Summary
At synapses, cell adhesion molecules (CAMs) provide the molecular framework for coordinating signaling events across the synaptic cleft. Amongst synaptic CAMs, the integrins, receptors for extracellular matrix proteins and counter-receptors on adjacent cells, are implicated in synapse maturation and plasticity, and memory formation. However, little is known about the molecular mechanisms of integrin action at central synapses. Here, we report that postsynaptic β3 integrins control synaptic strength by regulating AMPA receptors (AMPARs) in a subunit-specific manner. Pharmacological perturbation targeting β3 integrins promotes endocytosis of GluR2-containing AMPARs via Rap1 signaling, and expression of β3 integrins produces robust changes in the abundance and composition of synaptic AMPARs without affecting dendritic spine structure. Importantly, homeostatic synaptic scaling induced by activity deprivation elevates surface expression of β3 integrins, and in turn, β3 integrins are required for synaptic scaling. Our findings demonstrate a key role for integrins in the feedback regulation of excitatory synaptic strength.
Introduction
Activity-dependent synaptic plasticity is fundamental to the regulation and refinement of brain circuits. Despite our increasing knowledge of the mechanisms by which activity modulates synaptic efficacy, the underlying molecular basis remains to be fully elucidated. It has long been recognized that synapses contain a vast repertoire of cell adhesion molecules (CAMs). Once thought of as components required only for the structural and mechanical integrity of the connection, synaptic CAMs have been increasingly recognized as key players in signaling events regulating synapse function (Benson et al., 2000; Dityatev and Schachner, 2003; Yamagata et al., 2003). Amongst CAMs, the integrins, heterodimeric transmembrane receptors for extracellular matrix proteins and counter-receptors on adjacent cells, have received relatively little attention, especially in the central nervous system (CNS). However, several observations point to the integrins as potentially important regulators of synaptic transmission and plasticity. Many integrin subunits are expressed in the CNS, where some, such as β3, are enriched at synapses (Chan et al., 2003; Chavis and Westbrook, 2001; Pinkstaff et al., 1999; Shi and Ethell, 2006). Furthermore, the affinity of integrins for their ligands is finely modulated in response to intra- and extra-cellular signals; by interacting with the actin cytoskeleton and multiple intracellular signaling pathways, integrins can transduce extracellular mechanical and chemical stimuli into the cell (Hynes, 2002; Miranti and Brugge, 2002).
The Drosophila mutant Volado has provided the first persuasive evidence for a key role of integrins in the CNS. Ablation of Volado, which encodes for two α integrins, impairs short-term olfactory memory in flies (Grotewiel et al., 1998). Similarly, studies using mutant mice or specific inhibitors have shown that some integrins, such as β1, are involved in the early stabilization of long-term potentiation (LTP), a cellular model for learning and memory (Chan et al., 2003; Staubli et al., 1998). Further studies have however revealed that the function of integrins in synaptic transmission is more complex and extends beyond a specific requirement of integrins in LTP stabilization (Chan et al., 2006; Chavis and Westbrook, 2001; Huang et al., 2006).
In the present study, we address the role of integrins in excitatory synaptic transmission and plasticity at a cellular and molecular level by making use of a simple circuit formed by cultured hippocampal neurons. Specifically, we use acute pharmacological disruption of integrin-ligand interactions, molecular manipulations of integrin expression, and knockout mice to discriminate between pre- and post-synaptic actions of integrins. We find that postsynaptic β3 integrins rapidly adjust synaptic strength by regulating GluR2-containing AMPAR endocytosis via a Rap1 signaling pathway and that perturbing β3 integrin expression strongly affects synaptic AMPAR content. Remarkably, induction of homeostatic synaptic scaling by chronic blockade of network activity increases surface expression of endogenous β3 integrins, and in turn, β3 integrins are required for synaptic scaling. Our findings provide strong evidence that integrin signaling is tightly coupled to excitatory synaptic transmission and required for the homeostatic regulation of synaptic strength.
Results
Rapid Integrin-Mediated Modulation of mEPSCs
To investigate whether integrins regulate excitatory synaptic transmission on a rapid time scale, we used peptides containing the Arg-Gly-Asp (RGD) sequence found in the binding site of various integrin ligands. Such peptides disrupt the interactions of many integrins, including those present in neurons (Ruoslahti, 1996). We first examined the effects of acute bath application of RGD peptides on miniature excitatory postsynaptic currents (mEPSCs), which, in our recording conditions, represent the activation of synaptic AMPARs in response to the spontaneous release of neurotransmitter. A synthetic RGD peptide (GRGDSP) or the disintegrin echistatin, an RGD peptide from viper venom that targets β3- and β1-containing integrins with high affinity (Pfaff et al., 1994), reliably and reversibly reduced mEPSC amplitude by ~20% in all cells tested (Fig 1A&B; mEPSC amplitude after echistatin wash-out: 1.01 ± 0.01 of baseline [n=3]). The decrease in quantal size developed gradually, being maximal after 5 min (Fig 1D), and was specific as the control peptide (GRGESP) was ineffective (Fig 1E). Neither the initial mEPSC amplitude nor recording conditions promoting low or high release probability affected the efficacy of the peptides (Fig 1C; average mEPSC amplitude upon echistatin application: 0.82 ± 0.05 of baseline in both 2.2 mM Ca2+/1.5 mM Mg2+ [n=6] and 5 mM Ca2+/0.6 mM Mg2+ [n=7]). Moreover, the decrease in quantal size was not accompanied by changes in mEPSC waveform (Fig S1B). We also examined the effects on mEPSC frequency. Whereas the peptides had no effect on the overall mEPSC frequency, a transient burst of mEPSCs lasting a few seconds was observed after a variable time interval in many but not all cells tested (Fig S1A; 11 out of 13 for echistatin and 4 out of 5 for GRGDSP). The highly distinct time course of the change in mEPSC frequency compared to that of mEPSC amplitude and the differences in their Explorer.lnk occurrence and pharmacological profiles (Cingolani and Goda, unpublished observations) suggested that the two effects arose from distinct mechanisms. We next aimed to clarify the molecular mechanisms by which disrupting integrin interactions alters the amplitude of synaptic AMPAR currents.
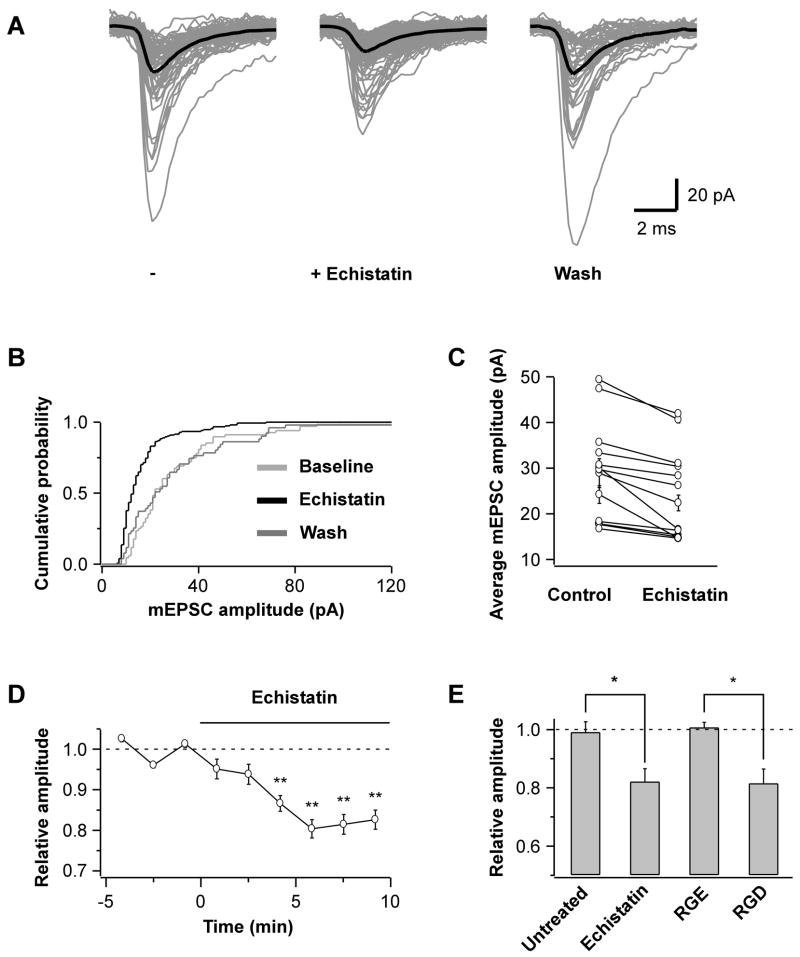
Fig 1. RGD Peptides Reduce mEPSC Amplitude.
(A) Overlay of mEPSC traces recorded from a hippocampal pyramidal neuron of rat primary cultures over a 5 min control period (−), the last 5 of 10 min bath application of echistatin (100 nM), and the last 5 of 10 min wash. Grey traces are aligned original mEPSCs and black traces represent the population average.
(B) Cumulative distribution plot of mEPSC amplitudes from the experiment shown in A (p<0.01, control vs. echistatin).
(C) Average mEPSC amplitude during control and echistatin application shown pairwise for each cell (p<0.05 for each pair).
(D) The reduction in mEPSC amplitude is maximal after 5 min bath application of echistatin (n=13). Each data point represents the mean amplitude over 100 s intervals normalized to baseline (**p<0.01 relative to baseline).
(E) Summary of the peptide effects on mEPSC amplitude. Mean amplitudes from the last 5 of 10 min application are plotted relative to 5 min baseline for untreated control cells (n=4) and echistatin (n=6); for the control GRGESP peptide (RGE, 200 μM; n=5) and GRGDSP (RGD, 200 μM; n=5) (*p<0.04).
Echistatin-Induced Reduction in Quantal Size Requires Elevation of Intracellular Ca2+
We first examined the involvement of the actin cytoskeleton, with which integrins dynamically interact via linker proteins such as talin and α-actinin (Liu et al., 2000). Depolymerizing actin filaments with latrunculin A increased the mEPSC frequency without affecting its amplitude (Fig 2B and data not shown) as previously reported (Morales et al., 2000). Moreover, latrunculin A did not prevent the echistatin-induced decrease in quantal size (Fig 2A–C), thus ruling out a major contribution of actin filaments in the integrin-mediated modulation of synaptic AMPAR currents. In contrast, buffering intracellular Ca2+ with BAPTA-AM or loading BAPTA into the postsynaptic neuron via the patch-pipette completely blocked the reduction in mEPSC amplitude (Fig 2A–C). The modulation of quantal size by integrins, therefore, requires an elevation of intracellular Ca2+ specifically in the postsynaptic cell.
