Abstract
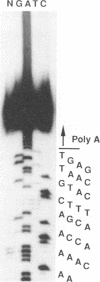
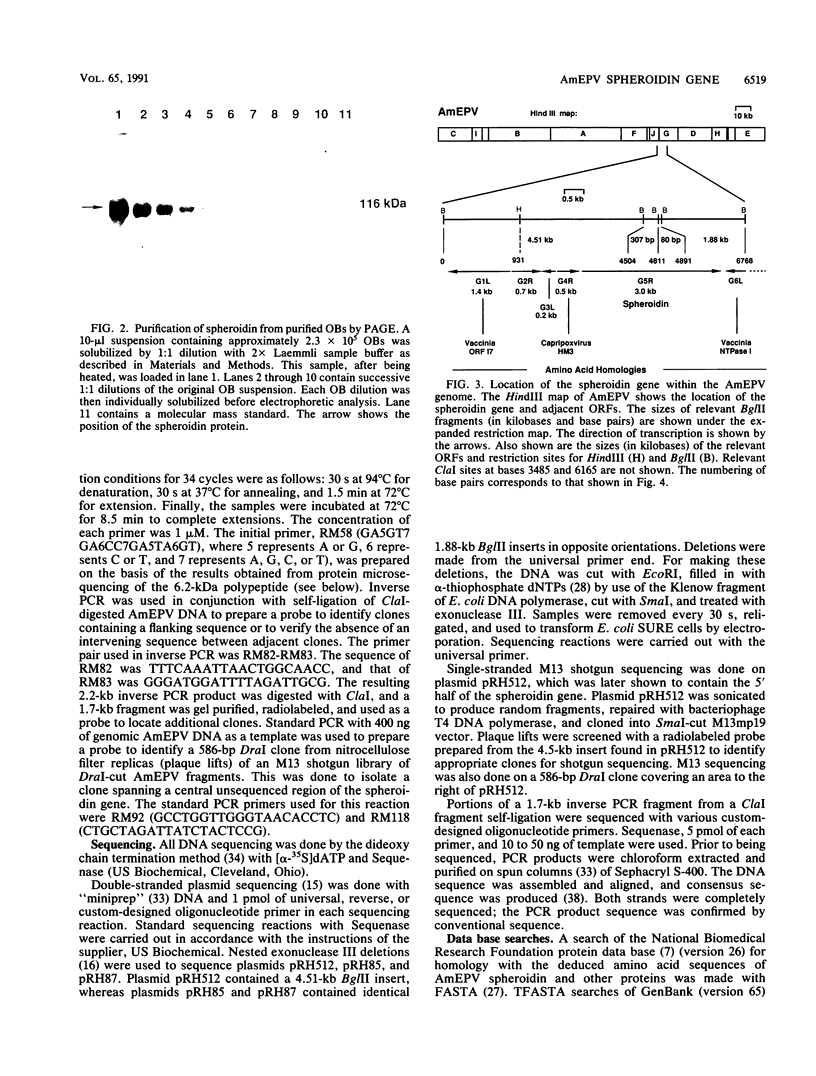
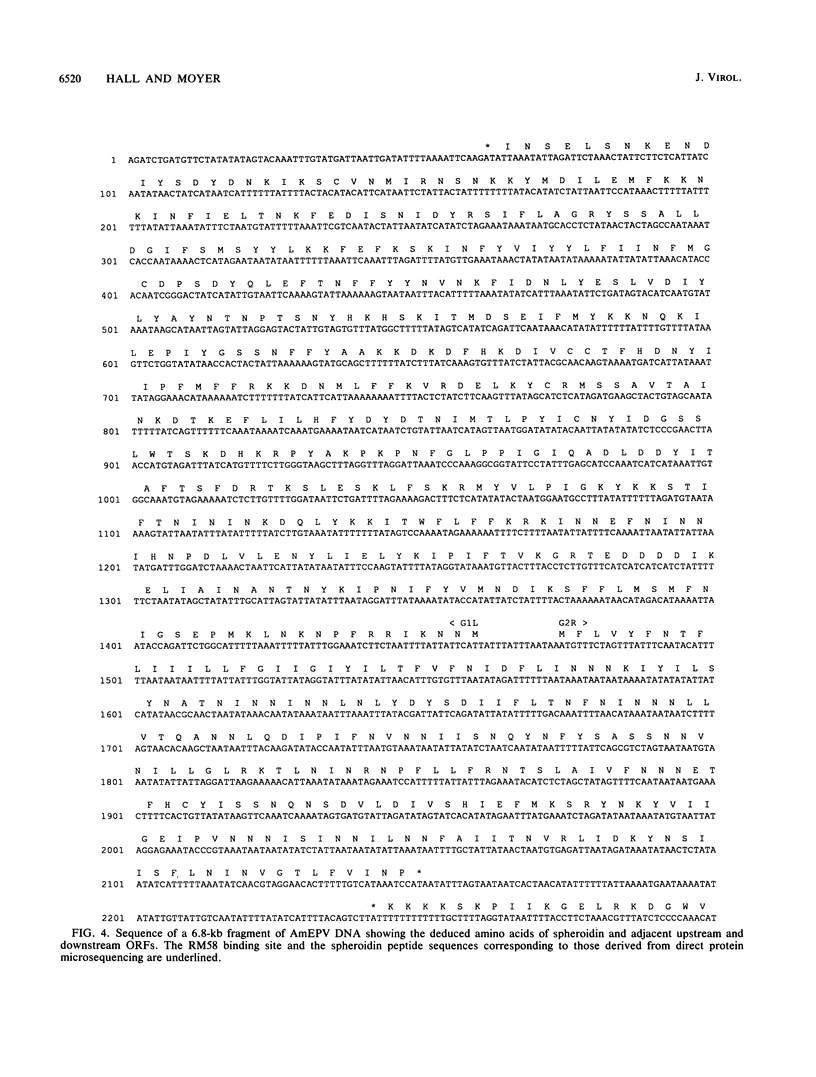
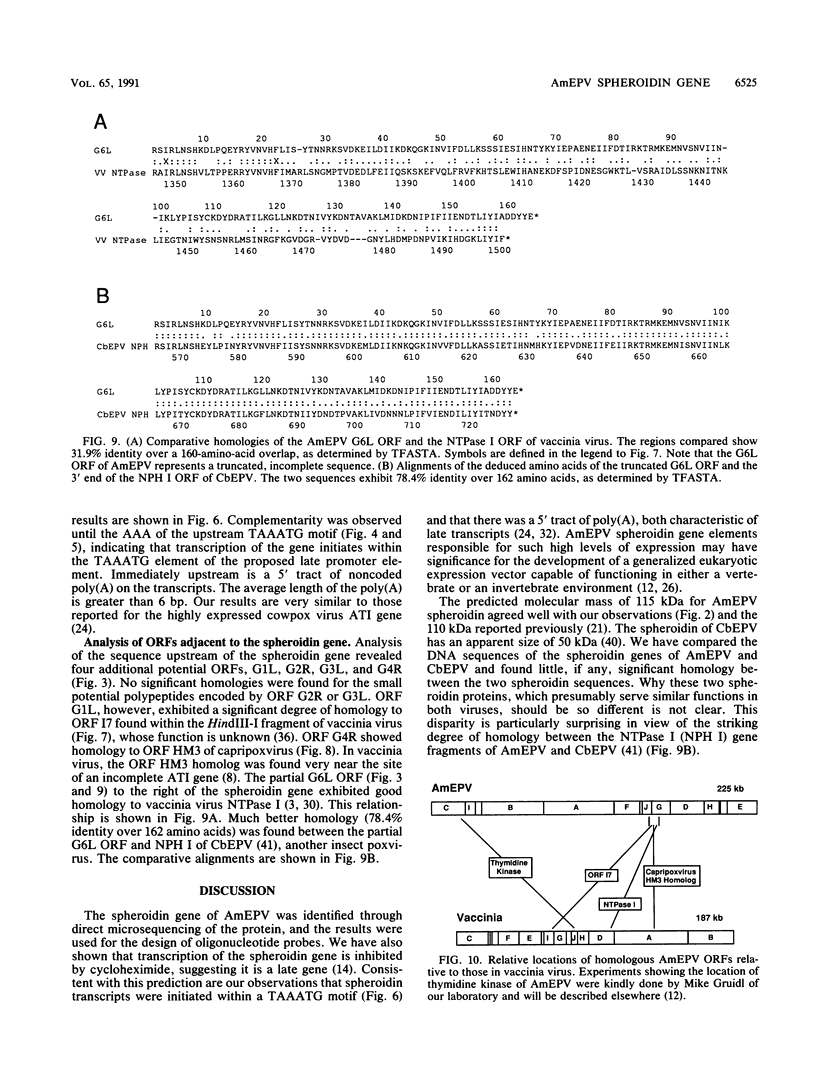
Entomopoxvirus virions are frequently contained within crystalline occlusion bodies, which are composed of primarily a single protein, spheroidin, which is analogous to the polyhedrin protein of baculovirus. The spheroidin gene of Amsacta moorei entomopoxvirus was identified following the microsequencing of polypeptides generated from cyanogen bromide treatment of spheroidin and the subsequent synthesis of oligonucleotide hybridization probes. DNA sequencing of a 6.8-kb region of DNA containing the spheroidin gene showed that the spheroidin protein is derived from a 3.0-kb open reading frame potentially encoding a protein of 115 kDa. Three copies of the heptanucleotide, TTTTTNT, a sequence associated with early gene transcription in the vertebrate poxviruses, and four in-frame translational termination signals were found within 60 bp upstream of the putative spheroidin gene promoter (TAAATG). The spheroidin gene promoter region contains the sequence TAAATG, which is found in many late promoters of the vertebrate poxviruses and which serves as the site of transcriptional initiation, as shown by primer extension. Primer extension experiments also showed that spheroidin gene transcripts contain 5' poly(A) sequences typical of vertebrate poxvirus late transcripts. The 92 bases upstream of the initiating TAAATG are unusually A + T rich and contain only 7 G or C residues. An analysis of open reading frames around the spheroidin gene suggests that the colinear core of "essential genes" typical of the vertebrate poxviruses is absent in A. moorei entomopoxvirus.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allington W. B., Cordry A. L., McCullough G. A., Mitchell D. E., Nelson J. W. Electrophoretic concentration of macromolecules. Anal Biochem. 1978 Mar;85(1):188–196. doi: 10.1016/0003-2697(78)90289-0. [DOI] [PubMed] [Google Scholar]
