Abstract
Insects have an open circulatory system in which the heart pumps blood (hemolymph) into the body cavity, where it directly bathes the internal organs and epidermis. The blood contains free and tissue-bound immune cells that function in the inflammatory response. Here, we use live imaging of transgenic Drosophila larvae with fluorescently labeled blood cells (hemocytes) to investigate the circulatory dynamics of larval blood cells and their response to tissue injury. We find that, under normal conditions, the free cells rapidly circulate, whereas the tissue-bound cells are sessile. After epidermal wounding, tissue-bound cells around the wound site remain sessile and unresponsive, whereas circulating cells are rapidly recruited to the site of damage by adhesive capture. After capture, these cells distribute across the wound, appear phagocytically active, and are subsequently released back into circulation by the healing epidermis. The results demonstrate that circulating cells function as a surveillance system that monitors larval tissues for damage, and that adhesive capture, an important mechanism of recruitment of circulating cells to inflammatory sites in vertebrates, is shared by insects and vertebrates despite the vastly different architectures of their circulatory systems.
Keywords: adhesion, inflammation, live-imaging, wound healing
The ability of blood cells to recognize and rapidly respond to tissue damage is an integral part of the tissue repair response. In vertebrates, this response has an early phase in which blood cells released from broken vessels bind directly to damaged extravascular tissue and a late phase in which blood cells adhere to activated local blood vessels and then diapedese through the vessel wall to reach the site of injury (1). The ability of blood cells to bind directly to “damaged self” tissue has been hypothesized to be an ancestral function of the immune system (2) but has not been studied extensively in organisms that possess only an innate immune system or simple open circulatory systems in which blood directly bathes the internal tissues.
Larval and adult Drosophila are capable of efficiently fighting infection (3) and repairing damaged tissue (4–7), and blood cells are a crucial cellular component of these responses. Embryonic blood cells differentiate in the head region and subsequently distribute throughout the body (8, 9). At this stage, they are attached to tissues but are highly motile and directly migrate to and scavenge apoptotic cells (9, 10) and damaged tissue (11) using chemotactic cues distinct from those that control their dispersal during development (12, 13).
Just before hatching and the beginning of larval life, the heart (dorsal vessel) begins to beat, and blood circulation is established. Although the open circulatory system of Drosophila and other insects has been classically viewed as a means of distributing nutrients and removing waste (14, 15), the blood also contains immune cells that battle microbial infections (16) and colonization by parasitoid wasps (17, 18). In Drosophila larvae, plasmatocytes, the major larval blood cell type, are phagocytic cells that are present both free in circulation and bound to tissues (19, 20). However, the circulatory dynamics of these cells and their response to tissue injury in vivo have not been systematically investigated.
Here, we use Drosophila larvae with fluorescently labeled blood cells and live imaging to investigate the circulatory dynamics of blood cells and their response to tissue injury. In unwounded larvae, free-circulating cells alternate between relatively slow posterior-directed flow within the open body cavity and much faster anterior-directed pumping through the heart, whereas resident tissue-bound cells are sessile. After epidermal wounding, tissue-bound cells remain sessile and unresponsive, whereas circulating cells are recruited to the site of damage by direct capture from circulation, a process we term wound-induced inflammation. After capture, the blood cells spread across the wound surface and assume an adhesive morphology, become phagocytically active and clear wound site debris, and are later released back into circulation by the healing epidermis. Thus, circulating blood cells in the larva serve a surveillance function, monitoring tissues for damage, and they are recruited to wound sites by direct capture from circulation, a mechanism reminiscent of the early response of blood cells to damaged tissue in vertebrates.
Results
Blood Cell Dynamics in the Larval Open Circulatory System.