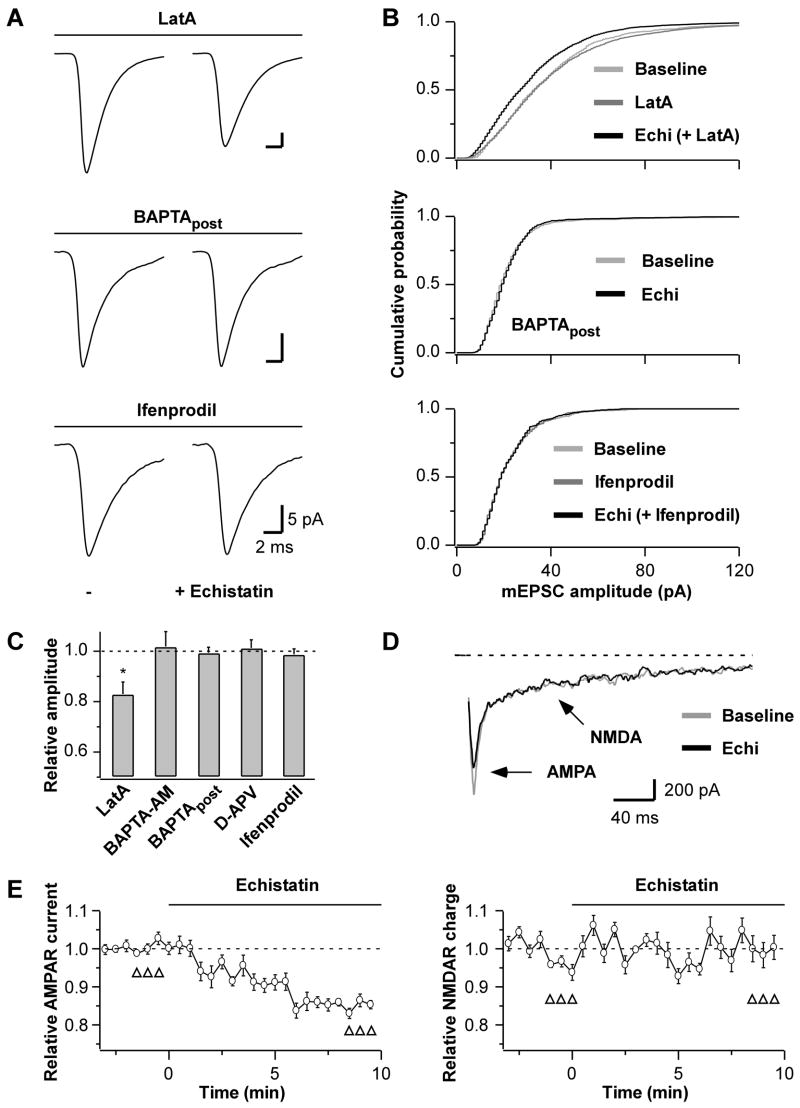
Fig 2. Echistatin-Mediated Reduction in mEPSC Amplitude Requires Elevation of Intracellular Ca2+.
(A) mEPSC population averages before (−) and after echistatin application (100 nM), and (B) cumulative distributions of mEPSC amplitudes from representative neurons where echistatin was applied at least 5 min after treatments with latrunculin A (LatA, top) or ifenprodil (bottom), or applied in the presence of BAPTA in the intracellular solution (BAPTApost, middle).
(C) Summary of the echistatin effects on mEPSC amplitude in the presence of blockers or Ca2+ chelators: LatA (20 μM; *p=0.04 relative to baseline, n=4), D-APV (50 μM; n=6), ifenprodil (3 μM; n=6), BAPTA-AM (100 μM, n=6), BAPTApost (37.5–50 mM, n=6). Echistatin does not reduce mEPSC amplitude in the presence of Ca2+ chelators or NMDAR blockers.
(D) Traces of evoked autaptic AMPAR and NMDAR synaptic currents from a representative experiment before (baseline) and after echistatin application (100 nM). The unclamped Na+ spikes have been blanked for clarity.
(E) Time courses of the echistatin effect on peak AMPAR current (left, n=5, p=0.03) and on NMDAR charge transfer (right, n=6, p=0.53; open triangles: responses used to evaluate statistical significance). Echistatin selectively reduces the AMPAR current.
We next tested the role of NMDA receptors (NMDARs), which are Ca2+-permeable and can be activated by a single quantum of neurotransmitter (Gomperts et al., 1998). Blocking NMDARs with either D-APV or the selective NR2B antagonist ifenprodil had no effect on mEPSC amplitude (Fig 2B) but prevented the decrease in quantal size induced by echistatin (Fig 2A–C; p=0.0002 vs. control without NMDAR antagonists [n=9]). Hence, acute modulation of synaptic AMPAR currents by integrins requires Ca2+ influx mediated by NMDARs.
The observed requirement for NMDARs raised the possibility that the reduction in AMPAR currents was a secondary effect of a direct action of integrins on NMDARs. Chronic incubation of hippocampal cultures with echistatin, for example, has been shown to affect NMDARs (Chavis and Westbrook, 2001). Therefore, to monitor the acute effects of echistatin on NMDAR currents, we elicited dual AMPAR and NMDAR synaptic currents from autapses in nominally 0 Mg2+. Evoked AMPAR currents were depressed by echistatin to a similar extent and with a similar time course as quantal AMPAR events. In contrast, the NMDAR component showed no change (Fig 2D&E). In addition, NMDAR currents evoked by local NMDA application (50 μM, 3 s-long puffs) were also unaffected by echistatin (100 nM; effect on charge transfer: 1.04±0.01 of baseline after 5–7 min [n=3, p=0.37]). Thus, although basal NMDAR activity is necessary for the integrin-mediated regulation of synaptic AMPAR currents, acute echistatin application does not affect NMDAR currents. The selective effect of echistatin on one of the two glutamatergic synaptic currents also excludes the presynaptic release machinery as the cause of reduced quantal size, as changes in neurotransmitter release would affect both AMPAR and NMDAR components.
While monitoring the effects of synthetic RGD peptides in parallel experiments, we found that GRGDSP (200–500 μM) rapidly and potently increased NMDAR currents, but only when the NMDAR co-agonist glycine was excluded from the extracellular recording solution; no effect was seen in the presence of saturating concentrations of glycine (20 μM; Fig S2C-E). In contrast, the more potent and specific peptide echistatin (100–300 nM) had no effect on NMDAR currents irrespective of the presence or absence of glycine (Fig 2D-E and Fig S2D). The EC50 of the glycine binding site of the NMDAR for glycine is less than 1 μM (Matsui et al., 1995). Therefore, in the absence of glycine, short RGD peptides, at the high concentrations used, may directly potentiate NMDAR currents either by interacting with the glycine binding site or because of glycine impurities in the peptide preparation. To avoid such complications, the effects of synthetic RGD peptides were always compared to those of echistatin.
The lack of involvement of actin filaments on integrin-modulation of synaptic AMPARs argues against the contribution of major structural alterations. A previous study using synthetic RGD peptides, however, reported of a change in dendritic protrusions (Shi and Ethell, 2006). The reduced quantal size could, therefore, result from postsynaptic structural modifications. To examine such a possibility, we carried out time lapse video recording of neurons expressing YFP-actin as a marker of dendrite and spine morphology. In agreement with previous studies (Shi and Ethell, 2006), high concentrations of short synthetic RGD peptides elongated dendritic protrusions and increased their mobility after 30 min of application; in contrast, echistatin treatment did not produce such changes (Fig S2A & B; dendritic protrusion length relative to baseline after 30 min with GRGDSP: +15.6 ± 2.3 %, p=0.002; with GRADSP: −3.1 ± 0.4 %, p=0.33; with echistatin: −1.8 ± 0.2 %, p=0.29; 33–63 protrusions from 3 cells each). The difference between the two RGD peptides could reflect their differential effect on NMDAR currents. Importantly, neither echistatin nor the synthetic RGD peptide produced a discernable structural change during the initial 10 min of application when the reduction in synaptic AMPAR currents was observed. These results suggest that the integrin-dependent reduction in mEPSC amplitude is not a secondary consequence of structural changes.
Integrins Regulate GluR2 Endocytosis
One of the major mechanisms for regulating synaptic strength involves changes in AMPAR trafficking. We therefore tested the possibility that disrupting integrin interactions reduced quantal size by affecting AMPAR endocytosis. Loading neurons with GTPγS via the patch-pipette to impair all GTP hydrolysis-dependent activities, including that of dynamin, prevented the reduction in mEPSC amplitude induced by echistatin (Fig 3B). To selectively impair dynamin-dependent endocytosis, we loaded neurons with the D15 peptide, which interferes with the binding of dynamin to amphiphysin (Luscher et al., 1999). As with GTPγS, the D15 peptide prevented the echistatin-induced reduction in mEPSC amplitude, whereas a scrambled version of the peptide (S15) did not (Fig 3A&B). The time course for mEPSC amplitudes in the presence of D15 was the same with or without echistatin (Fig 3B), thus excluding the possibility that a potential effect of echistatin was masked by a concomitant run up of the currents. Echistatin therefore reduces mEPSC amplitudes by affecting the exo-endocytic cycling of AMPARs.
