Abstract
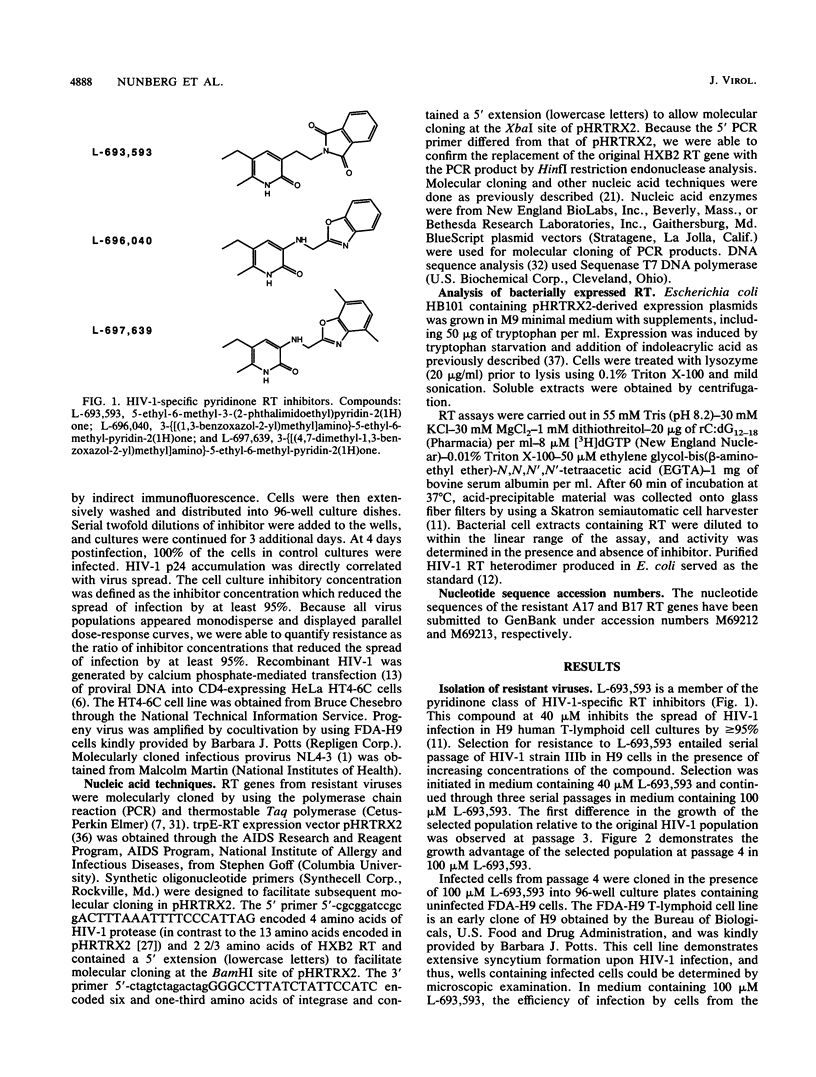
Human immunodeficiency virus type 1 (HIV-1)-specific pyridinone reverse transcriptase (RT) inhibitors prevent HIV-1 replication in cell culture (M. E. Goldman, J. H. Nunberg, J. A. O'Brien, J.C. Quintero, W. A. Schleif, K. F. Freund, S. L. Gaul, W. S. Saari, J. S. Wai, J. M. Hoffman, P. S. Anderson, D. J. Hupe, E. A. Emini, and A. M. Stern, Proc. Natl. Acad. Sci. USA 88:6863-6867, 1991). In contrast to nucleoside analog inhibitors, such as AZT, which need to be converted to triphosphates by host cells, these compounds act directly to inhibit RT via a mechanism which is noncompetitive with respect to deoxynucleoside triphosphates. As one approach to define the mechanism of action of pyridinone inhibitors, we isolated resistant mutants of HIV-1 in cell culture. Serial passage in the presence of inhibitor yielded virus which was 1,000-fold resistant to compounds of this class. Bacterially expressed RTs molecularly cloned from resistant viruses were also resistant. The resistant RT genes encoded two amino acid changes, K-103 to N and Y-181 to C, each of which contributed partial resistance. The mutation at amino acid 181 lies adjacent to the conserved YG/MDD motif found in most DNA and RNA polymerases. The mutation at amino acid 103 lies within a region of RT which may be involved in PPi binding. The resistant viruses, although sensitive to nucleoside analogs, were cross-resistant to the structurally unrelated RT inhibitors TIBO R82150 (R. Pauwels, K. Andries, J. Desmyter, D. Schols, M. J. Kukla, H. J. Breslin, A. Raeymaeckers, J. Van Gelder, R. Woestenborghs, J. Heykanti, K. Schellekens, M. A. C. Janssen, E. De Clercq, and P. A. J. Janssen, Nature [London] 343:470-474, 1990) and BI-RG-587 (V. J. Merluzzi, K. D. Hargrave, M. Labadia, K. Grozinger, M. Skoog, J. C. Wu, C.-K. Shih, K. Eckner, S. Hattox, J. Adams, A. S. Rosenthal, R. Faanes, R. J. Eckner, R. A. Koup, and J. L. Sullivan, Science 250:1411-1413, 1990). Thus, these nonnucleoside analog inhibitors may share a common binding site on RT and may all make up a single pharmacologic class of RT inhibitor. This observation may have important implications for the clinical development of these compounds.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos P. A sequence motif in many polymerases. Nucleic Acids Res. 1988 Nov 11;16(21):9909–9916. doi: 10.1093/nar/16.21.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A. M., Hizi A., Maizel J. V., Jr, Hughes S. H. HIV-1 reverse transcriptase: structure predictions for the polymerase domain. AIDS Res Hum Retroviruses. 1990 Sep;6(9):1061–1072. doi: 10.1089/aid.1990.6.1061. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Butler K. M., Husson R. N., Balis F. M., Brouwers P., Eddy J., el-Amin D., Gress J., Hawkins M., Jarosinski P., Moss H. Dideoxyinosine in children with symptomatic human immunodeficiency virus infection. N Engl J Med. 1991 Jan 17;324(3):137–144. doi: 10.1056/NEJM199101173240301. