Abstract
Replication of Cauliflower mosaic virus (CaMV), a plant double-stranded DNA virus, requires the viral translational transactivator protein P6. Although P6 is known to form cytoplasmic inclusion bodies (viroplasms) so far considered essential for virus biology, a fraction of the protein is also present in the nucleus. Here, we report that monomeric P6 is imported into the nucleus through two importin-α-dependent nuclear localization signals, and show that this process is mandatory for CaMV infectivity and is independent of translational transactivation and viroplasm formation. One nuclear function of P6 is to suppress RNA silencing, a gene regulation mechanism with antiviral roles, commonly counteracted by dedicated viral suppressor proteins (viral silencing suppressors; VSRs). Transgenic P6 expression in Arabidopsis is genetically equivalent to inactivating the nuclear protein DRB4 that facilitates the activity of the major plant antiviral silencing factor DCL4. We further show that a fraction of P6 immunoprecipitates with DRB4 in CaMV-infected cells. This study identifies both genetic and physical interactions between a VSR to a host RNA silencing component, and highlights the importance of subcellular compartmentalization in VSR function.
Keywords: Cauliflower mosaic virus, DRB4, P6, RNA silencing, suppression
Introduction
In RNA silencing, homologues of the RNase-III enzyme Dicer process double-stranded (ds)RNA molecules into 21–24 nt RNAs, called short interfering (si)RNAs and micro (mi)RNAs. Four specialized Dicer-like (DCL) proteins with specifically sized small RNA products define multiple endogenous RNA silencing pathways in Arabidopsis thaliana: DCL1 catalyses processing of fold-back precursors into miRNAs that repress expression of cellular transcripts; DCL3 produces 24 nt siRNAs guiding heterochromatin formation, whereas DCL4 converts non-coding RNA precursors into 21 nt trans-acting (ta)siRNAs controlling developmental timing and organ polarity (reviewed in Brodersen and Voinnet, 2006). The size of DCL products is likely influenced by dsRNA-binding proteins (DRBs) that physically and specifically interact with Dicers: Arabidopsis DRB1, known as HYL1, facilitates DCL1-directed miRNA synthesis, whereas DRB4 enhances DCL4-mediated tasiRNA processing (Adenot et al, 2006; Nakazawa et al, 2007). Besides its regulatory roles, RNA silencing confers sequence-specific antiviral immunity to plants and invertebrates through the action of virus-derived (vi) short interfering RNAs (reviewed in Ding and Voinnet, 2007). In Arabidopsis, DCL4 is the primary Dicer of RNA viruses, and produces 21-nt-long viRNAs; DCL2 rescues antiviral silencing if DCL4 is genetically inactivated or suppressed, producing diagnostic 22-nt-long viRNAs (Bouche et al, 2006; Deleris et al, 2006; Fusaro et al, 2006). viRNAs are then thought to guide endonucleolytic cleavage (‘slicing') of viral genomes/transcripts upon incorporation into an RNA-induced silencing complex (RISC) that likely contains AGO1 because viRNAs immunoprecipitate with AGO1 (Zhang et al, 2006).
This model for antiviral silencing is supported by the fact that most plant viruses produce proteins called viral silencing suppressors (VSRs) thought to target many steps involving DCL, RISC or small RNA activities (reviewed in Ding and Voinnet, 2007). Nonetheless, clear-cut examples of interactions between VSRs and host silencing components are scarce. The tombusviral P19 protein forms a viRNA calliper preventing RISC loading (Vargason et al, 2003), whereas the Cucumber mosaic virus (CMV) 2b protein interacts with AGO1 to inhibit slicing (Zhang et al, 2006). AGO1 was also characterized as a direct target of the polerovirus F-Box-like protein P0 (Baumberger et al, 2007; Bortolamiol et al, 2007). More recently, the geminiviral protein V2 was shown to interact with SGS3, involved in amplification of antiviral silencing. The only two available examples of genetic, rather than physical, interactions linking viral proteins to RNA silencing components were provided by studies of VSR-deficient carmoviruses and cucumoviruses (reviewed in Ding and Voinnet, 2007).
DNA caulimoviruses are sensitive to the four Arabidopsis DCLs, with a prevalent role for DCL4 and DCL3 (Blevins et al, 2006; Moissiard and Voinnet, 2006). The dsDNA genome of Cauliflower mosaic virus (CaMV), type member of the Caulimovirus genus is replicated by RNA reverse transcription, and is transcribed by RNA PolII into the 35S and 19S RNAs. The 35S RNA 5′ end forms an extensive fold-back structure known as translational leader, ensuring ribosomal shunting required for expression of all CaMV ORFs (Ryabova and Hohn, 2000). The leader is a major source of CaMV-derived viRNAs, which accumulate as 24-nt (DCL3-dependent) and 21-nt (DCL4-dependent) species (Moissiard and Voinnet, 2006). Among the CaMV-encoded proteins, the ORF6 product, called P6, is a symptom and host range determinant that was recently found to suppress transgene RNA silencing, suggesting that it is a VSR (Love et al, 2007). P6 is mandatory for translational transactivation of the 35S RNA, and is, therefore, vital for CaMV accumulation in single cells (Kobayashi et al, 1998). The most part of P6 aggregates into large, amorphous cytoplasmic bodies called viroplasms (Kobayashi et al, 1998; Haas et al, 2005), considered so far essential for virus assembly, replication, protein synthesis and, possibly, silencing suppression. Nonetheless, a small fraction of P6 is also found in the nucleus of infected cells, but its mode of import and function(s) into this organelle are unknown (Haas et al, 2005). Using a combination of cell biology, genetics and biochemistry, we address these questions and show that the small nuclear fraction of P6 is essential for CaMV infection, independently of its roles in translational transactivation or viroplasm formation. Our results indicate that nuclear P6 is a VSR that genetically and physically interacts with DRB4, a nuclear protein that interacts with, and facilitates the activity of the antiviral enzyme DCL4.
Results
Two nuclear localization signals are cooperatively required for nuclear import of monomeric P6 molecules through the importin α pathway
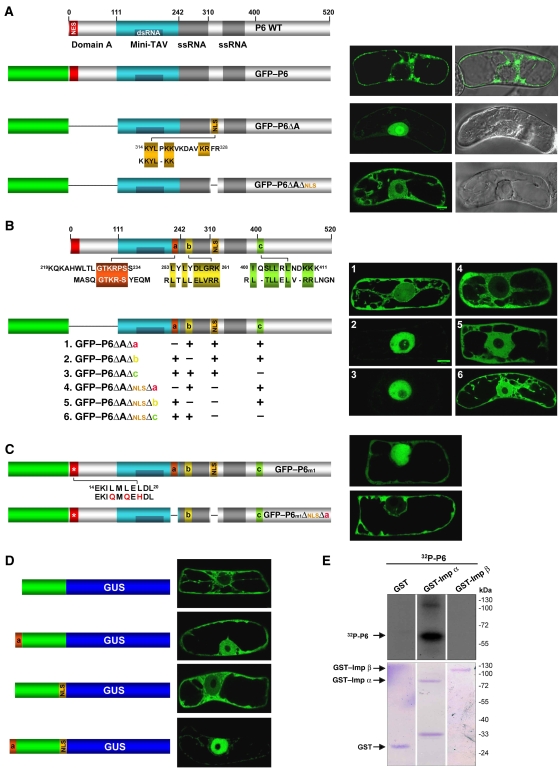
Use of N-terminal green fluorescent protein fusions (GFP–P6; Figure 1) shows that P6 shuttles between the cytoplasm and the nucleus of bombarded BY-2 cells and forms nuclear speckles in authentic infection context (Haas et al, 2005). Nonetheless, most of GFP–P6 is normally found in cytoplasmic, perinuclear bodies structurally identical to the viroplasms found in CaMV-infected cells (Figure 1A, top panel; Haas et al, 2005). This prevalent cytoplasmic P6 distribution is contributed by the N-terminal domain (domain A, aa 1–111), which contains a nuclear export signal (NES) overlapping with residues required for P6–P6 intermolecular interactions and viroplasm formation (Haas et al, 2005). To address the mechanisms of P6 import and its possible role(s) in the nucleus, we used GFP–P6ΔA, which localizes exclusively to the nucleus owing to a deletion of domain A (Figure 1A, middle panel; Haas et al, 2005). The molecular mass of GFP–P6ΔA (85 kDa) is incompatible with a passive diffusion across nuclear pores, suggesting that the region between aa 112 and 520 contains at least one nuclear localization signal (NLS) for P6 active import. Scanning P6 sequence, we found that the KYLPKKVKDAVKRFR motif between aa 314 and 328 defines a bipartite basic NLS frequently found in plant proteins, and shares homologies with the NLS of human ribosomal protein L22 (Figure 1A, middle panel; Shu-Nu et al, 2000). Deleting this motif in GFP–P6ΔA indeed caused cytoplasmic redistribution and partial loss of nuclear import of the resulting protein GFP–P6ΔAΔNLS (Figure 1A, lower panel).