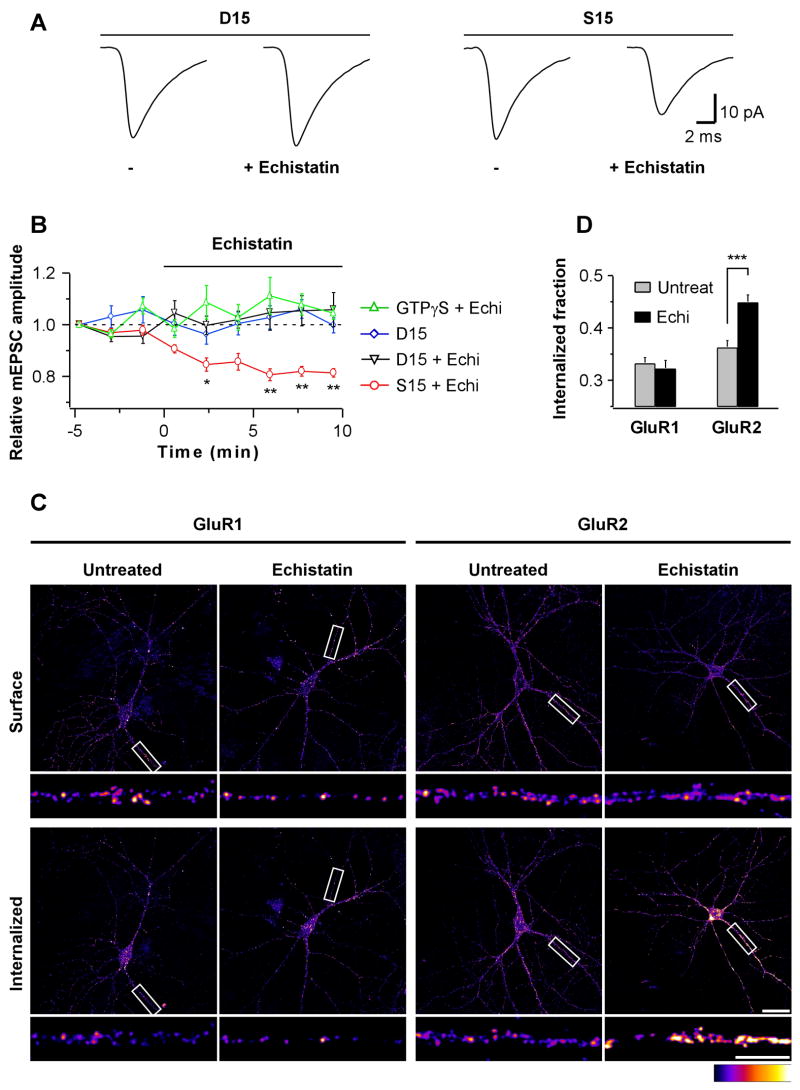
Fig 3. Integrins Regulate GluR2 Endocytosis.
(A) Neurons were loaded with D15 (left) or a scrambled S15 peptide (right) for at least 10 min prior to the baseline period. mEPSC population averages are from representative neurons during 5 min baseline (−) and the last 5 of 10 min application of echistatin (100 nM).
(B) Time course of mEPSC amplitudes from neurons loaded with GTPγS (0.5 mM; n=6), or D15 or S15 peptides (0.25–2 mM; D15, n=5; D15+echistatin, n=6; S15+echistatin, n=6). Each data point represents the average mEPSC amplitude over 100 s interval normalized to the first 100 s (*p=0.03, **p<0.01). Echistatin does not reduce mEPSC amplitudes under conditions that block endocytosis.
(C) Internalization assay for endogenous GluR1 (left) and GluR2 (right) subunits. Surface (top) vs. internalized fractions (bottom) from representative 10 min mock-(untreated) and echistatin-treated neurons (300 nM) are shown. Scale bars are 30 and 10 μm.
(D) Summary of GluR1 (n=21 images each from 4 independent cultures) and GluR2 (n=36 images each from 5 independent cultures) internalization assay. Internalized fraction indicates: (mean intensitysurface)/(mean intensitysurface + mean intensityinternalized). ***p=0.00002. Echistatin specifically increases GluR2 endocytosis.
To directly test whether disrupting integrin interactions has an effect on AMPAR endocytosis, we used an antibody-feeding fluorescence assay to quantify the degree of internalization of the endogenous AMPAR subunits GluR1 and GluR2. Acute treatment with echistatin increased the internalization of GluR2 by ~25% but did not affect that of GluR1 (Fig 3C&D). The synthetic RGD peptide but not the control peptide increased GluR2 internalization to a similar extent as echistatin (data not shown). We further investigated the specificity of echistatin for GluR2 by artificially changing the subunit composition of AMPARs by overexpressing either GluR1 or GluR2. Echistatin was no longer effective in reducing mEPSC amplitude in GluR1 overexpressing cells whereas GluR2 overexpressing neurons remained sensitive to echistatin (Fig S3). Taken together, these findings indicate that integrins affect quantal size by specifically regulating the endocytosis of GluR2-containing AMPARs.
Rap1 in Integrin Modulation of Synaptic AMPAR Currents
The small GTPase Rap1 is implicated in several aspects of integrin-mediated signaling in non-neuronal cells (Bos et al., 2001; Miranti and Brugge, 2002) and, in neurons, Rap1 is involved in regulating synaptic AMPAR content and trafficking (Fu et al., 2007; Xie et al., 2005; Zhu et al., 2002). Therefore, we asked whether the echistatin-dependent reduction in AMPAR currents by endocytic loss of GluR2 involved Rap1 activation. First, we examined whether Rap1 activity affected the surface expression of AMPAR subunits in our system. Consistent with previous reports (Fu et al., 2007; Zhu et al., 2002), inhibiting Rap1 activity by overexpressing a dominant-negative Rap1 (Rap1DN) significantly increased surface levels of endogenous GluR2 but not GluR1 in hippocampal pyramidal neurons (Fig S4). We next asked whether echistatin could activate Rap1 under conditions in which it induced GluR2 endocytosis. Following a 10 min incubation with echistatin, we found strong activation of endogenous Rap1 (Fig 4A). Thus, Rap1 activation is a potential signaling mechanism by which echistatin triggers the internalization of GluR2. To test whether the echistatin-induced decrease in quantal size requires Rap1 activation, we recorded mEPSCs from neurons overexpressing Rap1DN. Despite the increased GluR2 surface expression, echistatin was ineffective in reducing mEPSC amplitude (Fig 4B&C) and the lack of effect was not due to altered β3 surface levels, which remained unchanged (Fig S4). Rap2 is the most homologous small GTPase to Rap1 and, like Rap1, is present at excitatory synapses where it has also been implicated in AMPAR trafficking (Fu et al., 2007; Reuther and Der, 2000; Zhu et al., 2005). However, overexpression of Rap2 carrying a homologous mutation to that of Rap1DN did not impair the echistatin-mediated reduction in quantal size (Fig 4B&C), arguing for a specific involvement of Rap1. Most likely, therefore, disrupting integrin interactions leads to an activation of Rap1, which in turn, is required for the reduction in synaptic AMPAR currents.
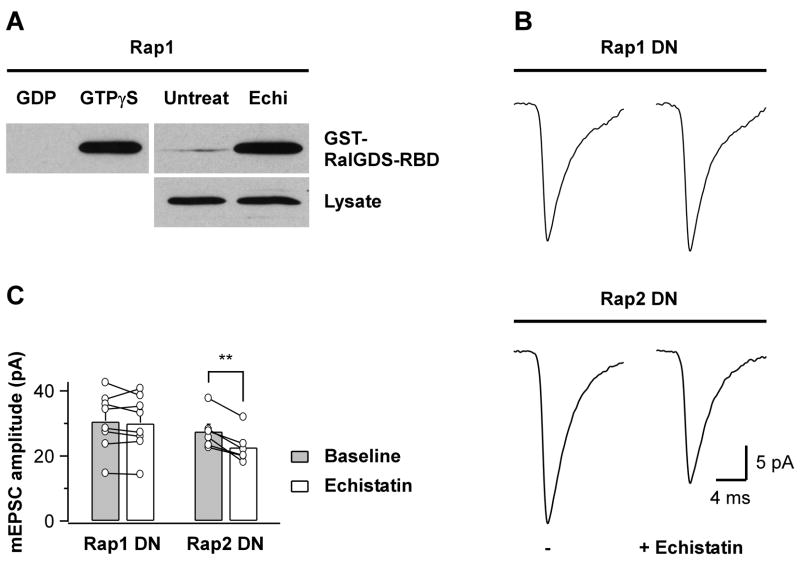
Fig 4. Echistatin-Mediated Activation of Rap1 Is Required for mEPSC Amplitude Decrease.
(A) Pulldown with GST-RalGDS-RBD from mock- (Untreat) and echistatin-treated (Echi, 300 nM, 10 min at 37°C) culture lysates (500 μg each) reveals activation of endogenous Rap1 by echistatin (top right). Comparable amounts of Rap1 were detected from the respective lysates (20 μg per lane, bottom right). Top left, lysates were treated with GDP (1 mM; negative control) or GTPγS (0.1 mM; positive control). Exposure times are different for the three panels shown. Similar results were obtained in four independent experiments.
(B) mEPSC population averages from representative neurons expressing Rap1DN (top) or Rap2DN (bottom), before (−) and after echistatin application (100 nM). Basal mEPSC amplitude is not significantly different between Rap1DN and Rap2DN transfected neurons (30.6 ± 3.1 pA vs. 27.5 ± 2.2 pA, respectively; n=6–8, p=0.44).
(C) Summary of experiments as in B shown pairwise for individual cells and as bar graph for the average cell population (**p=0.002). Echistatin does not reduce mEPSC amplitudes under conditions that prevent Rap1 activation.
Postsynaptic β3 Integrins Control Synaptic AMPAR currents
RGD-containing peptides pose some limitations in assessing the role of integrins. First, they target several integrin heterodimers, and therefore they alone cannot be used to identify the specific subunits involved in the biological response under investigation. Second, they do not discriminate between intra-cellular (cis) and cell-cell or cell-substrate (trans) actions of integrins when multi-cellular structures are involved. Thus, in order to determine the cellular location and subtypes of integrins involved in regulating glutamatergic quantal responses, we focused on β1 and β3 integrins, which are targeted by echistatin. We used either full-length wild-type β1 or β3 integrins (WTβ1 and WTβ3, respectively) or truncated forms of the two subunits in which the entire extracellular domain was replaced with EGFP (CTβ1 and CTβ3, respectively); previous studies have established that these chimeras localize with the respective endogenous integrins, and when expressed at high levels, act as specific dominant negative inhibitors of integrin function (LaFlamme et al., 1994; Smilenov et al., 1999). We transfected our cultures with these constructs after the initial wave of synaptogenesis and examined neurons after 2 days of expression. WTβ3, CTβ3, and CTβ1 showed punctate surface expression along the processes without an appreciable effect on the overall cell morphology and passive membrane properties (Fig S5A,E). In contrast, WTβ1 was toxic for neurons under a variety of conditions and could not be used.
