Abstract
Mounting evidence is demonstrating roles for the amyloid precursor protein (APP) and its proteolytic product Aβ in metal homeostasis. Furthermore, aberrant metal homeostasis is observed in patients with Alzheimer's disease (AD), and this may contribute to AD pathogenesis, by enhancing the formation of reactive oxygen species and toxic Aβ oligomers and facilitating the formation of the hallmark amyloid deposits in AD brain. Indeed, zinc released from synaptic activity has been shown to induce parenchymal and cerebrovascular amyloid in transgenic mice. On the other hand, abnormal metabolism of APP and Aβ may impair brain metal homeostasis as part of the AD pathogenic process. Aβ and APP expression have both been shown to decrease brain copper (Cu) levels, whereas increasing brain Cu availability results in decreased levels of Aβ and amyloid plaque formation in transgenic mice. Lowering Cu concentrations can downregulate the transcription of APP, strengthening the hypothesis that APP and Aβ form part of the Cu homeostatic machinery in the brain. This is a complex pathway, and it appears that when the sensitive metal balance in the brain is sufficiently disrupted, it can lead to the self-perpetuating pathogenesis of AD. Clinical trials are currently studying agents that can remedy abnormal Aβ–metal interactions.
Keywords: Alzheimer's disease, amyloid-β peptide, amyloid precursor protein, metal homeostasis, copper, zinc, brain, transgenic mice
Alzheimer's disease (AD) is a polygenic neurodegenerative disorder involving the abnormal accumulation and deposition of a copper–zinc metalloprotein Aβ. Whether the aggregation of the peptide into amyloid deposits constitutes clearance of a toxic soluble entity or whether the amyloid plaques themselves are toxic is still debated. The aggregation of Aβ is mediated by interaction with metals, in particular zinc (Zn), copper (Cu) and iron (Fe) (Bush et al. 1994b; Atwood et al. 1998; Atwood et al. 2000b). Therefore, altered metal homeostasis may be an important factor leading to AD pathogenesis. Aβ also catalyses the reduction of Cu2+ and Fe3+ (Huang et al. 1999a), which in the absence of sufficient antioxidant mechanisms, could lead to the production of toxic reactive oxygen species (ROS) that may contribute to the pathogenesis of AD. Oxidative stress in turn may contribute to Aβ accumulation by generating modified Aβ species that have a high tendency to aggregate and are resistant to clearance (Kuo et al. 1998; Stadtman & Oliver 1991). Aβ is generated by proteolytic cleavage of the amyloid precursor protein (APP). APP is processed by two competing pathways that either lead to (amyloidogenic pathway) or preclude (nonamyloidogenic pathway) Aβ production (Selkoe 1998). Aβ generation requires the consecutive action of two proteases, β- and γ-secretase, catalysing the release of the N- and C-termini of the Aβ molecule, respectively. The alternative α-cleavage of APP precludes Aβ formation by cleaving within the Aβ sequence. We review here the roles of metals in AD pathogenesis. In particular, we describe evidence for roles of APP and Aβ in Cu homeostasis, and how this may relate to the metal imbalances observed in AD brain, and the pathogenic process. The importance of brain metal homeostasis in Aβ accumulation and amyloid formation is also discussed.
Metals and amyloid formation
Metals have been postulated to play a role in the pathogenesis of AD (Atwood et al. 1999; Bush 2000, 2003). Cu, Zn and Fe are concentrated in and around amyloid plaques in AD brain (Smith et al. 1997; Lovell et al. 1998; Sayre et al. 2000; Suh et al. 2000; Dong et al. 2003). High levels of Zn (Lee et al. 1999) and Fe (Smith et al. 1998a) have also been reported in the amyloid plaques of the Tg2576 (APPsw) mouse model for AD; however, to our knowledge, Cu levels have not yet been reported. Aβ possesses selective high and low affinity Cu2+- and Zn2+-binding sites that mediate its aggregation via interaction with Cu2+, Zn2+ and to a lesser extent Fe3+in vitro (Bush et al. 1994b; Atwood et al. 1998; Atwood et al. 2000b). Electron paramagnetic resonance and nuclear magnetic resonance studies proposed a model of monomer Aβ binding to a Cu ion via three histidines and a tyrosine or via a bridging histidine for aggregated Aβ (Curtain et al. 2001). Recently, Raman spectroscopic analysis of senile plaque cores demonstrated that Cu and Zn ions are co-ordinated via histidine residues (Dong et al. 2003), which are located at the N-terminal end of the Aβ sequence. The affinity of Aβ variants for Cu2+ is greatest for Aβ1–42 > Aβ1–40 > > rat Aβ, correlating with redox activity and toxicity (Atwood et al. 1998; Huang et al. 1999b; Atwood et al. 2000b).
The exact role of metal ions in β-sheet formation and fibril assembly of Aβ peptides in vivo is unclear. Zinc is a more powerful inducer of Aβ aggregation than Cu or any other metal (Atwood et al. 1998; Atwood et al. 2000b). At neutral pH, Zn2+ binds to Aβ to form insoluble aggregates, while Cu2+ binding is competitive, inducing a soluble conformation (Clements et al. 1996; Miura et al. 2000). At mildly acidic pH, however, which occurs in an aged brain and in response to inflammation, Cu2+ induces the formation of insoluble Aβ aggregates (Atwood et al. 1998). Indeed, altered co-ordination of Cu by the Aβ-histidine residues has recently been demonstrated with the lowering of pH (Syme et al. 2004).
The balance of Zn and Cu concentrations, as well as the maintenance of physiological pH, may therefore be important to prevent Aβ aggregation and amyloid formation. The dynamics of these metal ion interactions in vivo, however, are unknown. Chelation of metal ions reverses the aggregation of synthetic Aβ peptide and dissolves amyloid in postmortem human brain specimens (Huang et al. 1997; Atwood et al. 1998; Cherny et al. 1999). Furthermore, the treatment of the Tg2576 transgenic mouse model for AD with clioquinol, an orally bioavailable metal chelator, induced a marked inhibition of cortical amyloid accumulation (Cherny et al. 2001). This effect was recently reproduced using another hydrophobic chelator DP-109 (Lee et al. 2004a).
A recent study found that the formation of Aβ deposits in rabbits that resulted from a high cholesterol diet could be prevented by feeding demineralized water, instead of tap water, which contained traces of Cu (0.12 ppm) and presumably a mixture of other metals (Sparks & Schreurs 2003). This suggested that dietary metals could influence Aβ deposition in the brain under certain conditions. Perhaps in a brain, where Aβ clearance is already challenged by other factors (such as high cholesterol), dietary metals may further promote Aβ accumulation.
Synaptic Zn is important for amyloid-β formation
A recent series of studies have demonstrated a key role for synaptic Zn in Aβ deposition and amyloid formation in Tg2576 mice (Lee et al. 2002; Friedlich et al. 2004; Lee et al. 2004b). Synaptic Zn is an exchangeable Zn2+ pool in synaptic vesicles that is externalized upon neurotransmission, resulting in transient elevations of Zn2+ in the extracellular spaces from a basal level of <0.5 µm[reviewed in (Bush 2000; Frederickson & Bush 2001)] to around 300 µm[reviewed in (Frederickson & Bush 2001)]. Mice deficient in synaptic Zn transporter (ZnT3) are deficient in synaptic Zn. Genetic ablation of ZnT3 in Tg2576 mice resulted in a approximately 50% reduction in amyloid burden compared with control Tg2576 mice (Lee et al. 2002). Interestingly, the highest levels of free or synaptic Zn are found in cortex and hippocampus, the regions most affected in AD (Frederickson et al. 1992; Bush 2003). Zn2+ reuptake after synaptic release is a rapid, energy-dependent process. Hence, energy depletion could cause a pooling of extracellular Zn2+, contributing to Aβ deposition (Bush 2003).
ZnT3 also regulates an exchangeable Zn2+ pool, recently identified in the cerebrovascular wall of mice (Friedlich et al. 2004). Cerebral amyloid angiopathy (CAA) is common in AD and may contribute to dementia and cerebral haemorrhage. The CAA in Tg2576 mice is enriched in this exchangeable Zn2+ pool, and depletion of this Zn2+ pool by genetic ablation of ZnT3 resulted in a dramatic reduction in CAA (Friedlich et al. 2004).
The ZnT3 regulatable synaptic Zn pool (which represents approximately 15% of total brain Zn) was shown to increase with age but only in female mice (Lee et al. 2002). Lee and co-workers subsequently demonstrated that synaptic Zn levels are regulated by oestrogen through the expression of an adaptor protein (Lee et al. 2004b). This age-dependent elevation in synaptic Zn accounted for the greater amyloid load in Tg2576 females, as genetic ablation of ZnT3 not only reduced the amyloid burden in both male and female Tg2576 mice but also removed the sex difference in amyloid load (Lee et al. 2002). Whether synaptic Zn also increases with age in humans is unknown. Furthermore, some (Sturchler-Pierrat & Staufenbiel 2000; Callahan et al. 2001; Wang et al. 2003) but not all (Oddo et al. 2003) transgenic mouse models of AD amyloidosis display enhanced pathology in females. This suggests that the age-dependent elevation in synaptic Zn may not be a phenomenon common to all mouse lines, or indeed across species.
