Abstract
Nucleotide excision repair (NER) is a multi-step DNA repair mechanism that removes helix-distorting modified nucleotides from the genome. NER is divided into two subpathways depending on the location of DNA damage in the genome and how it is first detected. Global genome NER identifies and repairs DNA lesions throughout the genome. This subpathway of NER primarily protects against the accumulation of mutations in the genome. Transcription-coupled (TC) NER rapidly repairs lesions in the transcribed strand of DNA that block transcription by RNA polymerase II. TC-NER prevents cell death in response to stalled transcription. Defects in NER cause three distinct human diseases: xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Each of these syndromes is characterized by premature onset of pathologies that overlap with those associated with old age in humans. This reveals the contribution of DNA damage to multiple age-related diseases. Tissues affected include the skin, eye, bone marrow, nervous system and endocrine axis. This review emphasizes accelerated aging associated with xeroderma pigmentosum and discusses the cause of these pathologies, either mutation accumulation or cell death as a consequence of failure to repair DNA damage.
Keywords: skin cancer, photoaging, neurodegeneration, bone marrow failure, progeria
Nucleotide excision repair
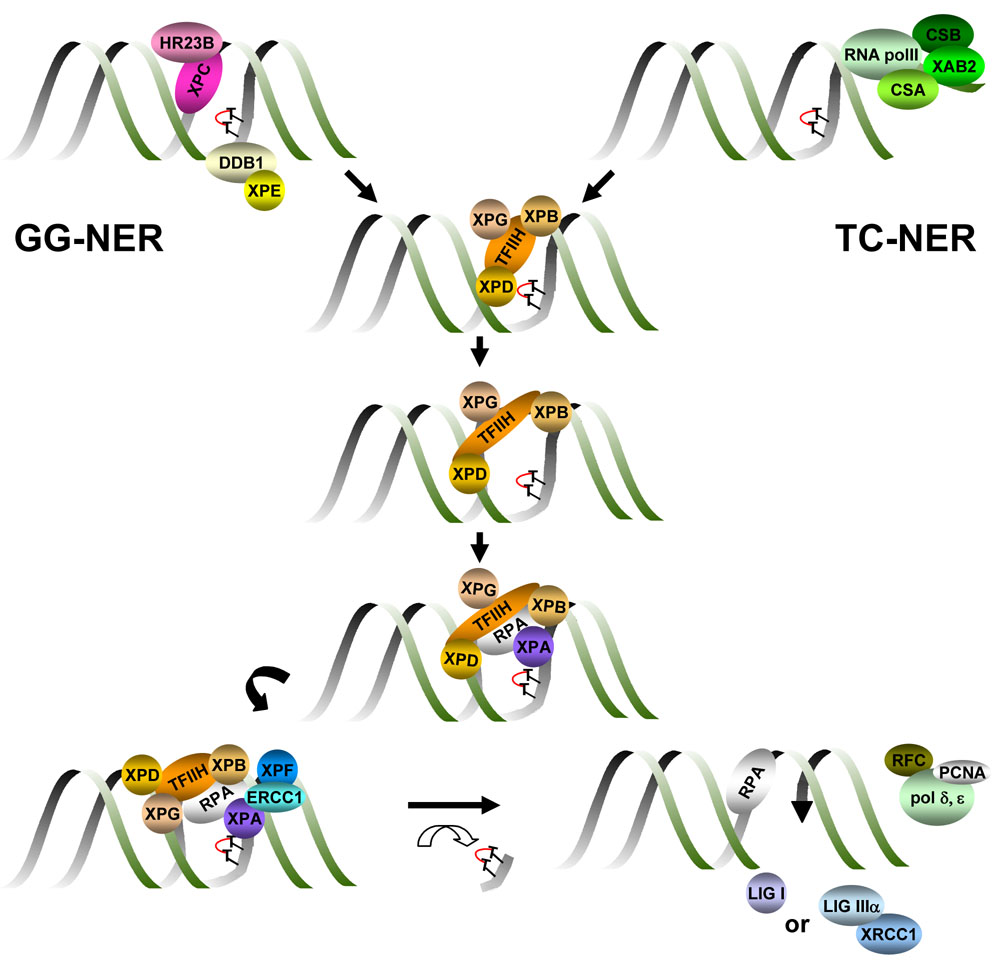
Nucleotide excision repair (NER) is an evolutionarily conserved mechanism that removes DNA lesions that distort the double helix (Figure 1). NER is commonly divided into two subpathways that differ mechanistically only in how the damaged nucleotides are identified in the genome. Helix-distortion is recognized throughout the genome by XPC in the global genome subpathway of NER (GG-NER). HR23B or its homolog HR23A facilitates damage recognition by stabilizing XPC (Sugasawa et al., 1997). Many of the DNA lesions removed by NER are caused by environmental genotoxins, including polycyclic aromatic hydrocarbons, psoralens, aromatic amines and chemotherapeutic agents such as cisplatin and mitomycin C (Sancar, 1996). For example, NER is the only repair mechanism for 6–4 photoproducts and cyclobutane pyrimidine dimers caused by ultraviolet (UV) radiation in placental mammals (Friedberg et al., 2006). A second protein complex, DDB1-DDB2/XPE, specifically facilitates the recognition of UV photolesions by XPC-HR23B (Sugasawa, 2006). DNA lesions that occur on the transcribed strand of a gene, which block transcription, are rapidly recognized and repaired by transcription-coupled NER (TC-NER). CSA, CSB and XAB2 facilitate TC-NER by stabilizing RNA polymerase II at or near the site of damage (Laine and Egly, 2006).
Figure 1. Schematic diagram of nucleotide excision repair.
Helix-distorting DNA lesions, for example UV-induced cyclopyrimidine dimes T∩T) are recognized throughout the genome by the protein complex XPC-HR23B. Lesion recognition is facilitated by the DDB complex (DDB1 and XPE/DDB2) specifically in the case of DNA damage caused by UV radiation. DDB is part of the Cul4A complex, which ubiquitylates XPC, leading to its stable association with damaged DNA. This subpathway of NER is called global genome NER (GG-NER). Lesions on the transcribed strand of DNA, which block RNA polymerase II-mediated transcription, activate transcription-coupled NER (TC-NER). TC-NER is facilitated by CSB, CSA and XAB2. Either XPC in GG-NER or CSB and CSA in TC-NER recruit the transcription factor TFIIH to the site of damage. XPG stabilizes TFIIH. The XPB and XPD subunits of TFIIH are helicases that unwind the DNA around the lesion. XPA and RPA then bind and stabilize this unwound intermediate and recruit XPF-ERCC1. This endonuclease incises the damaged strand of DNA 5’ to the lesion, while XPG makes the 3’ incision. The lesion is removed in a single-stranded oligonucleotide, leaving behind a gap, which is filled by the replication machinery comprised of polymerase δ and ε, PCNA, RPA and RFC. The DNA backbone is sealed by DNA Ligase I or DNA Ligase 3-XRCC1.
Once a lesion is identified, GG-NER and TC-NER use a common mechanism to excise the damage. The multi-protein basal transcription factor TFIIH is recruited to the damage site through interactions with XPC-HR23B or CSB and CSA. XPG binds TFIIH and is critical for the structural integrity of this multi-subunit complex (Ito et al., 2007). The XPB and XPD subunits of TFIIH are helicases that unwind the DNA on both sides of the lesion. XPA and RPA are then recruited to the site to stabilize this open repair intermediate (Patrick and Turchi, 2002). XPA facilitates the subsequent recruitment of XPF-ERCC1 nuclease, which incises the damaged strand of DNA 5’ to the lesion. XPG makes the 3’ incision. The lesion is therefore excised in a 26–29 base single-stranded oligonucleotide. This leaves a single-stranded gap, which is filled by the replication machinery (including polymerase δ and ε, PCNA, RPA and RFC) using the undamaged strand as a template. After DNA synthesis to fill the gap, DNA LigI or DNA Lig3-XRCC1 (Moser et al., 2007) restores the integrity of the backbone to complete the repair reaction.