Figure 1.
(A) Schematic of P6 and its functional domains (coloured boxes). NES: nuclear export signal; domain A: P6–P6 interaction; mini-TAV: minimal domain for translational transactivation; ssRNA: single-stranded RNA binding; dsRNA: double-stranded RNA binding. The various GFP–P6 alleles were transiently expressed into BY-2 tobacco cells and observed under confocal microscope 18 h post-bombardment. Images on the left show GFP detection and those on the right are composite images of green fluorescence and DIC. Position and amino-acid sequence of the putative basic bipartite NLS are indicated. Similarities with the NLS of human ribosomal protein L22 are shown in yellow. (B) P6 regions (a–c) showing homology with non-conventional, viral NLSs (red: influenza virus 1 NP protein; yellow and green: Borna disease virus P10 protein). The effects of their deletion (singly or in combination) on P6ΔA distribution are depicted on the right. (C) Cellular localization of GFP–P6m1 (leucine substitution in red) and P6m1ΔNLSΔa. (D) Cellular localization of GFP–GUS in the absence/presence of individual P6 NLS, or combination thereof. (E) GST pull down of P6 and rice importins. Radiolabelled P6 was incubated either with importin α (GST-Imp α), β (GST-Imp β) or GST alone (GST). Following SDS–PAGE, P6 was detected by autoradiography (upper panel) and total proteins by Coomassie blue staining (lower panel).
The residual nuclear GFP signal in GFP–P6ΔAΔNLS-bombarded cells suggested that additional sequences are required for complete P6 import, as often observed for viral nuclear proteins. Further inspection of P6 identified three regions with homologies to known, non-conventional NLSs of viruses. NLS-a (spanning aa 219–234) contains the GTKR-S motif recruited by importin α1 for nuclear import of influenza A virus NP protein (Wang et al, 1997). The second and third sequences (NLS-b and NLS-c, respectively) display similarities with the importin α-dependent NLS of Borna disease virus P10 protein (Figure 1B; Wolff et al, 2002). To test the contribution of NLS-a, -b and -c to P6 import, their individual deletions or combinations thereof were engineered into GFP–P6ΔA, and subcellular distribution of the resulting proteins monitored in bombarded BY-2 cells (Figure 1B). Deletion of NLS-a alone caused a nucleo-cytoplasmic redistribution resembling that of GFP–P6ΔAΔNLS, except for the nucleolus (Figure 1B, panel 1). Nuclear import of GFP–P6ΔA, remained, however, unaltered by NLS-b or NLS-c deletions (Figure 1B, panels 2 and 3), although nucleolar localization was partially prevented. Neither the NLS-b nor the NLS-c deletion caused a change in the nucleo-cytoplasmic distribution of GFP–P6ΔAΔNLS (Figure 1B, panels 5 and 6). By contrast, its nuclear localization was abolished by the NLS plus NLS-a deletion (GFP–P6ΔAΔNLSΔa; Figure 1B, panel 4). Collectively, the results suggest that, among the three predicted non-conventional NLSs, only NLS-a cooperates with the bipartite NLS for GFP–P6ΔA import. Additional experiments further indicated that P6 molecules are likely imported into the nucleus as monomers (Supplementary data).
To rule out that the large deletion in domain A artificially influenced localization of GFP–P6ΔA and its derivatives, we used construct GFP–P6m1 in which functionality of the NES within domain A is strongly reduced, albeit not eliminated, by three point mutations in essential leucine residues (Haas et al, 2005; Figure 1C). Consequently, GFP–P6m1 localizes to the cytoplasm and the nucleus. Deleting the bipartite NLS and NLS-a in GFP–P6m1 (leading to GFP–P6m1ΔNLSΔa) abolished nuclear localization, reinforcing the idea that both sequences are indeed necessary for P6 import. We then measured the ability of each sequence, or combination thereof, to promote nuclear import of the unrelated and strictly cytoplasmic GFP–GUS fusion protein (120 kDa; Figure 1D, top panel). Each sequence individually contributed to GFP–GUS import, but their effect was only partial and consistently stronger with NLS-a (Figure 1D, middle panels). By contrast, GFP was strictly nuclear when both sequences were introduced (Figure 1D, lower panel), indicating that the bipartite NLS and the non-conventional NLS-a are sufficient to cooperatively mediate full nuclear import.
NLS-dependent nuclear import in plants occurs mostly through importin α (Smith and Raikhel, 1998). To address this point, we used GST fusions of rice importin α1 and β1 immobilized onto glutathione-coupled sepharose (GST–importin (Imp) α and GST–Imp β, respectively). A P6 variant carrying an N-terminal hexapeptide allowing its phosphorylation was expressed in Escherichia coli and radiolabelled in vitro with 32P-γ-ATP. Previous work showed that P6 is specifically labelled among the proteins produced in this recombinant E. coli strain (Leh et al, 2000). Labelled P6 was incubated with sepharose beads coupled to GST alone, GST–Imp α or GST–Imp β, and protein complexes resolved on SDS–PAGE. As shown in Figure 1E, the P6 signal (62 kDa) and a fainter signal corresponding to P6 homodimers (∼120 kDa) were only detected in fractions incubated with GST–Imp α. Therefore, nuclear import of P6 by the bipartite NLS and the non-conventional NLS-a is likely through the importin α pathway. A minor part of P6 import could also occur through interactions between the miniTAV domain (Figure 1A) and the L13, L18 and presumably other ribosomal proteins. However, this process causes P6 retention within the nucleolar, as opposed to the nucleoplasmic compartment (Supplementary data).