Like Zn, Cu is also released with neurotransmission but at a concentration an order of magnitude less than Zn (Hartter &, Barnea 1988a,b). Synaptic Cu could potentially participate in the formation of extracellular Aβ aggregates. However, far less is known about this release mechanism, and data on its role in experimental AD models are not yet available.
Age-dependent elevations in Aβ-precipitating metal ions
Fe levels in the brain have been shown by several groups to rise markedly with age in both humans and mice (Drayer et al. 1986; Connor et al. 1992; Thomas et al. 1993; Bartzokis et al. 1997; Martin et al. 1998; Zecca et al. 2001; Maynard et al. 2002). Cu levels have also been shown to rise with age in mouse brain (Massie et al. 1979; Morita et al. 1994; Maynard et al. 2002), and therefore, probably also rise with age in human brain. Although the pools of Fe and Cu that increase with age have not been clearly defined, electron paramagnetic imaging has demonstrated clusters of both Cu and Fe ions in the brain that increase with age and are even further elevated in AD (Wender et al. 1992). This suggests the presence of redox-active metal ion stores with low bioavailability in the ageing brain. Together, a large body of data demonstrate an age-dependent breakdown of metal regulation that could be a common consequence of ageing. Increased levels of Cu, Fe and perhaps synaptic Zn levels may contribute to the age-dependent formation of amyloid pathology.
The literature available on the changes in total brain Zn levels with age is limited and inconsistent. However, the trend among reports on a range of mouse tissues including brain (Woodward et al. 1984; Morita et al. 1994) and human serum (Bohnen et al. 1994; Del Corso et al. 2000) is that Zn levels tend to either remain unchanged or show a slight decrease with age. Female mice have increasingly more exchangeable Zn2+ in the ZnT3-associated cortical synaptic vesicles with advancing age (Lee et al. 2002), possibly contributing to increased amyloid plaque burden in APP transgenic mice.
Metals and oxidative stress
Because of the redox-active nature of Cu and Fe, defective regulation of these metals can lead to reaction with O2 and the production of ROS, resulting in cellular toxicity. AD brain exhibits marked oxidative damage of proteins, lipids and nucleic acids (Pappolla et al. 1992; Smith et al. 1994; Sayre et al. 1997; Hensley et al. 1998; Pratico et al. 1998; Smith et al. 1998b; Nunomura et al. 1999; Smith et al. 2000). Oxidative damage is highly concentrated in and around amyloid plaques, but immunohistochemical analysis of Tg2576 brain has shown markers of an oxidative stress response also in neuropil devoid of Aβ deposits (Pappolla et al. 1998; Smith et al. 1998a). Markers of lipid peroxidation are also found in cerebrospinal fluid (CSF) and urine of patients with a clinical diagnosis of AD (Pratico et al. 2000a; Tuppo et al. 2001), and levels increase with the progression of the disease. Young patients with Down's syndrome, where APP/Aβ is overexpressed, also display increased lipid peroxidation markers in urine compared with age-matched controls (Pratico et al. 2000b). Analysis of Tg2576 mice, which display oxidative damage similar to that found in AD brain (Smith et al. 1998a), revealed an elevation in oxidative stress markers preceding amyloid formation and increasing with the age-dependent development of amyloid pathology (Pratico et al. 2001). Together, data from humans and transgenic mice indicate that elevated oxidative stress is an early event in AD pathogenesis and may therefore contribute to the range of pathological changes observed.
The production of oxygen radicals is a normal consequence of metabolic activity, but cellular antioxidant defences such as cytosolic Cu/Zn superoxide dismutase (SOD1), catalase, glutathione peroxidase, haemoxygenase and mitochondrial manganese (Mn) superoxide dismutase (SOD2), along with other nonenzymatic antioxidants, defend against oxidative damage [reviewed in (Perry et al. 2002b)]. The brain is particularly vulnerable to oxidative stress because of its high metabolic rate, utilizing 20% of basal oxygen consumption. In addition, the brain has limited antioxidant defences compared with other organs (Floyd 1999) and high levels of transition metals, which can generate oxygen radicals.
Metals and Aβ toxicity
Aβ is central to the pathogenesis of AD. The Aβ peptide is toxic to neurones in cell culture, and this toxicity has been shown to be mediated by the interaction of the peptide with Cu2+ and Fe3+ (Huang et al. 1999b; Rottkamp et al. 2001; Opazo et al. 2002). Aβ catalyses the reduction of Cu2+ to Cu+ and Fe3+ to Fe2+, generating H2O2 from molecular oxygen (O2) and available biological reducing agents such as vitamin C, cholesterol and catecholamines (Opazo et al. 2002). In the absence of sufficient detoxifying enzymes such as catalase and glutathione peroxidase, H2O2 will further react with reduced metal ions such as Fe2+ and Cu+ to generate toxic hydroxyl radicals (Fenton reaction). The toxicity of Aβ42 is greater than Aβ40 (Huang et al. 1999b; Cuajungco et al. 2000; Rottkamp et al. 2001) correlating with their relative Cu2+- and Fe3+-reducing potentials and the ability to catalytically generate H2O2 from biological reducing agents (Huang et al. 1999a; Opazo et al. 2002). Interestingly, murine APP–/– neurones are less susceptible to Cu-induced toxicity than wild-type neurones, whereas knockouts of APLP2 (which shares a homologous N-terminal metal-binding domain with APP but does not produce Aβ) are not protected against Cu-induced toxicity (White et al. 1999a).
The formation of amyloid deposits per se does not correlate with the clinical severity of AD. Instead, levels of soluble Aβ were found to correlate with the severity of the clinical symptoms of AD (Lue et al. 1999; McLean et al. 1999; Wang et al. 1999). AD brain has elevated levels of soluble Aβ as well as aggregated Aβ. Recent evidence suggests that soluble dimeric and oligomeric forms of Aβ are more toxic in cell culture than monomeric Aβ (Dahlgren et al. 2002; Walsh et al. 2002a) and may mediate toxicity in AD (Mucke et al. 2000; Walsh et al. 2002b).
Soluble Aβ oligomers that generate ROS by interaction with Cu and Fe may be a transient species that precede the formation of relatively inert amyloid complexes. A number of stress conditions upregulate APP expression and amyloidogenic processing of APP to generate Aβ (Misonou et al. 2000; Paola et al. 2000; Cheng & Trombetta 2004) [reviewed in detail in (Atwood et al. 2003)]. Evidence that Aβ can act as an antioxidant under certain conditions [summarized in (Atwood et al. 2003)] suggests that Aβ production, in conjunction with its neuroprotective and neurotrophic properties (Whitson et al. 1989; Whitson et al. 1990; Yankner et al. 1990; Koo et al. 1993; Luo et al. 1996; Chan et al. 1999), may be a stress response to minimize oxidative damage. If small Aβ–metal aggregates and oligomers that are formed during such stress responses are not rapidly degraded, they may cause further damage. With the further recruitment of metals, these oligomeric and aggregated Aβ species may become subsequently sequestered into amyloid which is less toxic than soluble Aβ oligomers.
The elevated oxidative stress levels in AD brain may operate in tandem with abnormalities in tissue Cu and Fe to engender Aβ oligomer formation and amyloid deposition. An oxidative environment has been demonstrated to result in the formation of a di-tyrosine linkage between two Aβ peptides (Galeazzi et al. 1999), hence promoting the irreversible oligomerization of Aβ peptides. Cu2+ directly interacts with Aβ to foster di-tyrosine cross-linking and covalent oligomerization (Atwood et al. 2004; Barnham et al. 2004). AD brain indeed possesses a high content of di-tyrosine (Atwood et al. 2000a). Oxidative reactions may also facilitate the accumulation of Aβ via the formation of covalent cross-links between Aβ peptides (Atwood et al. 2000a; Atwood et al. 2000b; Loske et al. 2000; Palmblad et al. 2002) and between other proteins [discussed in (Perry et al. 2002a)], generating larger protein aggregates that resist clearance (Stadtman & Oliver 1991; Friguet et al. 1994; Kuo et al. 1998). Interestingly, the oxidative modification of the sulphur atom of Met35 of Aβ by reaction with Cu2+ has been shown to reduce the affinity of Aβ for membranes (Barnham et al. 2003a). Oxidative modifications of Aβ may therefore also contribute to the increased levels of toxic, soluble Aβ oligomers, causing toxicity and neurodegeneration in AD, prior to the peptides becoming incorporated into relatively inert amyloid complexes.