Diseases caused by defective nucleotide excision repair
Inherited defects in NER cause three distinct human diseases. Xeroderma pigmentosum (XP) is characterized by photosensitivity, hyperpigmentation and ichthyosis (dry, scaly skin) in sun exposed areas, a 1000-fold increase in the risk of basal and squamous cell carcinomas and melanomas of the skin and eyes (Kraemer et al., 1984; Kraemer et al., 1987). Approximately 30% of XP patients develop progressive neurologic symptoms (Kraemer et al., 2007). There are seven complementation groups of XP (XP-A to –G) caused by defective NER, corresponding to mutations in the genes encoding XPA, XPB, XPC, XPD, XPE, XPF and XPG. The severity of symptoms in XP varies widely, with the age at diagnosis ranging from early infancy to adulthood, and for the most part correlates with the extent to which the mutation affects NER (Kraemer et al., 2007).
Cockayne syndrome (CS) is caused by mutations in CSA, CSB, XPB, XPD or XPG, leading to defective TC-NER. Symptoms include growth retardation, microcephaly, delayed psychomotor development, mental retardation, joint contractures, ataxia and abnormal gait, hypogonadism, muscle atrophy, cataracts, osteopenia, dental caries, cachexia, photosensitivity and a prematurely aged appearance (Nance and Berry, 1992). CS patients are not predisposed to cancer. Trichothiodystrophy (TTD), the third disease associated with defective NER, is identical to CS except the patients have additional cutaneous symptoms including brittle hair and nails and ichthyosis (dry, scaly skin). TTD is caused by mutations in XPB, XPD or TTDA, which encode subunits of TFIIH, resulting in destabilization or dysfunction of the transcription factor (Giglia-Mari et al., 2004). The cutaneous symptoms of TTD are caused by impaired transcription of skin-specific genes (de Boer et al., 1998).
Many symptoms of XP, CS and TTD are similar to those seen in very elderly people in the normal population. Thus defective NER may accelerate aging of several, but not all tissues (Table I). This review primarily focuses on premature aging associated with XP and caused by mutations in genes encoding core NER factors. For a discussion of aging and age-related diseases in CS, readers are referred to Stevnsner in this issue.
Table I.
Tissue-specific accelerated aging associated with xeroderma pigmentosum.
| Age-related pathology | Complementation groups affected | Pathway affected | Cause |
|---|---|---|---|
| Photoaging of the skin | XP-A, XP-C, XP-E and mild XP-F | GG-NER | Mutagenesis in keratinocytes and melanocytes |
| Photoaging of the eyes | XP-A others? | GG-NER in cancer TC-NER in leucoma |
Ocular tumors: mutagenesis in epithelial cells of the cornea, eyelid and conjunctiva or soft tissue; Leucoma: loss or replicative senescence of limbic stem cells |
| Hematopoietic failure | XpdTTD/TTD mice Ercc1−/− mice | TC-NER or interstrand crosslink repair | Replicative senescence of hematopoietic stem cells |
| Neuro-degeneration | XP-A, XP-B, XP-D, XP-F | TC-NER | Apoptosis of neurons |
| Endocrine changes | Xpdm/m;Xpa−/−, Csbm/m;Xpa/− and Ercc1−/− | TC-NER or interstrand crosslink repair | Apoptosis |
| Multiple organs | Severe depletion of XPF | interstrand crosslink repair | Apoptosis |
Skin
As we age, ours skin becomes more lax, causing wrinkling (Rabe et al., 2006). This is caused by progressive loss and disorganization of extracellular elastin and collagen fibers, reduction in extracellular glycosaminoglycans, senescence of dermal fibroblasts and decreased vascularization, collectively leading to atrophy of both the epidermis and dermis (Kligman and Kligman, 1986; Rabe et al., 2006). Aging of the skin is dramatically accelerated and exacerbated by UV radiation. Thus sun-exposed areas of the skin invariably appear “older” than unexposed areas due to photoaging. Photoaged skin is characterized by poikiloderma (dyspigmentation), laxity, deep wrinkles, telangiectasias and a yellowed, leathery appearance (Rabe et al., 2006). Although sun exposure accelerates aging of the skin, histopathologic changes caused by photoaging differ from age-associated changes of unexposed skin. Photoaging causes dramatic elastosis (accumulation of thick, tangled, degraded elastin fibers), loss of collagen fibers, increased glycosaminoglycans, disorganization and atypia of keratinocytes, inflammation of the dermis (heliodermatitis), hyperplasia of dermal fibroblasts and loss of vascularization with telangiectatic changes (Kligman and Kligman, 1986; Rabe et al., 2006).
With aging, the incidence of raised, irregular skin lesions increases exponentially. In sun-protected areas, benign cherry angiomas are the only growths that occur as we age (Kligman and Kligman, 1986). In contrast, skin that is exposed to UV radiation is highly prone to multiple benign (seborrheic keratoses, actinic keratoses) and malignant growths (squamous cells carcinoma, basal cell carcinoma and melanoma) (Rabe et al., 2006). The two major risk factors for skin cancer are UV exposure and age (Lewis and Weinstock, 2007).
All XP patients are photosensitive and have cutaneous abnormalities in sun-exposed areas of the skin (Kraemer et al., 1987). In patients with severe XP, in which NER is virtually absent, acute sunburn occurs after very brief or indirect sun exposure. Thus even a limited cumulative UV exposure leads to premature onset of poikiloderma, telangiectasias, actinic keratoses (pre-cancerous dry scaly lesions) and cutaneous neoplasms. The mean age at onset of skin cancer in XP is 8 years of age (Kraemer et al., 1987). Non-sun exposed areas of the skin are not affected in XP patients. Therefore XP represents dramatically accelerated photoaging of the skin.
The fact that XP is such a faithful model of photoaging indicates that UV-induced DNA damage, typically repaired by NER, is largely responsible for the degenerative and proliferative changes associated with photoaging. Terrestrial sunlight contains UV-B (295–320 nm) and UV-A (320–400 nm) wavelengths. UV is absorbed by DNA, causing direct DNA damage, such as 6–4 photoproducts and cyclopyrimidine dimers (Friedberg et al., 2006). These wavelengths can also damage DNA indirectly through the production of reactive oxygen species, which cause oxidative lesions and single-strand breaks. Cutaneous neoplasms are largely restricted to patients of complementation groups XP-A, XP-C, XP-E and mild XP-F patients (Friedberg et al., 2006). Patients with CS due to defects in TC-NER, although photosensitive, are not cancer-prone. This indicates that GG-NER is primarily responsible for protecting against UV-induced mutagenesis. In the absence of GG-NER, mutations accumulate in UV-exposed areas of skin leading to skin cancer.
In contrast, TC-NER is primarily responsible for protecting against a cytotoxic response to DNA damage (Mitchell et al., 2003; Schumacher et al., 2008). In the absence of TC-NER, UV induces erythema and edema (acute sunburn), but not skin cancer. This differential protective effect of GG-NER and TC-NER is supported by studies in mouse models of XP. Xpc−/− mice, defective only in GG-NER, are susceptible to UV-B induced skin cancer, but not erythema or edema (Berg et al., 1998). Xpa−/− and Ercc1flox/−;K5Cre mice (in which Ercc1 is knocked-out only in the skin), defective in both GG-NER and TC-NER, are exquisitely susceptible to UV-B induced erythema and skin cancer (de Vries et al., 1995; Doig et al., 2006). Surprisingly, mice harboring disease-causing human mutations in Csb and Xpd are also susceptible to UV-induced skin cancer, unlike patients with CS and TTD (de Boer et al., 1999; van der Horst et al., 2002; van der Horst et al., 1997). This can be explained by the fact that in the absence of TC-NER, excision of UV photolesions by GG-NER is more efficient in humans than in mice (van der Horst et al., 2002), reducing the risk of mutagenesis.