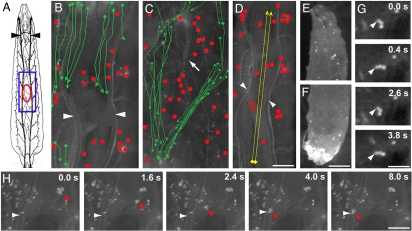
To visualize blood-cell dynamics, we constructed a Drosophila strain whose blood cells express a yellow fluorescent protein (YFP) driven by the blood cell-specific Peroxidasin (Pxn) promoter (11). Live imaging of these larvae (see schematic, Fig. 1A) revealed two populations of blood cells (Fig. 1 B–D). One was a stationary population of cells bound to the surface of internal organs and the barrier epidermis (19, 20). Most of these tissue-bound cells were sessile and appeared well anchored to their targets, although some were loosely tethered at just a single point around which they could swivel [Fig. 1G, supporting information (SI) Movie S1]. The other population was circulating cells and cell clusters that flowed posteriorly through the open body cavity at rates of 83 +/− 17.6 μm/second (Fig. 1 B and C, Movies S2 and S3). The internal organs provide barriers to flow that channel the circulating cells along certain predominant routes (Movies S2 and S3), mostly in the ventral part of the body. However, peristaltic motions of larval body-wall muscles could redirect circulating cells (Movie S4). Many cells reaching the larval posterior entered the heart and were recirculated anteriorly (Movie S5) at speeds of up to 3.2 mm/second (Fig. 1D, Movie S6), 40 × faster than the posterior-directed flow and similar to the rate of blood cell flow through vertebrate microvessels (21). There was some pooling of blood cells in the larval posterior (Fig. 1 E and F) (20), presumably due to a circulation bottleneck at reentry into the heart. Little interconversion was observed between the tissue-bound and circulating cell populations, although occasionally a tissue-bound cell detached from a tissue and transiently entered circulation before reattaching to another tissue (Fig. 1H, Movie S7).
Fig. 1.
Circulating and tissue-bound blood cell populations in Drosophila larvae. (A) Schematic of third-instar larva viewed dorsally showing tracheae (black) including dorsal trunks (arrowheads). Blue box, approximate area of view in B–D and Movies S2–S4. Red oval approximate position and size of pinch wounds shown in Figs. 23–4. (B–H) Composite trajectories from movies (B–D), micrographs (E and F) or still frames from movies (G and H) of Nrg-GFP;Pxn>YFP larvae with YFP-labeled blood cells. (B and C) Movement of blood cells in the dorsal (B, from Movie S2) and ventral (C, from Movie S3) sides tracked by videomicroscopy over an ≈8-s interval. Sessile tissue-bound blood cells (red dots) and the trajectories of circulating cells flowing posteriorly through the body cavity (green arrows) are shown. Arrowheads, tracheal dorsal trunks; arrow, ventral nerve cord. (D) Trajectories (yellow arrows) of cells (from Movie S5) pumped anteriorly through the heart and tracked over a 2-s interval. Tissue-bound cells indicated as above. Arrowheads, tracheal dorsal trunks. (E and F) Micrographs of larval anterior (E) and posterior (F) showing pooling of free posterior blood cells. (G) Frames from Movie S1 showing a cluster of three tissue-bound blood cells swivelling about its attachment (arrowhead) to the epidermis. Elapsed time is indicated. (H) Frames from Movie S6 showing transient release into circulation of a small cluster of tissue-bound blood cells that rebinds 4 s later to a tracheal dorsal trunk. Horizontal arrowhead in each frame, tracheal dorsal trunk; red arrowheads, released blood cell cluster. Elapsed time is indicated. [Scale bar (D) 100 μm for B–D; (F) 100 μm for E and F; (G) 50 μm; (H) 100 μm.]
Tissue Damage Induces a Rapid Inflammatory Response.
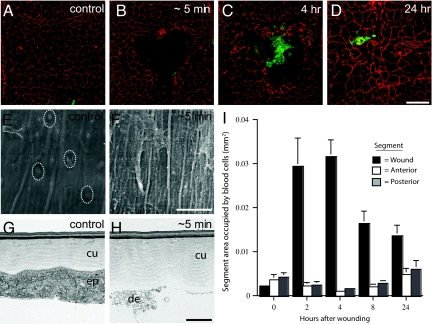
When early third-instar larvae are pinched with dissecting forceps, a gap is created in the epidermal sheet (Fig. 2 A and B), but the overlying cuticle remains intact (Fig. 2 E–H), preserving barrier function and preventing entry of microorganisms. Over the next 24 hours, the wound heals by spreading of the surrounding epidermal cells to close the wound gap (Fig. 2 C and D) (5). Immediately after wounding, the gap in the epidermal sheet was devoid of cells (Fig. 2B) but littered with cell debris (Fig. 2 F and H). Then, as early as 5 min after wounding, individual and small clusters of blood cells began to accumulate in the wound gap (Fig. S1 and data not shown). Over the next several hours, the number of cells increased until there were hundreds or, in some cases, thousands of blood cells covering one-third and sometimes more of the wound surface (Fig. 2 C and I), similar to what is seen at wounds in other insects (22–24).
