Abstract
During clathrin-mediated endocytosis, adaptor proteins recognize specific internalization signals on cargo receptors, either recruiting cargos into clathrin-coated pits (CCPs) or initiating clathrin-coat assembly around the cargo molecules. Here, we identify epsin 1, a clathrin-, ubiquitin-, and phospholipid-interacting protein, as a cargo-specific adaptor for influenza virus entry through the clathrin-mediated pathway. Using live-cell imaging to monitor the entry of individual virus particles, we observed recruitment of epsin 1 to the binding sites of influenza viruses in synchrony with the assembly of CCPs. Epsin 1 knockdown by siRNA significantly inhibited the clathrin-mediated endocytosis of the influenza virus and caused the majority of the virus particles to enter through a clathrin-independent pathway. The same treatment did not affect the entry of several classical ligands for clathrin-mediated endocytosis, including transferrin, LDL, and EGF. Overexpression of the dominant-negative epsin 1 mutant lacking the ubiquitin-interaction motifs nearly completely blocked the clathrin-mediated entry of the influenza virus without affecting transferrin uptake. These results suggest that epsin 1 functions as a cargo-specific adaptor for the clathrin-mediated entry of the influenza virus.
Adaptor proteins are key components of the clathrin-mediated endocytic machinery, bringing cargo molecules to the clathrin coat (1–4). During endocytosis, adaptors function to recognize cargo receptors selectively and to stimulate clathrin assembly. Among the known adaptor proteins, the AP-2 adaptor complex takes center stage because of its abundance in clathrin-coated pits (CCPs) and its ability to interact with many protein and lipid factors involved in clathrin-mediated endocytosis. However, increasing evidence has shown that not all cargo molecules are internalized through an interaction with AP-2 (3, 4). Both LDL and EGF are among the known examples of AP-2-independent cargos involved in clathrin-mediated endocytosis (5, 6). Various cargo-specific adaptor proteins have been proposed to function as alternative adaptors for clathrin-mediated endocytosis, but the exact adaptor proteins used by most of the AP-2-independent cargos are still unknown (3, 4).
We have recently shown that the clathrin-mediated endocytosis of the influenza virus is also independent of AP-2 (7). Influenza exploits multiple endocytic pathways for infection, and the majority of the virus particles enter cells through CCPs (8–10). AP-2 knockdown by siRNA does not inhibit the clathrin-mediated uptake of influenza (7), leaving open an important question for influenza infection: Which protein serves as the endocytic adaptor for influenza viral entry?
The ability of epsin to interact with multiple components of CCPs makes it a potential adaptor protein (11). Among the epsin family genes, epsin 1 and epsin 2 are more ubiquitously expressed, whereas epsin 3 is specifically expressed in keratinocytes induced by type I collagen (12). The C-terminal domain of epsin harbors several specific sequence motifs that bind to clathrin, AP-2 and Eps15 (13, 14). The N-terminal ENTH domain binds to PtdIns(4,5)P2 and induces membrane curvature (15, 16). Epsin also contains a few ubiquitin-interaction motifs (UIMs) (17, 18) that interact with polyubiquitins and may capture ubiquitinated cargo receptors for internalization (19, 20). Overexpression of epsin 1 fragments or mutants has been shown to inhibit internalization of classical clathrin-dependent endocytic ligands, such as transferrin and EGF (13, 15, 16), suggesting a possible role of epsin 1 in clathrin-mediated endocytosis. However, because these fragments and mutants retain their ability to interact with core components of CCPs, the caveat that they can deplete CCP components and thereby prevent normal clathrin-coat assembly leaves the verdict open for the requirement of epsin in clathrin-mediated endocytosis. The function of epsin is further mystified by two recent observations; that colocalization of epsin 1 with membrane-bound ubiquitin or clathrin appears to be mutually exclusive (21), and that epsin 1 is involved in the caveolin-mediated, instead of clathrin-dependent, internalization of EGF receptors (22). In this work, we investigate the role(s) of epsin in influenza viral entry and, more generally, in clathrin-mediated endocytosis.
Results
Imaging Epsin 1 in Live Cells.
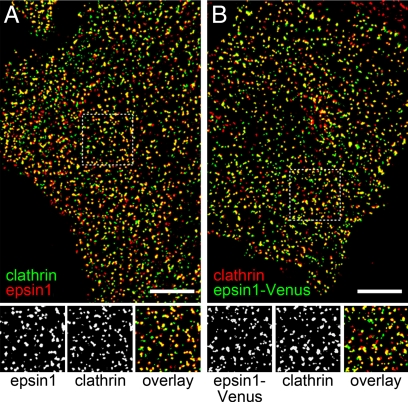
To detect the intracellular distribution of epsin 1, we imaged endogenous epsin 1 and clathrin in BS-C-1 cells by using immunofluorescence. Epsin 1 appeared as punctate structures and colocalized with clathrin extensively (Fig. 1A): 86 ± 3% of the epsin 1-stained structures showed a clathrin signal, and 75 ± 4% of the clathrin-stained structures displayed an epsin 1 signal, consistent with the observed colocalization between epsin 1 and clathrin-coated structures (13, 14). To facilitate live-cell imaging of epsin 1, we constructed a fusion protein containing epsin 1 and Venus, a variant of the enhanced yellow fluorescent protein (EYFP) (23), and transfected BS-C-1 cells with epsin 1-Venus. Epsin 1-Venus also displayed discrete structures and colocalized with endogenous clathrin to a similar extent (Fig. 1B): 86 ± 5% of the epsin 1-Venus structures colocalized with immunostained clathrin structures, and 83 ± 4% of the clathrin structures exhibited an epsin 1-Venus signal. These observations suggest that the fusion construct of epsin 1-Venus was targeted to the correct cellular locations and revealed nearly all epsin 1-containing structures.