Eyes
Approximately 40% of XP patients have ocular disease (Kraemer et al., 1987). This is restricted to the conjuctiva, eyelids and cornea; tissues, which are exposed to UV radiation. Symptoms are progressive and include photophobia, conjunctival injection (caused by vasodilation and telangiectasias of the conjunctiva), loss of eyelashes, pingueculae and pterygia (non-malignant conjunctival growths), corneal opacity (leucoma) and ocular neoplasms (squamous cell carcinoma, basal cell carcinoma, melanoma and atypical fibroxanthoma) (Kraemer et al., 1987; Kraemer et al., 2007; Shao et al., 2007). The iris, lens and retina are shielded from UV radiation by the cornea and are therefore unaffected in XP.
The ocular disease seen in XP can occur in normal individuals, where the greatest risk factors are sun exposure and age. Therefore XP represents accelerated photoaging of the eyes. Ocular tumors, like skin cancer are caused by accumulation of UV-induced mutations in the epithelial cells of the conjunctiva, cornea and eyelids, or superficial mesenchymal cells. However, leucoma appears to be driven by a deficiency of limbic stem cells (Fernandes et al., 2004). Limbic stem cells are tissue-specific stem cells that replenish damaged corneal epithelia. Therefore some of the accelerated ocular aging seen in XP is due to either cell death or replicative senescence of stem cells as a consequence of failure to repair DNA damage.
Nervous system
Neurological disease can emerge at any age in XP from the first to fourth decade of life (Robbins et al., 2002) depending upon the severity of the NER defect (Robbins et al., 1991). Neurological symptoms are progressive and include loss of fine motor control, choreoathetosis, ataxia, unsteady gait, spasticity, rigidity, hyporeflexia, loss of hearing, laryngeal dystonia and dysarthria, dysphagia, dementia and a peripheral sensory axonal neuropathy (Hayashi et al., 2004; Robbins et al., 1991). Neuropathological findings include microcephaly, cerebral cortical atrophy with enlarged ventricles. This is caused by neurodegeneration in the cerebral cortex, basal ganglia, cerebellum, brain stem, corticospinal tract, cochlea, dorsal root ganglia and peripheral nerves (Hayashi et al., 2004; Robbins et al., 1991; Robbins et al., 2002). Loss of neurons is due to apoptotic cell death (Kohji et al., 1998), presumably as a consequence of failure to repair endogenous DNA damage. Myelination, or white matter is not affected (Hentati et al., 1992) nor are glial cells (Kohji et al., 1998). Accordingly, in vitro primary neurons from Xpa−/− mice are significantly more sensitive to genotoxic stress than astroglial cells (Kisby et al., 2004).
The fact that neurodegeneration is a common feature of XP implies that NER is crucial for maintaining neurons in humans. Because NER functions exclusively on the nuclear genome, this further implies that nuclear DNA damage, if not repaired, can drive neurodegeneration. Endogenous DNA lesions that are substrates for NER include abasic sites and alkylated bases (Sancar, 1996; Wood, 1996), as well as some oxidative lesions including urea, 7,8-dihydro-8-oxoguanine and 8,5’-cyclopurines (Bjelland and Seeberg, 2003; Brooks, 2007). The high penetrance of neurodegeneration in severe XP, suggests that NER must be the only mechanism for the removal of some of these endogenous lesions, making cyclopurines the best candidate causative lesions identified to date (Brooks, 2007). For a complete discussion of the contribution of oxidative stress to neurodegeneration, the reader is referred to Englander in this issue.
Neurodegeneration occurs most frequently in XP complementation group A (XP-A patients) (Hentati et al., 1992). Because XPA functions exclusively in NER, this demonstrates that neurodegeneration is a direct consequence of loss of this DNA repair pathway. Neurologic disease can also occur in XP-B and XP-D patients. Milder, adult onset neurologic impairment can occur in XP-C and XP-F (Robbins et al., 1993; Sijbers et al., 1998), but has not been reported in XP-E(DDB2) patients (Rapic-Otrin et al., 2003). This indicates that TC-NER (requiring XPA, XPB, XPD and XPF among other proteins) is particularly important for neuronal protection, while GG-NER (requiring XPC and XPE) is less important for maintaining neurons. In accordance, GG-NER is decreased in human neurons relative to mitotically active cells, but TC-NER is sustained (Nouspikel and Hanawalt, 2000).
Surprisingly, in contrast to humans, neuropathology was not detected in Xpa−/− (de Vries et al., 1995; Nakane et al., 1995) or Xpc−/− mice (Cheo et al., 1997; Sands et al., 1995). However, under conditions of stress (traumatic brain injury), recovery is impaired in Xpa−/− mice (Wijnhoven et al., 2007), suggesting that there may be underlying deficits in the nervous system of these DNA repair-deficient mice, which are too subtle to observe directly. In general, mouse models of NER-deficiency syndromes (XP, CS or TTD) have milder neurologic disease than the patients they mimic (Niedernhofer, 2008), possibly because mice accumulate DNA damage more slowly than humans. Intercrossing mouse models of XP and CS (Xpa−/−;Csb−/− or Xpc−/−;Csb−/− mice) leads to a very severe phenotype with growth retardation, severely reduced lifespan and profound spontaneous neurodegeneration (Laposa et al., 2007; Murai et al., 2001; van der Pluijm et al., 2007). Neurologic symptoms include tremors, an abnormal gait, dystonia, poor balance and progressive ataxia beginning in the first week of life (Laposa et al., 2007; Murai et al., 2001; van der Pluijm et al., 2007). Neuronal proliferation is decreased, while apoptosis is increased in the cerebellum of these mice. This supports a critical role for CSB in protecting cells of the nervous system from a cytostatic and cytotoxic response to endogenous DNA damage. However in mice, unlike humans, this is not revealed until DNA damage levels are increased by simultaneously deleting NER.
Many of the neurologic symptoms seen in XP are also associated with old age in the normal population These include decreased fine motor control, unsteady gait, hyporeflexia, hearing loss, dementia and peripheral neuropathy. With aging, there is a progressive decrease in brain function and volume beginning in the 4th decade of life (Brazel and Rao, 2004). Similar to XP, this decrease is attributed to loss of neurons, either through apoptotic cell death or failure to replace neurons, while glial cells are spared (Brazel and Rao, 2004). Therefore at the level of the whole organism, tissue and cell, many aspects of XP represents accelerated aging of the nervous system. Oxidative DNA damage accumulates with age in the central nervous system (Weissman et al., 2007). In XP patients missing NER, this oxidative DNA damage presumably accumulates more rapidly or is more likely to trigger cell death.
Other neurologic symptoms seen in XP are hallmark features of age-related neurodegenerative diseases. Both Parkinson’s and XP patients may experience spasticity, rigidity, laryngeal dystonia and dysarthria, dysphagia and dementia. Cognitive decline is universal in Alzheimer’s disease and in XP patients with neurodegeneration. Oxidative DNA damage is implicated in the pathogenesis of both of these neurodegenerative diseases (Weissman et al., 2007). Thus there may be some overlap in the mechanism of pathogenesis between XP and Parkinson’s or Alzheimer’s disease. This is an important consideration in terms of disease prevention or therapy.