Fig. 2.
Accumulation and turnover of blood cells at larval wounds. (A–D) Unwounded segment (A) or pinch-wounded segments (B–D) in epidermal whole-mount preparations of Pxn>GFP third-instar larvae stained with anti-Fasciclin-III (red) to show epidermal membranes and anti-GFP (green) to show blood cells. (A) Unwounded control larva; (B) ≈5 min after wounding; (C) 4 h after wounding; (D) 24 h after wounding; (E and F) SEM; (E) unwounded epidermis (white dotted ovals, epidermal cell nuclei); (F) exposed cuticle and cell debris ≈5 min after wounding; (G and H) TEM; (G) unwounded epidermis (ep) and apical cuticle (cu); (H) intact cuticle and cell debris (de) ≈5 min after wounding; (I) area of epidermis (+/− SEM) occupied by blood cells in wounded dorsal segments (black bars), adjacent segments located anterior (white bars), and posterior (gray bars). [Scale bar (D) 100 μm for A–D;. (F) 33 μm for F, 26.5 μm for E; (H) 2 μm for G and H.]
Recruitment of Blood Cells to Wound Sites by Direct Capture from the Circulation.
Blood cells in Drosophila (9–13) and zebrafish embryos (25, 26) are highly motile and rapidly attracted to dead and dying cells by signals released by such cells. However, this is not the mechanism of blood-cell recruitment to larval wound sites. First, the small number of tissue-bound blood cells in the vicinity of the wound site was not sufficient to account for the large number of cells that rapidly accumulated at the wound during the inflammatory response (Fig. 2 B, C, and I). Second, blood-cell-specific expression of dominant-negative versions of the small GTPases Rac or Rho, which are thought to be universally required for cell migration (27), and which block blood cell recruitment to wound sites in embryos when expressed with the same Gal4 driver (11), had little or no effect on blood cell accumulation at larval wound sites (Fig. S2). Third, no local migration of nearby tissue-bound cells into wound sites was detected in the live imaging studies described below. Indeed, tissue-bound cells near the wound remained sessile and appeared completely unresponsive to the injury (see Fig. 3B′). The live imaging studies revealed that blood cells arrive instead by direct capture from circulation.
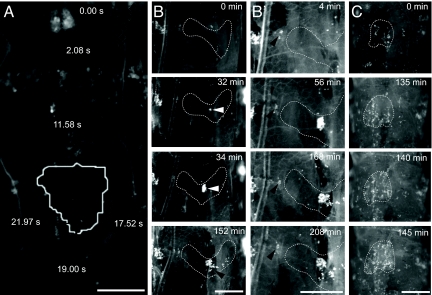
Fig. 3.
Direct capture of circulating blood cells at larval wound sites. (A–C) Composite trajectory (A) or still frames (B and C) from movies of wounded Nrg-GFP;Pxn>YFP larvae. Wound sites are outlined in white. Elapsed time is indicated; anterior is up. (A) Track of a circulating blood cell cluster (black line with diamonds) over a 22-s interval from a real-time movie (Movie S10). The cluster moves rapidly through the body cavity until docking abruptly at the wound site. (B) Frames from time-lapse movie (Movie S9). Few blood cells are present at the wound site <5 min after wounding (Upper). A cluster of ≈100 blood cells (white arrowhead) attaches to the wound site over a 2-min interval (32–34 min). Over the next 2 hr, the cluster shears apart, and some cells readhere to downstream (more posterior) portions of the wound (black arrowhead, 152 min). (B′) Closeups of frames from Movie S9 showing a group of sessile tissue-bound cells (arrowhead) near the wound edge (dashed white line) that fail to polarize or migrate to the wound. (C) Frames from time-lapse movie (Movie S11) showing direct docking of a large sheet of blood cells (right half of wound) within a 10-min interval.