- Bilimoria S. L., Arif B. M. Subunit protein and alkaline protease of entomopoxvirus spheroids. Virology. 1979 Jul 30;96(2):596–603. doi: 10.1016/0042-6822(79)90115-6. [DOI] [PubMed] [Google Scholar]
- Broyles S. S., Moss B. Identification of the vaccinia virus gene encoding nucleoside triphosphate phosphohydrolase I, a DNA-dependent ATPase. J Virol. 1987 May;61(5):1738–1742. doi: 10.1128/jvi.61.5.1738-1742.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Funahashi S., Sato T., Shida H. Cloning and characterization of the gene encoding the major protein of the A-type inclusion body of cowpox virus. J Gen Virol. 1988 Jan;69(Pt 1):35–47. doi: 10.1099/0022-1317-69-1-35. [DOI] [PubMed] [Google Scholar]
- George D. G., Barker W. C., Hunt L. T. The protein identification resource (PIR). Nucleic Acids Res. 1986 Jan 10;14(1):11–15. doi: 10.1093/nar/14.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon P. D., Ansell D. M., Black D. N. A comparison of the genome organization of capripoxvirus with that of the orthopoxviruses. J Virol. 1989 Nov;63(11):4703–4708. doi: 10.1128/jvi.63.11.4703-4708.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Hall R. L., Hink W. F. Physical mapping and field inversion gel electrophoresis of Amsacta moorei entomopoxvirus DNA. Arch Virol. 1990;110(1-2):77–90. doi: 10.1007/BF01310704. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Hukuhara T., Xu J. H., Yano K. Replication of an entomopoxvirus in two lepidopteran cell lines. J Invertebr Pathol. 1990 Sep;56(2):222–232. doi: 10.1016/0022-2011(90)90104-e. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langridge W. H., Bozarth R. F., Roberts D. W. The base composition of entomopoxvirus DNA. Virology. 1977 Feb;76(2):616–620. doi: 10.1016/0042-6822(77)90243-4. [DOI] [PubMed] [Google Scholar]