Hematopoiesis
Our hematopoietic system has a remarkably robust proliferative capacity, capable of producing 1012 blood cells per day throughout our lifespan (Effros and Globerson, 2002). Despite this, there is evidence that hematopoietic function declines with age. A significant fraction of healthy individuals over the age of 70 are mildly cytopenic, in particular anemic (Salive et al., 1992). Elderly individuals also have a reduced capacity to produce red blood cells in response to hypoxia (Udupa and Lipschitz, 1984) and neutrophils after endotoxin challenge (Timaffy, 1962). Age-associated loss of hematopoietic function is associated with decreased physical performance (Penninx et al., 2003) and increased risk of mortality (Wilkinson and Warren, 2003). Transplantation studies in mice indicate that the primary defect is loss of hematopoietic stem cell (HSC) function with age (Azuma et al., 2002; Liang et al., 2005).
Two very recent studies demonstrate that DNA repair mechanisms are critical for the preservation of HSC function with aging (Nijnik et al., 2007; Rossi et al., 2007). Specifically related to NER, Rossi et al. analyzed hematopoietic stem cells (HSCs) in a mouse model of TTD [XpdTTD mice (de Boer et al., 1998)]. The bone marrow of these mice remains normocellular until at least one year of age and the number of HSCs is preserved (Rossi et al., 2007). However, there is a progressive loss of bone marrow progenitor cells in these mice. Moreover, HSCs isolated from XpdTTD mice are impaired in their ability to repopulate the hematopoietic system of irradiated hosts in competitive bone marrow transplantation, relative to age-matched wild type mice. This directly demonstrates that HSC function is prematurely compromised in a model of TTD. Furthermore, Ercc1−/− mice undergo spontaneous bone marrow failure within their 4 wk lifespan (Prasher et al., 2005). Thus defects in at least two proteins required for NER accelerate aging of the hematopoietic system.
It is important to note that the XpdTTD and Ercc1−/− mice have defects in addition to decreased NER that influence their phenotypes (de Boer et al., 1998; McWhir et al., 1993; Weeda et al., 1997). Transcription is impaired in XpdTTD mice and DNA interstrand crosslink repair is defective in the absence of XPF-ERCC1 (McHugh et al., 2001; Niedernhofer et al., 2004), identical to what is seen in cells derived from patients that they model. Thus both animal models are exquisitely sensitive to genotoxic stress (de Boer et al., 2002; Niedernhofer et al., 2006). This sensitivity, or predisposition to a cytotoxic or cytostatic response to DNA damage, undoubtedly contributes to accelerated aging in these mice, including aging of the hematopoietic system. However, bone marrow failure was reported in an XP patient (Salob et al., 1992). Therefore NER may be important for maintaining hematopoietic function.
Accelerated aging of the hematopoietic system was also discovered in mice defective in nonhomologous end-joining of DNA double-strand breaks (Nijnik et al., 2007; Rossi et al., 2007), telomere maintenance (Rossi et al., 2007), the Fanconi anemia pathway of DNA interstrand crosslink repair (Carreau et al., 1999; Haneline et al., 1999; Navarro et al., 2006), mismatch repair (Reese et al., 2003), ATM (Ito et al., 2004) or RAD50 (Bender et al., 2002) [reviewed in (Niedernhofer, 2008)]. Many of the corresponding human genome instability disorders include prominent hematologic pathology (for a complete discussion of some of these diseases see Hasty in this issue). Therefore DNA damage clearly makes a substantial contribution to the loss of hematopoietic function associated with aging
Tissue-specific stem cells like HSCs are responsible for organ development, maintaining tissue homeostasis and regeneration after injury (Weissman, 2000). Preserving stem cells throughout the lifespan of an organism is therefore essential for maintaining tissue function and its ability to respond to stress (Rossi et al., 2007). Aging is defined as the loss of homeostatic reserve leading to degenerative changes and increased risk of morbidity and mortality (Kirkwood, 2005). Thus loss of tissue-specific stem cells is predicted to promote aging (Schlessinger and Van Zant, 2001; Van Zant and Liang, 2003). Genotoxic stress negatively affects HSCs and limbic stem cells of the eye, accelerating the onset of age-related disease. This is likely to extend to other tissue-specific stem cells (Sharpless and DePinho, 2007). The tissue-specific pattern of accelerated aging in XP, suggests that skin and neuronal stem cells are likely to be highly vulnerable to genotoxic stress.
Cancer
XP patients were reported to have an increased risk of non-cutaneous tumors (Kraemer et al., 1984), including astrocytoma, medulloblastoma and sarcomas of the brain, leukemia, pancreatic adenocarcinoma, testicular sarcoma, bronchogenic carcinoma and gastric cancer (Kraemer et al., 1987). In this study, the median age at onset of internal tumors in XP patients was in the third decade of life (ranging from the first to sixth decade). This is much later than the onset of skin cancer in XP, but much earlier than the onset of cancer in the normal population. Thus defective NER may accelerate the onset of the age-related disease cancer. The fact that there is no tissue-specificity to the predisposition to internal tumors in XP suggests that these malignancies could be caused by environmental exposures unique to individual patients rather than endogenous DNA damage.
Xpa−/− and Xpc−/− mice have a higher incidence and earlier onset of spontaneous, non-cutaneous tumors than wild type mice (Wijnhoven et al., 2007). Xpa−/− accumulate somatic mutations in liver as they age, but not in brain, and these mice have an increased incidence of spontaneous liver tumors (Giese et al., 1999). In contrast, mouse models of CS and TTD, with accelerated aging of multiple tissues, do not have an elevated spontaneous mutation frequency or an increased incidence of spontaneous internal tumors (Dolle et al., 2006). Thus mutations, arising from an NER defect, correlate with cancer but not aging, providing experimental evidence that mutations are not the driving force in aging (Mitchell et al., 2003; Schumacher et al., 2008)
XFE
In XP, accelerated aging is predominantly limited to the skin, eyes and nervous system and may be largely driven by environmental factors (UV exposure of skin and dietary or other factors that promote oxidative stress in the nervous system). However many proteins required for NER have additional functions, complicating interpretation of disease phenotypes. This is the case for XPB and XPD, which as subunits of TFIIH are required not only for NER but also for basal transcription. Another example is the nuclease XPF-ERCC1. Cells defective in XPF-ERCC1 are exquisitely sensitive to genotoxins that induced DNA interstrand crosslinks (ICLs) relative to other NER mutants (Niedernhofer et al., 2006). Thus XPF-ERCC1 is implicated in the repair of ICLs via a mechanism that is distinct from NER (McHugh et al., 2001; Niedernhofer et al., 2004). ICLs covalently link both strands of DNA together, preventing strand separation, transcription and replication, and therefore are extremely cytotoxic. Endogenous ICLs have not yet been detected in vivo, likely because the number of lesions required to kill a cell is below the limit of detection (Dronkert and Kanaar, 2001). However, there are numerous bifunctional electrophiles (e.g., malondialdehyde) produced endogenously in millimolar quantities (Largilliere and Melancon, 1988), which modify nuclear DNA (Chaudhary et al., 1994; Rouzer et al., 1997) and form crosslinks in vitro under physiological conditions (Kasai et al., 1998; Niedernhofer et al., 2003). Many of these endogenous genotoxins are produced via fragmentation of membrane fatty acids by reactive oxygen species (Marnett, 2002).
