Abstract
The establishment, characterization, and tumorigenicity of a new epithelial cell line (UOK 257) from human renal carcinoma of an individual with Birt-Hogg-Dubé (BHD) are reported. Unlike other established renal tumor cell lines from sporadic renal cell carcinoma, this is the first established renal tumor cell line of BHD, an inheritable neoplastic syndrome, from long-term tissue culture. The isolated tumor cells display loss of contact inhibition in vitro, and produce subcutaneous tumors in mouse xenografts. Histopathologic, ultrastructural, and cytogenetic characterizations of the established tumor cells are reported. Cytogenetic analysis using spectral karyotyping on UOK 257 cells revealed 17p loss and a near-triploid and aneuploid karyotype with multiple fluorescence in site hybridization analysis using a locus-specific gene probe for MYC. This result demonstrates that the established tumor cells consist of two cell populations, one containing four and one containing five copies of the MYC oncogene.
1. Introduction
Birt-Hogg-Dubé (BHD) is a rare, inherited autosomal dominant cancer syndrome caused by mutations in the FLCN gene (also known as BHD), which is located on the short arm of chromosome 17 (17p11.2)[1–3]. Patients affected with BHD are at risk to develop cutaneous fibrofolliculomas, pulmonary cysts, spontaneous pneumothorax, and bilateral renal tumors. As previously described, the renal tumors from BHD patients may display multiple histologies, including chromophobe, oncocytoma, clear cell, papillary, and hybrid oncocytic (a histology unique to BHD patients with features of both chromophobe RCC and oncocytoma)[2;4;5]. Furthermore, a mixture of several distinct populations of tumor cells can be seen in hybrid tumors[6]. The presence of these distinct histological types may reflect differences in germline and somatic mutations of the FLCN gene in the various BHD tumors[1;7;8].
Investigators have long recognized the potential value of a cell culture system as useful tool for studying hereditary human renal carcinomas, and the availability of human hereditary cancer cell cultures has led to a better understanding of the pathways affecting the growth and structure of those malignant cells [7;9]. From the current American Type Culture Collection (ATCC) online cell line indexes, 11 out of 950 described cancer cell lines have been derived from the human kidney (http://www.atcc.org/common/documents/pdf/CellCatalog/TumorLines.pdf). Currently, none of those are known to be derived directly from hereditary RCC[10;11].
Despite decades of effort, subsequent attempts to cultivate cells from hereditary renal carcinoma epithelial cells have been unsuccessful. Considering that the exact molecular and cellular events underlying the genesis of the BHD syndrome and the precise functions and pathways of the FLCN gene have yet to be established, there is a necessity for tumor cell lines established directly from BHD tumor samples. These cell lines could then serve as valuable tools for the study of the molecular mechanism of genesis, particularly for the BHD syndrome and the BHD/MTOR pathway, and ultimately become a useful preclinical model for molecular targeting by novel therapeutics.
Here we report the establishment and characterization of renal tumor cell line, UOK 257, by long term tissue culture derived from a tissue sample of a 46-year old male with a particularly aggressive BHD clinical manifestation. The traditional standard G-banding analysis revealed a complex karyotype. To better resolve the rearranged chromosomes, we employed molecular cytogenetic technologies, such as spectral karyotyping (SKY)[12] and fluorescence in situ hybridization (FISH) with gene-specific probes. We also utilized ultrastructural studies via electron microscopy to characterize sub-cellular morphology of organelles from the tumor cell. Specifically, we applied FISH with gene-specific probes to reveal the chromosomal abnormalities involving loss of heterozygosity for several loci on chromosome arm 17p, such as the TP53 and BHD genes[8]. It is our hope that the multilevel cytogenetic and ultrastructural characterization of the BHD tumor cell line provided herein will assist further studies on the molecular mechanism of BHD tumorigenesis, the mechanism of organ selective metastasis, the genotype-phenotype relationship, the identification of molecular markers for diagnosis and/or prognosis, and the discovery of novel therapeutic drugs for the BHD syndrome.