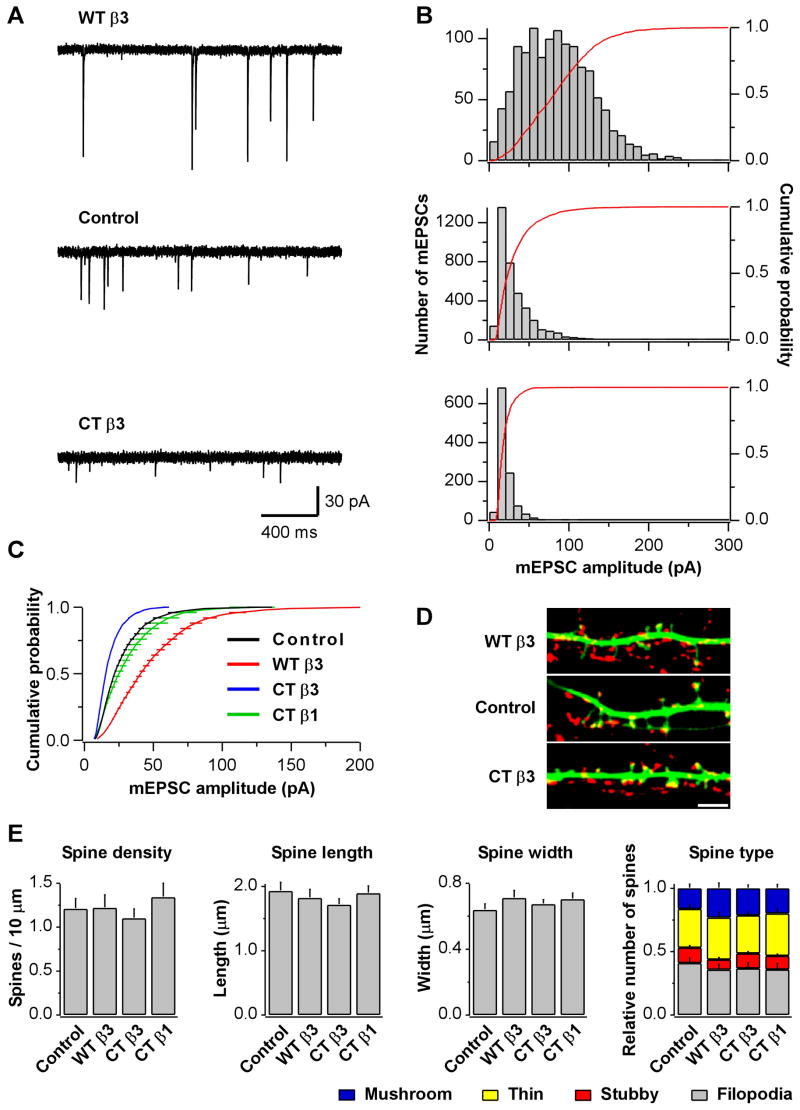
By taking advantage of the low transfection efficiency in primary neuronal cultures, we restricted mEPSC recordings to transfected neurons surrounded only by non-transfected cells. In this configuration, the patched neuron received synaptic inputs mostly from non-transfected cells, and we could consider the effects of postsynaptic expression of the constructs. Expression of β3 integrins resulted in a robust and bidirectional change in mEPSC amplitude relative to control cells: WTβ3 produced a large increase (mean: −48.6±5.5 pA for WTβ3 [n=25] vs. −27.9±3.0 pA for controls [n=22, p=0.001]) whereas CTβ3 produced a large decrease (mean: −18.9±1.0 pA for CTβ3 [n=26] vs. −27.9±3.0 for controls [n=22, p=0.002]) (Fig 5A–C). mEPSC waveform, however, remained unaltered (Fig S5C). Cells expressing CTβ3 showed a tendency towards lower mEPSC frequency, probably reflecting a larger number of mEPSCs falling below the detection threshold (Fig S5D). The effects of exogenous β3 integrins on quantal size were not influenced by chronic blockade of NMDAR activity (Fig S5F). Compared with the large changes induced by the expression of β3 constructs, CTβ1 had no effect on mEPSC amplitude (mean: −31.3±5.4 pA for CTβ1 [n=8] vs. −29.1±7.0 pA for controls [n=8, p=0.81]) (Fig 5C), despite being expressed at similar levels as CTβ3 (Fig S5A&B).
Fig 5. Postsynaptic Expression of β3 Integrins Alters mEPSC Amplitude.
(A) Sample mEPSCs and (B) histograms of mEPSC amplitudes from representative neurons overexpressing WTβ3 and mRFP (WTβ3, top), mRFP (control, middle), or CTβ3 and mRFP (CTβ3, bottom).
(C) Summary of group data from experiments as in A. WTβ3 increases and CTβ3 decreases mEPSC amplitudes.
(D) Images of dendrites from pyramidal neurons overexpressing WTβ3 and GFP (WTβ3, top), GFP (control, middle), or CTβ3 and GFP (CTβ3, bottom). Synapsin (red) was used as a presynaptic marker. Scale bar is 4 μm.
(E) Summary of spine measurements. None of the differences were statistically significant (n=19 cells for control and WTβ3; n=20 for CTβ3 and CTβ1).
Whereas acute application of RGD peptides did not induce structural changes over a timescale of minutes, expression of integrin constructs over a 2 day period could potentially affect spine morphology, especially given their large effects on quantal size. However, extensive morphological analyses failed to reveal significant alterations in spine density, length, or width in neurons expressing WTβ3, CTβ3, or CTβ1 relative to control GFP neurons. Moreover, the proportion of mushroom, thin, and stubby spines as well as filopodia also remained unchanged (Fig 5D&E). These results suggest that β3 integrin-dependent changes in quantal size are not accounted for by alterations in synapse morphology and that postsynaptic β3 integrins bi-directionally regulate AMPAR currents independently of structural changes.
Control of AMPAR Subunit Composition by β3 Integrins
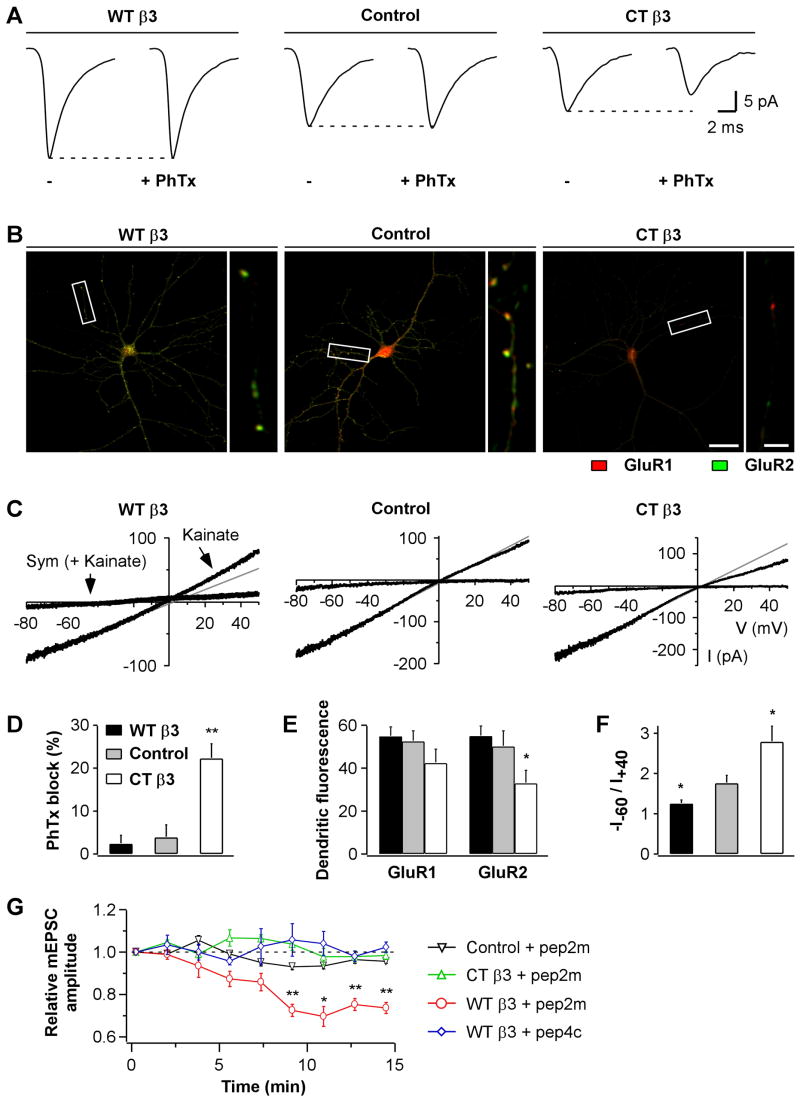
We used four complementary approaches to investigate whether the effects of β3 integrins on quantal size could be explained by changes in AMPAR subunit composition. First, we tested the efficacy of Philanthotoxin-433 (PhTx), a specific blocker of GluR2-lacking AMPARs, on mEPSC amplitudes. PhTx had no effect in control neurons as reported previously (Thiagarajan et al., 2005) nor in WTβ3 cells, but it decreased mEPSC amplitude in CTβ3 neurons, which already displayed severely reduced synaptic currents (Fig 6A&D). This selective effect of PhTx suggested that GluR2-containing AMPARs predominated in control and WTβ3 cells and that CTβ3 expression decreased quantal size by preferentially depleting GluR2-containing AMPARs.
Fig 6. Postsynaptic β3 Integrins Affect AMPAR Subunit Composition.
(A) mEPSC population averages from representative neurons overexpressing WTβ3 and mRFP (WTβ3, left), mRFP (Control, middle), or CTβ3 and mRFP (CTβ3, right), during 5 min baseline (−) and the last 5 of 10 min application of the GluR2-lacking AMPAR blocker PhTx (10 μM).
(B) Representative images of GluR1 (red) and GluR2 (green) surface labeling for neurons transfected with WTβ3 and GFP (WTβ3, left), GFP (Control, middle), or CTβ3 and GFP (CTβ3, right). GluR1 and GluR2 were stained with Cy3- and Cy5-conjugated secondary antibodies, respectively. GFP fluorescence is not shown. Scale bars are 30 and 5 μm.
(C) Agonist-evoked AMPAR I-V relationships from untransfected neurons (middle), and neurons overexpressing WTβ3 (left) or CTβ3 (right). Recordings were performed in outside-out somatic patches in response to a voltage-ramp from +60 to −90 mV delivered over 3 s. Currents were elicited by bath application of kainate (100 μM) and blocked by SYM 2206 (Sym; 25–50 μM).
(D) Summary of the effects of PhTx on mEPSC amplitudes. The reduction of mEPSC amplitudes is shown relative to baseline (n=6 for each condition, **p=0.01 relative to baseline). Only mEPSCs recorded from CTβ3-expressing neurons are reduced by PhTx.
(E) Summary of the GluR1 and GluR2 surface levels for experiments as in (B). Fluorescence intensity was quantified on the full dendritic arbor (n=27, 23, and 26 images for WTβ3, control, and CTβ3, respectively; *p=0.02 relative to control).