- Miller L. K. Baculoviruses as gene expression vectors. Annu Rev Microbiol. 1988;42:177–199. doi: 10.1146/annurev.mi.42.100188.001141. [DOI] [PubMed] [Google Scholar]
- Patel D. D., Pickup D. J., Joklik W. K. Isolation of cowpox virus A-type inclusions and characterization of their major protein component. Virology. 1986 Mar;149(2):174–189. doi: 10.1016/0042-6822(86)90119-4. [DOI] [PubMed] [Google Scholar]
- Patel D. D., Pickup D. J. Messenger RNAs of a strongly-expressed late gene of cowpox virus contain 5'-terminal poly(A) sequences. EMBO J. 1987 Dec 1;6(12):3787–3794. doi: 10.1002/j.1460-2075.1987.tb02714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson A., Richardson C., Yuen L. The 5' noncoding region sequence of the Choristoneura biennis entomopoxvirus spheroidin gene functions as an efficient late promoter in the mammalian vaccinia expression system. Virology. 1991 Feb;180(2):561–566. doi: 10.1016/0042-6822(91)90070-r. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney S. D., Benkovic S. J., Schimmel P. R. A DNA fragment with an alpha-phosphorothioate nucleotide at one end is asymmetrically blocked from digestion by exonuclease III and can be replicated in vivo. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7350–7354. doi: 10.1073/pnas.78.12.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Kahn J. S., Esteban M. Molecular cloning, encoding sequence, and expression of vaccinia virus nucleic acid-dependent nucleoside triphosphatase gene. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9566–9570. doi: 10.1073/pnas.83.24.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrmann G. F. Polyhedrin structure. J Gen Virol. 1986 Aug;67(Pt 8):1499–1513. doi: 10.1099/0022-1317-67-8-1499. [DOI] [PubMed] [Google Scholar]