2. Materials and methods
2.1 Case Report
The patient was a previously healthy 46 year old male who initially presented with acute onset gross hematuria. His past medical history was significant for history of two previous episodes spontaneous pneumothorax and the diagnosis of numerous fibrofolliculomas on the scalp, face, neck and trunk. During his workup for hematuria, the patient underwent computed tomography (CT) scan chest, abdomen, and pelvis revealing a 7 × 5 cm multi-nodular enhancing mass encompassing the upper pole of the left kidney, no solid lesions in the right kidney, and multiple pulmonary cysts. Otherwise, CT scan revealed no evidence of disease in the remainder of the chest, abdomen and retroperitoneum. Given the presence of both the dermatologic findings and the renal mass, the patient underwent genetic testing and was found to have a germline mutation in the FLCN gene and a diagnosis of BHD syndrome was made. Following diagnosis and evaluation, the patient underwent an open left radical nephrectomy via an 11th rib flank incision. Pathology revealed clear cell renal cell carcinoma (clear cell RCC), Fuhrman grade 3 of 4, with evidence of invasion into the hilar adipose tissue. Multiple hilar lymph nodes, removed at the time of resection, revealed no evidence of tumor. Furthermore, although the mass was predominantly clear cell RCC, the pathologist noted foci of tubular papillary and chromophobe histologies. Approximately eight months following surgery, the patient presented with a four day history of abdominal discomfort and diarrhea. He underwent a CT scan of the abdomen/pelvis, which revealed a 3 cm soft tissue mass in the left renal fossa. He subsequently underwent a percutaneous CT guided biopsy of the retroperitoneal mass, which revealed recurrent renal cell carcinoma that was morphologically consistent with the patient’s previous RCC tumor. Further metastatic imaging including a whole-body bone scan, MRI head, and PET scan, revealed foci of metastatic disease in the left renal fossa, left iliac crest, and left lesser trochanter. He subsequently underwent several cycles of systemic therapy including high dose interleukin 2 (IL 2), interferon, and an investigational agent (phase 1 clinical trial). The disease continued to progress through the therapies, was eventually was placed on palliative care measures, and, approximately 21 months after initial diagnosis and 13 months following the diagnosis of metastatic disease, he died of progressive metastatic renal cancer.
2.2 Tumor Origin and Pathologic Analysis
The surgical specimen from a left nephrectomy weighted 1,213.2 grams, consisting of a kidney and adrenal gland with overlying fascia and adipose tissue. The tumor mass measured approximately 8.0 × 8.0 × 8.0 cm and had completely distorted in the upper pole of kidney. The multiple nodules of friable tumor mass were present primarily in the superior pole of the kidney and were invading the collecting system. Further dissection revealed a yellow/tan homogeneous tumor mass within the nodule. A portion of the tumor was retained in the laboratory for viable in vitro culture. Portions were submitted for electron microscopy, and the remainder was retained for permanent histopathological diagnosis. Prior to surgery, the patient was consented on an internal review board approved tissue procurement protocol.
2.3 Tissue Culture, Establishment of Transplantable Cell Line and sub-clone lines
A portion of tissue was removed from the middle of the tumor mass approximately 10-min after nephrectomy and tissue extraction. Using sterile technique, the tissue was immersed into a small aliquot of 1x PBS buffer, minced with a sharp scalpel to obtain approximately 1 mm diameter fragments, and then subsequently plated onto a 60mm Petri dish. Dulbecco’s modified Eagle’s medium (DMEM) containing high glucose (4.5g/L), L-glutamine (2 mM), supplemented with penicillin (50 units/ml), streptomycin (100µg/ml), and MEM non essential amino acids (1x, from 100x solution. Invitrogen, MD), with 10% of heat-inactivated FBS was slowly added to the Petri dish. The volume of medium was adjusted so that there was enough media to cover the tissue fragments, but not enough to cause them to float. The culture was then incubated at 37°C in a humidified atmosphere containing 5% CO2. Approximately 36 hours after plating, adherent epithelial cell outgrowth from tissue debris was observed. An additional 1–2 ml of fresh DMEM was then added. The first passage was carried out three to five days after plating by a light treatment with 0.05% trypsin-EDTA, while monitoring the cells under an inverted tissue culture microscope. Subsequent passages were carried out every 3–5 days in same manner; the line was propagated for over 50 passages. The established primary line was designated UOK 257. From the parental stock of passage 6, limiting dilutions were performed, and resulting sub-clones were isolated and cultured for more than 15 passages. The sub-clones were numbered, following the name of the primary line (e.g. UOK 257 SC6 refers to subclone 6 of the primary cell line UOK 257).