(F) Summary of I-V rectification indices (−I−60/I+40) from recordings as in (C) (WTβ3, n=5; control, n=8; CTβ3, n=6; *p = 0.04 relative to control). WTβ3 expression decreases and CTβ3 expression increases the rectification of agonist-evoked AMPAR currents. (G) Time course of mEPSC amplitudes from untransfected neurons (Control), and neurons overexpressing WTβ3 or CTβ3, using intracellular solutions supplemented with pep2m or pep4c peptides (150 μM; Control+pep2m, n=4; CTβ3+pep2m, n=6; WTβ3+pep2m, n=6; WTβ3+pep4c, n=4). Each data point represents the average mEPSC amplitude over 100 s interval normalized to the first 100 s (*p=0.02 and **p<0.007). Only mEPSCs recorded from WTβ3 neurons are decreased by pep2m.
Second, we examined by immunofluorescence labeling if the expression of β3 integrins affected the surface level and distribution of endogenous GluR1 and GluR2 subunits (Fig 6B). In control neurons, both GluR1 and GluR2 signals were punctate although some GluR2 showed diffuse expression along the dendrites. In WTβ3 neurons, the diffuse GluR2 signal was less apparent, and instead, surface GluR2 fluorescence was highly punctate and bright. The total dendritic surface fluorescence for GluR2 and GluR1, however, was not measurably altered relative to controls (Fig 6E). In CTβ3 neurons, both fluorescence signals were generally weak, with the GluR2 signal being significantly reduced relative to control cells (Fig 6B,E), and the ratio of surface GluR2 to GluR1 was also decreased (1.07±0.10 in control cells vs. 0.81±0.06 in CTβ3 neurons; p=0.03). The specific reduction in the GluR2 to GluR1 ratio in CTβ3 neurons is in line with the PhTx-sensitivity, which was also exclusive to CTβ3 neurons.
Third, we recorded agonist-evoked AMPAR currents and studied their degree of rectification, which is highly sensitive to GluR2 content. WTβ3 neurons showed more outwardly rectifying current-voltage (I-V) relationships than controls, indicating enrichment in GluR2. In contrast, CTβ3 neurons exhibited inwardly rectifying I-V relationships, denoting paucity of GluR2 subunits (Fig 6C&F).
Last, to selectively target GluR2-containing AMPARs, we took advantage of a peptide, pep2m, containing the NSF binding sequence of GluR2. This peptide, when intracellularly perfused, compromises GluR2 surface delivery and thereby reduces currents mediated by GluR2-containing AMPARs (Luscher et al., 1999; Song et al., 1998). We found that intracellular dialysis of pep2m significantly decreased mEPSC amplitudes only in neurons overexpressing WTβ3 (Fig 6G). The reduction was specific to pep2m, because a homologous control peptide, pep4c, from GluR4, which is unable to bind NSF (Nishimune et al., 1998), had no effect. In agreement with a previous study that used concentrations of pep2m similar to ours (Noel et al., 1999), mEPSC amplitudes were not significantly reduced by pep2m in non-transfected cells. Since pep2m inhibits evoked EPSCs more potently than mEPSCs (Luscher et al., 1999; Nishimune et al., 1998), possibly because of its activity-dependent actions (Luscher et al., 1999), we confirmed the effectiveness of pep2m in our system using autaptic cultures. Evoked autaptic EPSCs declined in neurons loaded with pep2m but remained stable with pep4c (Fig S6) indicating that pep2m was active in our recording conditions.
Collectively, these results support a model in which β3 integrins increase quantal size by effectively stabilizing synaptic GluR2-containing AMPARs, whereas loss of contact with β3 extracellular ligands as a consequence of CTβ3 expression or application of RGD peptides depletes synapses preferentially of GluR2 subunits.
Postsynaptic β3 Integrins Mediate the Echistatin-Induced Reduction in Quantal Size
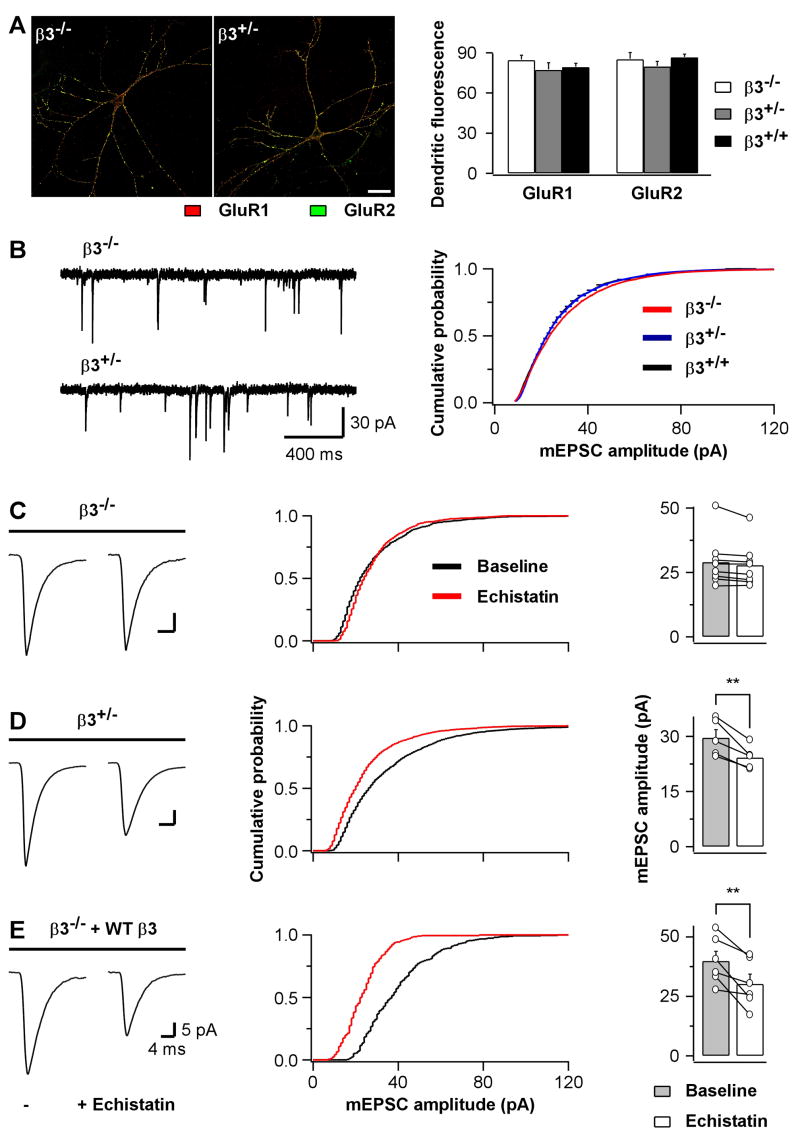
To further investigate the regulation of synaptic AMPARs by β3 integrins, we next made use of β3 integrin-deficient mice, which were viable and fertile (Hodivala-Dilke et al., 1999). Hippocampal pyramidal neurons from homozygous (β3−/−) and heterozygous (β3+/−) pups were plated on wild type glial cells. They grew normally in culture and developed morphologies comparable to neurons cultured from wild type littermates (β3+/+). The surface level and distribution of GluR1 and GluR2 subunits were not detectably different in β3−/−, β3+/−, and β3+/+ neurons (Fig 7A). Furthermore, patch clamp recordings revealed no significant differences in passive membrane properties of β3−/−and β3+/− mutant neurons compared to wild type cells (Fig S7C). Similarly, quantal excitatory synaptic transmission was normal in β3−/− and β3+/− cultures relative to β3+/+ cultures: no significant changes were detected for mEPSC amplitude, frequency, and waveform (Fig 7B and Fig S7A&B). The lack of an apparent phenotype suggests that β3 integrins are dispensable for the development and basic function of synapses. However, if β3 integrins are specifically involved in regulating synaptic AMPARs, then echistatin should be ineffective in modifying quantal size in neurons lacking β3 integrins. Indeed, echistatin application had no effect on mEPSC amplitude in β3−/− neurons whereas it reduced quantal size in β3+/− neurons as effectively as in rat cultures (Fig 7C&D; cf. Fig 1). Thus, β3 integrins are necessary for the echistatin-induced reduction in quantal size. To confirm the specific involvement of postsynaptic β3 integrins, we tested whether postsynaptic expression of WTβ3 in β3−/− neurons for 2 days could rescue the effect of echistatin. As shown in Fig 7E, in WTβ3-expressing neurons from β3 integrin-deficient cultures, echistatin reduced mEPSC amplitude (0.76 ± 0.07 of baseline, n=6) to an extent comparable to control mouse β3+/− cultures (0.82 ± 0.03 of baseline, n=5; p=0.40 compared to rescue) and rat cultures (0.82 ± 0.05 of baseline, n=13, p=0.42 compared to rescue). Moreover, postsynaptic expression of the dominant negative CTβ3 in rat cultures was sufficient to prevent the echistatin-dependent reduction in mEPSC amplitude (Fig S8). Taken together, these experiments indicate that postsynaptic β3 integrins are necessary and sufficient for the echistatin-induced down-regulation of synaptic AMPAR currents.
Fig 7. Postsynaptic β3 Integrins Mediate the Echistatin-Induced Reduction in mEPSC Amplitude.
(A) Left, representative images of GluR1 (red) and GluR2 (green) surface labeling for hippocampal pyramidal neurons from β3−/− and β3+/− mouse primary cultures. Scale bar is 30 μm. Right, summary of the surface levels of GluR1 and GluR2 (n=29, 24, and 16 images for β3−/−, β3+/−, and β3+/+, respectively).
(B) Left, sample traces of mEPSC recordings from hippocampal pyramidal neurons of β3−/− (top) and β3+/− (bottom) mouse primary cultures. Right, summary of group data from experiments as on the left but also including recordings from β3+/+ mouse cultures (average amplitude: −30.4.6±3.8 pA for β3−/− [n=21], −28.7±1.8 for β3+/− [n=16], and −28.4±2.3 pA for β3+/+ [n=13]). None of the differences were statistically significant. (C) Left, mEPSC population averages of a hippocampal pyramidal neuron from β3−/−mouse primary cultures before (left trace) and after (right trace) echistatin application (100 nM). Middle, cumulative distribution of mEPSC amplitudes for the experiment shown on the left. Right, summary for individual cells shown pairwise and for the average cell population shown as bar graph.
(D) As in C but for hippocampal pyramidal neurons from β3+/− mouse primary cultures. **p=0.009.
