Abstract
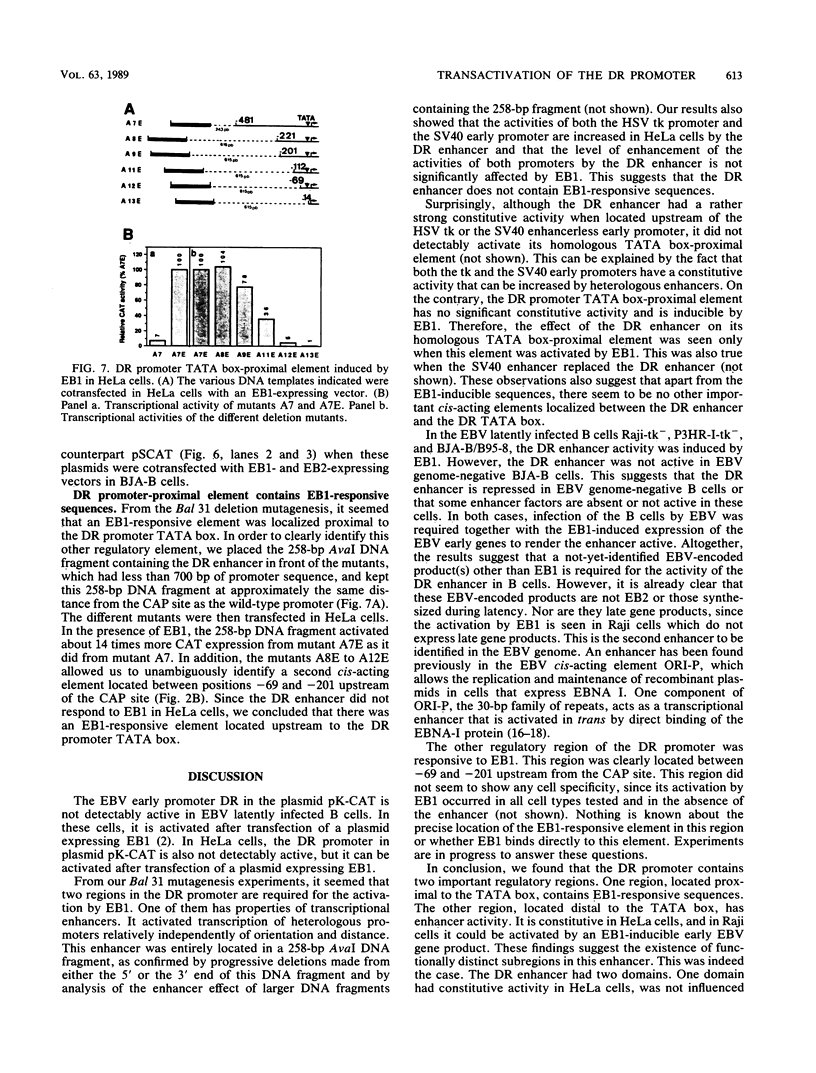
The Epstein-Barr Virus (EBV) DR promoter controlled the expression of the PstI repeat region IR4. This promoter was activated by the EBV trans-acting factor EB1, mainly at the transcriptional level, and the activation was mediated by the TATA box and two cis-acting regulatory regions, one proximal to the TATA box and one distal to the TATA box. The distal region had enhancer properties. In HeLa cells, it activated transcription from the herpes simplex virus type 1 thymidine kinase promoter linked to the chloramphenicol acetyltransferase gene when located in inverted orientation upstream of the thymidine kinase promoter or downstream of the chloramphenicol acetyltransferase gene coding sequence. This enhancer also activated transcription from the simian virus 40 early upstream regulatory elements. These results indicate that the DR These results indicate that the DR enhancer can constitutively activate heterologous promoters in HeLa cells. However, the DR enhancer was not active in EBV genome-negative B cell lines, but it became active when these cells were infected by EBV and when the expression of the EBV early genes was induced by EB1. This suggests that an EBV early gene product induces the DR enhancer activity. The DR promoter TATA box-proximal cis-acting regulatory element contained EB1-responsive sequences.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chevallier-Greco A., Gruffat H., Manet E., Calender A., Sergeant A. The Epstein-Barr virus (EBV) DR enhancer contains two functionally different domains: domain A is constitutive and cell specific, domain B is transactivated by the EBV early protein R. J Virol. 1989 Feb;63(2):615–623. doi: 10.1128/jvi.63.2.615-623.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier-Greco A., Manet E., Chavrier P., Mosnier C., Daillie J., Sergeant A. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 1986 Dec 1;5(12):3243–3249. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Countryman J., Miller G. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambaugh T. R., Kieff E. Identification and nucleotide sequences of two similar tandem direct repeats in Epstein-Barr virus DNA. J Virol. 1982 Dec;44(3):823–833. doi: 10.1128/jvi.44.3.823-833.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick J. M., Lieberman P. M., Hayward S. D. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J Virol. 1988 Jul;62(7):2274–2284. doi: 10.1128/jvi.62.7.2274-2284.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez N., Keller W. Splicing of in vitro synthesized messenger RNA precursors in HeLa cell extracts. Cell. 1983 Nov;35(1):89–99. doi: 10.1016/0092-8674(83)90211-8. [DOI] [PubMed] [Google Scholar]