Mutations in XPF can lead to either XP or a progeroid syndrome with dramatically accelerated aging of multiple tissues. XP-F patients typically have mild XP, with late onset of skin cancer and mild neurodegeneration (Sijbers et al., 1998). A mutation in XPF that severely affected XPF expression and its obligate binding partner ERCC1 caused accelerated aging of skin (not photoaging), nervous, cardiovascular, renal, hepatobiliary, hematopoietic and musculoskeletal systems (Niedernhofer et al., 2006). The neurologic symptoms in this single progeroid patient (hearing loss, tremors, ataxia and cerebral atrophy) are strikingly similar to the neurodegenerative disease in XP. Thus neurodegeneration in this patient may be caused by an NER defect. However, many of the other symptoms, including hypertension, impaired kidney and liver function, anemia, sarcopenia and osteoporosis are not commonly associated with XP, particular in the first decade of life. Thus accelerated aging of the kidney, liver and musculoskeletal system in this progeroid syndrome are ascribed to defective repair of DNA interstrand crosslinks (Niedernhofer et al., 2006).
Endocrine
A mouse model of the progeroid syndrome caused by XPF-ERCC1 deficiency revealed profound endocrine changes as a consequence of impaired DNA repair (Niedernhofer et al., 2006). Virtually identical endocrine changes were discovered in mouse models of TTD and CS crossed into an Xpa nullizygous background to increase the level of endogenous DNA damage (van de Ven et al., 2006; van der Pluijm et al., 2007). Ercc1−/−, Xpdm/m;Xpa−/−, and Csbm/m;Xpa−/− mice show defects in the somatotroph, thyrotroph and lactotroph hormonal axes. This is driven not by a pituitary defect, but by decreased expression of numerous effectors of the growth hormone (GH) axis, most notably insulin-like growth factor-1 (IGF-1). These endocrine changes, regulated by insulin/IGF-1, are an evolutionarily conserved, systemic response to stress activated by genotoxins, DNA repair deficiency or old age (Niedernhofer et al., 2006; van der Pluijm et al., 2007). Therefore premature onset of these endocrine changes in DNA repair deficient mice can be considered accelerated aging.
Importantly, this stress response is not activated in Xpa−/− mice, which are prone to UV-induced skin cancer (photoaging) but not other age-related diseases typical of XP (i.e. neurodegeneration). Therefore these age-related endocrine changes are only detected in mice defective in DNA repair mechanisms that protect against cell death and as a consequence have prominent features of accelerated aging.
Premature onset of these age-related endocrine changes is commonly seen in patients with genome instability syndromes associated with prominent symptoms of accelerated aging. Circulating levels of GH and IGF-1 are decreased in patients with Werner’s syndrome (Rubin and Reed, 1996), Fanconi anemia (Giri et al., 2007) and Rothmund-Thomson syndrome (Pujol et al., 2000). Furthermore, diabetes mellitus is common in Fanconi anemia (Giri et al., 2007) and ataxia telangiectasia (Bar et al., 1978). DNA repair pathways affected in these human syndromes protect against cytotoxic DNA lesions including stalled replication forks, DNA interstrand crosslinks and double-strand breaks. Therefore, aging of the endocrine system, like the nervous system, cornea and hematopoietic system is accelerated by cell death in response to genotoxic stress.
SUMMARY
Many aspects of XP parallel age-associated disease and pathology in the normal population. Photoaging of the skin and eyes, early onset cancer and neurodegeneration represent accelerated aging in XP. In addition, mice harboring mutations in genes encoding proteins required for NER display hematopoietic failure and endocrinopathies associated with advanced age in humans. Skin, ocular and internal tumors characteristic of XP are caused by loss of global genome-NER of DNA damage incurred from environmental exposures, leading to mutation accumulation. In contrast, neurodegeneration, bone marrow failure and age-associated endocrine changes are caused by failure of transcription-coupled NER or other DNA repair mechanisms to remove endogenous, cytotoxic DNA damage. Analysis of the accelerated degenerative changes in the bone marrow and eyes caused by defects in NER, indicate that loss of function of tissue-specific stem cells is a primary cause of accelerated aging in response to unrepaired DNA damage.
ACKNOWLEDGEMENTS
L.J.N. is supported by The Ellison Medical Foundation (AG-NS-0303) the NCI (CA103730, CA121411 and CA111525) and the NIEHS (ES016114).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Azuma E, Hirayama M, Yamamoto H, Komada Y. The role of donor age in naive T-cell recovery following allogeneic hematopoietic stem cell transplantation: the younger the better. Leuk Lymphoma. 2002;43:735–739. doi: 10.1080/10428190290016827. [DOI] [PubMed] [Google Scholar]
- Bar RS, Levis WR, Rechler MM, Harrison LC, Siebert C, Podskalny J, Roth J, Muggeo M. Extreme insulin resistance in ataxia telangiectasia: defect in affinity of insulin receptors. N Engl J Med. 1978;298:1164–1171. doi: 10.1056/NEJM197805252982103. [DOI] [PubMed] [Google Scholar]
- Bender CF, Sikes ML, Sullivan R, Huye LE, Le Beau MM, Roth DB, Mirzoeva OK, Oltz EM, Petrini JH. Cancer predisposition and hematopoietic failure in Rad50(S/S) mice. Genes Dev. 2002;16:2237–2251. doi: 10.1101/gad.1007902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg RJ, Ruven HJ, Sands AT, de Gruijl FR, Mullenders LH. Defective global genome repair in XPC mice is associated with skin cancer susceptibility but not with sensitivity to UVB induced erythema and edema. J Invest Dermatol. 1998;110:405–409. doi: 10.1111/j.1523-1747.1998.00173.x. [DOI] [PubMed] [Google Scholar]
- Bjelland S, Seeberg E. Mutagenicity, toxicity and repair of DNA base damage induced by oxidation. Mutat Res. 2003;531:37–80. doi: 10.1016/j.mrfmmm.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Brazel CY, Rao MS. Aging and neuronal replacement. Ageing Res Rev. 2004;3:465–483. doi: 10.1016/j.arr.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Brooks PJ. The case for 8,5′-cyclopurine-2′-deoxynucleosides as endogenous DNA lesions that cause neurodegeneration in xeroderma pigmentosum. Neuroscience. 2007;145:1407–1417. doi: 10.1016/j.neuroscience.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreau M, Gan OI, Liu L, Doedens M, Dick JE, Buchwald M. Hematopoietic compartment of Fanconi anemia group C null mice contains fewer lineage-negative CD34+ primitive hematopoietic cells and shows reduced reconstruction ability. Exp Hematol. 1999;27:1667–1674. doi: 10.1016/s0301-472x(99)00102-2. [DOI] [PubMed] [Google Scholar]
