacteria. J. Clin. Invest. 101:2065–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fort, M.M., A. Mozaffarian, A.G. Stover, S. Correia Jda, D.A. Johnson, R.T. Crane, R.J. Ulevitch, D.H. Persing, H. Bielefeldt-Ohmann, P. Probst, et al. 2005. A synthetic TLR4 antagonist has anti-inflammatory effects in two murine models of inflammatory bowel disease. J. Immunol. 174:6416–6423. [DOI] [PubMed] [Google Scholar]
- 16.Daubeuf, B., J. Mathison, S. Spiller, S. Hugues, S. Herren, W. Ferlin, M. Kosco-Vilbois, H. Wagner, C.J. Kirschning, R. Ulevitch, and G. Elson. 2007. TLR4/MD-2 monoclonal antibody therapy affords protection in experimental models of septic shock. J. Immunol. 179:6107–6114. [DOI] [PubMed] [Google Scholar]
- 17.Pound, M.W., and D.B. May. 2005. Proposed mechanisms and preventative options of Jarisch-Herxheimer reactions. J. Clin. Pharm. Ther. 30:291–295. [DOI] [PubMed] [Google Scholar]
- 18.Munford, R.S. 2006. Severe sepsis and septic shock: the role of Gram-negative bacteremia. Annu. Rev. Pathol. 1:467–496. [DOI] [PubMed] [Google Scholar]
- 19.Meng, G., M. Rutz, M. Schiemann, J. Metzger, A. Grabiec, R. Schwandner, P.B. Luppa, F. Ebel, D.H. Busch, S. Bauer, et al. 2004. Antagonistic antibody prevents toll-like receptor 2-driven lethal shock-like syndromes. J. Clin. Invest. 113:1473–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haraga, A., M.B. Ohlson, and S.I. Miller. 2008. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 6:53–66. [DOI] [PubMed] [Google Scholar]
- 21.Fan, J., Y. Li, Y. Vodovotz, T.R. Billiar, and M.A. Wilson. 2006. Hemorrhagic shock-activated neutrophils augment TLR4 signaling-induced TLR2 upregulation in alveolar macrophages: role in hemorrhage-primed lung inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 290:L738–L746. [DOI] [PubMed] [Google Scholar]
- 22.Car, B.D., V.M. Eng, B. Schnyder, L. Ozmen, S. Huang, P. Gallay, D. Heumann, M. Aguet, and B. Ryffel. 1994. Interferon γ receptor deficient mice are resistant to endotoxic shock. J. Exp. Med. 179:1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wittebole, X., S.M. Coyle, A. Kumar, M. Goshima, S.F. Lowry, and S.E. Calvano. 2005. Expression of tumour necrosis factor receptor and Toll-like receptor 2 and 4 on peripheral blood leucocytes of human volunteers after endotoxin challenge: a comparison of flow cytometric light scatter and immunofluorescence gating. Clin. Exp. Immunol. 141:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maris, N.A., M.C. Dessing, A.F. de Vos, P. Bresser, J.S. van der Zee, H.M. Jansen, C.A. Spek, and T. van der Poll. 2006. Toll-like receptor mRNA levels in alveolar macrophages after inhalation of endotoxin. Eur. Respir. J. 28:622–626. [DOI] [PubMed] [Google Scholar]
- 25.Lauener, R.P., T. Birchler, J. Adamski, C. Braun-Fahrlander, A. Bufe, U. Herz, E. von Mutius, D. Nowak, J. Riedler, M. Waser, and F.H. Sennhauser. 2002. Expression of CD14 and Toll-like receptor 2 in farmers' and non-farmers' children. Lancet. 360:465–466. [DOI] [PubMed] [Google Scholar]
- 26.Calandra, T., B. Echtenacher, D.L. Roy, J. Pugin, C.N. Metz, L. Hultner, D. Heumann, D. Mannel, R. Bucala, and M.P. Glauser. 2000. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat. Med. 6:164–170. [DOI] [PubMed] [Google Scholar]
- 27.Lotze, M.T., and K.J. Tracey. 2005. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 5:331–342. [DOI] [PubMed] [Google Scholar]
- 28.Spiller, S., S. Dreher, G. Meng, A. Grabiec, W. Thomas, T. Hartung, K. Pfeffer, H. Hochrein, H. Brade, W. Bessler, et al. 2007. Cellular recognition of trimyristoylated peptide or enterobacterial lipopolysaccharide via both TLR2 and TLR4. J. Biol. Chem. 282:13190–13198. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 301:640–643. [DOI] [PubMed] [Google Scholar]
- 30.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R.M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-gamma receptor. Science. 259:1742–1745. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.