- Kenney S., Kamine J., Markovitz D., Fenrick R., Pagano J. An Epstein-Barr virus immediate-early gene product trans-activates gene expression from the human immunodeficiency virus long terminal repeat. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1652–1656. doi: 10.1073/pnas.85.5.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman P. M., O'Hare P., Hayward G. S., Hayward S. D. Promiscuous trans activation of gene expression by an Epstein-Barr virus-encoded early nuclear protein. J Virol. 1986 Oct;60(1):140–148. doi: 10.1128/jvi.60.1.140-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthman H., Nilsson M. G., Magnusson G. Non-contiguous segments of the polyoma genome required in cis for DNA replication. J Mol Biol. 1982 Nov 15;161(4):533–550. doi: 10.1016/0022-2836(82)90406-5. [DOI] [PubMed] [Google Scholar]
- Miksicek R., Heber A., Schmid W., Danesch U., Posseckert G., Beato M., Schütz G. Glucocorticoid responsiveness of the transcriptional enhancer of Moloney murine sarcoma virus. Cell. 1986 Jul 18;46(2):283–290. doi: 10.1016/0092-8674(86)90745-2. [DOI] [PubMed] [Google Scholar]
- Rawlins D. R., Milman G., Hayward S. D., Hayward G. S. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell. 1985 Oct;42(3):859–868. doi: 10.1016/0092-8674(85)90282-x. [DOI] [PubMed] [Google Scholar]
- Reisman D., Sugden B. trans activation of an Epstein-Barr viral transcriptional enhancer by the Epstein-Barr viral nuclear antigen 1. Mol Cell Biol. 1986 Nov;6(11):3838–3846. doi: 10.1128/mcb.6.11.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman D., Yates J., Sugden B. A putative origin of replication of plasmids derived from Epstein-Barr virus is composed of two cis-acting components. Mol Cell Biol. 1985 Aug;5(8):1822–1832. doi: 10.1128/mcb.5.8.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant A., Bohmann D., Zentgraf H., Weiher H., Keller W. A transcription enhancer acts in vitro over distances of hundreds of base-pairs on both circular and linear templates but not on chromatin-reconstituted DNA. J Mol Biol. 1984 Dec 15;180(3):577–600. doi: 10.1016/0022-2836(84)90028-7. [DOI] [PubMed] [Google Scholar]
- Takada K., Shimizu N., Sakuma S., Ono Y. trans activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J Virol. 1986 Mar;57(3):1016–1022. doi: 10.1128/jvi.57.3.1016-1022.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Wong K. M., Levine A. J. Identification and mapping of Epstein-Barr virus early antigens and demonstration of a viral gene activator that functions in trans. J Virol. 1986 Oct;60(1):149–156. doi: 10.1128/jvi.60.1.149-156.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Rosser D. S., Schmidt M. C., Berk A. A TATA box implicated in E1A transcriptional activation of a simple adenovirus 2 promoter. Nature. 1987 Apr 2;326(6112):512–515. doi: 10.1038/326512a0. [DOI] [PubMed] [Google Scholar]