- Chaudhary AK, Nokubo M, Reddy GR, Yeola SN, Morrow JD, Blair IA, Marnett LJ. Detection of endogenous malondialdehyde-deoxyguanosine adducts in human liver. Science. 1994;265:1580–1582. doi: 10.1126/science.8079172. [DOI] [PubMed] [Google Scholar]
- Cheo DL, Ruven HJ, Meira LB, Hammer RE, Burns DK, Tappe NJ, van Zeeland AA, Mullenders LH, Friedberg EC. Characterization of defective nucleotide excision repair in XPC mutant mice. Mutat Res. 1997;374:1–9. doi: 10.1016/s0027-5107(97)00046-8. [DOI] [PubMed] [Google Scholar]
- de Boer J, Andressoo JO, de Wit J, Huijmans J, Beems RB, van Steeg H, Weeda G, van der Horst GT, van Leeuwen W, Themmen AP, Meradji M, Hoeijmakers JH. Premature aging in mice deficient in DNA repair and transcription. Science. 2002;296:1276–1279. doi: 10.1126/science.1070174. [DOI] [PubMed] [Google Scholar]
- de Boer J, de Wit J, van Steeg H, Berg RJ, Morreau H, Visser P, Lehmann AR, Duran M, Hoeijmakers JH, Weeda G. A mouse model for the basal transcription/DNA repair syndrome trichothiodystrophy. Mol Cell. 1998;1:981–990. doi: 10.1016/s1097-2765(00)80098-2. [DOI] [PubMed] [Google Scholar]
- de Boer J, van Steeg H, Berg RJ, Garssen J, de Wit J, van Oostrum CT, Beems RB, van der Horst GT, van Kreijl CF, de Gruijl FR, Bootsma D, Hoeijmakers JH, Weeda G. Mouse model for the DNA repair/basal transcription disorder trichothiodystrophy reveals cancer predisposition. Cancer Res. 1999;59:3489–3494. [PubMed] [Google Scholar]
- de Vries A, van Oostrom CT, Hofhuis FM, Dortant PM, Berg RJ, de Gruijl FR, Wester PW, van Kreijl CF, Capel PJ, van Steeg H, et al. Increased susceptibility to ultraviolet-B and carcinogens of mice lacking the DNA excision repair gene XPA. Nature. 1995;377:169–173. doi: 10.1038/377169a0. [DOI] [PubMed] [Google Scholar]
- Doig J, Anderson C, Lawrence NJ, Selfridge J, Brownstein DG, Melton DW. Mice with skin-specific DNA repair gene (Ercc1) inactivation are hypersensitive to ultraviolet irradiation-induced skin cancer and show more rapid actinic progression. Oncogene. 2006;25:6229–6238. doi: 10.1038/sj.onc.1209642. [DOI] [PubMed] [Google Scholar]
- Dolle ME, Busuttil RA, Garcia AM, Wijnhoven S, van Drunen E, Niedernhofer LJ, van der Horst G, Hoeijmakers JH, van Steeg H, Vijg J. Increased genomic instability is not a prerequisite for shortened lifespan in DNA repair deficient mice. Mutat Res. 2006;596:22–35. doi: 10.1016/j.mrfmmm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Dronkert ML, Kanaar R. Repair of DNA interstrand cross-links. Mutat Res. 2001;486:217–247. doi: 10.1016/s0921-8777(01)00092-1. [DOI] [PubMed] [Google Scholar]
- Effros RB, Globerson A. Hematopoietic cells and replicative senescence. Exp Gerontol. 2002;37:191–196. doi: 10.1016/s0531-5565(01)00183-8. [DOI] [PubMed] [Google Scholar]
- Fernandes M, Sangwan VS, Vemuganti GK. Limbal stem cell deficiency and xeroderma pigmentosum: a case report. Eye. 2004;18:741–743. doi: 10.1038/sj.eye.6700717. [DOI] [PubMed] [Google Scholar]
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. Washington, D.C.: American Society for Microbiology; 2006. [Google Scholar]
- Giese H, Dolle ME, Hezel A, van Steeg H, Vijg J. Accelerated accumulation of somatic mutations in mice deficient in the nucleotide excision repair gene XPA. Oncogene. 1999;18:1257–1260. doi: 10.1038/sj.onc.1202404. [DOI] [PubMed] [Google Scholar]
- Giglia-Mari G, Coin F, Ranish JA, Hoogstraten D, Theil A, Wijgers N, Jaspers NG, Raams A, Argentini M, van der Spek PJ, Botta E, Stefanini M, Egly JM, Aebersold R, Hoeijmakers JH, Vermeulen W. A new, tenth subunit of TFIIH is responsible for the DNA repair syndrome trichothiodystrophy group A. Nat Genet. 2004;36:714–719. doi: 10.1038/ng1387. [DOI] [PubMed] [Google Scholar]
- Giri N, Batista DL, Alter BP, Stratakis CA. Endocrine abnormalities in patients with Fanconi anemia. J Clin Endocrinol Metab. 2007;92:2624–2631. doi: 10.1210/jc.2007-0135. [DOI] [PubMed] [Google Scholar]
- Haneline LS, Gobbett TA, Ramani R, Carreau M, Buchwald M, Yoder MC, Clapp DW. Loss of FancC function results in decreased hematopoietic stem cell repopulating ability. Blood. 1999;94:1–8. [PubMed] [Google Scholar]
- Hayashi M, Araki S, Kohyama J, Shioda K, Fukatsu R, Tamagawa K. Brainstem and basal ganglia lesions in xeroderma pigmentosum group A. J Neuropathol Exp Neurol. 2004;63:1048–1057. doi: 10.1093/jnen/63.10.1048. [DOI] [PubMed] [Google Scholar]
- Hentati F, Ben Hamida C, Zeghal M, Kamoun M, Fezaa B, Ben Hamida M. Age-dependent axonal loss in nerve biopsy of patients with xeroderma pigmentosum. Neuromuscul Disord. 1992;2:361–369. doi: 10.1016/s0960-8966(06)80007-6. [DOI] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, Ikeda Y, Mak TW, Suda T. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- Ito S, Kuraoka I, Chymkowitch P, Compe E, Takedachi A, Ishigami C, Coin F, Egly JM, Tanaka K. XPG stabilizes TFIIH, allowing transactivation of nuclear receptors: implications for Cockayne syndrome in XP-G/CS patients. Mol Cell. 2007;26:231–243. doi: 10.1016/j.molcel.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Kasai H, Iwamoto-Tanaka N, Fukada S. DNA modifications by the mutagen glyoxal: adduction to G and C, deamination of C and GC and GA cross-linking. Carcinogenesis. 1998;19:1459–1465. doi: 10.1093/carcin/19.8.1459. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Kisby GE, Lesselroth H, Olivas A, Samson L, Gold B, Tanaka K, Turker MS. Role of nucleotide- and base-excision repair in genotoxin-induced neuronal cell death. DNA Repair (Amst) 2004;3:617–627. doi: 10.1016/j.dnarep.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Kligman LH, Kligman AM. The nature of photoaging: its prevention and repair. Photodermatol. 1986;3:215–227. [PubMed] [Google Scholar]
- Kohji T, Hayashi M, Shioda K, Minagawa M, Morimatsu Y, Tamagawa K, Oda M. Cerebellar neurodegeneration in human hereditary DNA repair disorders. Neurosci Lett. 1998;243:133–136. doi: 10.1016/s0304-3940(98)00109-8. [DOI] [PubMed] [Google Scholar]
- Kraemer KH, Lee MM, Scotto J. DNA repair protects against cutaneous and internal neoplasia: evidence from xeroderma pigmentosum. Carcinogenesis. 1984;5:511–514. doi: 10.1093/carcin/5.4.511. [DOI] [PubMed] [Google Scholar]
- Kraemer KH, Lee MM, Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987;123:241–250. doi: 10.1001/archderm.123.2.241. [DOI] [PubMed] [Google Scholar]
- Kraemer KH, Patronas NJ, Schiffmann R, Brooks BP, Tamura D, DiGiovanna JJ. Xeroderma pigmentosum, trichothiodystrophy and Cockayne syndrome: a complex genotype-phenotype relationship. Neuroscience. 2007;145:1388–1396. doi: 10.1016/j.neuroscience.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine JP, Egly JM. When transcription and repair meet: a complex system. Trends Genet. 2006;22:430–436. doi: 10.1016/j.tig.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Laposa RR, Huang EJ, Cleaver JE. Increased apoptosis, p53 up-regulation, and cerebellar neuronal degeneration in repair-deficient Cockayne syndrome mice. Proc Natl Acad Sci U S A. 2007;104:1389–1394. doi: 10.1073/pnas.0610619104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largilliere C, Melancon SB. Free malondialdehyde determination in human plasma by high-performance liquid chromatography. Anal Biochem. 1988;170:123–126. doi: 10.1016/0003-2697(88)90098-x. [DOI] [PubMed] [Google Scholar]