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K. Development of a sensitive quantitative focal assay for human immunodeficiency virus infectivity. J Virol. 1988 Oct;62(10):3779–3788. doi: 10.1128/jvi.62.10.3779-3788.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. E., Stacy-Phipps S., Nunberg J. H. Recombinant feline herpesviruses expressing feline leukemia virus envelope and gag proteins. J Virol. 1990 Oct;64(10):4930–4938. doi: 10.1128/jvi.64.10.4930-4938.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Fischl M. A., Richman D. D., Grieco M. H., Gottlieb M. S., Volberding P. A., Laskin O. L., Leedom J. M., Groopman J. E., Mildvan D., Schooley R. T. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Goldman M. E., Nunberg J. H., O'Brien J. A., Quintero J. C., Schleif W. A., Freund K. F., Gaul S. L., Saari W. S., Wai J. S., Hoffman J. M. Pyridinone derivatives: specific human immunodeficiency virus type 1 reverse transcriptase inhibitors with antiviral activity. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6863–6867. doi: 10.1073/pnas.88.15.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M. E., Salituro G. S., Bowen J. A., Williamson J. M., Zink D. L., Schleif W. A., Emini E. A. Inhibition of human immunodeficiency virus-1 reverse transcriptase activity by rubromycins: competitive interaction at the template.primer site. Mol Pharmacol. 1990 Jul;38(1):20–25. [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hizi A., Barber A., Hughes S. H. Effects of small insertions on the RNA-dependent DNA polymerase activity of HIV-1 reverse transcriptase. Virology. 1989 May;170(1):326–329. doi: 10.1016/0042-6822(89)90389-9. [DOI] [PubMed] [Google Scholar]
- Hizi A., Hughes S. H., Shaharabany M. Mutational analysis of the ribonuclease H activity of human immunodeficiency virus 1 reverse transcriptase. Virology. 1990 Apr;175(2):575–580. doi: 10.1016/0042-6822(90)90444-v. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Darby G., Richman D. D. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science. 1989 Mar 31;243(4899):1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT). Science. 1989 Dec 1;246(4934):1155–1158. doi: 10.1126/science.2479983. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D., Purifoy D. J. Infectious potential of human immunodeficiency virus type 1 reverse transcriptase mutants with altered inhibitor sensitivity. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4803–4807. doi: 10.1073/pnas.86.13.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Purifoy D. J., Powell K. L., Darby G. Site-specific mutagenesis of AIDS virus reverse transcriptase. 1987 Jun 25-Jul 1Nature. 327(6124):716–717. doi: 10.1038/327716a0. [DOI] [PubMed] [Google Scholar]