Blood cell infiltration of pinch wounds was imaged in Pxn>YFP transgenic larvae that also carried a Neuroglian–GFP (Nrg-GFP) transgene (28) that labels epidermal cell membranes to permit simultaneous visualization of the wound site borders (Fig. 3 and Movies S8–S11). Time-lapse and real-time imaging studies showed that within an hour or two after wounding individual circulating blood cells (Movie S8) or, more commonly, circulating clusters of cells (Fig. 3B, Movie S9) abruptly arrived at the wound site. Arriving cells directly docked on the exposed cuticle and debris in the wound gap (Fig. 3, Movies S8–S11). A blood cell capture event recorded in real time (Fig. 3A, Movie S10) showed that a circulating cell initially bound loosely to the wound surface and pivoted around a single tether point for several seconds before attaching more securely. When a cluster of cells docked at a wound site, cells in the cluster subsequently dispersed (Fig. 3B Bottom) apparently by flow-induced shearing of the attached cluster and reattachment of the separated cells to more downstream (posterior) positions in the wound gap (Movie S9). However, dispersal was not always necessary, as one recorded capture event involved direct docking in the wound gap of several large clusters or sheets of blood cells that almost completely filled the gap (Fig. 3C, Movie S11). Inhibiting larval peristalsis and mobility dampened wound-induced inflammation (Fig. S3 and Fig. S4), providing evidence that blood cell circulation facilitates the inflammatory response.
Wound-Adherent Blood Cells Undergo Morphological Changes and Appear Phagocytically Active.
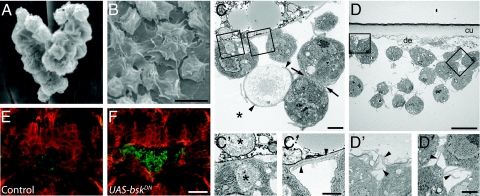
Ultrastructural analysis showed that in contrast to the rounded morphology of sessile blood cells bound to the epidermis in unwounded larvae (Fig. 4A), blood cells bound to epidermal wound sites had a spread morphology (Fig. 4B). Cells bound at wound sites where more tightly adherent than tissue-bound cells as they persisted during dissection and staining whereas tissue-bound cells generally did not (data not shown). Cells bound at the wound were commonly arranged in loose clusters, with each cell extending many long cytoplasmic processes (Fig. 4 C and D), some of which contacted neighboring cells in the cluster (Fig. 4 C″ and D″). The layering of attached cells suggests that later capture events might occur by binding of blood cells to previously attached cells, as occurs in the response to parasitoid wasps (17, 18). Cell debris were present at most sites of contact between blood cells and the wound (Fig. 4 C and D′), and the debris were typically surrounded by cellular processes (Fig. 4C) or present within intracellular vesicles (Fig. 4C′), implying that the blood cells were phagocytically active (16, 29, 30).
Fig. 4.
Morphology of wound-adherent blood cells and release of bound cells by the spreading epidermis. (A–D) SEM (A and B) and TEM (C and D) of blood cells bound at wound sites. (A) Cluster of tissue-bound blood cells in control unwounded larva; (B) blood cells attached to wound site 4 h after wounding; (C) 2 h postwounding. Phagocytic processes (arrowheads) extend from blood cells to engulf cellular debris (asterisk). Arrows, close apposition of blood cells; (C′) closeup of left box in C. Vesciculate cell debris at wound site (upper asterisk) and within a blood cell vesicle surrounded by healthy dark gray cytoplasm (lower asterisk); (C″) closeup of right box in C. Arrowheads, overlapping cell extensions along wound-site debris; (D) 4 h postwounding. Cluster of blood cells under exposed cuticle (cu) of pinch wound. Cell debris (de); (D′) closeup of left box in D. Fine cell processes (arrowheads) attached to debris beneath the cuticle; (D″) closeup of right box in D. Fine cell extensions (arrowheads) between blood cells; (E and F) epidermal whole-mount preparations of pinch-wounded UAS-srcGFP, A58-Gal4 larvae immunostained with anti-GFP (red) to label epidermal cell membranes and anti-Peroxidasin (green) to label blood cells. (E) Control larva lacking UAS-bskDN transgene, 24 h after wounding. (F) Larva carrying a UAS-bskDN transgene, 24 h after wounding. [Scale bar (B) 10 μm for A and B; (C) 10 μm; (C″), 2 μm for C′, C″; (D) 2 μm; (D″), 1 μm for D′, D″); (F) 100 μm for E and F.