- Lewis KG, Weinstock MA. Trends in nonmelanoma skin cancer mortality rates in the United States, 1969 through 2000. J Invest Dermatol. 2007;127:2323–2327. doi: 10.1038/sj.jid.5700897. [DOI] [PubMed] [Google Scholar]
- Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106:1479–1487. doi: 10.1182/blood-2004-11-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnett LJ. Oxy radicals, lipid peroxidation and DNA damage. Toxicology. 2002;181–182:219–222. doi: 10.1016/s0300-483x(02)00448-1. [DOI] [PubMed] [Google Scholar]
- McHugh PJ, Spanswick VJ, Hartley JA. Repair of DNA interstrand crosslinks: molecular mechanisms and clinical relevance. Lancet Oncol. 2001;2:483–490. doi: 10.1016/S1470-2045(01)00454-5. [DOI] [PubMed] [Google Scholar]
- McWhir J, Selfridge J, Harrison DJ, Squires S, Melton DW. Mice with DNA repair gene (ERCC-1) deficiency have elevated levels of p53, liver nuclear abnormalities and die before weaning. Nat Genet. 1993;5:217–224. doi: 10.1038/ng1193-217. [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Hoeijmakers JH, Niedernhofer LJ. Divide and conquer: nucleotide excision repair battles cancer and ageing. Curr Opin Cell Biol. 2003;15:232–240. doi: 10.1016/s0955-0674(03)00018-8. [DOI] [PubMed] [Google Scholar]
- Moser J, Kool H, Giakzidis I, Caldecott K, Mullenders LH, Fousteri MI. Sealing of chromosomal DNA nicks during nucleotide excision repair requires XRCC1 and DNA ligase III alpha in a cell-cycle-specific manner. Mol Cell. 2007;27:311–323. doi: 10.1016/j.molcel.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Murai M, Enokido Y, Inamura N, Yoshino M, Nakatsu Y, van der Horst GT, Hoeijmakers JH, Tanaka K, Hatanaka H. Early postnatal ataxia and abnormal cerebellar development in mice lacking Xeroderma pigmentosum Group A and Cockayne syndrome Group B DNA repair genes. Proc Natl Acad Sci U S A. 2001;98:13379–13384. doi: 10.1073/pnas.231329598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane H, Takeuchi S, Yuba S, Saijo M, Nakatsu Y, Murai H, Nakatsuru Y, Ishikawa T, Hirota S, Kitamura Y, et al. High incidence of ultraviolet-B-or chemical-carcinogen-induced skin tumours in mice lacking the xeroderma pigmentosum group A gene. Nature. 1995;377:165–168. doi: 10.1038/377165a0. [DOI] [PubMed] [Google Scholar]
- Nance MA, Berry SA. Cockayne syndrome: review of 140 cases. Am J Med Genet. 1992;42:68–84. doi: 10.1002/ajmg.1320420115. [DOI] [PubMed] [Google Scholar]
- Navarro S, Meza NW, Quintana-Bustamante O, Casado JA, Jacome A, McAllister K, Puerto S, Surralles J, Segovia JC, Bueren JA. Hematopoietic dysfunction in a mouse model for Fanconi anemia group D1. Mol Ther. 2006;14:525–535. doi: 10.1016/j.ymthe.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Niedernhofer LJ. DNA repair is crucial for maintaining hematopoietic stem cell function. DNA Repair 2008 Jan 7. 2008 Jan 7; doi: 10.1016/j.dnarep.2007.11.012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedernhofer LJ. Nucleotide excision repair deficient mouse models and neurological disease. DNA Repair (Amst) 2008 doi: 10.1016/j.dnarep.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J Biol Chem. 2003;278:31426–31433. doi: 10.1074/jbc.M212549200. [DOI] [PubMed] [Google Scholar]
- Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, Theil AF, Vermeulen W, van der Horst GT, Meinecke P, Kleijer WJ, Vijg J, Jaspers NG, Hoeijmakers JH. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- Niedernhofer LJ, Odijk H, Budzowska M, van Drunen E, Maas A, Theil AF, de Wit J, Jaspers NG, Beverloo HB, Hoeijmakers JH, Kanaar R. The structure-specific endonuclease Ercc-1-Xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol Cell Biol. 2004;24:5776–5787. doi: 10.1128/MCB.24.13.5776-5787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijnik A, Woodbine L, Marchetti C, Dawson S, Lambe T, Liu C, Rodrigues NP, Crockford TL, Cabuy E, Vindigni A, Enver T, Bell JI, Slijepcevic P, Goodnow CC, Jeggo PA, Cornall RJ. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–690. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- Nouspikel T, Hanawalt PC. Terminally differentiated human neurons repair transcribed genes but display attenuated global DNA repair and modulation of repair gene expression. Mol Cell Biol. 2000;20:1562–1570. doi: 10.1128/mcb.20.5.1562-1570.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick SM, Turchi JJ. Xeroderma pigmentosum complementation group A protein (XPA) modulates RPA-DNA interactions via enhanced complex stability and inhibition of strand separation activity. J Biol Chem. 2002;277:16096–16101. doi: 10.1074/jbc.M200816200. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Guralnik JM, Onder G, Ferrucci L, Wallace RB, Pahor M. Anemia and decline in physical performance among older persons. Am J Med. 2003;115:104–110. doi: 10.1016/s0002-9343(03)00263-8. [DOI] [PubMed] [Google Scholar]
- Prasher JM, Lalai AS, Heijmans-Antonissen C, Ploemacher RE, Hoeijmakers JH, Touw IP, Niedernhofer LJ. Reduced hematopoietic reserves in DNA interstrand crosslink repair-deficient Ercc1−/− mice. Embo J. 2005;24:861–871. doi: 10.1038/sj.emboj.7600542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol LA, Erickson RP, Heidenreich RA, Cunniff C. Variable presentation of Rothmund-Thomson syndrome. Am J Med Genet. 2000;95:204–207. [PubMed] [Google Scholar]
- Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol. 2006;55:1–19. doi: 10.1016/j.jaad.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Rapic-Otrin V, Navazza V, Nardo T, Botta E, McLenigan M, Bisi DC, Levine AS, Stefanini M. True XP group E patients have a defective UV-damaged DNA binding protein complex and mutations in DDB2 which reveal the functional domains of its p48 product. Hum Mol Genet. 2003;12:1507–1522. doi: 10.1093/hmg/ddg174. [DOI] [PubMed] [Google Scholar]
- Reese JS, Liu L, Gerson SL. Repopulating defect of mismatch repair-deficient hematopoietic stem cells. Blood. 2003;102:1626–1633. doi: 10.1182/blood-2002-10-3035. [DOI] [PubMed] [Google Scholar]
- Robbins JH, Brumback RA, Mendiones M, Barrett SF, Carl JR, Cho S, Denckla MB, Ganges MB, Gerber LH, Guthrie RA, et al. Neurological disease in xeroderma pigmentosum. Documentation of a late onset type of the juvenile onset form. Brain. 1991;114(Pt 3):1335–1361. doi: 10.1093/brain/114.3.1335. [DOI] [PubMed] [Google Scholar]