The role of Zn2+ in Aβ toxicity is complex. In vitro, Zn2+ inhibits Aβ toxicity in cell cultures (Lovell et al. 1999; Cuajungco et al. 2000), possibly by quenching H2O2 production from Aβ:Cu2+ complexes (Cuajungco et al. 2000). As Zn2+ forms plaques in vivo, the quenching of Aβ redox activity is consistent with the observation that oxidation damage in the brain in AD inversely correlates with the plaque burden (Cuajungco et al. 2000). However, even in the most heavily plaque-burdened brains, there is still marked oxidation damage, which may be mediated by soluble or diffuse forms of Aβ (Cuajungco et al. 2000).
Abnormal metal homeostasis in AD
Numerous reports have demonstrated transition metal imbalances in AD brain, such as increased Fe, Zn and Mn (Samudralwar et al. 1995; Deibel et al. 1996; Danscher et al. 1997; Cornett et al. 1998; Rao et al. 1999), and importantly, decreased Cu (Deibel et al. 1996; Plantin et al. 1987; Loeffler et al. 1996; Rao et al. 1999). CSF and serum of AD patients tend to show the opposite trend to brain, with increased serum and CSF Cu levels (Basun et al. 1991; Gonzalez et al. 1999; Squitti et al. 2002) and decreased serum Fe and Zn (Loeffler et al. 1994; Molina et al. 1998), suggesting a redistribution of metals between the brain and the fluids responsible for supplying and clearing excreted metals from the brain.
Imbalances in metal levels in the AD brain may reflect deficiencies or excesses of particular metalloproteins or defective metal transporters. Indeed, the levels of several Cu and Fe regulatory and storage proteins are altered in AD brain (Basun et al. 1991; Connor et al. 1993; Loeffler et al. 1994; Loeffler et al. 1996; Smith et al. 1998c; Castellani et al. 1999) as are several essential Cu-dependent enzymes (see next section). Iron and Cu form essential components of several enzymes required for vital brain functions including energy production, neurotransmitter synthesis and antioxidant function.
AD brain is deficient in bioavailable copper and cupro-enzymes
Several important enzymes that are altered in AD brain require Cu for their catalytic activity. Cytochrome C-oxidase (COX), a Cu-dependent enzyme involved in mitochondrial respiration, is essential for energy production in the brain. Several studies have shown decreased COX levels (Cottrell et al. 2001) or activity (Maurer et al. 2000) and defective energy metabolism (Duara et al. 1986; McGeer et al. 1990; Mielke et al. 1992; Mielke et al. 1996) in AD brain. In fact, inherited mutations in mitochondrial COX genes have been shown to segregate with greater frequency with late onset AD cases (Davis et al. 1997). This supports defective COX activity as a risk factor for AD. The APP mismetabolism that occurs in AD may impact upon the ability of COX to obtain adequate copper.
Ceruloplasmin comprises a major reservoir of the body's copper. Ceruloplasmin levels in AD patients have also been found to be decreased in the brain tissue (Connor et al. 1993) and increased in the CSF (Basun et al. 1991; Loeffler et al. 1994). Ceruloplasmin is a key regulator of Fe transport and is important for the inhibition of Fe-induced lipid peroxidation of proteins by oxidizing Fe2+ to Fe3+ leading to the incorporation of Fe into ferritin (Samokyszyn et al. 1991; de Silva & Aust 1993).
The activity of peptidylglycine α-amidating mono-oxygenase (PAM), a Cu-dependent enzyme involved in neuropeptide and peptide hormone processing, has been shown to decrease by 16% per year in the CSF of AD patients, and lower PAM activity levels are also found in temporal lobe of AD brain at postmortem (Wand et al. 1987). The possibility that these decreases are due to a broad cell loss is negated by the findings that other markers, such as oxidative stress-handling enzymes (SOD1, HO-1, catalase, glutathione peroxidase and glutathione reductase) are elevated in AD brain (Pappolla et al. 1992; Aksenov et al. 1998; Omar et al. 1999).
Expression of the highly abundant antioxidant enzyme Cu/Zn-superoxide dismutase (SOD1) has been shown to be elevated in AD brain (Omar et al. 1999) and in transgenic APP mice (Bayer et al. 2003). However, in both cases, SOD1 activity is decreased, suggesting that the decreased SOD1 activity is due to a reduction in the active site Cu occupancy. Interestingly, dietary supplementation with Cu was found to restore SOD1 activity in transgenic APP mice and improve survival of the mice (Bayer et al. 2003). This suggests that Cu deficiency in AD may play a role in the disease development.
The mechanism of the Cu depletion and the pools of Cu affected in AD remain largely unknown. Free Cu is not normally found in vivo (Rae et al. 1999), as its highly reactive nature can participate in oxygen radical formation via Fenton and Haber-Weiss reactions. Cu is delivered to its target proteins, transported across cell membranes and between cellular compartments by specific Cu chaperones [reviewed in (Shim & Harris 2003)]. Although several Cu chaperones have been identified, much remains to be known about the proteins and mechanisms involved in Cu transport and regulation, particularly in the brain.
Roles of APP and Aβ in metal homeostasis
APP and Aβ impact upon metal homeostasis: a possible metal transport mechanism
The high energy, positively cooperative hexameric structure adopted by Aβ when binding Cu2+ and Zn2+ (Curtain et al. 2001; Curtain et al. 2003) implies that the complex subserves a possible physiological function. There is an interdependent relationship between Cu levels, APP expression and Aβ production supporting a role for APP and Aβ in Cu efflux. The initial finding showed that the APP and amyloid precursor-like protein 2 (APLP2) knockout mice have specific elevations in brain and liver Cu levels (White et al. 1999b). APLP2 shares a homologous N-terminal Cu-binding domain with APP but does not produce Aβ (Bush et al. 1993; Hesse et al. 1994). Primary cortical neurones and embryonic fibroblasts from APP and APLP2 double knockout mice also display significantly elevated Cu levels (Bellingham et al. 2004a). Conversely, overexpression of APP has been consistently shown to result in decreased Cu levels in three independent transgenic mouse lines (Maynard et al. 2002; Bayer et al. 2003; Phinney et al. 2003) and in primary cortical neurones (Bellingham et al. 2004a).
Overexpression of APP-C100, which contains Aβ but not the N-terminal Zn2+-and Cu2+-binding domain of APP, results in decreased brain Cu levels as well as Fe levels (Maynard et al. 2002). This suggests a role for Aβ in both Cu and Fe homeostasis. The effect of Aβ on Fe levels supports the previous proposal that the APP/Aβ system may play a role in the removal of excess Fe from the cell (Bush 2003). This stems from the identification of an iron-regulatory element (IRE-Type II) in the 5′-untranslated region of APP (Rogers et al. 2002). Alternatively, the decrease in Fe may reflect a homeostatic adjustment to the reduction in Cu levels.
Although Cu is the trace metal most strikingly affected in these transgenic and knockout mouse models, other less pronounced metal level changes may have some importance. The Cu deficit in Tg2576 mice was accompanied by a small but significant decrease in Zn levels (Maynard et al. 2002), whereas the Cu increases in APP–/– mice were accompanied by nonsignificant increases in Zn (White et al. 1999b). Interestingly, in both studies, the molar Zn changes were of similar magnitude to the molar Cu changes. However, because of the greater abundance of Zn in the brain, these changes corresponded to a smaller percentage of total Zn than Cu. The effects on Zn could therefore be a direct result of APP/Aβ expression.
Both APPsw (Tg2576) and APP-C100 mice displayed significant elevations in Mn levels (Maynard et al. 2002). Because Mn does not appreciably interact with Aβ or APP (Bush et al. 1993; Bush et al. 1994a; Bush et al. 1994b; Atwood et al. 1998), the increased Mn levels are more likely a secondary effect of altered metal homeostasis. Elevated Mn levels have also been found in AD brain (Rao et al. 1999). Most Mn in the brain is bound to metalloproteins such as glutamine synthetase and mitochondrial Mn superoxide dismutase (SOD2). A portion of Mn also exists in the synaptic vesicles of glutamatergic neurones and is released into the synaptic cleft along with glutamate, participating in the regulation of synaptic neurotransmission (Takeda 2003).
Abnormally high concentrations of brain Mn have been shown to cause an irreversible neurological syndrome similar to Parkinson's disease (Aschner 1997), hence, the elevated Mn in AD brain may contribute to the pathology. Decreased Cu and increased Mn levels have been observed in other neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS) and Creutzfeldt–Jakob disease (CJD) (Kapaki et al. 1997; Wong et al. 2001). Brain tissue from CJD patients displays decreased Cu and increased Mn levels. Furthermore, prion protein extracted from CJD brain displays a decreased Cu and increased Mn contents (Wong et al. 2001). Interestingly, replacement of Cu by Mn facilitates misfolding of the prion protein in favour of higher β-sheet content, protease resistance and loss of antioxidant function (Brown et al. 2000). However, Mn-induced aggregation of Aβ has not been demonstrated by in vitro studies using synthetic peptides (Bush et al. 1994b; Atwood et al. 1998). Decreased Cu and increased Mn levels have also been found in the serum and CSF (Mn increase nonsignificantly) of ALS patients (Kapaki et al. 1997). Although the relationship between Cu and Mn changes is not well understood, opposing changes in the levels of Cu and Mn are emerging as a common trait of neurological diseases.