A nuclear function of P6 distinct from translational transactivation is essential for CaMV infectivity
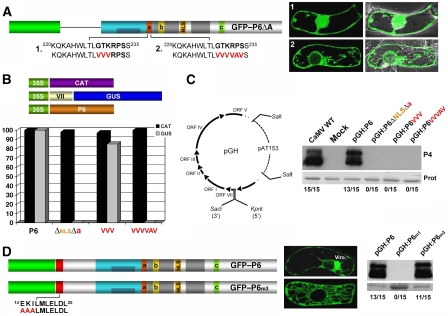
An essential function of P6 in CaMV biology is to transactivate translation of the polycistronic 35S RNA and its derivatives through the mini-TAV domain (Pooggin et al, 2000; Figure 1A). To get insights into possible nuclear role(s) of P6 in this process, we created additional alleles of GFP–P6ΔA carrying point mutations in conserved amino acids of the non-conventional NLS-a. Some, or all of the GTKRPS residues were replaced by valines or alanines in constructs GFP–P6ΔAVVV and GFP–P6ΔAVVVVAV, respectively (Figure 2A). Compared to the strict nuclear localization of GFP–P6ΔA (Figure 1A, middle panel), GFP–P6ΔAVVV had a nucleo-cytoplasmic pattern, whereas nuclear import was nearly completely abolished with GFP–P6ΔAVVVVAV (Figure 2A), resembling GFP–P6ΔAΔNLSΔa distribution (Figure 1B, panel 4). We then re-inserted the N-terminal A domain into the above P6 alleles to generate P6VVV and P6VVVVAV and test their ability to re-initiate translation of a bicistronic GUS reporter gene, using a transactivation assay in Nicotiana plumbaginifolia protoplasts (Pooggin et al, 2000). In this assay, a second, monocistronic reporter gene encoding the chloramphenicol acetyl transferase (CAT) is used as an internal transfection and translation control. Transactivation values obtained with wild-type (WT) P6, arbitrarily set to 100%, served as a reference for the activity of P6 variants. In two independent experiments, neither P6ΔNLSΔa nor P6VVVVAV could transactivate GUS expression, whereas transactivation by P6VVV was 82% that of WT P6 (Figure 2B).
Figure 2.
(A) Effect of point mutations in NLS-a (red) on GFP–P6ΔA localization. (B). N. plumbaginifolia protoplasts were co-transfected with constructs expressing P6, CAT and GUS, under the control of the CaMV 35S promoter. The GUS ORF cloned downstream of the CaMV ORF VII is used to monitor translational transactivation. CAT expression is an internal control for translation and transfection efficacy. The effects of P6, or mutated version thereof, on GUS expression are represented by histograms. GUS activity in the presence of WT P6 was arbitrarily set to 100%. The data are from two independent experiments. (C) Schematic of the pGH recombinant viral vector. ORF VI variants can be inserted owing to the SacI/KpnI restriction sites. The indicated P6 variants were cloned in pGH and inoculated to Arabidopsis upon linearization with SalI. Plants were monitored for symptom formation over an 18-day period, upon which tissues were collected for analysis of the CaMV coat protein P4. The ratio of infected to inoculated plants is shown. Coomassie blue staining shows equal protein loading. (D) Schematic of the GFP–P6 and GFP–P6m3 proteins and analysis of their localization in BY-2 cells 18 h post-bombardment. Both alleles were introduced into pGH and infections were monitored as in (C).
To test the three P6 alleles in infection contexts, we engineered a DNA-based viral vector (pGH) allowing insertion of ORF VI variants (Figure 2C, diagram). Arabidopsis plants inoculated with pGH:P6, carrying the WT P6 allele, showed typical chlorotic and leaf curling symptoms. Moreover P4 (coat-protein) accumulation in systemically infected tissues was the same in pGH:P6 as it was in CaMV-infected plants, confirming infectivity of the pGH:P6-derived virus (Figure 2C). By contrast, plants inoculated with pGH carrying either of the three P6 mutant alleles remained symptomless, and P4 was undetectable in systemic tissues (Figure 2C). This was notably the case for pGH:P6VVV, carrying a transactivation-proficient P6 allele (Figure 2B). Thus, partial loss of P6 nuclear localization caused by the GTK → VVV substitution in NLS-a (Figure 2A) was sufficient to abolish CaMV infectivity without significantly altering transactivation.
Lack of infectivity was also observed with pGH:P6m1 (Figure 2D, right panel), carrying a triple mutation that compromises P6 nuclear export (Figure 1C). Unlike mutations affecting P6 nuclear import (Figures 1 and 2), m1 also alleviates viroplasm formation (Figure 1C). Viroplasms normally contain the vast majority of P6 molecules and have been so far considered essential for viral replication and particle morphogenesis or storage (Haas et al, 2002). Thus, to discriminate which impaired function (viroplasm formation or nuclear export) accounted for the lack of pGH:P6m1 infectivity, we used P6m3, carrying the EKI → AAA amino-acid substitution in the NES motif of domain A (Figure 2D, left panel). GFP–P6 fusions carrying this mutation (GFP–P6m3) display intact nuclear export but fail to form viroplasms in BY-2 cells (Haas et al, 2005; Figure 2D). In contrast to pGH:P6m1, pGH:P6m3 was fully infectious in Arabidopsis despite complete absence of detectable viroplasms in infected tissues (Figure 2D; Haas et al, in preparation). Therefore, although viroplasms are apparently dispensable for CaMV infection, P6 nuclear export is essential for this process. We conclude from the compared analysis of its import- and export-deficient alleles that nucleo-cytoplasmic shuttling of P6 is mandatory for infection, and that P6 exerts in the nucleus one or several essential functions distinct from its role in translational transactivation.
Nuclear import is required for RNA silencing suppression by transgenic P6
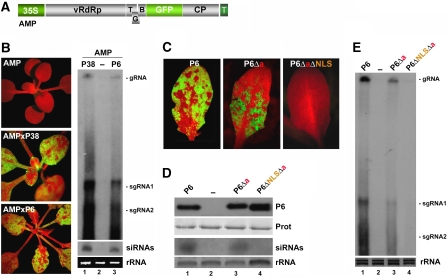
Recently, P6 was found to suppress transgene silencing (Love et al, 2007). Therefore, we set out to determine whether P6 nuclear localization is required for its VSR function, which could explain the loss of infectivity of pGH variants expressing nuclear import-deficient P6 alleles (Figure 2C and D). WT P6 was cloned under the CaMV 35S promoter and transformed into the Arabidopsis AMPLICON (AMP) line, in which a transgene expressing GFP-tagged and replicating PVX RNA (PVX–GFP; Figure 3A) is both an initiator and a target of RNA silencing (Dalmay et al, 2000). In the AMP line (ecotype C24), PVX–GFP RNA levels are vastly reduced, plants appear uniformly red under UV illumination, owing to chlorophyll fluorescence (Figure 3B, upper panel); and viral-derived siRNAs remain undetectable (Figure 3B, lane 2).
Figure 3.
(A) Schematic of the PVX–GFP transgene in the Arabidopsis AMP line. (B). Restauration of GFP accumulation upon transgenic expression of P6 and P38 in the AMP line (left panel). The red colour is from chlorophyll autofluorescence under UV and signifies GFP silencing. High molecular weight (HMW) RNA and siRNA were extracted and detected using a GFP-specific probe (right panel). rRNA: ethidium bromide staining of ribosomal RNA; gRNA and sgRNA: genomic and subgenomic PVX–GFP RNA respectively. (C) Leaves of AMP plants expressing P6, P6Δa or P6ΔaΔNLS, under UV light. (D) Accumulation of P6 or its variants was detected by western blot analysis using a P6 antiserum (P6, upper panel). Prot: Coomassie blue staining of total protein. PVX–GFP-derived siRNA were detected as in (B) (lower panel). (E) HMW RNA analysis of PVX gRNA and sgRNA in AMP lines expressing P6 and its variants, as in (B).