Dispersal of Wound-Adherent Cells Requires Wound Closure.
As wounds healed and the epidermis spread to close the wound gap, the number of blood cells at the wound site gradually declined (Fig. 2 D and I). This decline was not likely due to programmed cell death, because no antiactivated caspase 3 immunoreactivity was detected in any blood cells up to 24 h after wounding (data not shown). When wound closure was blocked by expression in epidermal cells of a dominant-negative basket (Jun N-terminal kinase) transgene (31), blood cells persisted within the wound gap for at least 24 h (Fig. 4 E and F). Thus, spreading of the epidermis is necessary for turnover of blood cells at the wound site, suggesting that the spreading epidermal sheet may physically displace blood cells or induce their release from the wound site. Genetic ablation of the blood cells before wounding did not impair wound closure (Fig. S5).
Discussion
Visualization of blood-cell dynamics in living Drosophila larvae revealed that tissue-bound and circulating blood cells have distinct mobility and responsiveness to injury. Tissue-bound cells are sessile and do not respond to local tissue damage. By contrast, circulating cells cycle between anteriorly directed movement through the heart, slower posteriorly directed flow through the open body cavity and peristaltic redistribution, and these cells are rapidly recruited to sites of tissue injury. The results suggest that circulating blood cells serve as a surveillance system, continuously monitoring larval tissues for damage. After injury, these cells are recruited to the site of damage by direct capture from circulation. Initially, captured cells or cell clusters can be tenuously bound to the wound surface, but shortly thereafter, clusters fragment and distribute across the wound surface, and the bound cells become tightly adherent and phagocytically active. Although recruitment is dispensable for normal healing, as the wound heals, the spreading epidermis releases bound blood cells back into circulation to rejoin other cells in surveillance. The function of tissue-bound cells is less clear, although they might provide a reservoir of cells that can be mobilized under specific global immune challenges (20).
Adhesive capture of circulating blood cells by damaged tissue is completely different from the mechanism of blood cell recruitment to sites of damage and cell death in the Drosophila embryo, before circulation is established. In the embryo, tissue-bound blood cells are highly motile and are attracted to injured or dying cells by short range chemotactic signals (9–11, 13). Tissue-bound cells in the larva were never observed crawling along the body wall as they do in the embryo, even when located right next to the wound (Fig. 3B′). This implies there is a developmental change at or near hatching that results in the loss of the ability of tissue-bound blood cells to sense and respond to injury. The transition from migration-directed wound responsiveness to direct capture of circulating blood cells is physiologically appropriate, because it allows a relatively small number of cells to continuously survey the much larger larval body for tissue damage.
Drosophila blood cells use pattern-recognition receptors to detect foreign or “nonself” objects, such as invading pathogens (3). Seong and Matzinger (2) hypothesize that an ancestral function of the immune system is to recognize “damaged self” and propose that pattern recognition receptors that promiscuously bind to exposed hydrophobic “danger” signals might mediate this recognition. Consistent with this, the first blood cells at larval wound sites almost always bind to sites of cell debris (Fig. 4 C and D). Alternatively, the wound site might first be coated with a blood-borne factor such as a complement-like opsonin (32) or spreading peptide (33) that promotes blood cell adhesion.
There are significant parallels between the direct-capture mechanism described here and the initial phase of blood-cell recruitment during wound-induced inflammation in mammals, where blood cells released from disrupted vessels at the wound site directly adhere to damaged extravascular tissue (1). This is consistent with the hypothesis that the ability to recognize and adhere to damaged or “nonself” tissue is an ancestral feature of blood cells that predates evolution of a closed circulatory system. If so, then up-regulation of selectins and other adhesion molecules on the endothelial cell lining of activated blood vessels (34) is likely a vertebrate evolutionary adaptation that allowed capture of circulating blood cells at injury sites where vessels remain intact. Genetic dissection of the Drosophila immune surveillance system described here should lead to identification of both wound site- and blood cell-specific factors involved in recognition of damaged tissue and in the attachment, activation, and release of circulating cells.
Materials and Methods
Fly Stocks and Genetics.