The Cu-binding domain of APP shows structural homology to Cu chaperones (Barnham et al. 2003b), supporting the notion that the APP (and APLP2) Cu-binding domain functions as a neuronal metal transporter and/or chaperone to modulate Cu homeostasis. Furthermore, APP gene expression has recently been shown to be downregulated by Cu depletion (Bellingham et al. 2004b), suggesting a negative feedback mechanism evolved to preserve intracellular Cu levels. The Cu stores depleted by APP and Aβ overexpression remain unknown, as is the mechanism by which this occurs.
The main problem with distinguishing whether the effects on Cu homeostasis are elicited by the APP N-terminal Cu-binding domain or Aβ, both of which bind and catalyse the reduction of Cu2+, is that most of the models studied to date utilize the overexpression or ablation of APP, resulting in the joint alterations in the levels of both APP and Aβ. The only direct evidence for Aβ causing Cu efflux, independently of APP, is that Cu levels are decreased in mice overexpressing APP-C100 (Maynard et al. 2002). Furthermore, the magnitude of the Cu elevation in APP–/– mice is greater than that in APLP2–/– mice (White et al. 1999b), suggesting that the increased Cu may be due to the joint effects of the APP N-terminal Cu-binding domain and Aβ rather than the action of the N-terminal copper-binding domain alone, because this domain is homologous in both APP and APLP2. No consensus has been reached as to whether APP expression is altered in AD, except in cases resulting from Down's syndrome or head trauma. The majority of genetic and biochemical evidence links increased the production of Aβ or Aβ42 with AD, rather than increased APP expression. Hence, APP-overexpressing models may not be an accurate reflection of the Cu depletion observed in AD. These models do however increase our understanding of the roles of APP and Aβ in metal homeostasis.
Cu promotes the nonamyloidogenic APP processing pathway
An important link between Cu levels and amyloid formation has recently been unveiled by two independent and complementary studies using two different transgenic APP mouse models. Both studies reported decreased constitutive brain Cu levels (Bayer et al. 2003; Phinney et al. 2003) in agreement with earlier findings (Maynard et al. 2002). Elevation of brain Cu levels, either by dietary Cu supplementation (Bayer et al. 2003) or by the introduction of a mutant allele of the CuATPase7b Cu transporter (Phinney et al. 2003), improved the survival of the mice and resulted in a marked decrease in Aβ and amyloid plaque load. These findings suggest that elevated Cu may drive nonamyloidogenic processing of APP, as demonstrated previously in vitro (Borchardt et al. 1999).
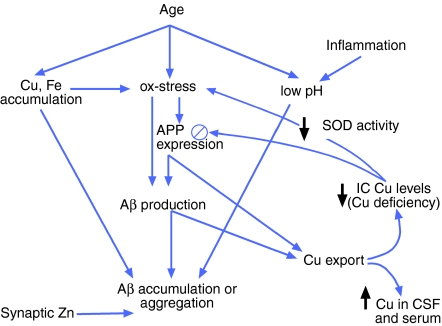
This is important, as it suggests that the decreased Cu levels in AD brain could further increase Aβ production, perpetuating a pathogenic cascade of events. However, if low Cu availability in the brain signals the downregulation of APP expression as occurs in vitro (Bellingham et al. 2004b), this may provide a protective mechanism to limit the amount of Aβ production in an environment where amyloidogenic APP processing is favoured. However, in the transgenic mouse models, which use non-APP promotors, this protective mechanism is absent. Hence, Cu supplementation in humans may not be expected to exert such a profound effect on Aβ levels and amyloid formation. Replenishment of deficient Cu stores in humans may decrease the proportion of APP that undergoes amyloidogenic processing; however, concurrent upregulation of APP expression may further add to the net Aβ burden (Figure 1).
Figure 1.
Proposed mechanism for copper (Cu) depletion and β-amyloid formation in humans. Increasing age leads to the accumulation of Cu and iron (Fe) deposits, elevated oxidative stress and reduced pH in the brain, all of which may promote the aggregation of Aβ. Oxidative stress and other metabolic stresses may also promote Aβ production by jointly upregulating amyloid precursor protein (APP) expression and driving amyloidogenic processing of APP (Misonou et al. 2000; Paola et al. 2000; Atwood et al. 2003; Cheng & Trombetta 2004). Higher APP expression and elevated Aβ levels cause greater than required Cu export, leading to increased Cu in cerebrospinal fluid (CSF) and serum, and an intracellular (IC) Cu deficiency in the brain. Cu-deficient superoxide dismutase (SOD1) contributes to the reduced antioxidant capacity of the brain, allowing further oxidative stress. Unlike in transgenic mouse models, decreased intracellular Cu levels may downregulate APP expression (Bellingham et al. 2004a) in an attempt to conserve Cu levels. In Alzheimer's disease (AD) brain, this negative feedback must be insufficient to counteract other factors that promote APP expression, Aβ production and Aβ accumulation. Consequently, a Cu deficiency develops along with the classic Aβ-amyloid pathology of AD. Zn – zinc.
When attempting to predict the effects of Cu supplementation on human AD patients, the potential benefits of restoring the apparent Cu deficiency on enzyme activities and perhaps nonamyloidogenic processing of APP must be weighed against the potential risk of exacerbating the condition by increasing the availability of Cu for the formation of toxic Aβ oligomers and the generation of ROS, as well as possibly increasing the APP expression. The correlation of the findings obtained from mice to humans is limited not only by the non-native regulation of transgenic APP expression and the lack of the full spectrum of AD pathology in transgenic APP mice but also by the differences between humans and mice in their metal-regulatory machinery.
A recent clinical trial of the Cu/Zn chelator clioquinol has revealed promising effects, with a modest slowing of cognitive decline and a parallel decrease in serum Aβ42 (Ritchie et al. 2003). The effects of oral clioquinol treatment on Tg2576 mice were a striking reduction in brain Aβ levels and amyloid plaque load (Cherny et al. 2001), as has been since achieved with another hydrophobic chelator DP-109 (Lee et al. 2004a). Although clioquinol treatment results in decreased Cu, Fe and cobalt levels in nontransgenic mice (Yassin et al. 2000), Tg2576 mice treated with clioquinol displayed paradoxical elevations in brain Cu and Zn levels (Cherny et al. 2001). A plausible explanation for the findings is that clioquinol treatment loosens amyloid plaques and aggregates and solubilizes smaller Aβ oligomers that would otherwise exert toxicity and become sequestered into amyloid deposits. The liberation of Aβ into more soluble forms allows more efficient clearance of Aβ. Hence, with prolonged treatment, much of the existing amyloid burden has been cleared, and the continued growth of amyloid deposits is prevented. The observation that Cu and Zn levels were elevated suggests that by achieving lower total Aβ levels, the excessive Cu export that occurs in Tg2576 mice is attenuated. The elevation in Zn levels, however, cannot be explained by the reduction in Aβ levels, as APP-C100 mice do not display decreased Zn levels (Maynard et al. 2002). The Cu and Zn elevations could be due in part to Cu and Zn ions liberated from amyloid plaques that are still bound to clioquinol in the brain. However, the net increase in Cu and Zn levels in the brain in the presence of fewer amyloid deposits indicates either greater uptake or reduced export. Hence, the Cu and Zn elevations may be a result of favourable changes to metal homeostasis resulting from the clioquinol treatment. Approximately, 15% of plasma Zn in mice is in communication with Zn released in cortical synapses associated with ZnT3 activity (Friedlich et al. 2004). The normalization of plasma zinc (rising from below normal baseline levels) as a result of clioquinol treatment in AD patients might be explained by the dissolution of parenchymal and cerebrovascular amyloid permitting the re-establishment of communication between plasma and synaptic Zn (Ritchie et al. 2003).
Concluding remarks
Experimental evidence from transgenic mouse models that the APP/Aβ system forms part of the brain's Cu-regulatory machinery has revealed the possibility that the corruption of Aβ metabolism in AD brain may not only result in an intracellular Cu deficiency, but this Cu deficiency may further propel amyloidogenesis. Future research will elucidate the mechanisms by which APP and Aβ regulate Cu transport and the pools of Cu and other metal ions that are affected. These findings will help predict the efficacy of therapeutic strategies targeting metals in the brain, such as chelation therapy or metal supplementation.