(E) As in C but for β3−/− hippocampal pyramidal neurons transfected with WT β3. **p=0.01.
Postsynaptic β3 Integrins Are Required for Homeostatic Synaptic Scaling
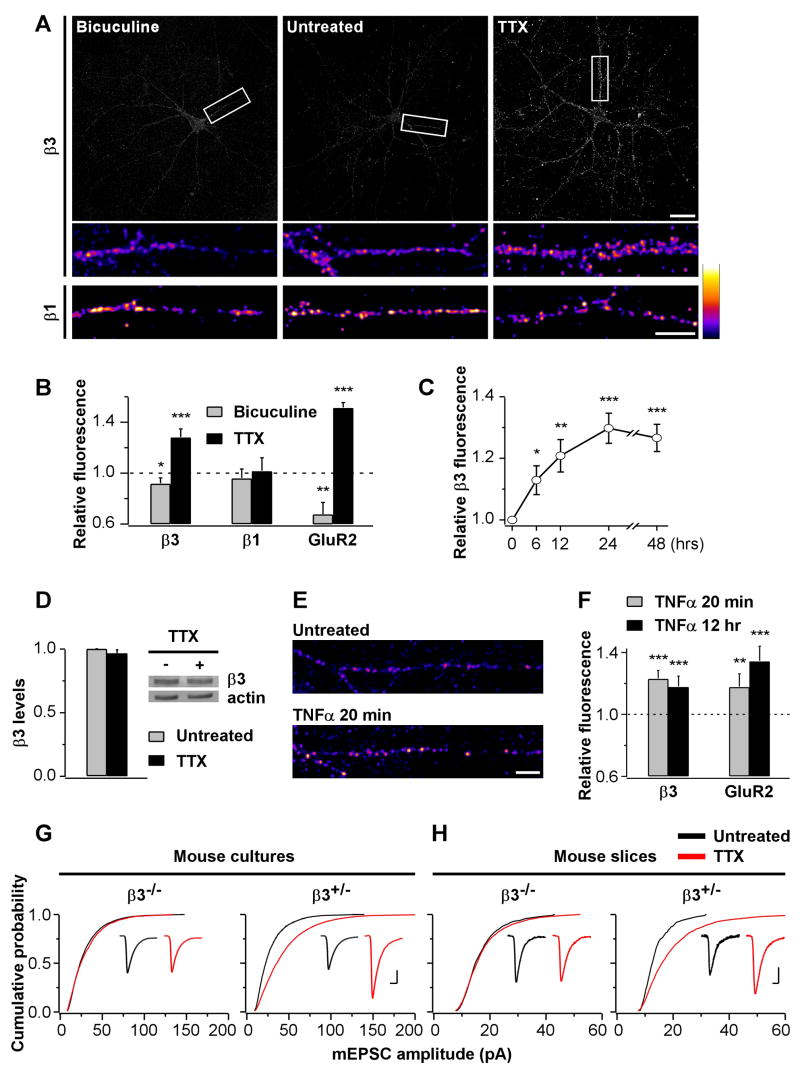
Thus far our findings identify the contribution of postsynaptic β3 integrins in regulating synaptic strength and AMPAR subunit composition. We next asked whether β3 integrins could play a role in activity-dependent changes in synaptic strength at mature synapses. Such a function for this particular integrin subtype has not been explored to date. Notably, homeostatic synaptic plasticity induced by chronic modifications of neuronal network activity scales quantal size by changing the abundance and composition of AMPARs (O'Brien et al., 1998; Sutton et al., 2006; Thiagarajan et al., 2005; Turrigiano et al., 1998; Wierenga et al., 2005). However, the underlying molecular mechanisms are still poorly understood. In order to determine whether integrins are involved in synaptic scaling, we first examined if the surface expression of endogenous integrins are affected by chronic changes in neuronal network activity. Live antibody labeling revealed significant changes in the level of β3 but not β1 integrins in response to chronic treatments with bicuculline or tetrodotoxin (TTX) to enhance or reduce network activity, respectively. TTX was especially effective in increasing β3 surface levels (Fig 8A&B). The effect developed gradually over 24 h (Fig 8C) without a detectable change in total protein expression (Fig 8D).
Fig 8. Postsynaptic β3 Integrins are Required for Synaptic Scaling.
(A) Surface labeling of endogenous β3 (top) and β1 (bottom) integrins for untreated (middle) and bicuculline- (20 μM, 48 h; left) or TTX-treated (1 μM, 48 h; right) hippocampal cultures. Scale bars are 30 and 10 μm.
(B) Summary of the effects of 48 h bicuculline or TTX treatments on the surface expression of GluR2 and β1 and β3 integrins (bicuculline, β3, n=28 images; TTX, β3, n=65; bicuculline, β1, n=30; TTX, β1, n=30; bicuculline, GluR2, n=16; TTX, GluR2, n=18; *p=0.03, **p=0.006, ***p<0.0002 relative to untreated controls). Chronic changes in neuronal network activity alter surface expression of β3 integrins and GluR2 but not β1 integrins.
(C) β3 integrin surface expression in response to TTX treatments of different durations (6 h, n=25; 12 h, n=24; 24 h, n=26; 48 h, n=30; *p=0.05, **p=0.004, ***p<0.00001 relative to untreated controls).
(D) Western blot of protein extracts (20 μg per lane) from untreated (−) and TTX-treated (1 μM, 48 h) sister cortical cultures reveals no changes in the total amount of β3 integrins. The same membrane was re-probed with an anti-actin antibody. Bar graphs indicate quantification of three independent experiments.
(E) Surface labeling of β3 integrins for untreated (top) and TNFα-treated (0.1 μg/μl, 20 min; bottom) hippocampal cultures. Surface β3 levels are higher in TNFα-treated cultures. Scale bar is 10 μm.
(F) Summary of the increase in β3 and GluR2 surface expression induced by TNFα (n=27 each for β3 and n=39 each for GluR2; **p=0.002, ***p<0.0004 relative to untreated controls).
(G) Summary of mEPSC amplitudes from hippocampal pyramidal neurons of β3−/− and β3+/− mouse primary cultures incubated with (red) or without (black) TTX (1 μM, 48 h; β3−/−, n=21; β3−/−+TTX, n=21; β3+/−, n=16; β3+/−+TTX, n=18; p=0.008 between β3+/− and β3+/− +TTX). Insets, mEPSC population averages from representative experiments. Scale bars are 4 ms and 10 pA.
(H) As in G but for CA1 pyramidal neurons of mouse hippocampal slice cultures (β3−/−, n=18; β3−/−+TTX, n=19; β3+/−, n=18; β3+/−+TTX, n=18; p=0.000003 between β3+/− and β3+/−+TTX). Scale bars for insets are 8 ms and 5 pA.
Next, we wanted to gain an insight into how chronic changes in network activity modified surface expression of β3 integrins. Recently, synaptic scaling induced by TTX has been shown to require the progressive accumulation in the extracellular medium of tumor-necrosis factor-α (TNFα) derived from glia (Stellwagen and Malenka, 2006). Interestingly, exposure to TNFα is known to increase αVβ3 integrin levels in non-neuronal cells (Gao et al., 2002; Hynes, 2002). We therefore examined whether TNFα affected surface expression of β3 integrins in hippocampal pyramidal neurons. Acute treatment with TNFα, within 20 min, increased β3 levels nearly as much as chronic incubation with TTX (~23% vs. ~28% increase, Fig 8E&F), and the enhancement was maintained at least up to 12 h of TNFα treatment. Therefore, TNFα is a plausible candidate for mediating the observed increase in surface β3 integrins upon activity deprivation by TTX.
If the increase in β3 is instrumental in the homeostatic regulation of synaptic AMPAR currents, then TTX-induced synaptic scaling should be blocked in neurons overexpressing dominant negative CTβ3. Also, TTX might be ineffective in triggering additional quantal scaling in WTβ3 cells with elevated surface β3 integrins. As summarized in Fig S9A, chronic TTX treatment scaled mEPSCs in control neurons but not in CTβ3 cells suggesting that β3 integrins are required for synaptic scaling. Moreover, TTX did not further increase mEPSC amplitude in WTβ3 neurons. This result is consistent with the effects of TTX being occluded by WTβ3 expression, suggestive of shared mechanisms involving up-regulation of surface β3. In further support, exogenous expression of β3 integrin constructs scaled mEPSCs multiplicatively (Fig S9B), which is a hallmark feature of homeostatic synaptic plasticity (Turrigiano et al., 1998).
Our results thus far are consistent with TTX-induced synaptic scaling being dependent on an up-regulation of postsynaptic β3 integrins. To directly assess the requirement of β3 integrins for this form of synaptic plasticity, we compared synaptic scaling in β3−/− and β3+/− neurons in dissociated cultures and in slice cultures, a preparation that largely retains the native hippocampal circuitry. In both preparations, TTX treatment scaled mEPSCs in β3+/− neurons to a similar extent as was observed in rat cultures. In contrast, scaling was prevented in β3−/− cultures (Fig 8G). Altogether, these results demonstrate that β3 integrins are required for homeostatic synaptic scaling induced by activity deprivation.
Discussion
Previous studies using RGD peptides have demonstrated that synaptic transmission and ion channels can be influenced by integrins on a rapid timescale. For example, at the neuromuscular junction, integrins modulate neurotransmitter release induced by muscle stretch by directly translating mechanical changes in membrane tension across the synaptic cleft (Chen and Grinnell, 1995; Chen and Grinnell, 1997). Furthermore, RGD peptides affect the activity of high voltage-activated Ca2+ channels within minutes (Wildering et al., 2002; Wu et al., 1998). In our experiments, echistatin increased dynamin-dependent GluR2 endocytosis also within minutes, leading to a reduction in synaptic AMPAR currents. To our knowledge, this is the first study showing an involvement of integrins in dynamin-dependent endocytosis. It is unlikely that the effect was a consequence of a generalized increase in endocytosis, as it was specific to GluR2 over GluR1, and NMDAR currents were not affected. Given that AMPAR endocytosis likely occurs mainly at extrasynaptic sites (Ashby et al., 2004; Blanpied et al., 2002), integrin-mediated cell adhesion may gate the lateral diffusion of GluR2-containing AMPARs between synaptic sites and extrasynaptic endocytic hotspots. Alternatively, the increase in GluR2 endocytosis may be due to a modification in integrin-dependent signaling that directly converges on the endocytic machinery.