Fig. 1.
Intracellular distribution of epsin 1. (A Upper) Immunofluorescence image of endogenous epsin 1 (red) and endogenous clathrin (green). (Lower) Magnified view of the boxed region. Shown are the epsin 1(Left), clathrin (Center), and overlaid (Right) images. (B Upper) Overlay of the Venus fluorescence image of epsin 1-Venus (green) and the immunofluorescence image of endogenous clathrin (red). The cell was transiently transfected with epsin 1-Venus. (Lower) Magnified view of the boxed region. Shown are the epsin 1-Venus (Left), clathrin (Center), and overlaid (Right) images. (Scale bars,10 μm.)
Influenza Virus Colocalizes with Epsin 1 during Clathrin-Mediated Endocytosis.
The influenza virus enters cells through multiple endocytic pathways (8–10). About 60% of the virus particles enter by clathrin-mediated endocytosis, and the remaining 40% enter through a clathrin- and caveolin-independent pathway (10). Upon internalization, the virus releases its genome into the cytoplasm via pH-dependent membrane fusion of the viral envelope with the acidic endosome (7, 24). To probe the involvement of epsin 1 in influenza viral entry, we tracked individual virus particles in live cells expressing epsin 1-Venus. For virus imaging, the virus was labeled with a red lipophilic dye, DiD, which did not perturb influenza infectivity (25) and was added to cells in situ at 37 °C. Upon binding and internalization, the virus particles exhibited rapid and directed movements in a microtubule-dependent manner [supporting information (SI) Movie S1]. Pretreatment of cells with nocodazole, a microtubule-depolymerizing drug, abolished these movements. Eventually the virus particles fused with the endosomal membrane, as indicated by a strong increase in the DiD signal because of the spreading of DiD molecules into the larger endosomal membrane and the resulting dequenching of DiD fluorescence (Movie S1) (25). As a control, dequenching of DiD fluorescence was not observed in cells treated with ammonium chloride, a lipophilic base known to raise endosomal pH and inhibit pH-dependent viral fusion (25).
In the following experiments, we used rapid microtubule-dependent movement and viral fusion as the criteria to indicate that viral entry occurred successfully. Viral fusion is a critical step in the influenza infection pathway, allowing the genetic material of the virus to be released into the cell. It has been shown that influenza infection is inhibited when endosomal pH is raised to prevent fusion (8, 24), and that isolated genetic materials of influenza are capable of inducing infection when microinjected into the cytosol (26, 27). Thus, a criterion that involves viral fusion likely represents infectious entry. When virus particles displaying rapid microtubule-dependent movement and viral fusion were identified, we then traced the virus particles back to analyze the colocalization of the virus particles with epsin 1-Venus before their entry. Quantitative analysis showed that 56 ± 6% of the internalized virus particles colocalized with epsin 1-Venus before entry, whereas the remaining 44 ± 6% entered cells without overlapping with epsin 1-Venus (n = 71; Fig. S1). The ratio is similar to the determined partition of influenza virus particles between the clathrin-mediated and clathrin-independent endocytic pathways (10), leading to a hypothesis that the virus particles internalized through clathrin-mediated endocytosis also colocalized with epsin 1.
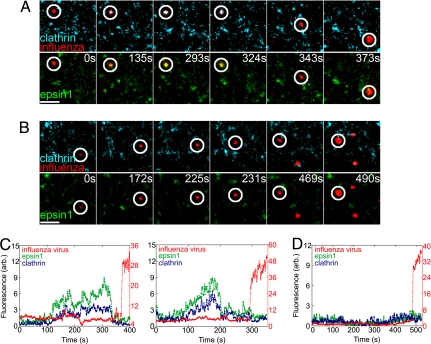
To test this hypothesis, we performed three-color imaging experiments in which the virus particles were tracked in cells that coexpressed epsin 1-Venus and clathrin ECFP, the fusion protein of clathrin light chain and the enhanced cyan fluorescent protein (ECFP). It has been shown that the clathrin fusion protein functions as normally as its endogenous counterpart (10, 28), and that the fluorescent clathrin signal reveals >96% of the clathrin-coated structures (10). In cells coexpressing epsin 1-Venus and clathrin-ECFP, extensive colocalization was observed between the two fluorescent proteins (Fig. S2A). Two classes of influenza entry behaviors were observed in these cells. Among the virus particles that successfully entered cells (n = 73), 52 ± 6% appeared to recruit both epsin 1 and clathrin to the virus-binding sites (Fig. 2 A and C and Movie S2), whereas the remaining 48 ± 6% entered cells without colocalizing with either clathrin or epsin 1 (Fig. 2 B and D and Movie S3). Interestingly, the recruitment of epsin 1 and clathrin always appeared to occur simultaneously within the time resolution (≈1 s) of our experiments (Fig. 2C). The fluorescence intensities of epsin 1 and clathrin then increased in a synchronized manner before coat disassembly occurred, suggesting a potential cooperative recruitment of the two proteins. These observations suggest the participation of epsin 1 in the clathrin-mediated endocytosis of the influenza virus.