- Robbins JH, Brumback RA, Moshell AN. Clinically asymptomatic xeroderma pigmentosum neurological disease in an adult: evidence for a neurodegeneration in later life caused by defective DNA repair. Eur Neurol. 1993;33:188–190. doi: 10.1159/000116932. [DOI] [PubMed] [Google Scholar]
- Robbins JH, Kraemer KH, Merchant SN, Brumback RA. Adult-onset xeroderma pigmentosum neurological disease--observations in an autopsy case. Clin Neuropathol. 2002;21:18–23. [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- Rouzer CA, Chaudhary AK, Nokubo M, Ferguson DM, Reddy GR, Blair IA, Marnett LJ. Analysis of the malondialdehyde-2′-deoxyguanosine adduct in human leuokocyte DNA by gas chromatography/electron capture negative chemical ionization/mass spectrometry. Chem Res Toxicol. 1997;10:181–188. doi: 10.1021/tx9601216. [DOI] [PubMed] [Google Scholar]
- Rubin CD, Reed B. Twenty-four hour growth hormone secretion in a patient with Werner's syndrome. Exp Gerontol. 1996;31:557–561. doi: 10.1016/0531-5565(96)00031-9. [DOI] [PubMed] [Google Scholar]
- Salive ME, Cornoni-Huntley J, Guralnik JM, Phillips CL, Wallace RB, Ostfeld AM, Cohen HJ. Anemia and hemoglobin levels in older persons: relationship with age, gender, and health status. J Am Geriatr Soc. 1992;40:489–496. doi: 10.1111/j.1532-5415.1992.tb02017.x. [DOI] [PubMed] [Google Scholar]
- Salob SP, Webb DK, Atherton DJ. A child with xeroderma pigmentosum and bone marrow failure. Br J Dermatol. 1992;126:372–374. doi: 10.1111/j.1365-2133.1992.tb00681.x. [DOI] [PubMed] [Google Scholar]
- Sancar A. DNA excision repair. Annu Rev Biochem. 1996;65:43–81. doi: 10.1146/annurev.bi.65.070196.000355. [DOI] [PubMed] [Google Scholar]
- Sands AT, Abuin A, Sanchez A, Conti CJ, Bradley A. High susceptibility to ultraviolet-induced carcinogenesis in mice lacking XPC. Nature. 1995;377:162–165. doi: 10.1038/377162a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger D, Van Zant G. Does functional depletion of stem cells drive aging? Mech Ageing Dev. 2001;122:1537–1553. doi: 10.1016/s0047-6374(01)00299-8. [DOI] [PubMed] [Google Scholar]
- Schumacher B, Garinis GA, Hoeijmakers JH. Age to survive: DNA damage and aging. Trends Genet. 2008;24:77–85. doi: 10.1016/j.tig.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Shao L, Newell B, Quintanilla N. Atypical fibroxanthoma and squamous cell carcinoma of the conjunctiva in xeroderma pigmentosum. Pediatr Dev Pathol. 2007;10:149–152. doi: 10.2350/06-06-0103.1. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8:703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- Sijbers AM, van Voorst Vader PC, Snoek JW, Raams A, Jaspers NG, Kleijer WJ. Homozygous R788W point mutation in the XPF gene of a patient with xeroderma pigmentosum and late-onset neurologic disease. J Invest Dermatol. 1998;110:832–836. doi: 10.1046/j.1523-1747.1998.00171.x. [DOI] [PubMed] [Google Scholar]
- Sugasawa K. UV-induced ubiquitylation of XPC complex, the UV-DDB-ubiquitin ligase complex, and DNA repair. J Mol Histol. 2006;37:189–202. doi: 10.1007/s10735-006-9044-7. [DOI] [PubMed] [Google Scholar]
- Sugasawa K, Ng JM, Masutani C, Maekawa T, Uchida A, van der Spek PJ, Eker AP, Rademakers S, Visser C, Aboussekhra A, Wood RD, Hanaoka F, Bootsma D, Hoeijmakers JH. Two human homologs of Rad23 are functionally interchangeable in complex formation and stimulation of XPC repair activity. Mol Cell Biol. 1997;17:6924–6931. doi: 10.1128/mcb.17.12.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timaffy M. A comparative study of bone marrow function in young and old individuals. Gerontol Clin (Basel) 1962;4:13–18. doi: 10.1159/000244707. [DOI] [PubMed] [Google Scholar]
- Udupa KB, Lipschitz DA. Erythropoiesis in the aged mouse: I. Response to stimulation in vivo. J Lab Clin Med. 1984;103:574–580. [PubMed] [Google Scholar]
- van de Ven M, Andressoo JO, Holcomb VB, von Lindern M, Jong WM, De Zeeuw CI, Suh Y, Hasty P, Hoeijmakers JH, van der Horst GT, Mitchell JR. Adaptive stress response in segmental progeria resembles long-lived dwarfism and calorie restriction in mice. PLoS Genet. 2006;2:e192. doi: 10.1371/journal.pgen.0020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst GT, Meira L, Gorgels TG, de Wit J, Velasco-Miguel S, Richardson JA, Kamp Y, Vreeswijk MP, Smit B, Bootsma D, Hoeijmakers JH, Friedberg EC. UVB radiation-induced cancer predisposition in Cockayne syndrome group A (Csa) mutant mice. DNA Repair (Amst) 2002;1:143–157. doi: 10.1016/s1568-7864(01)00010-6. [DOI] [PubMed] [Google Scholar]
- van der Horst GT, van Steeg H, Berg RJ, van Gool AJ, de Wit J, Weeda G, Morreau H, Beems RB, van Kreijl CF, de Gruijl FR, Bootsma D, Hoeijmakers JH. Defective transcription-coupled repair in Cockayne syndrome B mice is associated with skin cancer predisposition. Cell. 1997;89:425–435. doi: 10.1016/s0092-8674(00)80223-8. [DOI] [PubMed] [Google Scholar]
- van der Pluijm I, Garinis GA, Brandt RM, Gorgels TG, Wijnhoven SW, Diderich KE, de Wit J, Mitchell JR, van Oostrom C, Beems R, Niedernhofer LJ, Velasco S, Friedberg EC, Tanaka K, van Steeg H, Hoeijmakers JH, van der Horst GT. Impaired genome maintenance suppresses the growth hormone--insulin-like growth factor 1 axis in mice with Cockayne syndrome. PLoS Biol. 2007;5:e2. doi: 10.1371/journal.pbio.0050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zant G, Liang Y. The role of stem cells in aging. Exp Hematol. 2003;31:659–672. doi: 10.1016/s0301-472x(03)00088-2. [DOI] [PubMed] [Google Scholar]
- Weeda G, Donker I, de Wit J, Morreau H, Janssens R, Vissers CJ, Nigg A, van Steeg H, Bootsma D, Hoeijmakers JH. Disruption of mouse ERCC1 results in a novel repair syndrome with growth failure, nuclear abnormalities and senescence. Curr Biol. 1997;7:427–439. doi: 10.1016/s0960-9822(06)00190-4. [DOI] [PubMed] [Google Scholar]
- Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- Weissman L, Jo DG, Sorensen MM, de Souza-Pinto NC, Markesbery WR, Mattson MP, Bohr VA. Defective DNA base excision repair in brain from individuals with Alzheimer's disease and amnestic mild cognitive impairment. Nucleic Acids Res. 2007;35:5545–5555. doi: 10.1093/nar/gkm605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnhoven SW, Hoogervorst EM, de Waard H, van der Horst GT, van Steeg H. Tissue specific mutagenic and carcinogenic responses in NER defective mouse models. Mutat Res. 2007;614:77–94. doi: 10.1016/j.mrfmmm.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Wilkinson TJ, Warren MR. What is the prognosis of mild normocytic anaemia in older people? Intern Med J. 2003;33:14–17. doi: 10.1046/j.1445-5994.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- Wood RD. DNA repair in eukaryotes. Annu Rev Biochem. 1996;65:135–167. doi: 10.1146/annurev.bi.65.070196.001031. [DOI] [PubMed] [Google Scholar]