- Merluzzi V. J., Hargrave K. D., Labadia M., Grozinger K., Skoog M., Wu J. C., Shih C. K., Eckner K., Hattox S., Adams J. Inhibition of HIV-1 replication by a nonnucleoside reverse transcriptase inhibitor. Science. 1990 Dec 7;250(4986):1411–1413. doi: 10.1126/science.1701568. [DOI] [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Pauwels R., Andries K., Desmyter J., Schols D., Kukla M. J., Breslin H. J., Raeymaeckers A., Van Gelder J., Woestenborghs R., Heykants J. Potent and selective inhibition of HIV-1 replication in vitro by a novel series of TIBO derivatives. Nature. 1990 Feb 1;343(6257):470–474. doi: 10.1038/343470a0. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Ratner L., Fisher A., Jagodzinski L. L., Mitsuya H., Liou R. S., Gallo R. C., Wong-Staal F. Complete nucleotide sequences of functional clones of the AIDS virus. AIDS Res Hum Retroviruses. 1987 Spring;3(1):57–69. doi: 10.1089/aid.1987.3.57. [DOI] [PubMed] [Google Scholar]
- Remington K. M., Chesebro B., Wehrly K., Pedersen N. C., North T. W. Mutants of feline immunodeficiency virus resistant to 3'-azido-3'-deoxythymidine. J Virol. 1991 Jan;65(1):308–312. doi: 10.1128/jvi.65.1.308-312.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman D., Rosenthal A. S., Skoog M., Eckner R. J., Chou T. C., Sabo J. P., Merluzzi V. J. BI-RG-587 is active against zidovudine-resistant human immunodeficiency virus type 1 and synergistic with zidovudine. Antimicrob Agents Chemother. 1991 Feb;35(2):305–308. doi: 10.1128/aac.35.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Annau T. M., Chandrasegaran S. Finding sequence motifs in groups of functionally related proteins. Proc Natl Acad Sci U S A. 1990 Jan;87(2):826–830. doi: 10.1073/pnas.87.2.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. S., Brian E. L., Pagano J. S. Resumption of virus production after human immunodeficiency virus infection of T lymphocytes in the presence of azidothymidine. J Virol. 1987 Dec;61(12):3769–3773. doi: 10.1128/jvi.61.12.3769-3773.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanese N., Goff S. P. Domain structure of the Moloney murine leukemia virus reverse transcriptase: mutational analysis and separate expression of the DNA polymerase and RNase H activities. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1777–1781. doi: 10.1073/pnas.85.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanese N., Prasad V. R., Goff S. P. Structural requirements for bacterial expression of stable, enzymatically active fusion proteins containing the human immunodeficiency virus reverse transcriptase. DNA. 1988 Jul-Aug;7(6):407–416. doi: 10.1089/dna.1.1988.7.407. [DOI] [PubMed] [Google Scholar]
- Tanese N., Roth M., Goff S. P. Expression of enzymatically active reverse transcriptase in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4944–4948. doi: 10.1073/pnas.82.15.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]