References
- Aksenov MY, Tucker HM, Nair P, et al. The expression of key oxidative stress-handling genes in different brain regions in Alzheimer's disease. J Mol Neurosci. 1998;11:151–164. doi: 10.1385/JMN:11:2:151. [DOI] [PubMed] [Google Scholar]
- Aschner M. Manganese neurotoxicity and oxidative damage. In: Aschner M, editor. Metal and Oxidative Damage in Neurological Disorders. New York, USA: Plenum Press; 1997. pp. 77–93. [Google Scholar]
- Atwood CS, Moir RD, Huang X, et al. Dramatic aggregation of Alzheimer abeta by Cu(II) is induced by conditions representing physiological acidosis. J Biol Chem. 1998;273:12817–12826. doi: 10.1074/jbc.273.21.12817. [DOI] [PubMed] [Google Scholar]
- Atwood CS, Huang X, Moir RD, Tanzi RE, Bush AI. Role of free radicals and metal ions in the pathogenesis of Alzheimer's disease. Met Ions Biol Syst. 1999;36:309–364. [PubMed] [Google Scholar]
- Atwood CS, Huang X, Khatri A, et al. Copper catalyzed oxidation of Alzheimer Abeta. Cell Mol Biol. 2000a;46:777–783. (Noisy-le-Grand) [PubMed] [Google Scholar]
- Atwood CS, Scarpa RC, Huang X, et al. Characterization of copper interactions with alzheimer amyloid beta peptides: identification of an attomolar-affinity copper binding site on amyloid beta1-42. J Neurochem. 2000b;75:1219–1233. doi: 10.1046/j.1471-4159.2000.0751219.x. [DOI] [PubMed] [Google Scholar]
- Atwood CS, Obrenovich ME, Liu T, et al. Amyloid-beta: a chameleon walking in two worlds: a review of the trophic and toxic properties of amyloid-beta. Brain Res Brain Res Rev. 2003;43:1–16. doi: 10.1016/s0165-0173(03)00174-7. [DOI] [PubMed] [Google Scholar]
- Atwood CS, Perry G, Zeng H, et al. Copper mediates dityrosine cross-linking of Alzheimer's amyloid-beta. Biochemistry. 2004;43:560–568. doi: 10.1021/bi0358824. [DOI] [PubMed] [Google Scholar]
- Barnham KJ, Ciccotosto GD, Tickler AK, et al. Neurotoxic, redox-competent Alzheimer's beta-amyloid is released from lipid membrane by methionine oxidation. J Biol Chem. 2003a;278:42959–42965. doi: 10.1074/jbc.M305494200. [DOI] [PubMed] [Google Scholar]
- Barnham KJ, McKinstry WJ, Multhaup G, et al. Structure of the Alzheimer's disease amyloid precursor protein copper binding domain. A regulator of neuronal copper homeostasis. J Biol Chem. 2003b;278:17401–17407. doi: 10.1074/jbc.M300629200. [DOI] [PubMed] [Google Scholar]
- Barnham KJ, Haeffner F, Ciccotosto GD, et al. Tyrosine gated electron transfer is key to the toxic mechanism of Alzheimer's disease beta-amyloid. FASEB J. 2004;18:1427–1429. doi: 10.1096/fj.04-1890fje. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Hance DB, Marx P, Foster JA, Marder SR. MR evaluation of age-related increase of brain iron in young adult and older normal males. Magn Reson Imaging. 1997;15:29–35. doi: 10.1016/s0730-725x(96)00234-2. [DOI] [PubMed] [Google Scholar]
- Basun H, Forssell LG, Wetterberg L, Winblad B. Metals and trace elements in plasma and cerebrospinal fluid in normal aging and Alzheimer's disease. J Neural Transm Park Dis Dement Sect. 1991;3:231–258. [PubMed] [Google Scholar]
- Bayer TA, Schafer S, Simons A, et al. Dietary Cu stabilizes brain superoxide dismutase 1 activity and reduces amyloid Abeta production in APP23 transgenic mice. Proc Natl Acad Sci USA. 2003;100:14187–14192. doi: 10.1073/pnas.2332818100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham SA, Ciccotosto GD, Needham E, et al. Gene knockout amyloid precursor protein amyloid precursor-like protein-2 increases cellular copper levels primary mouse cort neurons embryonic fibroblasts. J Neurochem. 2004a;91:423–428. doi: 10.1111/j.1471-4159.2004.02731.x. [DOI] [PubMed] [Google Scholar]
- Bellingham SA, Lahiri DK, Maloney B, La Fontaine S, Multhaup G, Camakaris J. Copper depletion down-regulates expression of the Alzheimer's disease amyloid-{beta} precursor protein gene. J Biol Chem. 2004b;279:20378–20386. doi: 10.1074/jbc.M400805200. [DOI] [PubMed] [Google Scholar]
- Bohnen N, Jolles J, Degenaar CP. Levels of trace elements in blood in healthy aging subjects. Z Gerontol. 1994;27:324–327. [PubMed] [Google Scholar]
- Borchardt T, Camakaris J, Cappai R, Masters CL, Beyreuther K, Multhaup G. Copper inhibits beta-amyloid production and stimulates the non-amyloidogenic pathway of amyloid-precursor-protein secretion. Biochem J. 1999;344(Pt 2):461–467. [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Hafiz F, Glasssmith LL, et al. Consequences of manganese replacement of copper for prion protein function and proteinase resistance. EMBO J. 2000;19:1180–1186. doi: 10.1093/emboj/19.6.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush AI, Multhaup G, Moir RD, et al. A novel zinc(II) binding site modulates the function of the beta A4 amyloid protein precursor of Alzheimer's disease. J Biol Chem. 1993;268:16109–16112. [PubMed] [Google Scholar]
- Bush AI, Pettingell WH, Jr, Paradis MD, Tanzi RE. Modulation of A beta adhesiveness and secretase site cleavage by zinc. J Biol Chem. 1994a;269:12152–12158. [PubMed] [Google Scholar]
- Bush AI, Pettingell WH, Multhaup G, et al. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science. 1994b;265:1464–1467. doi: 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- Bush AI. Metals and neuroscience. Curr Opin Chem Biol. 2000;4:184–191. doi: 10.1016/s1367-5931(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Bush AI. The metallobiology of Alzheimer's disease. Trends Neurosci. 2003;26:207–214. doi: 10.1016/S0166-2236(03)00067-5. [DOI] [PubMed] [Google Scholar]
- Callahan MJ, Lipinski WJ, Bian F, Durham RA, Pack A, Walker LC. Augmented senile plaque load in aged female beta-amyloid precursor protein-transgenic mice. Am J Pathol. 2001;158:1173–1177. doi: 10.1016/s0002-9440(10)64064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani RJ, Smith MA, Nunomura A, Harris PL, Perry G. Is increased redox-active iron in Alzheimer disease a failure of the copper-binding protein ceruloplasmin? Free Radic Biol Med. 1999;26:1508–1512. doi: 10.1016/s0891-5849(99)00016-7. [DOI] [PubMed] [Google Scholar]
- Chan C-W, Dharmarajan A, Atwood CS, et al. Anti-apoptotic action of Alzheimer A-beta. Alzheimer's Reports. 1999;2:1–6. [Google Scholar]
- Cheng SY, Trombetta LD. The induction of amyloid precursor protein and alpha-synuclein in rat hippocampal astrocytes by diethyldithiocarbamate and copper with or without glutathione. Toxicol Lett. 2004;146:139–149. doi: 10.1016/j.toxlet.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Cherny RA, Legg JT, McLean CA, et al. Aqueous dissolution of Alzheimer's disease Abeta amyloid deposits by biometal depletion. J Biol Chem. 1999;274:23223–23228. doi: 10.1074/jbc.274.33.23223. [DOI] [PubMed] [Google Scholar]
- Cherny RA, Atwood CS, Xilinas ME, et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron. 2001;30:665–676. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- Clements A, Allsop D, Walsh DM, Williams CH. Aggregation and metal-binding properties of mutant forms of the amyloid A beta peptide of Alzheimer's disease. J Neurochem. 1996;66:740–747. doi: 10.1046/j.1471-4159.1996.66020740.x. [DOI] [PubMed] [Google Scholar]
- Connor JR, Snyder BS, Beard JL, Fine RE, Mufson EJ. Regional distribution of iron and iron-regulatory proteins in the brain in aging and Alzheimer's disease. J Neurosci Res. 1992;31:327–335. doi: 10.1002/jnr.490310214. [DOI] [PubMed] [Google Scholar]
- Connor JR, Tucker P, Johnson M, Snyder B. Ceruloplasmin levels in the human superior temporal gyrus in aging and Alzheimer's disease. Neurosci Lett. 1993;159:88–90. doi: 10.1016/0304-3940(93)90805-u. [DOI] [PubMed] [Google Scholar]
- Cornett CR, Markesbery WR, Ehmann WD. Imbalances of trace elements related to oxidative damage in Alzheimer's disease brain. Neurotoxicology. 1998;19:339–345. [PubMed] [Google Scholar]
- Cottrell DA, Blakely EL, Johnson MA, Ince PG, Turnbull DM. Mitochondrial enzyme-deficient hippocampal neurons and choroidal cells in AD. Neurology. 2001;57:260–264. doi: 10.1212/wnl.57.2.260. [DOI] [PubMed] [Google Scholar]