As a positive control, we used line AMP-P38 (Moissiard et al, 2007) in which green fluorescence, PVX–GFP and 21-nt-long viral siRNA accumulation are all restored by expression of the DCL4 antagonist P38 VSR of Turnip crinkle virus (Figure 3B, middle panel, lane 1). Whereas P38, similar to P6 (see below), strongly reduces DCL4-dependent tasiRNA accumulation, its paradoxical enhancing effect on the levels of AMP-derived siRNA is likely due to the dramatic increase in PVX–GFP replication and subsequent dicing, by DCL4, of highly abundant viral dsRNA (Moissiard et al, 2007). Independent T2 AMP-P6 lines were isolated and P6 expression was confirmed by western blot analysis. The results in Figure 3 are representative of several independent P6 lines showing comparable expression levels. In all cases, AMP-P6 plants had visual and molecular phenotypes resembling those of AMP-P38 plants (Figure 3B, lower panel, lane 3), indicating that transgenic P6 suppresses AMP silencing nearly to the same extent as transgenic P38. To test if P6 nuclear import is required for silencing suppression, we transformed the AMP line with the P6Δa or P6ΔNLSΔa alleles, expected to be partially and completely excluded from the nucleus, respectively (Figure 1). Transgenic lines were selected to express similar levels of P6 and its variants (Figure 3D). Of note, only two lines expressing the P6ΔNLSΔa allele to significant levels could be obtained in multiple transformation attempts, suggesting cellular toxicity. The silencing suppression phenotype of AMP-P6Δa lines was consistently less pronounced than in AMP-P6 lines: green fluorescence was less extensive in plant tissues (Figure 3C, middle panel compared to left panel) and AMP-derived siRNAs accumulated slightly less than in AMP-P6 plants (Figure 3D, lane 3 compared to lane 1). Accordingly, reactivation of PVX–GFP replication was less pronounced in AMP-P6Δa compared with AMP-P6 plants (Figure 3E, lanes 1 and 3). The AMP-P6ΔNLSΔa lines remained completely red fluorescent (Figure 3C, right panel), whereas siRNAs and PVX–GFP replication products were below detection limit (Figure 3D and E, lane 4). We conclude that silencing suppression by transgenic P6 requires its nuclear import.
P6-mediated suppression of the tasiRNA pathway requires nuclear import, but none of the previously characterized features of the protein
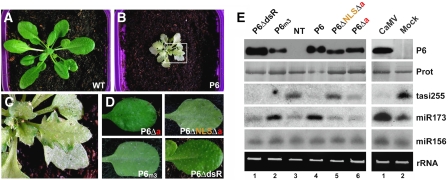
Viral symptom expression, viroplasm formation and dsRNA binding through the mini-TAV domain are verified or suspected biochemical features of P6 that could all contribute to its VSR function (discussed in Ding and Voinnet, 2007). To address this issue, we established transgenic lines in WT Arabidopsis (ecotype Col-0) expressing either P6, P6ΔdsR, P6m3, P6Δa or P6ΔNLSΔa from the CaMV 35S promoter, and tested the effect of these various alleles on accumulation of endogenous small silencing RNA. These experiments were not carried out in the AMP background because high PVX–GFP replication caused by silencing suppression (Figure 3) results in the accumulation of the PVX-encoded VSR protein P25, which can alter endogenous silencing pathways (Moissiard et al, 2007). Plants expressing WT P6 displayed strong stunting, leaf serration and a ‘silvering' chlorosis resembling CaMV-elicited symptoms (Figure 4A–C). This agrees with work in ecotype Ler (Love et al, 2007) and confirms that P6 is a major symptom determinant. By contrast, none of the transgenic lines expressing the various P6 mutant alleles displayed these phenotypic defects (Figure 4D), despite accumulation of comparable P6 levels (Figure 4E, left panel).
Figure 4.
(A, B) P6 transgenic Arabidopsis ecotype Col-0 (B) are stunted, chlorotic and exhibit leaf serration compared with their non-transgenic counterparts (A). (C) Magnified view of (B) showing the ‘silvery' chlorosis developing on older leaves. (D) None of the P6 mutants induce developmental anomalies when expressed transgenically in the Col-0 ecotype. (E) Upper panel: western blot analysis of P6 accumulation in plants depicted in (A–D), as in Figure 3D. Lower panel: LMW RNA analysis in the corresponding genotypes using probes for tasiRNA255, miR173 and miR156.
We then measured accumulation of endogenous tasiRNAs in the various transgenic lines. In tasiRNA biogenesis, the initial miRNA-guided cleavage of primary tasiRNA transcripts sets a defined point for RDR6-directed complementary strand synthesis, followed by a phased DCL4-dependent processing reaction that generates mature tasiRNAs (Yoshikawa et al, 2005; Adenot et al, 2006). Accumulation of tasiRNA255 from the TAS1 locus was decreased but still detectable in the P6Δa transgenic lines compared with non-transgenic plants (Figure 4E, left panel, lanes 3 and 6). It was strongly reduced in the P6 lines, and abolished in the P6ΔdsR and P6m3 lines (Figure 4E, left panel, lanes 1, 2 and 4). These results could not be ascribed to altered accumulation of miR173, which is required for tasiRNA255 biogenesis: its levels were either unaffected in P6ΔdsR and P6Δa lines, or enhanced by approximately 2.5-fold in P6 and P6m3 lines (Figure 4E, left panel, lanes 1, 2, 4 and 6). None of the above P6 alleles caused significant changes in the levels of miR156, unrelated to tasiRNA biogenesis (Figure 4E, left panel, lanes 1, 2, 4 and 6). miR173 and miR156 levels were also unaltered in transgenic lines expressing the P6ΔNLSΔa allele, which is completely excluded from the nucleus (Figure 1C). However, unlike all the other P6 alleles, P6ΔNLSΔa had no effect on tasiRNA255 accumulation (Figure 4E, left panel, lane 5).
Therefore, transgenic P6 suppresses tasiRNA, but not miRNA accumulation. This mimics the effects of authentic CaMV infections because CaMV-infected and P6 transgenic Arabidopsis had similar molecular phenotypes (Figure 4E, right panel). Moreover, high molecular weight RNA species, which presumably correspond to tasiRNA255 dsRNA precursors, overaccumulated in both P6 transgenic and CaMV-infected plants, as previously reported (Blevins et al, 2006; Supplementary data). The lack of effect of P6m3 and P6ΔdsR on symptom formation indicates that dsRNA binding, and viroplasm formation are all dispensable for suppression. Moreover, because the P6ΔdsR deletion disrupts the mini-TAV domain, neither its transactivation property nor its interaction with L13–L18 is required by P6 to suppress the tasiRNA pathway. By contrast, nuclear import is essential for this process, agreeing with the AMP results (Figure 3). We conclude that P6 VSR function entails an interaction with a nuclear factor specifically required for siRNA, but not miRNA biogenesis or accumulation.
The nuclear protein DRB4 is a genetic and physical target of nuclear P6
On the basis of our current knowledge of Arabidopsis post-transcriptional siRNA pathways and on the results of Figures 3 and 4, possible nuclear targets of P6 include the DCL4 RNase-III and the dsRNA-binding protein DRB4: both localize to, and interact within the nucleus (Nakazawa et al, 2007), and both are required downstream of RDR6 for tasiRNA synthesis. Of the two possibilities, an effect of P6 on DCL4 was unlikely, as genetic lesions or VSRs that compromise DCL4 activity generally cause a diagnostic 21 nt → 22 nt shift in tasiRNA size (Adenot et al, 2006; Deleris et al, 2006; Moissiard et al, 2007), not observed in any of our P6 transgenic lines. By contrast, mutations in DRB4 decrease the levels of some tasiRNAs, including tasiRNA255, without size shifting (Adenot et al, 2006). We could not formally rule out an effect of P6 on RDR6, which also localizes into the nucleus (Luo and Chen, 2007), although the extensive suppression phenotype in AMP-P6 lines made this possibility unlikely, as green fluorescence in AMP lines is only modestly re-activated by the rdr6 mutation (Moissiard et al, 2007).