The GAL4/UAS system (35) was used to drive expression of UAS transgenes in blood cells (Pxn-Gal4) (11) and larval epidermis (A58-Gal4) (5). w;Pxn-Gal4 8.1.1, UAS-GFP (Pxn>GFP) (11) was used to label blood cells and drive expression of UAS-rhoL.N25DN, UAS-rac1.N17DN, UAS-cdc42.N17DN, which encode dominant-negative forms of the respective proteins (36). For TEM analysis, Pxn>GFP drove expression of a UAS-lacZ.NZ transgene (Bloomington) to allow identification of wound-associated blood cells in X-Gal- (5-bromo-4-chloro-3-indolyl-d-galactopyranoside) stained samples (see below). For live-imaging experiments, w, Nrg-GFPG00305;Pxn-Gal4, UAS-2xeYFP larvae were used that carry a UAS-2xeYFP transgene (37) to label blood cells and a protein-trap GFP insertion (Nrg-GFPG00305) in neuroglian (28), which expresses a GFP fusion protein localized to epithelial septate junctions. w;UAS-src-GFP, A58-Gal4/TM6b line was used to express a dominant-negative form of Basket (31) in GFP-labeled larval epidermis.
Wounding Procedure.
Pinch wounds were done as described (5), except that wounds were centered in a single abdominal segment, usually A4, -5, or -6. Larvae were maintained at 25°C except during wounding or live imaging, which was performed at room temperature.
Whole-Mount Immunofluorescence.
Dissection and immunostaining of larval epidermal whole-mount preparations were done as described (5). Primary antibodies were anti-Fasciclin III (38) (Developmental Studies Hybridoma Bank, 1:50), anti-Peroxidasin (39) (1:3,000), and anti-GFP (Molecular Probes, 1:500). Secondary antibodies (Jackson ImmunoResearch) were goat-anti-mouse Cy3 (1:1,000) and goat-anti-rabbit-FITC (1:300).
Live Imaging.
Third-instar w, Nrg-GFP;Pxn-Gal4, UAS-2xeYFP larvae were wounded and mounted dorsal side up on a glass slide with the anterior and posterior ends of the larvae taped down to varying degrees to prevent locomotion and constrain body peristalsis. Larvae were imaged on a Leica MZ16FA microscope using a Planapo 1.0× objective, and images were captured on a monochrome Leica DFC350FX digital camera. For real-time recording of larval circulation or heartbeat, frames were captured every ≈400 ms over a 4- to 40-s period and for time-lapse imaging, frames were captured every 2 or 5 min over a 2- to 4-h period. Images were obtained by using the Scope-Pro Advanced Acquisition plug-in, and image analysis was carried out with Image-Pro AMS ver. 5.1 Software (Media Cybernetics). The object-tracking tool was used to manually track specific blood cells in a time series. Velocities of tracked cells were calculated by using coordinates provided by the software.
Electron Microscopy.
For TEM, larvae were prepared as described (5), except that SPURR resin (Electron Microscopy Sciences) was used. Ninety-nanometer sections were observed in a JEOL JEM 1010 transmission electron microscope. Digital photos were obtained by using an AMT (Advanced Microscopy Techniques) imaging system. For SEM, dissected larvae were fixed in 3% glutaraldehyde/2% paraformaldehyde with 2.5% DMSO in 0.2 M sodium phosphate buffer, dehydrated in graded ethanol concentrations, and immersed in hexamethyldisilazane before vacuum drying, mounting on conductive carbon tabs (Electron Microscopy Sciences), and sputter-coating with gold to 0.1 kÅ. Samples were imaged with a Philips 525 scanning electron microscope and photographed with a Semicaps digital camera.
Blocking Larval Peristalsis and Mobility.
Two-hour immobilization of w,Nrg-GFP;Pxn-Gal4,UAS-2xeYFP larvae was accomplished by anesthetizing with 250 μl Flynap (Carolina Biological Supply) for 5 min and mounting securely on double-sided tape subsequent to wounding. Wounded and mounted larvae were maintained at room temperature under light humidity. Control groups were anesthetized with ether and put on food in a 25°C incubator.
Supplementary Material
Acknowledgments.