Fig. 2.
Influenza colocalized with epsin 1 during clathrin-mediated endocytosis. (A) Snapshots of a virus (red, circled) internalized through a CCP, colocalizing with both epsin 1 (green) and clathrin (cyan), and fused with an endosome. (Top) Clathrin and the virus channels. (Bottom) Epsin 1 and the virus channels of the three-color movie. Time indicates how long the virus was bound to the cell. (B) Snapshots of a virus (red, circled) that was internalized without colocalizing with either epsin 1 (green) or clathrin (cyan) and fused with an endosome. (Scale bars, 2.5 μm) (C) Fluorescence time traces of the DiD (red), epsin 1-Venus (green), and clathrin-ECFP (blue) signals associated with two virus particles internalized through clathrin-mediated endocytosis. (Left) Corresponds with the virus shown in A. (Right) Fluorescence time traces of a different virus, of which the snapshots are not shown. (D) Fluorescence time traces of the DiD, epsin 1-Venus, and clathrin-ECFP signals associated with a virus particle internalized through clathrin-independent mechanism, the snapshots of which are shown in B.
Epsin 1 Is Required for the Clathrin-Mediated Endocytosis of Influenza.
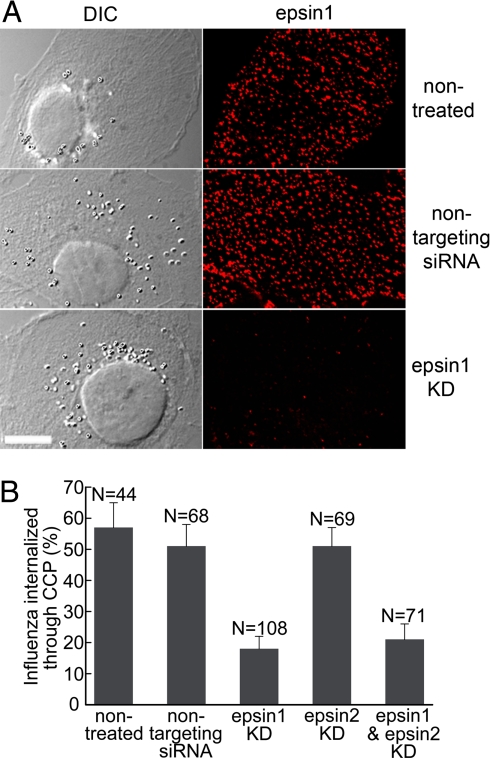
Epsin 1 has a multidomain structure that interacts with several components of CCPs (11). To test whether epsin 1 plays a critical role in the clathrin-mediated endocytosis of the influenza virus or simply appears in the CCPs enclosing virus particles by a “piggyback” mechanism, we knocked down epsin 1 in cells by using siRNA. The knockdown efficiency was assessed by immunofluorescence and Western blot. In the immunofluorescence image of the siRNA knockdown cells, the number of epsin 1-stained spots and the total epsin 1 fluorescence were reduced by 84% and 87%, respectively, compared with those in the nontreated or nontargeting siRNA treated cells (Fig. 3A). A dramatic reduction in the expression of epsin 1 was also observed by Western blot analyses (Fig. S3A).
Fig. 3.
Epsin 1 knockdown inhibited the clathrin-mediated endocytosis of the influenza virus. (A) Efficient epsin 1 knockdown by siRNA. (Left) Differential interference contrast images of the cells. (Right) Corresponding immunofluorescence images of endogenous epsin 1. Shown are nontreated cells (Top), nontargeting siRNA-treated cells (Middle), and epsin 1 siRNA treated cells (Bottom). (Scale bar, 10 μm.) (B) The fraction of successfully internalized influenza virus particles that entered through clathrin-mediated endocytosis in nontreated, nontargeting siRNA treated, epsin 1 knockdown, epsin 2 knockdown, and epsin 1 and epsin 2 double knockdown cells.
We then tested the entry mechanism of the influenza virus by tracking individual virus particles in the epsin 1 knockdown cells expressing clathrin-EYFP. Among the virus particles that successfully entered cells (n = 108), only 18 ± 4% were internalized through CCPs, whereas the great majority (82 ± 4%) of the virus particles were internalized without colocalization with any clathrin-coated structures. This partition ratio was in contrast to those observed in nontreated cells and nontargeting siRNA treated cells, where the majority of the internalized virus particles entered through CCPs (Fig. 3B), suggesting that epsin 1 was important for the clathrin-mediated endocytosis of the influenza virus.
The total percentage of the virus particles that entered and fused per cell, however, did not change, and the infectivity of the virus was also uninhibited by epsin 1 knockdown (Fig. S4), suggesting that the virus did not immediately commit to one of the two pathways upon binding. Thus, when the clathrin-dependent, entry pathway was inhibited in the absence of epsin 1, most of the virus particles were routed to the clathrin-independent entry pathway. This property would have made it difficult to dissect the role of specific proteins in each individual entry pathway in a conventional biochemical assay demonstrating the power of the single-particle tracking approach.