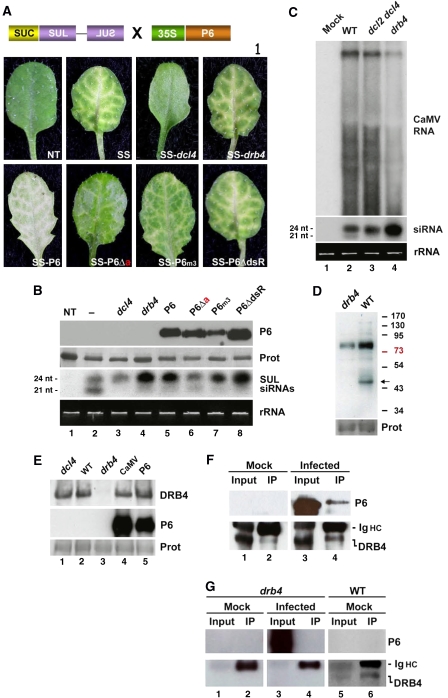
To get insights into the nature of the nuclear silencing factor(s) targeted by P6, we used the SUC-SULPHUR (SUL) (SS) reporter line, in which phloem-specific expression of an inverted repeat transgene triggers non-cell-autonomous RNAi of endogenous SUL transcripts, causing a vein-centred chlorotic phenotype (Figure 1A; Dunoyer et al, 2007). We showed that SUL silencing is RDR6 independent (Himber et al, 2003) and that, of the 21 nt (DCL4-dependent) and 24 nt (DCL3-dependent) SUL siRNA species accumulating in the SS reference line, only the former is required for RNAi in a strict AGO1-dependent manner (Dunoyer et al, 2007). Nonetheless, introduction of specific RNA silencing mutations in the SS system unravelled the existence of redundant pathways that can compensate for reduced DCL4 activities. Notably, null mutations in DRB4 reduce the levels of 21 nt SUL siRNAs, but simultaneously dramatically elevate those of DCL3-dependent 24 nt SUL siRNAs. We showed that these abnormally high levels of DCL3 products fully rescue the SUL silencing phenotype upon recruitment of AGO1 (Dunoyer et al, 2007; Figure 5A, upper panel; Figure 5B, lane 4).
Figure 5.
(A) Schematic of the SUC-SUL transgene and phenotype of progenies expressing the P6 alleles used in Figure 4. (B) Upper panel: accumulation of P6 and its variants, as in Figures 3D and 4E. Lower panel: LMW RNA analysis in the corresponding genotypes, using a DNA probe specific for the SUL region. SUL siRNAs accumulate as 21 and 24 nt species. (C) Detection of CaMV-derived HMW (upper panel) and LMW (lower panel) RNA species in WT, dcl2 dcl4 and drb4 mutant plants. The DNA probe covers the CaMV genome. (D) Western blot analysis of DRB4 accumulation from WT and drb4 mutant plants. The arrow indicates the expected migration of DRB4 (predicted molecular mass: 43 kDa). (E) Western blot analysis of DRB4 accumulation in Arabidopsis plants infected by CaMV or expressing P6. drb4 and dcl4 mutant plants were used as controls. (F) DRB4 immunoprecipitation in mock-inoculated (left) and CaMV-infected plants (right). Immunoprecipitates were subjected to western blot analysis using a P6 antibody (upper panel). The filter was then re-hybridized with the DRB4 antibody (lower panel). The ∼50 kDa nonspecific signal is from the immunoglobulin heavy chain (Ig HC). (G) Same as in (F) but in the drb4 mutant background. DRB4 immunoprecipitates from drb4 plants are devoid of P6.
The transgenic plants described in Figure 4 were crossed to the SS reference line (Col-0 ecotype). SUL silencing and siRNA accumulation were then assayed in progenies, in which P6 or its variants accumulated to comparable levels (Figure 5B). Note that we repeatedly failed to produce viable plants out of many SS-P6ΔNLSΔa crosses, or by direct transformation of the SS line. As in the AMP system, exclusive accumulation of P6ΔNLSΔa into the cytoplasm likely caused cellular toxicity, probably exacerbated by the chlorosis developing in the SS background. The previously described SS-dcl4 and SS-drb4 plants, carrying null mutations in DCL4 and DRB4, respectively (Dunoyer et al, 2007), were used as references (Figure 5A, upper panel). SS-P6ΔdsR plants had a phenotype resembling that of SS or SS-drb4 plants. SS-P6 plants also displayed unaltered SUL silencing superimposed onto the silvery chlorosis caused by WT P6 (Figure 5A, lower panel). SS plants expressing the P6m3 allele also showed the vein-chlorotic phenotype, but it was somewhat less pronounced than in SS-P6 and SS-P6ΔdsR, owing, presumably, to slightly lower expression (Figure 5A, lower panel; Figure 5B, lane 7). By contrast, the extent of SUL silencing was significantly reduced in the SS-P6Δa crosses (Figure 5A, lower panel), but it was still visible compared to the complete lack of RNAi in SS-dcl4 plants (Figure 5A, upper panel).
All P6 alleles, except P6Δa, had a molecular phenotype resembling strikingly that of the drb4 mutation: there were reduced levels of DCL4-dependent 21 nt siRNAs, whereas those of DCL3-dependent 24 nt siRNAs were dramatically enhanced (Figure 5B). The latter effect was particularly pronounced with the P6ΔdsR allele (Figure 5B, lane 8). SS-P6Δa plants also had reduced 21 nt siRNA levels but accumulated normal levels of 24 nt siRNAs (Figure 5B), resembling the effect of weak dcl4 alleles (Dunoyer et al, 2007). As reported previously, there was a complete lack of 21 nt siRNAs and unaltered 24 nt siRNA levels in the dcl4 null background (Dunoyer et al, 2007). These results agree with those in Figure 4, and indicate that expression of P6 alleles with unaltered nuclear import properties, on the one hand, and null mutations in DRB4, on the other hand, are genetically equivalent. Moreover, like P6 transgenic and CaMV-infected plants (Supplementary data), SS plants carrying the drb4 mutation, but not the dcl4 mutation, accumulated high molecular weight dsRNA species that derive from tasiRNA255 precursors (Supplementary data). Thus, the visual phenotypes of SS-P6Δa plants, expressing a P6 allele partially impaired for nuclear import, likely results from a interaction with only a fraction of DRB4 preventing overaccumulation of 24 nt siRNAs and rescue of compromised DCL4 activity.
We previously showed that the vast majority of 21 and 24 nt siRNAs accumulating during CaMV infection is produced by DCL4 and DCL3, respectively, using the highly structured 35S RNA leader as a template (Figure 5C, lane 2; Moissiard and Voinnet, 2006). Transposed into infection contexts, the results in the SS system (Figure 5B) thus predicted that the 35S leader should be accessed by DRB4. To test this idea, viral RNA and siRNA levels were measured in CaMV-infected drb4 mutants of Arabidopsis. dcl2 dcl4 mutant plants were also infected in parallel, because this specific combination of dcl lesions promotes the exclusive accumulation of CaMV-derived 24 nt siRNAs, which are predominantly affected by the drb4 mutation in RNAi (Figure 5B). The effects of the drb4 mutation on accumulation of leader-derived siRNAs were strikingly similar to its effects on SUL siRNAs: the levels of the 21 nt species were reduced and those of the 24 nt species strongly enhanced (Figure 5C). Viral RNA levels were also slightly reduced (Figure 5C), presumably because 24 nt siRNAs were shown to impact CaMV accumulation negatively (Moissiard and Voinnet, 2006). We conclude that the nuclear DRB4 protein is a genetic target of nuclear P6.