We thank John Perrino of the Stanford Cell Sciences Imaging Facility for assistance with SEM; Kenn Dunner of the MD Anderson electron microscopy core facility for assistance with TEM; John Fessler for anti-Peroxidasin antibody; and Miles Wilkinson, Brian Stramer, Paul Martin, and Yujane Wu for comments on the manuscript. The MD Anderson electron microscopy facility is supported by a Cancer Center Core Grant (CA16672). D.T.B. was supported by National Institutes of Health predoctoral training grant T32-HD07325-16. M.J.G. was supported by a Beckman Scholar Award, American Heart Association Grant 0730258N, and University of Texas MD Anderson Cancer Center institutional startup funds. M.A.K. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709951105/DCSupplemental.
References
- 1.Martin P, Leibovich SJ. Inflammatory cells during wound repair: The good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Seong SY, Matzinger P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 3.Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: Sensing and signaling during bacterial and fungal infections. Nat Rev Immunol. 2007;7:862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- 4.Bosch M, Serras F, Martin-Blanco E, Baguna J. JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. Dev Biol. 2005;280:73–86. doi: 10.1016/j.ydbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Galko MJ, Krasnow MA. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2004;2:E239. doi: 10.1371/journal.pbio.0020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattila J, et al. Role of Jun N-terminal Kinase (JNK) signaling in the wound healing and regeneration of a Drosophila melanogaster wing imaginal disc. Int J Dev Biol. 2005;49:391–399. doi: 10.1387/ijdb.052006jm. [DOI] [PubMed] [Google Scholar]
- 7.Ramet M, Lanot R, Zachary D, Manfruelli P. JNK signaling pathway is required for efficient wound healing in Drosophila. Dev Biol. 2002;241:145–156. doi: 10.1006/dbio.2001.0502. [DOI] [PubMed] [Google Scholar]
- 8.Paladi M, Tepass U. Function of Rho GTPases in embryonic blood cell migration in Drosophila. J Cell Sci. 2004;117:6313–6326. doi: 10.1242/jcs.01552. [DOI] [PubMed] [Google Scholar]
- 9.Tepass U, Fessler LI, Aziz A, Hartenstein V. Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development. 1994;120:1829–1837. doi: 10.1242/dev.120.7.1829. [DOI] [PubMed] [Google Scholar]
- 10.Franc NC, Heitzler P, Ezekowitz RA, White K. Requirement for croquemort in phagocytosis of apoptotic cells in Drosophila. Science. 1999;284:1991–1994. doi: 10.1126/science.284.5422.1991. [DOI] [PubMed] [Google Scholar]
- 11.Stramer B, et al. Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J Cell Biol. 2005;168:567–573. doi: 10.1083/jcb.200405120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho NK, et al. Developmental control of blood cell migration by the Drosophila VEGF pathway. Cell. 2002;108:865–876. doi: 10.1016/s0092-8674(02)00676-1. [DOI] [PubMed] [Google Scholar]
- 13.Wood W, Faria C, Jacinto A. Distinct mechanisms regulate hemocyte chemotaxis during development and wound healing in Drosophila melanogaster. J Cell Biol. 2006;173:405–416. doi: 10.1083/jcb.200508161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller TA. In: Comprehensive Insect Physiology Biochemistry and Pharmacology. Kerkut GA, Gilbert LI, editors. Vol 3. Oxford: Pergamon; 1985. pp. 289–354. [Google Scholar]
- 15.Wigglesworth VB. Principles of Insect Physiology. London: Chapman and Hall; 1982. [Google Scholar]
- 16.Elrod-Erickson M, Mishra S, Schneider D. Interactions between the cellular and humoral immune responses in Drosophila. Curr Biol. 2000;10:781–784. doi: 10.1016/s0960-9822(00)00569-8. [DOI] [PubMed] [Google Scholar]
- 17.Pech LL, Strand MR. Granular cells are required for encapsulation of foreign targets by insect haemocytes. J Cell Sci. 1996;109:2053–2060. doi: 10.1242/jcs.109.8.2053. [DOI] [PubMed] [Google Scholar]
- 18.Russo J, et al. Insect immunity: Early events in the encapsulation process of parasitoid (Leptopilina boulardi) eggs in resistant and susceptible strains of Drosophila. Parasitology. 1996;112:135–142. doi: 10.1017/s0031182000065173. [DOI] [PubMed] [Google Scholar]