As previously reported for AMPA-, NMDA-, and insulin-induced AMPAR internalization (Beattie et al., 2000; Carroll et al., 1999; Ehlers, 2000; Lin et al., 2000; Lissin et al., 1999; Man et al., 2000), GluR2 endocytosis induced by echistatin required the elevation of intracellular Ca2+. Moreover, echistatin activated the small GTPase Rap1, and active Rap1 was necessary for the reduction in AMPAR currents. While these observations are consistent with the reported involvement of Rap1 in AMPAR trafficking (Fu et al., 2007; Thomas and Huganir, 2004; Zhu et al., 2002) and the requirement of NMDARs and intracellular Ca2+ elevation for Rap1 activation (Franke et al., 1997; Kennedy et al., 2005; Xie et al., 2005), our findings identify a novel link between integrin signaling and Rap1-dependent regulation of AMPAR trafficking. Notably, while basal NMDAR activity was necessary for the echistatin-induced decrease in AMPAR currents, probably as a source of Ca2+, echistatin application by itself did not alter NMDAR currents. It is therefore likely that integrin- and NMDAR-dependent signaling pathways converge on Rap1 to exert joint control on AMPARs.
How do the effects of acute pharmacological treatments relate with those of chronic manipulations of β3 integrin expression? At least part of the mechanisms appears to be shared since echistatin required functional postsynaptic β3 integrins in order to reduce synaptic AMPAR currents. Moreover, both acute RGD peptide application and chronic expression of β3 integrin constructs affected quantal size by preferentially targeting GluR2-containing AMPARs (Fig S10). Previous work has shown that PICK1, which binds to the GluR2 C-terminus and PKC, also controls the GluR2 content of synaptic AMPARs (Terashima et al., 2004). It will be of interest to see whether PICK and PKC are involved in mediating the effects β3 integrins on synaptic AMPAR currents.
Surprisingly, echistatin-mediated reduction of mEPSC amplitude was not dependent on the polymerization state of actin. Moreover, neither echistatin application nor overexpression of β3 integrins significantly affected dendrite and spine morphology. Application of short synthetic RGD peptides at high doses have been reported to both lengthen and shorten dendritic protrusions (Bourgin et al., 2007; Shi and Ethell, 2006). Because, at the concentrations used in the above studies, RGD peptides may have integrin-independent effects (Fig S2; Buckley et al., 1999), the results based on high concentrations of short synthetic RGD peptides should be interpreted with caution. Studies in which various integrin subunits were genetically ablated also reported no effect on neuronal or synaptic structure (Chan et al., 2003; Chan et al., 2006; Grotewiel et al., 1998; Huang et al., 2006; but see Rohrbough et al., 2000). While our data indicate a specific role for β3 integrins in regulating synaptic AMPARs independently of postsynaptic structure, other integrin subtypes may be involved in spine morphogenesis (cf. (Webb et al., 2007).
We find that integrins are critical for homeostatic synaptic plasticity, a previously unrecognized function for this class of cell adhesion molecules in the CNS. In particular, our experiments point to a specific role for β3 integrins. Whereas exogenous expression of β3 integrins was sufficient to scale synaptic AMPAR currents, chronic modification of network activity also altered surface expression of endogenous β3. Importantly, TTX was ineffective in scaling synaptic AMPAR currents when β3 integrins were genetically ablated, demonstrating their requirement for this form of synaptic plasticity.
Although expression of synaptic scaling is thought to be caused, at least in part, by changes in the abundance and composition of AMPARs, the molecular mechanisms involved are still poorly understood (Davis, 2006). Furthermore, different induction protocols for homeostatic plasticity may achieve scaling by targeting different AMPAR subunits (Ju et al., 2004; Thiagarajan et al., 2005; Wierenga et al., 2005). For instance, a recent report demonstrated that homeostatic synaptic scaling, similarly to LTP, involves a transient incorporation of GluR2-lacking AMPARs, which are subsequently replaced by GluR2-containing receptors (Plant et al., 2006; Sutton et al., 2006; but see Adesnik and Nicoll, 2007). Without this exchange in subunit composition, the increase in synaptic strength would eventually decline. Amongst potential molecular mediators of homeostatic synaptic plasticity, the activity-dependent release of a cytokine, TNFα, from glial cells has been shown to be critical for TTX-induced synaptic scaling (Rutherford et al., 1998; Stellwagen and Malenka, 2006). Our study identifies a novel role for β3 integrins in TNFα-dependent synaptic scaling. Notably, in non-neuronal cells, exposure to TNFα augments αVβ3 integrin levels (Gao et al., 2002; Hynes, 2002). Similarly, in neurons, we find that acute treatment with TNFα increases surface β3 expression nearly as much as chronic incubation with TTX. As previously shown (Stellwagen et al., 2005), we noticed that acute application of TNFα induced a modest increase in GluR2 surface levels (~18%). However, prolonged treatment with TNFα (12h) augmented GluR2 surface expression more robustly (~34%). The progressive accumulation of glial TNFα in the extracellular medium in response to activity deprivation may therefore control synaptic scaling by first increasing GluR1 expression (Stellwagen et al., 2005; Stellwagen and Malenka, 2006) and subsequently up-regulating GluR2 levels on a longer time scale and in an integrin-dependent manner. In this way, TTX-induced synaptic scaling will eventually result in an increase in quantal size without an apparent shift in subunit composition (Wierenga et al., 2005). We thus propose that postsynaptic β3 integrins are specifically involved in the late GluR2-dependent phase of homeostatic synaptic scaling that is necessary to maintain enhanced synaptic strength.
In summary, our findings are consistent with a model in which β3 integrins function as a negative feedback regulator of synaptic strength. Synaptic AMPARs are effectively stabilized by β3 integrins whereas loss or lack of contact with β3 extracellular ligands is associated with a reduction in AMPAR content. We propose that synapses exploit the state of β3 integrin interactions with their extracellular ligands to fine tune AMPAR currents according to the level of neuronal network activity (Fig S10). Trans-synaptic interactions between pre- and post-synaptic cell adhesion molecules, for example between postsynaptic β3 integrins and presynaptic L1 or semaphorin proteins (Blaess et al., 1998; Pasterkamp et al., 2003), would provide the most direct way to detect changes in presynaptic activity. However, equally important roles might be played by indirect interactions across the synaptic cleft and between neurons and glial cells via extracellular matrix proteins and the integrins that interact with them.
Experimental procedures
(Detailed methods are described in Supplementary Experimental Procedures.)
Neuronal Cultures and Transfection
Dissociated and slice hippocampal cultures were prepared as previously described (De Simoni et al., 2003; Morales et al., 2000). Dissociated rat hippocampal cultures were transfected at 8–12 DIV using a Ca2+ phosphate protocol (Xia et al., 1996) and used for experiments after 1–3 days. For transmembrane proteins, surface expression of the constructs was confirmed by live labeling with antibodies against extracellular epitopes.
Electrophysiology
Whole-cell recordings were performed from pyramidal neurons of dissociated hippocampal cultures and CA1 pyramidal neurons of mouse slice cultures as previously described (De Simoni et al., 2003; Morales et al., 2000). Recordings from experimental and control cells were interleaved and sister cultures were used between groups. Each set of experiments was performed on at least two independent cultures.
Imaging
Internalization of endogenous GluR1 and GluR2 was determined using an antibody-feeding assay as previously described (Passafaro et al., 2001). Confocal images were analyzed blindly to the experimental condition using ImageJ (NIH). For each channel, dendritic regions were separately thresholded based on the grey level histogram of a neuron-free area in the same field of view (mode plus 3 times the standard deviation). The ratio between the thresholded dendritic fluorescence of the red channel and the sum of the values for green and red channels was defined as “internalized fraction”. Surface expression of GluR1, GluR2, β1, β3, CTβ1, and CTβ3 was evaluated by live labeling. For untransfected neurons, analysis was performed on the full field of view; for transfected cells, only the fluorescence signal colocalizing with the GFP or mRFP counterstains was considered. Images were thresholded as above. Spine analysis was carried out according to De Simoni et al. (2003). Controls were always performed in parallel on sister cultures.
Rap1 Activation Assay
Activation of endogenous Rap1 in neurons was examined using the EZ-Detect™ Rap1 Activation Kit (Pierce, Rockford, IL, USA) according to the manufacture’s instructions.
Statistical Analysis
For normally distributed data (normality determined by the one-sample Kolmogorov-Smirnov test), statistical differences were assessed using the paired and unpaired two-tailed Student’s t-test as required. The Mann-Whitney test was used when the criteria for normality were not met and the two-sample Kolmogorov-Smirnov test was applied to mEPSC amplitude distributions (Igor Pro 4.07; Instat 3, GraphPad Software Inc., San Diego, CA, USA). In Figures, statistical significance is indicted by (*) for 0.05<p<0.01, (**) for 0.01<p<0.001, and (***) for p<0.001. Average data are expressed as mean±SEM.