To test if P6 expression alters DRB4 integrity, we employed a DRB4 antibody previously used in immunoprecipitation experiments (Nakazawa et al, 2007). First, we verified that the antibody could be used in western blot analyses of Arabidopsis protein extracts. As shown in Figure 5D, a signal consistent with the predicted molecular mass of DRB4 (approximately 43 kDa) was detected in WT, but not in drb4 mutant plants. We then carried out similar experiments with protein extracts isolated from P6 transgenic Arabidopsis (Figure 4B) or CaMV-infected plants. In both cases, neither the levels nor the electrophoretic mobility of DRB4 was compromised by P6 (Figure 5E, lanes 4 and 5). The same result was obtained in dcl4 mutants, used as controls (Figure 5E, lane 1). The integrity of DRB4 being not apparently altered by P6, we then assayed for possible interactions between the two proteins in authentic infection contexts. The DRB4 antibody was used to immunoprecipitate total proteins extracted from either mock-inoculated or CaMV-infected Arabidopsis (Figure 5F, upper panel, lanes 1 and 3). Immunoprecipitates were then assayed for the presence of P6 and DRB4 in western blot analyses (Figure 5F). We found that a fraction of P6 does indeed co-immunoprecipitate with DRB4 in CaMV-infected plants (Figure 5F, upper panel, lane 4). Presumably, this fraction corresponds to nuclear P6, which accounts for only a small proportion of the protein in infected cells (Haas et al, 2005). As expected, the DRB4 immunoprecipitates were devoid of P6 in mock-inoculated plants (Figure 5F, upper panel, lane 2), and P6 could not be immunoprecipitated from CaMV-infected plants with the drb4 null mutation (Figure 5G, lower panel, lanes 1–4 compared to 5 and 6). We conclude that P6 and DRB4 interact, either directly or indirectly, in authentic infection contexts. Therefore, DRB4 is not only a genetic but also a physical target of P6.
Discussion
Although RNA silencing suppression is recognized as a widespread strategy of plant and invertebrate viruses (Ding and Voinnet, 2007), the mode of action and molecular targets of VSRs remain largely unknown. The present study uncovers a previously unappreciated nuclear function of the CaMV-encoded P6 protein. This function, although independent of the established role of P6 in translational transactivation, is essential for virus infectivity. We show that at least one nuclear activity of P6 is to suppress RNA silencing through inhibition of DRB4, a factor required for optimal dicing of CaMV-derived dsRNA by DCL4.
Monomeric P6 is imported into the nucleus (Supplementary data) through the cooperative action of two NLSs that likely recruit importin α. Nuclear import might be facilitated by interactions between the P6 dsRNA-binding domain and ribosomal proteins L13 and L18, but this results in P6 nucleolar retention and is completely dispensable for its VSR function (Supplementary data; Figure 4E). We propose, therefore, that P6-mediated silencing suppression is predominantly nucleoplasmic. Accordingly, silencing suppression by the P6ΔdsR allele was consistently stronger than with WT P6 (Figures 4E and 5B). Presumably, the loss of L13–L18 interaction reduces P6ΔdsR access to the nucleolus and, thus, increases its availability in the nucleoplasm for enhanced VSR activity. The viroplasm-deficient P6m3 allele also displays an enhanced suppression phenotype, at least in terms of tasiRNA accumulation (Figure 4E). The finding that viroplasms are dispensable not only for silencing suppression but also for CaMV infectivity in whole plants, thus raises the possibility that P6 aggregation in amorphous cytoplasmic bodies might, in fact, restrain nuclear silencing suppression. We consistently experienced difficulties in generating plants expressing high levels of strictly cytoplasmic P6 (i.e. P6ΔNLSΔa), suggesting that its full cytoplasmic retention is toxic. Conversely, all attempts to generate transgenic plants expressing the strictly nuclear P6m1 allele (Figure 1C) were unsuccessful (data not shown). Moreover, we failed to express to significant levels an allele derived from P6ΔAΔNLSΔa, whose nuclear localization and retention are restored by addition of an NLS from SV40 (Supplementary data). Taking also into account the data in Figure 2C and D, these results suggest that constant shuttling of P6 between the nucleus and the cytoplasm is a prerequisite to successful virus infection. This nucleo-cytoplasmic shuttling might represent an original viral strategy to modulate P6 VSR activity and, consequently, to preserve host cell integrity. Nonetheless, silencing suppression is only one of several possible nuclear functions of P6. Additional functions include splicing or export of viral transcripts, as well as recruitment of host factors required for virus transcription and translation.
Among the several biochemical properties of P6 tested in this study, nuclear import appears crucial for its transgenic VSR function. Notably, silencing suppression and chlorotic symptom expression could be readily uncoupled, illustrating that this feature alone cannot be reliably used in VSR identification. Similar observations were made with natural variants of the 2b protein from related cucumoviruses: these variants elicited symptoms of drastically different severity when expressed from recombinant PVX, yet they suppressed silencing to the same extent (Li et al, 1999). Incidentally, 2b nuclear import was also found mandatory for VSR function (Lucy et al, 2000), a feature somewhat difficult to reconcile with the recent demonstration that 2b inhibits AGO1-mediated slicing (Zhang et al, 2006), which is expected to occur in the cytoplasm where cucumoviral transcripts accumulate. Most likely, 2b and other VSRs have multiple targets in host cells, and the results presented here with P6 provide an example as to how inhibiting RNA silencing factors in the nucleus might contribute to the effects of some VSRs, including 2b.
So far, clear-cut genetic interactions between VSRs and host silencing components have been only established in the case of the TCV P38 and the CMV 2b proteins (reviewed in Ding and Voinnet, 2007). These experiments, which entail the rescue of VSR-deficient viruses in plants carrying specific lesions in RNA silencing components, are often challenging because many VSR proteins have additional functions that are essential for virus replication or movement (Ding and Voinnet, 2007). This caveat obviously applies to the P6 protein, which is indispensable for translational transactivation of the CaMV 35S RNA, required for replication (Kobayashi et al, 1998). Nonetheless, the present study illustrates an alternative approach taking advantage of the highly sensitive and discriminative SUC-SUL RNAi reporter system. The SS line has been already successfully used to decipher the impact of many RNA silencing mutations, including drb4, whose effects on RNAi had not been appreciated using other available silencing systems (Dunoyer et al, 2007). Similarly, the diagnostic gain in 24-nt-long SUL siRNAs and rescue of vein-centred chlorosis in SS-P6 lines strongly suggest that constitutive expression of P6 is molecularly equivalent to the genetic inactivation of DRB4, but not of DCL4, although additional interactions with other nuclear silencing components cannot yet be excluded. Importantly, the elevated levels of leader-derived siRNAs in the drb4 mutant (Figure 5C) indicates that DRB4 interacts with dsRNA produced during CaMV infection. This provides a molecular explanation as to why CaMV-derived siRNAs are in their majority 24 nt in size in WT infected plants (Figure 5C; Blevins et al, 2006; Moissiard and Voinnet, 2006), as this probably reflects inhibition of DRB4 by P6.
Neither the accumulation nor the electrophoretic mobility of DRB4 is modified by P6. However, a fraction of P6—presumably nuclear—co-immunoprecipitates with DRB4 in vivo, indicating that the P6–DRB4 interaction is not only genetic, but also physical. Although a direct interaction between the two proteins remains to be formally established, it is possible that its association with P6 promotes nucleo-cytoplasmic shuttling of DRB4, thereby compromising its nuclear RNA silencing function. Alternatively, P6–DRB4 nuclear interaction might induce conformational changes or post-translational protein modifications that prevent DRB4 function. Targeting of DRB4 by P6 may seem at odd considering that this protein has only an accessory function in viral siRNA biogenesis by DCL4. DCL4 is the major antiviral Dicer of Arabidopsis and is thus expected to be a preferred target of VSRs, as shown with TCV P38, for instance (Deleris et al, 2006). However, we have shown that several DCL4-dependent siRNAs derived from the CaMV 35S leader exhibit near-complete complementarity to dozens of host transcripts that are likely degraded during infection, possibly to the benefit of the virus (Moissiard and Voinnet, 2006). Decreasing rather than eliminating DCL4 activity through DRB4 inhibition may therefore constitute a compromise whereby 21 nt siRNA levels are sufficiently reduced to prevent antiviral defence, but high enough to target host gene expression. Alternatively, targeting of DRB4 by P6 could allow for high accumulation of 24 nt siRNAs that may be required for heterochromatin formation and transcriptional silencing of CaMV minichromosomes (Al-Kaff et al, 1998), potentially preventing virus overaccumulation and preserving host cell integrity.