- Cuajungco MP, Goldstein LE, Nunomura A, et al. Evidence that the beta-amyloid plaques of Alzheimer's disease represent the redox-silencing and entombment of abeta by zinc. J Biol Chem. 2000;275:19439–19442. doi: 10.1074/jbc.C000165200. [DOI] [PubMed] [Google Scholar]
- Curtain CC, Ali F, Volitakis I, et al. Alzheimer's disease amyloid-beta binds copper and zinc to generate an allosterically ordered membrane-penetrating structure containing superoxide dismutase-like subunits. J Biol Chem. 2001;276:20466–20473. doi: 10.1074/jbc.M100175200. [DOI] [PubMed] [Google Scholar]
- Curtain CC, Ali FE, Smith DG, Bush AI, Masters CL, Barnham KJ. Metal ions, pH, and cholesterol regulate the interactions of Alzheimer's disease amyloid-beta peptide with membrane lipid. J Biol Chem. 2003;278:2977–2982. doi: 10.1074/jbc.M205455200. [DOI] [PubMed] [Google Scholar]
- Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- Danscher G, Jensen KB, Frederickson CJ, et al. Increased amount of zinc in the hippocampus and amygdala of Alzheimer's diseased brains: a proton-induced X-ray emission spectroscopic analysis of cryostat sections from autopsy material. J Neurosci Methods. 1997;76:53–59. doi: 10.1016/s0165-0270(97)00079-4. [DOI] [PubMed] [Google Scholar]
- Davis RE, Miller S, Herrnstadt C, et al. Mutations in mitochondrial cytochrome c oxidase genes segregate with late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1997;94:4526–4531. doi: 10.1073/pnas.94.9.4526. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Deibel MA, Ehmann WD, Markesbery WR. Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer's disease: possible relation to oxidative stress. J Neurol Sci. 1996;143:137–142. doi: 10.1016/s0022-510x(96)00203-1. [DOI] [PubMed] [Google Scholar]
- Del Corso L, Pastine F, Protti MA, et al. Blood zinc, copper and magnesium in aging. A study in healthy home-living elderly. Panminerva Med. 2000;42:273–277. [PubMed] [Google Scholar]
- Dong J, Atwood CS, Anderson VE, et al. Metal binding and oxidation of amyloid-beta within isolated senile plaque cores: Raman microscopic evidence. Biochemistry. 2003;42:2768–2773. doi: 10.1021/bi0272151. [DOI] [PubMed] [Google Scholar]
- Drayer B, Burger P, Darwin R, Riederer S, Herfkens R, Johnson GA. MRI of brain iron. Am J Roentgenol. 1986;147:103–110. doi: 10.2214/ajr.147.1.103. [DOI] [PubMed] [Google Scholar]
- Duara R, Grady C, Haxby J, et al. Positron emission tomography in Alzheimer's disease. Neurology. 1986;36:879–887. doi: 10.1212/wnl.36.7.879. [DOI] [PubMed] [Google Scholar]
- Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999;222:236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Rampy BA, Reamy-Rampy S, Howell GA. Distribution of histochemically reactive zinc in the forebrain of the rat. J Chem Neuroanat. 1992;5:521–530. doi: 10.1016/0891-0618(92)90007-d. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Bush AI. Synaptically released zinc: physiological functions and pathological effects. Biometals. 2001;14:353–366. doi: 10.1023/a:1012934207456. [DOI] [PubMed] [Google Scholar]
- Friedlich AL, Lee JY, van Groen T, et al. Neuronal zinc exchange with the blood vessel wall promotes cerebral amyloid angiopathy in an animal model of Alzheimer's disease. J Neurosci. 2004;24:3453–3459. doi: 10.1523/JNEUROSCI.0297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friguet B, Stadtman ER, Szweda LI. Modification of glucose-6-phosphate dehydrogenase by 4-hydroxy-2-nonenal. Formation of cross-linked protein that inhibits the multicatalytic protease. J Biol Chem. 1994;269:21639–21643. [PubMed] [Google Scholar]
- Galeazzi L, Ronchi P, Franceschi C, Giunta S. In vitro peroxidase oxidation induces stable dimers of beta-amyloid (1–42) through dityrosine bridge formation. Amyloid. 1999;6:7–13. doi: 10.3109/13506129908993282. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Martin T, Cacho J, et al. Serum zinc, copper, insulin and lipids in Alzheimer's disease epsilon 4 apolipoprotein E allele carriers. Eur J Clin Invest. 1999;29:637–642. doi: 10.1046/j.1365-2362.1999.00471.x. [DOI] [PubMed] [Google Scholar]
- Hartter DE, Barnea A. Brain tissue accumulates 67copper by two ligand-dependent saturable processes. A high affinity, low capacity and a low affinity, high capacity process. J Biol Chem. 1988a;263:799–805. [PubMed] [Google Scholar]
- Hartter DE, Barnea A. Evidence for release of copper in the brain: depolarization-induced release of newly taken-up 67copper. Synapse. 1988b;2:412–415. doi: 10.1002/syn.890020408. [DOI] [PubMed] [Google Scholar]
- Hensley K, Maidt ML, Yu Z, Sang H, Markesbery WR, Floyd RA. Electrochemical analysis of protein nitrotyrosine and dityrosine in the Alzheimer brain indicates region-specific accumulation. J Neurosci. 1998;18:8126–8132. doi: 10.1523/JNEUROSCI.18-20-08126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse L, Beher D, Masters CL, Multhaup G. The beta A4 amyloid precursor protein binding to copper. FEBS Lett. 1994;349:109–116. doi: 10.1016/0014-5793(94)00658-x. [DOI] [PubMed] [Google Scholar]
- Huang X, Atwood CS, Hartshorn MA, et al. The A beta peptide of Alzheimer's disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry. 1999a;38:7609–7616. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- Huang X, Cuajungco MP, Atwood CS, et al. Cu(II) potentiation of alzheimer abeta neurotoxicity. Correlation with cell-free hydrogen peroxide production and metal reduction. J Biol Chem. 1999b;274:37111–37116. doi: 10.1074/jbc.274.52.37111. [DOI] [PubMed] [Google Scholar]
- Huang X, Atwood CS, Moir RD, et al. Zinc-induced Alzheimer's Abeta1–40 aggregation is mediated by conformational factors. J Biol Chem. 1997;272:26464–26470. doi: 10.1074/jbc.272.42.26464. [DOI] [PubMed] [Google Scholar]
- Kapaki E, Zournas C, Kanias G, Zambelis T, Kakami A, Papageorgiou C. Essential trace element alterations in amyotrophic lateral sclerosis. J Neurol Sci. 1997;147:171–175. doi: 10.1016/s0022-510x(96)05334-8. [DOI] [PubMed] [Google Scholar]
- Koo EH, Park L, Selkoe DJ. Amyloid beta-protein as a substrate interacts with extracellular matrix to promote neurite outgrowth. Proc Natl Acad Sci USA. 1993;90:4748–4752. doi: 10.1073/pnas.90.10.4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo YM, Webster S, Emmerling MR, De Lima N, Roher AE. Irreversible dimerization/tetramerization and post-translational modifications inhibit proteolytic degradation of A beta peptides of Alzheimer's disease. BiochimBiophysActa. 1998;1406:291–298. doi: 10.1016/s0925-4439(98)00014-3. [DOI] [PubMed] [Google Scholar]
- Lee JY, Mook-Jung I, Koh JY. Histochemically reactive zinc in plaques of the Swedish mutant beta- amyloid precursor protein transgenic mice. J Neurosci. 1999;19:RC10. doi: 10.1523/JNEUROSCI.19-11-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Cole TB, Palmiter RD, Suh SW, Koh JY. Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc Natl Acad Sci USA. 2002;99:7705–7710. doi: 10.1073/pnas.092034699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Friedman JE, Angel I, Kozak A, Koh J-Y. The lipophilic metal chelator DP-109 reduces amyloid pathology in brains of human beta-amyloid precursor protein transgenic mice. Neurobiol Aging. 2004a. doi: 10.1016/j.neurobiolaging.2004.01.005. [DOI] [PubMed]
- Lee JY, Kim JH, Hong SH, et al. Estrogen decreases zinc transporter 3 expression and synaptic vesicle zinc levels in mouse brain. J Biol Chem. 2004b;279:8602–8607. doi: 10.1074/jbc.M309730200. [DOI] [PubMed] [Google Scholar]
- Loeffler DA, DeMaggio AJ, Juneau PL, et al. Ceruloplasmin is increased in cerebrospinal fluid in Alzheimer's disease but not Parkinson's disease. Alzheimer Dis Assoc Disord. 1994;8:190–197. doi: 10.1097/00002093-199408030-00005. [DOI] [PubMed] [Google Scholar]