Supplementary Material
Acknowledgments
We thank Mark Ginsberg, Jean de Gunzburg, Alan Hall, Yasunori Hayashi, Eugene Marcantonio, Roger Tsien, and Fiona Watt for constructs, Martin Raff, Karine Pozo, and Andrew McGeachie for comments on the manuscript, and David Elliott for technical assistance. This work was supported by the Medical Research Council and the National Institutes of Health (RO1MH66676). L.Y. was funded by a PhD studentship from the Eisai London Research Laboratories.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adesnik H, Nicoll RA. Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci. 2007;27:4598–4602. doi: 10.1523/JNEUROSCI.0325-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby MC, De La Rue SA, Ralph GS, Uney J, Collingridge GL, Henley JM. Removal of AMPA receptors (AMPARs) from synapses is preceded by transient endocytosis of extrasynaptic AMPARs. J Neurosci. 2004;24:5172–5176. doi: 10.1523/JNEUROSCI.1042-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Benson DL, Schnapp LM, Shapiro L, Huntley GW. Making memories stick: cell-adhesion molecules in synaptic plasticity. Trends Cell Biol. 2000;10:473–482. doi: 10.1016/s0962-8924(00)01838-9. [DOI] [PubMed] [Google Scholar]
- Blaess S, Kammerer RA, Hall H. Structural analysis of the sixth immunoglobulin-like domain of mouse neural cell adhesion molecule L1 and its interactions with alpha(v)beta3, alpha(IIb)beta3, and alpha5beta1 integrins. J Neurochem. 1998;71:2615–2625. doi: 10.1046/j.1471-4159.1998.71062615.x. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Scott DB, Ehlers MD. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron. 2002;36:435–449. doi: 10.1016/s0896-6273(02)00979-0. [DOI] [PubMed] [Google Scholar]
- Bos JL, de Rooij J, Reedquist KA. Rap1 signalling: adhering to new models. Nat Rev Mol Cell Biol. 2001;2:369–377. doi: 10.1038/35073073. [DOI] [PubMed] [Google Scholar]
- Bourgin C, Murai KK, Richter M, Pasquale EB. The EphA4 receptor regulates dendritic spine remodeling by affecting beta1-integrin signaling pathways. J Cell Biol. 2007;178:1295–1307. doi: 10.1083/jcb.200610139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CD, Pilling D, Henriquez NV, Parsonage G, Threlfall K, Scheel-Toellner D, Simmons DL, Akbar AN, Lord JM, Salmon M. RGD peptides induce apoptosis by direct caspase-3 activation. Nature. 1999;397:534–539. doi: 10.1038/17409. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci. 1999;2:454–460. doi: 10.1038/8123. [DOI] [PubMed] [Google Scholar]
- Chan CS, Weeber EJ, Kurup S, Sweatt JD, Davis RL. Integrin requirement for hippocampal synaptic plasticity and spatial memory. J Neurosci. 2003;23:7107–7116. doi: 10.1523/JNEUROSCI.23-18-07107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Weeber EJ, Zong L, Fuchs E, Sweatt JD, Davis RL. Beta 1-integrins are required for hippocampal AMPA receptor-dependent synaptic transmission, synaptic plasticity, and working memory. J Neurosci. 2006;26:223–232. doi: 10.1523/JNEUROSCI.4110-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavis P, Westbrook G. Integrins mediate functional pre- and postsynaptic maturation at a hippocampal synapse. Nature. 2001;411:317–321. doi: 10.1038/35077101. [DOI] [PubMed] [Google Scholar]
- Chen BM, Grinnell AD. Integrins and modulation of transmitter release from motor nerve terminals by stretch. Science. 1995;269:1578–1580. doi: 10.1126/science.7667637. [DOI] [PubMed] [Google Scholar]
- Chen BM, Grinnell AD. Kinetics, Ca2+ dependence, and biophysical properties of integrin-mediated mechanical modulation of transmitter release from frog motor nerve terminals. J Neurosci. 1997;17:904–916. doi: 10.1523/JNEUROSCI.17-03-00904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- De Simoni A, Griesinger CB, Edwards FA. Development of rat CA1 neurones in acute versus organotypic slices: role of experience in synaptic morphology and activity. J Physiol. 2003;550:135–147. doi: 10.1113/jphysiol.2003.039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A, Schachner M. Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci. 2003;4:456–468. doi: 10.1038/nrn1115. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Franke B, Akkerman JW, Bos JL. Rapid Ca2+-mediated activation of Rap1 in human platelets. Embo J. 1997;16:252–259. doi: 10.1093/emboj/16.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Lee SH, Simonetta A, Hansen J, Sheng M, Pak DT. Differential roles of Rap1 and Rap2 small GTPases in neurite retraction and synapse elimination in hippocampal spiny neurons. J Neurochem. 2007;100:118–131. doi: 10.1111/j.1471-4159.2006.04195.x. [DOI] [PubMed] [Google Scholar]
- Gao B, Saba TM, Tsan MF. Role of alpha(v)beta(3)-integrin in TNF-alpha-induced endothelial cell migration. Am J Physiol Cell Physiol. 2002;283:C1196–1205. doi: 10.1152/ajpcell.00064.2002. [DOI] [PubMed] [Google Scholar]
- Gomperts SN, Rao A, Craig AM, Malenka RC, Nicoll RA. Postsynaptically silent synapses in single neuron cultures. Neuron. 1998;21:1443–1451. doi: 10.1016/s0896-6273(00)80662-5. [DOI] [PubMed] [Google Scholar]
- Grotewiel MS, Beck CD, Wu KH, Zhu XR, Davis RL. Integrin-mediated short-term memory in Drosophila. Nature. 1998;391:455–460. doi: 10.1038/35079. [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Shimazu K, Woo NH, Zang K, Muller U, Lu B, Reichardt LF. Distinct roles of the beta 1-class integrins at the developing and the mature hippocampal excitatory synapse. J Neurosci. 2006;26:11208–11219. doi: 10.1523/JNEUROSCI.3526-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- Kennedy MB, Beale HC, Carlisle HJ, Washburn LR. Integration of biochemical signalling in spines. Nat Rev Neurosci. 2005;6:423–434. doi: 10.1038/nrn1685. [DOI] [PubMed] [Google Scholar]
- LaFlamme SE, Thomas LA, Yamada SS, Yamada KM. Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration, and matrix assembly. J Cell Biol. 1994;126:1287–1298. doi: 10.1083/jcb.126.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- Lissin DV, Carroll RC, Nicoll RA, Malenka RC, von Zastrow M. Rapid, activation-induced redistribution of ionotropic glutamate receptors in cultured hippocampal neurons. J Neurosci. 1999;19:1263–1272. doi: 10.1523/JNEUROSCI.19-04-01263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Calderwood DA, Ginsberg MH. Integrin cytoplasmic domain-binding proteins. J Cell Sci. 2000;113(Pt 20):3563–3571. doi: 10.1242/jcs.113.20.3563. [DOI] [PubMed] [Google Scholar]
- Luscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- Matsui T, Sekiguchi M, Hashimoto A, Tomita U, Nishikawa T, Wada K. Functional comparison of D-serine and glycine in rodents: the effect on cloned NMDA receptors and the extracellular concentration. J Neurochem. 1995;65:454–458. doi: 10.1046/j.1471-4159.1995.65010454.x. [DOI] [PubMed] [Google Scholar]
- Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83–90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- Morales M, Colicos MA, Goda Y. Actin-dependent regulation of neurotransmitter release at central synapses. Neuron. 2000;27:539–550. doi: 10.1016/s0896-6273(00)00064-7. [DOI] [PubMed] [Google Scholar]
- Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- Noel J, Ralph GS, Pickard L, Williams J, Molnar E, Uney JB, Collingridge GL, Henley JM. Surface expression of AMPA receptors in hippocampal neurons is regulated by an NSF-dependent mechanism. Neuron. 1999;23:365–376. doi: 10.1016/s0896-6273(00)80786-2. [DOI] [PubMed] [Google Scholar]
- O'Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- Pfaff M, McLane MA, Beviglia L, Niewiarowski S, Timpl R. Comparison of disintegrins with limited variation in the RGD loop in their binding to purified integrins alpha IIb beta 3, alpha V beta 3 and alpha 5 beta 1 and in cell adhesion inhibition. Cell Adhes Commun. 1994;2:491–501. doi: 10.3109/15419069409014213. [DOI] [PubMed] [Google Scholar]
- Pinkstaff JK, Detterich J, Lynch G, Gall C. Integrin subunit gene expression is regionally differentiated in adult brain. J Neurosci. 1999;19:1541–1556. doi: 10.1523/JNEUROSCI.19-05-01541.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci. 2006;9:602–604. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]
- Reuther GW, Der CJ. The Ras branch of small GTPases: Ras family members don't fall far from the tree. Curr Opin Cell Biol. 2000;12:157–165. doi: 10.1016/s0955-0674(99)00071-x. [DOI] [PubMed] [Google Scholar]
- Rohrbough J, Grotewiel MS, Davis RL, Broadie K. Integrin-mediated regulation of synaptic morphology, transmission, and plasticity. J Neurosci. 2000;20:6868–6878. doi: 10.1523/JNEUROSCI.20-18-06868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- Rutherford LC, Nelson SB, Turrigiano GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Shi Y, Ethell IM. Integrins control dendritic spine plasticity in hippocampal neurons through NMDA receptor and Ca2+/calmodulin-dependent protein kinase II-mediated actin reorganization. J Neurosci. 2006;26:1813–1822. doi: 10.1523/JNEUROSCI.4091-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilenov LB, Mikhailov A, Pelham RJ, Marcantonio EE, Gundersen GG. Focal adhesion motility revealed in stationary fibroblasts. Science. 1999;286:1172–1174. doi: 10.1126/science.286.5442.1172. [DOI] [PubMed] [Google Scholar]
- Song I, Kamboj S, Xia J, Dong H, Liao D, Huganir RL. Interaction of the N-ethylmaleimide-sensitive factor with AMPA receptors. Neuron. 1998;21:393–400. doi: 10.1016/s0896-6273(00)80548-6. [DOI] [PubMed] [Google Scholar]
- Staubli U, Chun D, Lynch G. Time-dependent reversal of long-term potentiation by an integrin antagonist. J Neurosci. 1998;18:3460–3469. doi: 10.1523/JNEUROSCI.18-09-03460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Terashima A, Cotton L, Dev KK, Meyer G, Zaman S, Duprat F, Henley JM, Collingridge GL, Isaac JT. Regulation of synaptic strength and AMPA receptor subunit composition by PICK1. J Neurosci. 2004;24:5381–5390. doi: 10.1523/JNEUROSCI.4378-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Zhang H, Majumdar D, Horwitz AF. alpha5 integrin signaling regulates the formation of spines and synapses in hippocampal neurons. J Biol Chem. 2007;282:6929–6935. doi: 10.1074/jbc.M610981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CJ, Ibata K, Turrigiano GG. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci. 2005;25:2895–2905. doi: 10.1523/JNEUROSCI.5217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildering WC, Hermann PM, Bulloch AG. Rapid neuromodulatory actions of integrin ligands. J Neurosci. 2002;22:2419–2426. doi: 10.1523/JNEUROSCI.22-07-02419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Mogford JE, Platts SH, Davis GE, Meininger GA, Davis MJ. Modulation of calcium current in arteriolar smooth muscle by alphav beta3 and alpha5 beta1 integrin ligands. J Cell Biol. 1998;143:241–252. doi: 10.1083/jcb.143.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Dudek H, Miranti CK, Greenberg ME. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J Neurosci. 1996;16:5425–5436. doi: 10.1523/JNEUROSCI.16-17-05425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Huganir RL, Penzes P. Activity-dependent dendritic spine structural plasticity is regulated by small GTPase Rap1 and its target AF-6. Neuron. 2005;48:605–618. doi: 10.1016/j.neuron.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol. 2003;15:621–632. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Pak D, Qin Y, McCormack SG, Kim MJ, Baumgart JP, Velamoor V, Auberson YP, Osten P, van Aelst L, et al. Rap2-JNK removes synaptic AMPA receptors during depotentiation. Neuron. 2005;46:905–916. doi: 10.1016/j.neuron.2005.04.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.