Materials and methods
Construction of pGH and derivatives
Plasmid pMD324 (M Delseny, Perpignan, France) was PCR-amplified using oligonucleotides with KpnI or SacI sites. The mixture was incubated with DpnI for 2 h at 37°C. Upon 5′ end phosphorylation, PCR products were ligated, resulting in plasmid pGH. WT ORF VI or mutated versions thereof were PCR-amplified using primers with 5′ KpnI and SacI sites, respectively, digested and cloned into pGH. Point mutations and small deletions in ORF VI were introduced either in pCK-EGFP–P6 (Haas et al, 2005) for localization studies, or in pETK-ORFVI (Leh et al, 2000) for protein expression in E. coli.
Biolistic and fluorescence analysis
eGFP fusions of P6 and its variants were transiently expressed in BY-2 cells (Nicotiana tabacum cv Bright Yellow 2). Cells were subcultured weekly and harvested 3 days after medium renewal for biolistic transfection. Particle preparation and bombardment were as in Haas et al (2005). After bombardment, cells were transferred to 0.8% agar MS media, incubated in the dark at 28°C and collected under binoculars for confocal microscopy (Zeiss LSM510). eGFP and RFP imaging was by excitation at 488 and 568 nm, respectively.
Translational transactivation assays
pmonoCAT and pbiGUS were as described (Bonneville et al, 1989). PCR products for P6 and derivatives were mobilized under the CaMV 35S RNA promoter of pTAV (p35S-P6; Kobayashi et al, 1998). N. plumbaginifolia leaf protoplasts were prepared and protein extracts were analysed as described by Pooggin et al (2000).
Protein analyses
Protein extracts were prepared from inflorescences according to Hurkman and Tanaka (1986). Protein concentrations were determined using the Bio-Rad RCDC protein assay (standard: BSA). After SDS–PAGE separation and transfer to PVDF membranes (Immobilon-P; Millipore) proteins were stained by Coomassie blue and analysed for antibody reaction (Lumi-light plus ECL; Roche). CaMV P6 and P4 proteins were detected using polyclonal antisera at 1:15 000 dilution. DRB4 was detected using a polyclonal antibody at 1:10 000 dilution (Nakazawa et al, 2007).
Immunoprecipitation
Proteins were extracted from inflorescences in 3 ml/g fresh material of extraction buffer EB (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 5 mM MgCl2, glycerol 10%, complete inhibitors protease cocktail (Roche), MG132 10 μM (Sigma)). Insoluble material was separated (30 min, 12 000 g, 4°C) and lysate was precleaned by incubation with proteinA-agarose beads (Roche) at 4°C for 30 min. The supernatant was then incubated with anti-DRB4 serum (1:500 dilution) overnight at 4°C. After incubation with 25 μl of proteinA-agarose beads, immunoprecipitates were washed three times with EB, and eluted from beads with SDS loading buffer.
RNA preparation and hybridization
Total RNA was extracted using TRIzol (Invitrogen), precipitated with isopropanol and redissolved in 50% formamide. Northern analyses of LMW and HMW RNA were performed with 10 and 5 μg of total RNA, respectively (Deleris et al, 2006). Radiolabelled probes for detection of CaMV or SUL RNAs were made by [α-32P]dCTP random priming reactions, whereas DNA oligonucleotides complementary to miRNAs or tasiRNAs were end-labelled with [γ-32P]ATP (Deleris et al, 2006).
Expression and phosphorylation of recombinant proteins
GST and GST–Imp were expressed in E. coli BL21/DE3(pLys S; Novagen) from pGEX-2TK, and P6 from pETKaks (Leh et al, 2000). Protein expression was induced at an exponential phase with 1 mM IPTG for 3 h. Bacteria were pelleted (4000 g, 10 min), resuspended in HMK buffer (20 mM Tris–HCl pH 7·5, 100 mM NaCl, 12 mM MgCl2), and lysed by sonication (two pulses of 20 s, 50 W). After centrifugation (12 000 g, 10 min), the supernatant was frozen at −20°C. Protein aliquots were separated by SDS–PAGE and protein expression was controlled by Coomassie blue staining for GST and GST–Imp, and by western blot analysis for P6. P6 was end-labelled with [γ-32P]ATP using 20 U bovine heart muscle protein kinase (Sigma). Non-incorporated radioactive ATP was eliminated by Sephadex G-50 filtration (Amersham Pharmacia Biotech). The eluate (300 μl) was treated for 30 min with a mixture of RNase A (40 μg) and DNase (100 U) at 37°C. Degradation of nucleic acids was verified on 1.5% agarose and ethidium bromide staining.
In vitro GST pull down
GST or GST–Imp fusion proteins (1 μg) (Leh et al, 2000) was mixed with 50 μl glutathione–Sepharose 4B beads, previously washed and resuspended in PBS buffer, and incubated in PBS containing 1% Tween 20 for 1 h at 4°C with gentle shaking. After three washes, beads were mixed with 30 μl bacterial protein containing 32P-labelled P6. The mixture was adjusted to a final volume of 500 μl and incubated for 3 h at 4°C with gentle shaking. Beads were washed three times and resuspended in 20 μl dissociation buffer, boiled for 5 min and pelleted (10 000 g, 2 min). The supernatant was immediately resolved by SDS–PAGE and 32P-labelled P6 was detected by autoradiography.
Transgenic plants
Arabidopsis AMP and SUC-SUL (SS) lines were described previously (Dalmay et al, 2000; Dunoyer et al, 2007). The P6 ORF and derivatives were cloned into the pBin61 binary vector, mobilized into Agrobacterium strain GV3101 and transformed into WT Arabidopsis (ecotype Col-0) or AMP line using the floral dip method. Upon kanamycin selection, stable T2 lines were established from representative individuals showing comparable P6 expression levels. Single and combination silencing mutants, and SS-dcl4 and SS-drb4 were as described (Dunoyer et al, 2007). Plants were germinated under selective media in standard growth chamber for 3 weeks and subsequently transferred into soil under short-day conditions.
Infection assays
Infections of A. thaliana (Col-0) and turnip (Brassica rapa, Just Right) were carried out with SalI-linearized pMD324 or pGH-derived plasmids. Turnip (∼3 weeks old) and Arabidopsis (rosette leaves, ∼4–5 weeks old) plants were inoculated with 2 μg of viral DNA diluted in 20 μl of sterile water per plant, or with a sap extract from CaMV-infected turnip. Infected leaves were harvested, ground in liquid nitrogen and stored at −80°C.
Supplementary Material
Supplementary data 1
Supplementary data 2
Supplementary data 3
Supplementary data 4
Acknowledgments
We thank members of the Voinnet and Keller laboratories for helpful comments on the manuscript. Supported by grants from the French Ministry of Research (GH and GM), as well as grants ‘AKKROSS' from I'Agence Nationale pour la Recherche (JA) and ‘SIROCCO' LSHG-CT+2006-037900 from the European Union (OV). The Lilliane Bettencourt Fundation is also acknowledged for its financial support to OV's laboratory. Richard Wagner and his team are thanked for plant care.