- Loeffler DA, LeWitt PA, Juneau PL, et al. Increased regional brain concentrations of ceruloplasmin in neurodegenerative disorders. Brain Res. 1996;738:265–274. doi: 10.1016/s0006-8993(96)00782-2. [DOI] [PubMed] [Google Scholar]
- Loske C, Gerdemann A, Schepl W, et al. Transition metal-mediated glycoxidation accelerates cross-linking of beta-amyloid peptide. Eur J Biochem. 2000;267:4171–4178. doi: 10.1046/j.1432-1327.2000.01452.x. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer's disease senile plaques. J Neurol Sci. 1998;158:47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Xie C, Markesbery WR. Protection against amyloid beta peptide toxicity by zinc. Brain Res. 1999;823:88–95. doi: 10.1016/s0006-8993(99)01114-2. [DOI] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, et al. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Sunderland T, Roth GS, Wolozin B. Physiological levels of beta-amyloid peptide promote PC12 cell proliferation. Neurosci Lett. 1996;217:125–128. [PubMed] [Google Scholar]
- Martin WR, Ye FQ, Allen PS. Increasing striatal iron content associated with normal aging. Mov Disord. 1998;13:281–286. doi: 10.1002/mds.870130214. [DOI] [PubMed] [Google Scholar]
- Massie HR, Aiello VR, Iodice AA. Changes with age in copper and superoxide dismutase levels in brains of C57BL/6J mice. Mech Ageing Dev. 1979;10:93–99. doi: 10.1016/0047-6374(79)90073-3. [DOI] [PubMed] [Google Scholar]
- Maurer I, Zierz S, Moller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol Aging. 2000;21:455–462. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Maynard CJ, Cappai R, Volitakis I, et al. Overexpression of Alzheimer's disease amyloid-beta opposes the age-dependent elevations of brain copper and iron. J Biol Chem. 2002;4:4. doi: 10.1074/jbc.M204379200. [DOI] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL, Harrop R, Akiyama H, Kamo H. Correlations of regional postmortem enzyme activities with premortem local glucose metabolic rates in Alzheimer's disease. J Neurosci Res. 1990;27:612–619. doi: 10.1002/jnr.490270422. [DOI] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Mielke R, Herholz K, Grond M, Kessler J, Heiss WD. Differences of regional cerebral glucose metabolism between presenile and senile dementia of Alzheimer type. Neurobiol Aging. 1992;13:93–98. doi: 10.1016/0197-4580(92)90015-p. [DOI] [PubMed] [Google Scholar]
- Mielke R, Schroder R, Fink GR, Kessler J, Herholz K, Heiss WD. Regional cerebral glucose metabolism and postmortem pathology in Alzheimer's disease. Acta Neuropathol. 1996;91:174–179. doi: 10.1007/s004010050410. (Berl.) [DOI] [PubMed] [Google Scholar]
- Misonou H, Morishima-Kawashima M, Ihara Y. Oxidative stress induces intracellular accumulation of amyloid beta-protein (Abeta) in human neuroblastoma cells. Biochemistry. 2000;39:6951–6959. doi: 10.1021/bi000169p. [DOI] [PubMed] [Google Scholar]
- Miura T, Suzuki K, Kohata N, Takeuchi H. Metal binding modes of Alzheimer's amyloid beta-peptide in insoluble aggregates and soluble complexes. Biochemistry. 2000;39:7024–7031. doi: 10.1021/bi0002479. [DOI] [PubMed] [Google Scholar]
- Molina JA, Jimenez-Jimenez FJ, Aguilar MV, et al. Cerebrospinal fluid levels of transition metals in patients with Alzheimer's disease. J Neural Transm. 1998;105:479–488. doi: 10.1007/s007020050071. [DOI] [PubMed] [Google Scholar]
- Morita A, Kimura M, Itokawa Y. The effect of aging on the mineral status of female mice. Biol Trace Elem Res. 1994;42:165–177. doi: 10.1007/BF02785387. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, et al. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Pappolla MA, et al. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer's disease. JNeurosci. 1999;19:1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Omar RA, Chyan YJ, Andorn AC, Poeggeler B, Robakis NK, Pappolla MA. Increased expression but reduced activity of antioxidant enzymes in Alzheimer's disease. JAlzheimers Dis. 1999;1:139–145. doi: 10.3233/jad-1999-1301. [DOI] [PubMed] [Google Scholar]
- Opazo C, Huang X, Cherny RA, et al. Metalloenzyme-like activity of Alzheimer's disease beta-amyloid. Cu-dependent catalytic conversion of dopamine, cholesterol, and biological reducing agents to neurotoxic H(2)O(2) J Biol Chem. 2002;277:40302–40308. doi: 10.1074/jbc.M206428200. [DOI] [PubMed] [Google Scholar]
- Palmblad M, Westlind-Danielsson A, Bergquist J. Oxidation of methionine 35 attenuates formation of amyloid beta-peptide 1–40 oligomers. J Biol Chem. 2002;277:19506–19510. doi: 10.1074/jbc.M112218200. [DOI] [PubMed] [Google Scholar]
- Paola D, Domenicotti C, Nitti M, et al. Oxidative stress induces increase in intracellular amyloid beta-protein production and selective activation of betaI and betaII PKCs in NT2 cells. Biochem Biophys Res Commun. 2000;268:642–646. doi: 10.1006/bbrc.2000.2164. [DOI] [PubMed] [Google Scholar]
- Pappolla MA, Omar RA, Kim KS, Robakis NK. Immunohistochemical evidence of oxidative [corrected] stress in Alzheimer's disease. Am J Pathol. 1992;140:621–628. [PMC free article] [PubMed] [Google Scholar]
- Pappolla MA, Chyan YJ, Omar RA, et al. Evidence of oxidative stress and in vivo neurotoxicity of beta-amyloid in a transgenic mouse model of Alzheimer's disease: a chronic oxidative paradigm for testing antioxidant therapies in vivo. Am J Pathol. 1998;152:871–877. [PMC free article] [PubMed] [Google Scholar]
- Perry G, Cash AD, Smith MA. Alzheimer disease and oxidative stress. J Biomed Biotechnol. 2002a;2:120–123. doi: 10.1155/S1110724302203010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G, Sayre LM, Atwood CS, et al. The role of iron and copper in the aetiology of neurodegenerative disorders: therapeutic implications. CNS Drugs. 2002b;16:339–352. doi: 10.2165/00023210-200216050-00006. [DOI] [PubMed] [Google Scholar]
- Phinney AL, Drisaldi B, Schmidt SD, et al. In vivo reduction of amyloid-beta by a mutant copper transporter. ProcNatl Acad Sci USA. 2003;100:14193–14198. doi: 10.1073/pnas.2332851100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantin L-O, Lysing-Tunnell U, Kristensson K. Trace elements in the human central nervous system studied with neutron activation analysis. Biol Trace Elem Res. 1987;13:69–75. doi: 10.1007/BF02796622. [DOI] [PubMed] [Google Scholar]
- Pratico D, Lee VM, Trojanowski JQ, Rokach J, Fitzgerald GA. Increased F2-isoprostanes in Alzheimer's disease: evidence for enhanced lipid peroxidation in vivo. FASEB J. 1998;12:1777–1783. doi: 10.1096/fasebj.12.15.1777. [DOI] [PubMed] [Google Scholar]
- Pratico D, Clark CM, Lee VM, Trojanowski JQ, Rokach J, FitzGerald GA. Increased 8,12-iso-iPF2alpha-VI in Alzheimer's disease: correlation of a noninvasive index of lipid peroxidation with disease severity. Ann Neurol. 2000a;48:809–812. [PubMed] [Google Scholar]
- Pratico D, Iuliano L, Amerio G, et al. Down's syndrome is associated with increased 8,12-iso-iPF2alpha-VI levels: evidence for enhanced lipid peroxidation in vivo. Ann Neurol. 2000b;48:795–798. [PubMed] [Google Scholar]
- Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001;21:4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O'Halloran TV. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science. 1999;284:805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- Rao KS.J, Rao RV, Shanmugavelu P, Menon RB. Trace elements in Alzheimer's disease brain: a new hypothesis. Alzheimer's Reports. 1999;2:241–246. [Google Scholar]
- Ritchie CW, Bush AI, Mackinnon A, et al. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Abeta amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch Neurol. 2003;60:1685–1691. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]
- Rogers JT, Randall JD, Cahill CM, et al. An iron-responsive element type II in the 5′-untranslated region of the Alzheimer's amyloid precursor protein transcript. J Biol Chem. 2002;277:45518–45528. doi: 10.1074/jbc.M207435200. [DOI] [PubMed] [Google Scholar]