Interestingly, despite the structural similarity to epsin 1 (29), epsin 2 knockdown did not have a similar effect on influenza viral entry. A nearly identical fraction of virus particles entered through the clathrin pathway in epsin 2 knockdown cells as in control cells (Fig. 3B), despite efficient epsin 2 knockdown (Fig. S3B). Consistent with this result, the double knockdown of both epsin 1 and epsin 2 showed a similar effect as epsin 1 single knockdown (Fig. 3B and Fig. S3C).
The UIM Motifs of Epsin 1 Are Critical for the Clathrin-Mediated Endocytosis of Influenza.
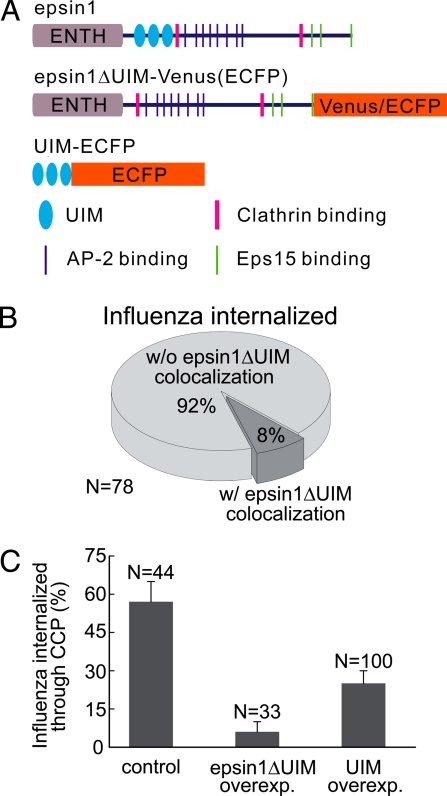
Epsin 1 has several UIMs that interact with polyubiquitin chains and ubiquitinated receptors (Fig. 4A), potentially allowing epsin 1 to capture endocytic cargo receptors by using this mechanism (17, 18, 20, 30). To test whether the UIMs play a role in the clathrin-mediated entry of influenza, we constructed an epsin 1 mutant that lacked the UIM domains (epsin1ΔUIM), fused the mutant construct to either Venus or ECFP, and transfected the cells with the fluorescent-protein-tagged epsin1ΔUIM (Fig. 4A). At low expression levels, epsin1ΔUIM-Venus appeared as discrete spots that colocalized extensively with endogenous clathrin (Fig. S2B), indicating that epsin1ΔUIM was still able to interact with CCPs efficiently. However, when we tracked influenza virus particles that successfully entered cells expressing epsin1ΔUIM-Venus, only 8 ± 3% of the virus particles colocalized with epsin1ΔUIM before their entry, whereas the remaining 92 ± 3% did not show any colocalization with epsin1ΔUIM before internalization (n = 78; Fig. 4B). This is in contrast to the major fraction of virus particles (56 ± 6%) observed to colocalize with epsin 1 before their entry in epsin 1-Venus expressing cells, suggesting that the removal of the UIMs significantly reduced the interaction between epsin 1 and influenza virus receptors.
Fig. 4.
Overexpression of epsin1ΔUIM or tandem UIM motifs inhibited the clathrin-mediated endocytosis of the influenza virus. (A) Schematic illustration of wild-type epsin 1, the epsin 1 mutant lacking the UIM motifs (epsin1ΔUIM) fused with Venus or ECFP, and the fragment that contains only the three tandem UIM motifs fused with ECFP. (B) The UIMs of epsin 1 is critical for its recruitment to the virus binding sites. In cells expressing epsin1ΔUIM-Venus, most of the virus particles did not show colocalization with epsin1ΔUIM-Venus before their internalization. (C) The fraction of influenza virus particles that entered through clathrin-mediated endocytosis in cells overexpressing epsin1ΔUIM or UIM. Here, cells were cotransfected with clathrin-EYFP and one of the two proteins: epsin1ΔUIM-ECFP or UIM-ECFP. Cells that showed overexpression of epsin1ΔUIM-ECFP or UIM-ECFP were chosen for analysis, and the results were compared with that observed in control cells not expressing epsin1ΔUIM-ECFP or UIM-ECFP.
Next, we overexpressed epsin1ΔUIM-ECFP in clathrin-EYFP-expressing cells. Overexpression of epsin1ΔUIM-ECFP was indicated by a strong and diffuse ECFP signal. The density of clathrin-coated structures in epsin1ΔUIM-ECFP overexpressing cells was similar to that observed in cells not expressing epsin1ΔUIM-ECFP (Fig. S5A). Moreover, when transferrin was added to these cells, 88% of the transferrin colocalized with clathrin-EYFP-labeled structures, and 70% of the clathrin-EYFP structures were labeled with transferrin (Fig. S5B). These results suggest that the overexpression of epsin1ΔUIM-ECFP did not perturb the overall distribution of CCPs, and that nearly all CCPs were labeled with clathrin-EYFP. However, when we tracked influenza virus particles in these cells, only 6 ± 4% of the successfully internalized virus particles entered through CCPs, whereas the overwhelming majority (94 ± 4%) took the nonclathrin pathway (n = 33). This is in stark contrast to the cells not expressing epsin1ΔUIM, where 57% of the internalized virus particles (n = 44) entered through clathrin-mediated endocytosis (Fig. 4C). These results indicate that overexpression of epsin1ΔUIM severely inhibit the clathrin-mediated entry of the influenza virus.