- Rosel J. L., Earl P. L., Weir J. P., Moss B. Conserved TAAATG sequence at the transcriptional and translational initiation sites of vaccinia virus late genes deduced by structural and functional analysis of the HindIII H genome fragment. J Virol. 1986 Nov;60(2):436–449. doi: 10.1128/jvi.60.2.436-449.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt J. F., Stunnenberg H. G. Sequence and transcriptional analysis of the vaccinia virus HindIII I fragment. J Virol. 1988 Jun;62(6):1889–1897. doi: 10.1128/jvi.62.6.1889-1897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Lawrance S. K., Gillespie G. A., Cantor C. R., Weissman S. M., Collins F. S. Strategies for mapping and cloning macroregions of mammalian genomes. Methods Enzymol. 1987;151:461–489. doi: 10.1016/s0076-6879(87)51038-2. [DOI] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialard J. E., Yuen L., Richardson C. D. Identification and characterization of a baculovirus occlusion body glycoprotein which resembles spheroidin, an entomopoxvirus protein. J Virol. 1990 Dec;64(12):5804–5811. doi: 10.1128/jvi.64.12.5804-5811.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen L., Dionne J., Arif B., Richardson C. Identification and sequencing of the spheroidin gene of Choristoneura biennis entomopoxvirus. Virology. 1990 Apr;175(2):427–433. doi: 10.1016/0042-6822(90)90427-s. [DOI] [PubMed] [Google Scholar]
- Yuen L., Noiseux M., Gomes M. DNA sequence of the nucleoside triphosphate phosphohydrolase I (NPH I) of the Choristoneura biennis entomopoxvirus. Virology. 1991 May;182(1):403–406. doi: 10.1016/0042-6822(91)90690-d. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]