References
- Adenot X, Elmayan T, Lauressergues D, Boutet S, Bouche N, Gasciolli V, Vaucheret H (2006) DRB4-dependent TAS3 trans-acting siRNAs control leaf morphology through AGO7. Curr Biol 16: 927–932 [DOI] [PubMed] [Google Scholar]
- Al-Kaff NS, Covey SN, Kreike MM, Page AM, Pinder R, Dale PJ (1998) Transcriptional and posttranscriptional plant gene silencing in response to a pathogen. Science 279: 2113–2115 [DOI] [PubMed] [Google Scholar]
- Baumberger N, Tsai CH, Lie M, Havecker E, Baulcombe DC (2007) The polerovirus silencing suppressor P0 targets ARGONAUTE proteins for degradation. Curr Biol 17: 1609–1614 [DOI] [PubMed] [Google Scholar]
- Blevins T, Rajeswaran R, Shivaprasad PV, Beknazariants D, Si-Ammour A, Park HS, Vazquez F, Robertson D, Meins F Jr, Hohn T, Pooggin MM (2006) Four plant Dicers mediate viral small RNA biogenesis and DNA virus induced silencing. Nucleic Acids Res 34: 6233–6246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneville JM, Sanfaçon H, Futterer J, Hohn T (1989) Posttranscriptional trans-activation in cauliflower mosaic virus. Cell 59: 1135–1143 [DOI] [PubMed] [Google Scholar]
- Bortolamiol D, Pazhouhandeh M, Marrocco K, Genschik P, Ziegler-Graff V (2007) The polerovirus F box protein P0 targets ARGONAUTE1 to suppress RNA silencing. Curr Biol 17: 1615–1621 [DOI] [PubMed] [Google Scholar]
- Bouche N, Lauressergues D, Gasciolli V, Vaucheret H (2006) An antagonistic function for Arabidopsis DCL2 in development and a new function for DCL4 in generating viral siRNAs. EMBO J 25: 3347–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen P, Voinnet O (2006) The diversity of RNA silencing pathways in plants. Trends Genet 22: 268–280 [DOI] [PubMed] [Google Scholar]
- Dalmay T, Hamilton AJ, Mueller E, Baulcombe DC (2000) Potato virus X amplicons in Arabidopsis mediate genetic and epigenetic gene silencing. Plant Cell 12: 369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleris A, Gallego-Bartolome J, Bao J, Kasschau KD, Carrington JC, Voinnet O (2006) Hierarchical action and inhibition of plant Dicer-like proteins in antiviral defense. Science 313: 68–71 [DOI] [PubMed] [Google Scholar]
- Ding SW, Voinnet O (2007) Antiviral immunity directed by small RNAs. Cell 130: 413–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunoyer P, Himber C, Ruiz-Ferrer V, Alioua A, Voinnet O (2007) Intra- and intercellular RNA interference in Arabidopsis thaliana requires components of the microRNA and heterochromatic silencing pathways. Nat Genet 39: 848–856 [DOI] [PubMed] [Google Scholar]
- Fusaro AF, Matthew L, Smith NA, Curtin SJ, Dedic-Hagan J, Ellacott GA, Watson JM, Wang MB, Brosnan C, Carroll BJ, Waterhouse PM (2006) RNA interference-inducing hairpin RNAs in plants act through the viral defence pathway. EMBO Rep 7: 1168–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M, Bureau M, Geldreich A, Yot P, Keller M (2002) Cauliflower mosaic virus: still in the news. Mol Plant Pathol 3: 419–429 [DOI] [PubMed] [Google Scholar]
- Haas M, Geldreich A, Bureau M, Dupuis L, Leh V, Vetter G, Kobayashi K, Hohn T, Ryabova L, Yot P, Keller M (2005) The open reading frame VI product of cauliflower mosaic virus is a nucleocytoplasmic protein: its N terminus mediates its nuclear export and formation of electron-dense viroplasms. Plant Cell 17: 927–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himber C, Dunoyer P, Moissiard G, Ritzenthaler C, Voinnet O (2003) Transitivity-dependent and -independent cell-to-cell movement of RNA silencing. EMBO J 22: 4523–4533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurkman WJ, Tanaka CK (1986) Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol 81: 802–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Tsuge S, Nakayashiki H, Mise K, Furusawa I (1998) Requirement of cauliflower mosaic virus open reading frame VI product for viral gene expression and multiplication in turnip protoplasts. Microbiol Immunol 42: 377–386 [DOI] [PubMed] [Google Scholar]
- Leh V, Yot P, Keller M (2000) The cauliflower mosaic virus translational transactivator interacts with the 60S ribosomal subunit protein L18 of Arabidopsis thaliana. J Virol 266: 1–7 [DOI] [PubMed] [Google Scholar]
- Li HW, Lucy AP, Guo HS, Li WX, Ji LH, Wong SM, Ding SW (1999) Strong host resistance targeted against a viral suppressor of the plant gene silencing defence mechanism. EMBO J 18: 2683–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucy AP, Guo HS, Li WX, Ding SW (2000) Suppression of post-transcriptional gene silencing by a plant viral protein localized in the nucleus. EMBO J 19: 1672–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Chen Z (2007) Improperly terminated, unpolyadenylated mRNA of sense transgenes is targeted by RDR6-mediated RNA silencing in Arabidopsis. Plant Cell 19: 943–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love AJ, Laird J, Holt J, Hamilton AJ, Sadanandom A, Milner JJ (2007) Cauliflower mosaic virus protein P6 is a suppressor of RNA silencing. J Gen Virol 88: 3439–3444 [DOI] [PubMed] [Google Scholar]
- Moissiard G, Parizotto EA, Himber C, Voinnet O (2007) Transitivity in Arabidopsis can be primed, requires the redundant action of the antiviral Dicer-like 4 and Dicer-like 2, and is compromised by viral-encoded suppressor proteins. RNA 13: 1268–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moissiard G, Voinnet O (2006) RNA silencing of host transcripts by cauliflower mosaic virus requires coordinated action of the four Arabidopsis Dicer-like proteins. Proc Natl Acad Sci USA 103: 19593–19598 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nakazawa Y, Hiraguri A, Moriyama H, Fukuhara T (2007) The dsRNA-binding protein DRB4 interacts with the Dicer-like protein DCL4 in vivo and functions in the trans-acting siRNA pathway. Plant Mol Biol 63: 777–785 [DOI] [PubMed] [Google Scholar]
- Pooggin MM, Hohn T, Futterer J (2000) Role of a short open reading frame in ribosome shunt on the cauliflower mosaic virus RNA leader. J Biol Chem 275: 17288–17296 [DOI] [PubMed] [Google Scholar]
- Ryabova LA, Hohn T (2000) Ribosome shunting in the cauliflower mosaic virus 35S RNA leader is a special case of reinitiation of translation functioning in plant and animal systems. Genes Dev 14: 817–829 [PMC free article] [PubMed] [Google Scholar]
- Smith HM, Raikhel NV (1998) Nuclear localization signal receptor importin alpha associates with the cytoskeleton. Plant Cell 10: 1791–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu-Nu C, Lin CH, Lin A (2000) An acidic amino acid cluster regulates the nucleolar localization and ribosome assembly of human ribosomal protein L22. FEBS Lett 484: 22–28 [DOI] [PubMed] [Google Scholar]
- Vargason JM, Szittya G, Burgyan J, Tanaka Hall TM (2003) Size selective recognition of siRNA by an RNA silencing suppressor. Cell 115: 799–811 [DOI] [PubMed] [Google Scholar]
- Wang P, Palese P, O'Neill RE (1997) The NPI-1/NPI-3 (karyopherin alpha) binding site on the influenza a virus nucleoprotein NP is a nonconventional nuclear localization signal. J Virol 71: 1850–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff T, Unterstab G, Heins G, Richt JA, Kann M (2002) Characterization of an unusual importin alpha binding motif in the borna disease virus p10 protein that directs nuclear import. J Biol Chem 277: 12151–12157 [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Peragine A, Park MY, Poethig RS (2005) A pathway for the biogenesis of trans-acting siRNAs in Arabidopsis. Genes Dev 19: 2164–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yuan YR, Pei Y, Lin SS, Tuschl T, Patel DJ, Chua NH (2006) Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev 20: 3255–3268 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data 1
Supplementary data 2
Supplementary data 3
Supplementary data 4