We also tested whether the tandem UIM motifs themselves can serve as a dominant-negative mutant by constructing a mutant protein containing only the three UIMs fused with ECFP (UIM-ECFP) (Fig. 4A). In cells overexpressing UIM-ECFP, a significant reduction was also observed in the fraction of the virus particles taking the clathrin pathway, although the reduction was not as strong as that induced by the epsin1ΔUIM dominant-negative mutant (Fig. 4C). A plausible explanation for the difference is that epsin1ΔUIM, with all of the CCP-interacting motifs of epsin 1 retained, can efficiently compete with the endogenous epsin 1 for clathrin recruitment but does not interact with influenza receptors, thus potently depleting clathrin from being available for the influenza receptors. However, the UIM motifs likely do not bind influenza receptors as well as full-length epsin 1, considering that the lipid-, clathrin-, AP-2-, and Eps15-interacting domains of epsin 1 may effectively increase the avidity between epsin 1 and influenza receptors and thus have a weaker inhibitory effect.
The above results indicate a critical role for the UIM motifs of epsin 1 in clathrin-mediated influenza viral entry. To test whether this effect was specific to epsin 1, we sought to probe the role of other UIM-containing CCP components, such as Eps15 and Eps15R (31), in influenza uptake. Efficient double knockdown of both Eps15 and Eps15R was achieved with siRNAs (Fig. S6A). In contrast to epsin 1 knockdown, the fraction of the virus particles entering through the clathrin pathway was not significantly affected by the Eps15 and Eps15R double knockdown (Fig. S6B).
Epsin 1 Is Not Required for the Clathrin-Mediated Endocytosis of Transferrin, EGF, and LDL.
We noticed that the overexpression of epsin1ΔUIM did not perturb the cellular distribution of clathrin (Fig. S5 A and B). Moreover, in cells overexpressing epsin1ΔUIM-ECFP, transferrin was internalized to the same extent as in control cells (Fig. S5 C and D), suggesting that the dominant-negative effect of epsin1ΔUIM is cargo-specific. One possible explanation of the different effect of epsin1ΔUIM on the uptake of transferrin versus influenza is that the clathrin-binding affinity of epsin1ΔUIM, although similar to that of the endogenous epsin 1, may be lower than that of other CCP adaptors, such as AP-2. As a result, overexpression of epsin1ΔUIM does not efficiently deplete clathrin for transferrin endocytosis, which uses AP-2 as the adaptor.
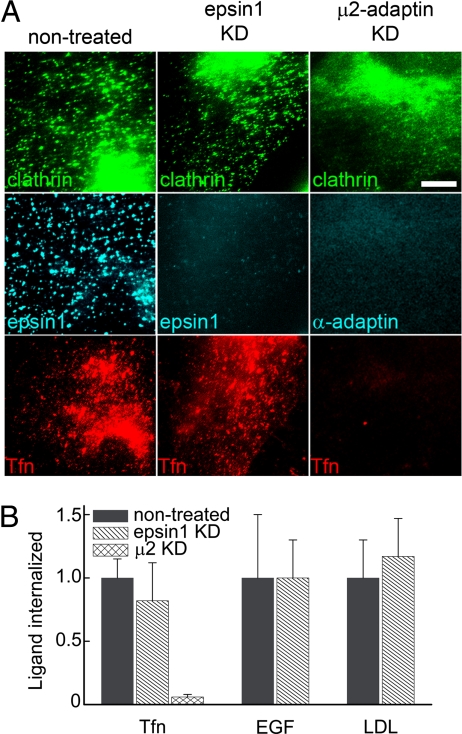
To test whether epsin 1 is indeed a cargo-specific adaptor, we measured the effect of epsin 1 knockdown on the internalization of transferrin, EGF, and LDL. Highly efficient epsin 1 knockdown was achieved by using siRNA (Fig. 5A and Fig. S3A). The knockdown did not affect clathrin distribution; a similar density of fluorescent clathrin spots was observed by immunofluorescence in both the nontreated and the epsin 1 knockdown cells (Fig. 5A). The uptake of transferrin was not affected by epsin 1 knockdown (Figs. 5 A and B), and extensive colocalization (approximately 70%) was observed between transferrin and clathrin before the internalization of transferrin (Fig. S7), indicating that transferrin entered through the clathrin pathway. In contrast, the knockdown of AP-2 by μ2-adaptin siRNA efficiently blocked the internalization of transferrin (Figs. 5 A and B). Similar to transferrin, EGF and LDL also entered the epsin 1 knockdown cells as efficiently as in the nontreated cells (Fig. S8 and Fig. 5B). Because EGF may explore alternative clathrin-independent endocytic pathways (22, 32, 33), we tested whether EGF still entered cells through CCPs by adding EGF to the epsin 1 knockdown cells that expressed clathrin-EYFP. In these cells, EGF quickly clustered, and over 90% of the EGF clusters colocalized with clathrin-EYFP before entry (Fig. S9 A and C). Similarly, approximately 90% of the LDL particles were internalized after colocalizing with clathrin-EYFP (Fig. S9 B and C), consistent with a previous report on normal LDL entry in epsin 1 knockdown cells (34). In addition, all three ligands showed essentially the same entry kinetics in epsin 1 knockdown cells as in control cells (Fig. S10). These results indicate that the clathrin-mediated endocytosis of transferrin, EGF, and LDL did not require epsin 1.