- 19.Lanot R, Zachary D, Holder F, Meister M. Postembryonic hematopoiesis in Drosophila. Dev Biol. 2001;230:243–257. doi: 10.1006/dbio.2000.0123. [DOI] [PubMed] [Google Scholar]
- 20.Zettervall CJ, et al. A directed screen for genes involved in Drosophila blood cell activation. Proc Natl Acad Sci USA. 2004;101:14192–14197. doi: 10.1073/pnas.0403789101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zharov VP, Galanzha EI, Tuchin VV. In vivo photothermal flow cytometry: Imaging and detection of individual cells in blood and lymph flow. J Cell Biochem. 2006;97:916–932. doi: 10.1002/jcb.20766. [DOI] [PubMed] [Google Scholar]
- 22.Lai-Fook J. Haemocytes in the repair of wounds in an insect (Rhodnius prolixus) J Morphol. 1970;130:297–314. doi: 10.1002/jmor.1051300304. [DOI] [PubMed] [Google Scholar]
- 23.Rowley AF, Ratcliffe NA. A histological study of wound healing and hemocyte function in the wax moth, Galleria mellonella. J Morphol. 1978;157:181–200. doi: 10.1002/jmor.1051570206. [DOI] [PubMed] [Google Scholar]
- 24.Wigglesworth VB. Wound healing in an insect (Rhodnius prolixus Hemiptera) J Exp Biol. 1937;14:364–381. [Google Scholar]
- 25.Mathias JR, et al. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukocyte Biol. 2006;80:1281–1288. doi: 10.1189/jlb.0506346. [DOI] [PubMed] [Google Scholar]
- 26.Redd MJ, et al. Imaging macrophage chemotaxis in vivo: Studies of microtubule function in zebrafish wound inflammation. Cell Motil Cytoskeleton. 2006;63:415–422. doi: 10.1002/cm.20133. [DOI] [PubMed] [Google Scholar]
- 27.Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Morin X, Daneman R, Zavortink M, Chia W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc Natl Acad Sci USA. 2001;98:15050–15055. doi: 10.1073/pnas.261408198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson AM, et al. Identification of cytoskeletal regulatory proteins required for efficient phagocytosis in Drosophila. Microbes Infect. 2003;5:815–824. doi: 10.1016/s1286-4579(03)00157-6. [DOI] [PubMed] [Google Scholar]
- 30.Shrestha R, Gateff E. Ultrastructure and Cytochemistry of the cell types in the larval hematopoietic organs and hemolymph of Drosophila melanogaster. Dev Growth Differ. 1982;24:65–82. doi: 10.1111/j.1440-169X.1982.00065.x. [DOI] [PubMed] [Google Scholar]
- 31.Adachi-Yamada T, et al. p38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis. Mol Cell Biol. 1999;19:2322–2329. doi: 10.1128/mcb.19.3.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lagueux M, et al. Constitutive expression of a complement-like protein in Toll and JAK gain-of-function mutants of Drosophila. Proc Natl Acad Sci USA. 2000;97:11427–11432. doi: 10.1073/pnas.97.21.11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark KD, Pech LL, Strand MR. Isolation and identification of a plasmatocyte-spreading peptide from the hemolymph of the Lepidopteran insect Pseudoplusia includens. J Biol Chem. 1997;272:23440–23447. doi: 10.1074/jbc.272.37.23440. [DOI] [PubMed] [Google Scholar]
- 34.Simon SI, Green CE. Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu Rev Biomed Eng. 2005;7:151–185. doi: 10.1146/annurev.bioeng.7.060804.100423. [DOI] [PubMed] [Google Scholar]
- 35.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 36.Luo L, Liao YJ, Jan LY, Jan YN. Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- 37.Halfon MS, et al. New fluorescent protein reporters for use with the Drosophila Gal4 expression system and for vital detection of balancer chromosomes. Genesis. 2002;34:135–138. doi: 10.1002/gene.10136. [DOI] [PubMed] [Google Scholar]
- 38.Patel NH, Snow PM, Goodman CS. Characterization and cloning of fasciclin III: A glycoprotein expressed on a subset of neurons and axon pathways in Drosophila. Cell. 1987;48:975–988. doi: 10.1016/0092-8674(87)90706-9. [DOI] [PubMed] [Google Scholar]
- 39.Nelson RE, et al. Peroxidasin: A novel enzyme-matrix protein of Drosophila development. EMBO J. 1994;13:3438–3447. doi: 10.1002/j.1460-2075.1994.tb06649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.