- Rottkamp CA, Raina AK, Zhu X, et al. Redox-active iron mediates amyloid-beta toxicity. Free Radic Biol Med. 2001;30:447–450. doi: 10.1016/s0891-5849(00)00494-9. [DOI] [PubMed] [Google Scholar]
- Samokyszyn VM, Reif DW, Miller DM, Aust SD. Effects of ceruloplasmin on superoxide-dependent iron release from ferritin and lipid peroxidation. Free Radic Res Commun. 1991;12–13(Pt 1):153–159. doi: 10.3109/10715769109145780. [DOI] [PubMed] [Google Scholar]
- Samudralwar DL, Diprete CC, Ni B-F, Ehmann WD, Markesbery WR. Elemental imbalances in the olfactory pathway in Alzheimer's disease. J Neurol Sci. 1995;130:139–145. doi: 10.1016/0022-510x(95)00018-w. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer's disease. J Neurochem. 1997;68:2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Perry G, Harris PL, Liu Y, Schubert KA, Smith MA. In situ oxidative catalysis by neurofibrillary tangles and senile plaques in Alzheimer's disease: a central role for bound transition metals. J Neurochem. 2000;74:270–279. doi: 10.1046/j.1471-4159.2000.0740270.x. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer's disease. Trends Cell Biol. 1998;8:447–453. doi: 10.1016/s0962-8924(98)01363-4. [DOI] [PubMed] [Google Scholar]
- Shim H, Harris ZL. Genetic defects in copper metabolism. J Nutr. 2003;133:1527S–1531S. doi: 10.1093/jn/133.5.1527S. [DOI] [PubMed] [Google Scholar]
- de Silva DM, Aust SD. Ferritin and ceruloplasmin in oxidative damage: review and recent findings. Can J Physiol Pharmacol. 1993;71:715–720. doi: 10.1139/y93-107. [DOI] [PubMed] [Google Scholar]
- Smith MA, Taneda S, Richey PL, et al. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci USA. 1994;91:5710–5714. doi: 10.1073/pnas.91.12.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci USA. 1997;94:9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Hirai K, Hsiao K, et al. Amyloid-beta deposition in Alzheimer transgenic mice is associated with oxidative stress. J Neurochem. 1998a;70:2212–2215. doi: 10.1046/j.1471-4159.1998.70052212.x. [DOI] [PubMed] [Google Scholar]
- Smith MA, Sayre LM, Anderson VE, et al. Cytochemical demonstration of oxidative damage in Alzheimer disease by immunochemical enhancement of the carbonyl reaction with 2,4-dinitrophenylhydrazine. J Histochem Cytochem. 1998b;46:731–735. doi: 10.1177/002215549804600605. [DOI] [PubMed] [Google Scholar]
- Smith MA, Wehr K, Harris PL, Siedlak SL, Connor JR, Perry G. Abnormal localization of iron regulatory protein in Alzheimer's disease. Brain Res. 1998c;788:232–236. doi: 10.1016/s0006-8993(98)00002-x. [DOI] [PubMed] [Google Scholar]
- Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G. Oxidative stress in Alzheimer's disease. Biochim Biophys Acta. 2000;1502:139–144. doi: 10.1016/s0925-4439(00)00040-5. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Schreurs BG. Trace amounts of copper in water induce {beta}-amyloid plaques and learning deficits in a rabbit model of Alzheimer's disease. Proc Natl Acad Sci USA. 2003. [DOI] [PMC free article] [PubMed]
- Squitti R, Lupoi D, Pasqualetti P, et al. Elevation of serum copper levels in Alzheimer's disease. Neurology. 2002;59:1153–1161. doi: 10.1212/wnl.59.8.1153. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Oliver CN. Metal-catalyzed oxidation of proteins. Physiological consequences. J Biol Chem. 1991;266:2005–2008. [PubMed] [Google Scholar]
- Sturchler-Pierrat C, Staufenbiel M. Pathogenic mechanisms of Alzheimer's disease analyzed in the APP23 transgenic mouse model. Ann NY Acad Sci. 2000;920:134–139. doi: 10.1111/j.1749-6632.2000.tb06915.x. [DOI] [PubMed] [Google Scholar]
- Suh SW, Jensen KB, Jensen MS, et al. Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer's diseased brains. Brain Res. 2000;852:274–278. doi: 10.1016/s0006-8993(99)02096-x. [DOI] [PubMed] [Google Scholar]
- Syme CD, Nadal RC, Rigby SE, Viles JH. Copper binding to the amyloid-beta (Abeta) peptide associated with Alzheimer's disease. Folding, coordination geometry, pH dependence, stoichiometry and affinity of Abeta (1–28); insights from a range of complementary spectroscopic techniques. J Biol Chem. 2004;279:18169–18177. doi: 10.1074/jbc.M313572200. [DOI] [PubMed] [Google Scholar]
- Takeda A. Manganese action in brain function. Brain Res Brain Res Rev. 2003;41:79–87. doi: 10.1016/s0165-0173(02)00234-5. [DOI] [PubMed] [Google Scholar]
- Thomas LO, Boyko OB, Anthony DC, Burger PC. MR detection of brain iron. Am J Neuroradiol. 1993;14:1043–1048. [PMC free article] [PubMed] [Google Scholar]
- Tuppo EE, Forman LJ, Spur BW, Chan-Ting RE, Chopra A, Cavalieri TA. Sign of lipid peroxidation as measured in the urine of patients with probable Alzheimer's disease. Brain Res Bull. 2001;54:565–568. doi: 10.1016/s0361-9230(01)00450-6. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002a;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Rowan MJ, Selkoe DJ. Amyloid-beta oligomers: their production, toxicity and therapeutic inhibition. Biochem Soc Trans. 2002b;30:552–557. doi: 10.1042/bst0300552. [DOI] [PubMed] [Google Scholar]
- Wand GS, May C, May V, Whitehouse PJ, Rapoport SI, Eipper BA. Alzheimer's disease: low levels of peptide alpha-amidation activity in brain and CSF. Neurology. 1987;37:1057–1061. doi: 10.1212/wnl.37.6.1057. [DOI] [PubMed] [Google Scholar]
- Wang J, Dickson DW, Trojanowski JQ, Lee VM. The levels of soluble versus insoluble brain Abeta distinguish Alzheimer's disease from normal and pathologic aging. Exp Neurol. 1999;158:328–337. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- Wang J, Tanila H, Puolivali J, Kadish I, van Groen T. Gender differences in the amount and deposition of amyloidbeta in APPswe and PS1 double transgenic mice. Neurobiol Dis. 2003;14:318–327. doi: 10.1016/j.nbd.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Wender M, Szczech J, Hoffmann S, Hilczer W. Electron paramagnetic resonance analysis of heavy metals in the aging human brain. Neuropatol Pol. 1992;30:65–72. [PubMed] [Google Scholar]
- White AR, Multhaup G, Maher F, et al. The Alzheimer's disease amyloid precursor protein modulates copper-induced toxicity and oxidative stress in primary neuronal cultures. J Neurosci. 1999a;19:9170–9179. doi: 10.1523/JNEUROSCI.19-21-09170.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AR, Reyes R, Mercer JF, et al. Copper levels are increased in the cerebral cortex and liver of APP and APLP2 knockout mice. Brain Res. 1999b;842:439–444. doi: 10.1016/s0006-8993(99)01861-2. [DOI] [PubMed] [Google Scholar]
- Whitson JS, Selkoe DJ, Cotman CW. Amyloid beta protein enhances the survival of hippocampal neurons in vitro. Science. 1989;243:1488–1490. doi: 10.1126/science.2928783. [DOI] [PubMed] [Google Scholar]
- Whitson JS, Glabe CG, Shintani E, Abcar A, Cotman CW. Beta-amyloid protein promotes neuritic branching in hippocampal cultures. Neurosci Lett. 1990;110:319–324. doi: 10.1016/0304-3940(90)90867-9. [DOI] [PubMed] [Google Scholar]
- Wong BS, Chen SG, Colucci M, et al. Aberrant metal binding by prion protein in human prion disease. J Neurochem. 2001;78:1400–1408. doi: 10.1046/j.1471-4159.2001.00522.x. [DOI] [PubMed] [Google Scholar]
- Woodward WD, Filteau SM, Allen OB. Decline in serum zinc level throughout adult life in the laboratory mouse. J Gerontol. 1984;39:521–524. doi: 10.1093/geronj/39.5.521. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- Yassin MS, Ekblom J, Xilinas M, Gottfries CG, Oreland L. Changes in uptake of vitamin B(12) and trace metals in brains of mice treated with clioquinol. J Neurol Sci. 2000;173:40–44. doi: 10.1016/s0022-510x(99)00297-x. [DOI] [PubMed] [Google Scholar]
- Zecca L, Gallorini M, Schunemann V, et al. Iron, neuromelanin and ferritin content in the substantia nigra of normal subjects at different ages: consequences for iron storage and neurodegenerative processes. J Neurochem. 2001;76:1766–1773. doi: 10.1046/j.1471-4159.2001.00186.x. [DOI] [PubMed] [Google Scholar]