Fig. 5.
Epsin 1 knockdown did not affect transferrin (TFn), EGF, or LDL uptake. (A) Fluorescence images of clathrin (Top), epsin 1 or AP-2 (Middle), and transferrin (Bottom) in nontreated (Left), epsin 1 knockdown (Center), and μ2-adaptin knockdown (Right) cells. The clathrin, epsin 1, and AP-2 signals were detected by immunofluorescence. To image internalized transferrin, cells were incubated with Alexa 633-labeled transferrin at 37°C for 15 min to allow internalization, and then cell-surface transferrin was removed by acid buffer wash. (Scale bar, 10 μm.) Similar assays were used for EGF and LDL (Fig. S8). (B) The amount of internalized transferrin, EGF, and LDL in epsin 1 or μ2-adaptin knockdown cells in comparison with that in control cells. The total fluorescence intensity of the ligands was normalized against that in control cells and used to quantify the internalization amount. Results were averaged over 10–15 cells in each case.
Discussion
Viruses must deliver their genome into host cells to initiate infection. For the influenza virus, a pathogen that has caused some of the biggest pandemics in human history, the viral entry mechanism remains incompletely understood. The influenza virus infects cells through both clathrin-mediated and clathrin-independent endocytic pathways (8–10), with the majority of the virus particles taking the former pathway (10). We have recently shown that the clathrin-dependent entry of influenza does not require AP-2 (7), a primary adaptor for clathrin-mediated endocytosis. This led to an important question in influenza virology: What is the endocytic adaptor protein exploited by the influenza virus for entering host cells? In this work, we showed that epsin 1 is a cargo-specific adaptor required for the clathrin-mediated endocytosis of the influenza virus by using live-cell imaging and single-virus tracking in combination with RNA interference and dominant-negative mutants.
Epsin 1 contains specific sequence motifs that interact directly with clathrin (14), several repetitive DPW and NPF motifs that bind to AP-2 and Eps15 (13), respectively, and an ENTH domain that binds to the phospholipid PtdIns(4,5)P2 (15, 16). This multidomain structure allows epsin 1 to serve as a linchpin between the clathrin coat and the plasma membrane. By tracking individual influenza viruses that successfully entered cells, we observed the recruitment of epsin 1 to the virus particles that entered through CCPs. For every virus particle that showed colocalization with clathrin before its entry, the recruitment of epsin 1 occurs simultaneously with that of clathrin. Similar correlation has been observed between clathrin and the AP-2 adaptor (35), suggesting that clathrin-coat assembly may occur through cooperative interactions between adaptor proteins and clathrin.
Furthermore, knockdown of epsin 1 by siRNA largely inhibited the clathrin-mediated endocytosis of influenza. Whereas the majority of the virus particles entered normal cells through clathrin-mediated endocytosis, in the epsin 1 knockdown cells, only a small fraction (18%) entered through clathrin-coated pits, and most (82%) were routed to the clathrin-independent entry pathway. This result indicates that epsin 1 did not simply appear at the virus-binding sites coincidentally because of its interactions with constitutive CCP components but is actually required for the clathrin-mediated entry of the influenza virus. Interestingly, knockdown of epsin 2 did not have a similar effect, potentially because of the relatively low expression level of epsin 2 in comparison with epsin 1 or a lower affinity of epsin 2 to influenza receptors compared with epsin 1. In contrast to the influenza virus, the clathrin-mediated internalization of transferrin, EGF, and LDL was unperturbed by epsin 1 knockdown. Therefore, epsin 1 is not generally required for clathrin-mediated endocytosis but rather is a cargo-specific adaptor.
To probe which part of epsin 1 potentially recognizes influenza receptors, we turned to the UIMs of epsin 1 (17, 18), which exhibit strong affinity to ubiquitin chains and have been suggested to recognize ubiquitinated cargo receptors (19, 20). Ubiquitination has been shown to play a key role in the internalization and endocytic sorting of membrane receptors (36, 37). To test the involvement of the UIMs of epsin 1 in influenza entry, we generated an epsin 1 mutant that lacks the UIM motifs (epsin1ΔUIM). The deletion of the UIMs substantially reduced the recruitment of epsin1ΔUIM to the virus-binding sites. Overexpression of epsin1ΔUIM nearly completely blocked the clathrin-mediated endocytosis of the influenza virus and resulted in the vast majority of the virus particles (92%) taking the clathrin-independent pathway. Corroboratively, the overexpression of UIM domains alone also significantly inhibited the clathrin-mediated endocytosis of the virus. Although the UIM overexpression may affect many ubiquitin-dependent processes nonspecifically, the effect of the epsin1Δ UIM suggests the specific importance of the UIM domains of epsin 1 in the clathrin-mediated entry of influenza. Knocking down other UIM-containing CCP cofactors, such as Eps15 and Eps15R, did not cause a similar effect, presumably because the lack of the phospholipid-interacting ENTH domain and the clathrin-interacting sequences in Eps15 and Eps15R makes them less efficient as adaptors, compared with epsin 1 (13–16, 38, 39).
In summary, we have shown that epsin 1 is a cargo-specific adaptor protein for the clathrin-mediated internalization of the influenza virus. Epsin 1 was recruited to the virus-binding sites simultaneously with clathrin. Knockdown of epsin 1 inhibited the clathrin-mediated endocytosis of influenza but had no effect on the entry of transferrin, EGF, and LDL. The UIMs of epsin 1 play a critical role in influenza uptake: overexpression of the UIM fragments or epsin 1 lacking the UIMs inhibited influenza entry through the clathrin pathway. Based on these observations, we propose the following hypothesis for the receptor-mediated endocytosis of the influenza virus: The cellular receptors of the virus are recognized by epsin 1, most likely through interaction with the UIMs, and the epsin 1 adaptor then initiates the formation of clathrin-coated pits, ultimately resulting in the internalization of the virus.
Materials and Methods
Cells, Viruses, Antibodies, Plasmids, and siRNAs.
Procedures for cell culture, virus labeling, immunofluorescence, transfection, siRNA knockdown, and ligand uptake are described in SI Text.
Live-Cell Fluorescence Imaging.
Tracking of the influenza virus, EGF, and LDL in live cells was performed at 37°C on an inverted Olympus IX-71 microscope. ECFP was excited by a 457-nm Ar ion laser (Melles-Griot), EYFP or Venus was excited by a 532 nm Nd:YAG laser (Crystalaser), and DiD, Alexa633, or Alexa647 was excited by a 633-nm He-Ne laser (Melles-Griot). Multicolor imaging was performed as described (7). All emissions were collected by a 1.4 N.A. objective (Olympus). The short- and long-wavelength emissions were separated by 650-nm long-pass dichroic mirrors (Chroma) and imaged by a CCD camera (CoolSNAP HQ, Roper Scientific). The emission filters used are a 665- nm long-pass filter (Chroma) for DiD, Alexa633, or Alexa647; a 570/40- or 585/70-nm band-pass filter (Chroma) for EYFP or Venus; and a 480/40-nm band-pass filter (Chroma) for ECFP. The image acquisition rates were 0.5–2 Hz. Image analysis and single-particle tracking were carried out by using custom-written software as described (7, 10).
Supplementary Material
Acknowledgments.
We thank P. De Camilli (Yale University, New Haven, CT) for providing plasmids containing rat epsin 1 cDNA and antibodies against epsin 2, J. H. Keen (Thomas Jefferson University, Philadelphia) for providing plasmids containing clathrin light chain cDNA, and A. Miyawaki (RIKEN, Japan) for providing plasmids containing Venus cDNA. This work is supported in part by the National Institutes of Health and a Packard Science and Engineering Fellowship to X.Z. X.Z. is a Howard Hughes Medical Institute investigator.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803711105/DCSupplemental.
References
- 1.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 2.Bonifacino JS, Lippincott-Schwartz J. Coat proteins: Shaping membrane transport. Nat Rev Mol Cell Biol. 2003;4:409–414. doi: 10.1038/nrm1099. [DOI] [PubMed] [Google Scholar]
- 3.Traub LM. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol. 2003;163:203–208. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson MS. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Conner SD, Schmid SL. Differential requirements for AP-2 in clathrin-mediated endocytosis. J Cell Biol. 2003;162:773–779. doi: 10.1083/jcb.200304069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motley A, Bright NA, Seaman MN, Robinson MS. Clathrin-mediated endocytosis in AP-2-depleted cells. J Cell Biol. 2003;162:909–918. doi: 10.1083/jcb.200305145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakadamyali M, Rust MJ, Zhuang X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell. 2006;124:997–1009. doi: 10.1016/j.cell.2005.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matlin KS, Reggio H, Helenius A, Simons K. Infectious entry pathway of influenza virus in a canine kidney cell line. J Cell Biol. 1981;91:601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sieczkarski SB, Whittaker GR. Influenza virus can enter and infect cells in the absence of clathrin-mediated endocytosis. J Virol. 2002;76:10455–10464. doi: 10.1128/JVI.76.20.10455-10464.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rust MJ, Lakadamyali M, Zhang F, Zhuang X. Assembly of endocytic machinery around individual influenza viruses during viral entry. Nat Struct Mol Biol. 2004;11:567–573. doi: 10.1038/nsmb769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wendland B. Epsins: Adaptors in endocytosis? Nat Rev Mol Cell Biol. 2002;3:971–977. doi: 10.1038/nrm970. [DOI] [PubMed] [Google Scholar]
- 12.Spradling KD, McDaniel AE, Lohi J, Pilcher BK. Epsin 3 is a novel extracellular matrix-induced transcript specific to wounded epithelia. J Biol Chem. 2001;276:29257–29267. doi: 10.1074/jbc.M101663200. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, et al. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–797. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- 14.Drake MT, Downs MA, Traub LM. Epsin binds to clathrin by associating directly with the clathrin-terminal domain. Evidence for cooperative binding through two discrete sites. J Biol Chem. 2000;275:6479–6489. doi: 10.1074/jbc.275.9.6479. [DOI] [PubMed] [Google Scholar]
- 15.Itoh T, et al. Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis. Science. 2001;291:1047–1051. doi: 10.1126/science.291.5506.1047. [DOI] [PubMed] [Google Scholar]
- 16.Ford MG, et al. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- 17.Polo S, et al. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature. 2002;416:451–455. doi: 10.1038/416451a. [DOI] [PubMed] [Google Scholar]
- 18.Shih SC, et al. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat Cell Biol. 2002;4:389–393. doi: 10.1038/ncb790. [DOI] [PubMed] [Google Scholar]
- 19.Barriere H, et al. Molecular basis of oligoubiquitin-dependent internalization of membrane proteins in mammalian cells. Traffic. 2006;7:282–297. doi: 10.1111/j.1600-0854.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 20.Hawryluk MJ, et al. Epsin 1 is a polyubiquitin-selective clathrin-associated sorting protein. Traffic. 2006;7:262–281. doi: 10.1111/j.1600-0854.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, De Camilli P. The association of epsin with ubiquitinated cargo along the endocytic pathway is negatively regulated by its interaction with clathrin. Proc Natl Acad Sci USA. 2005;102:2766–2771. doi: 10.1073/pnas.0409719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sigismund S, et al. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci USA. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagai T, et al. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimura A, Ohnishi S. Uncoating of influenza virus in endosomes. J Virol. 1984;51:497–504. doi: 10.1128/jvi.51.2.497-504.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakadamyali M, Rust MJ, Babcock HP, Zhuang X. Visualizing infection of individual influenza viruses. Proc Natl Acad Sci USA. 2003;100:9280–9285. doi: 10.1073/pnas.0832269100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kemler I, Whittaker G, Helenius A. Nuclear import of microinjected influenza virus ribonucleoproteins. Virology. 1994;202:1028–1033. doi: 10.1006/viro.1994.1432. [DOI] [PubMed] [Google Scholar]
- 27.Babcock HP, Chen C, Zhuang X. Using single-particle tracking to study nuclear trafficking of viral genes. Biophys J. 2004;87:2749–2758. doi: 10.1529/biophysj.104.042234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaidarov I, Santini F, Warren RA, Keen JH. Spatial control of coated-pit dynamics in living cells. Nat Cell Biol. 1999;1:1–7. doi: 10.1038/8971. [DOI] [PubMed] [Google Scholar]
- 29.Rosenthal JA, et al. The epsins define a family of proteins that interact with components of the clathrin coat and contain a new protein module. J Biol Chem. 1999;274:33959–33965. doi: 10.1074/jbc.274.48.33959. [DOI] [PubMed] [Google Scholar]
- 30.Sugiyama S, Kishida S, Chayama K, Koyama S, Kikuchi A. Ubiquitin-interacting motifs of epsin are involved in the regulation of insulin-dependent endocytosis. J Biochem (Tokyo) 2005;137:355–364. doi: 10.1093/jb/mvi044. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann K, Falquet L. A ubiquitin-interacting motif conserved in components of the proteasomal and lysosomal protein degradation systems. Trends Biochem Sci. 2001;26:347–350. doi: 10.1016/s0968-0004(01)01835-7. [DOI] [PubMed] [Google Scholar]
- 32.Yamazaki T, et al. Role of Grb2 in EGF-stimulated EGFR internalization. J Cell Sci. 2002;115:1791–1802. doi: 10.1242/jcs.115.9.1791. [DOI] [PubMed] [Google Scholar]
- 33.Jiang X, Sorkin A. Epidermal growth factor receptor internalization through clathrin-coated pits requires Cbl RING finger and proline-rich domains but not receptor polyubiquitylation. Traffic. 2003;4:529–543. doi: 10.1034/j.1600-0854.2003.t01-1-00109.x. [DOI] [PubMed] [Google Scholar]
- 34.Keyel PA, et al. A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors. Mol Biol Cell. 2006;17:4300–4317. doi: 10.1091/mbc.E06-05-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ehrlich M, et al. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 36.Hicke L. A new ticket for entry into budding vesicles-ubiquitin. Cell. 2001;106:527–530. doi: 10.1016/s0092-8674(01)00485-8. [DOI] [PubMed] [Google Scholar]
- 37.Staub O, Rotin D. Role of ubiquitylation in cellular membrane transport. Physiol Rev. 2006;86:669–707. doi: 10.1152/physrev.00020.2005. [DOI] [PubMed] [Google Scholar]
- 38.Fazioli F, Minichiello L, Matoskova B, Wong WT, Di Fiore PP. Eps15, a novel tyrosine kinase substrate, exhibits transforming activity. Mol Cell Biol. 1993;13:5814–5828. doi: 10.1128/mcb.13.9.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong WT, et al. A protein-binding domain, EH, identified in the receptor tyrosine kinase substrate Eps15 and conserved in evolution. Proc Natl Acad Sci USA. 1995;92:9530–9534. doi: 10.1073/pnas.92.21.9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.