Approximately 3 ×106 cells in 1x PBS 0.2ml each volume was subcutaneously injected into SCID/BEIG mice to determine the tumorigenic potential of the cells. Xenograft tumor specimens were reimplanted into new mice for multiple generations, and xenografts from the mice were also cultured in the laboratory. Animal care was approved and was in accordance with the guidelines of the National Cancer Institute.
2.4 Cell Proliferation and Metaphase Preparation
When the UOK 257 line culture reached the proliferation phase (i.e. seeded at ~40% confluence and grown and harvested on third day at ~ 90% confluence), the proliferative activity and cellular kinetics were analyzed using automated flow-through cytometer (BD FACScalibur E1806, BD Biosciences, San Jose, CA). The protocol and technical control used was as previously described[13] with some modifications. Briefly, the cells (106/ml) were washed with PBS and fixed in 70% ethanol for at least 1 hour, or the cells were incubated at 4 ° C overnight, washed twice with PBS, and incubated with 50 µl of RNase in 1 mL PBS plus 0.1% bovine serum albumin for 30 minutes. A DNA histogram was obtained by staining cells with 7-Aminoactinomycin D (5 µl (0.25 µg) / 106 cells) (BD Biosciences Pharmingen) and incubating 10 minutes before analysis. Data was analyzed using FlowJo version 6.3.2 software (Tree Star, OR).
At passage 18, UOK 257 cells were treated with Colcemid (10 µl/ml, Boehringer Mannheim [Roche Diagnostics Corporation, USA]) for 1 hr, subjected to hypotonic treatment (0.075 M KCl) and fixative (methanol-acetic acid, 3:1) as described[12]. Chromosome spreads were prepared in a humidity chamber (Thermotron, Model CDS-5, Holland, MI). Metaphase chromosome preparation was as previously described[14]. See: http://www.nature.com/nprot/journal/v1/n6/abs/nprot.2006.358.html]
2.5 Cell Pellet Fixation and Thin Section for Ultrastructural Studies
Cell monolayers were washed with PBS, scraped and fixed in 2.5% PBS-buffered glutaraldehyde, post-fixed in osmium tetroxide, dehydrated and embedded in Spurr’s epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate and viewed in a Philips CM10 transmission electron microscope (Philips Electronics, Mahway, NJ).
2.6 DNA Probes Preparation and DNA Sequencing Analysis
BAC based DNA hybridization probes for FISH were prepared by using Vysis Nick-translation Kit (Abbott Molecular Inc./Vysis, IL) incorporated with Spectrum-Orange or Spectrum-Green. Sky painting probes were prepared in-house, as detailed in: http://www.riedlab.nci.nih.gov/protocols.asp. Germline mutation sequencing analysis for the cell line was performed on our lab ABI PRISM™ 310 Genetic Analyzer (Perkin Elmer, CA).
2.7 Sky Analysis and FISH with Chromosome-specific Probes
Fifteen UOK 257 metaphase spreads were analyzed and scored for the following: chromosome number (ploidy), numerical and structural aberrations. Spectrum-based classification and analysis of the fluorescent images (SKY) was achieved using SkyView™ software (Applied Spectral Imaging, Vista, CA). The chromosome complements (karyotypes) of every metaphase spread were analyzed using human chromosome nomenclature rules described in ISCN, 2005[15]. FISH analysis using a chromosome painting probe for chromosome 8, and a gene loci probe for MYC (8 q24.3) (Abbott Molecular Inc./Vysis, IL) was used to assess the copy number of this oncogene and to better define aberrant chromosomes containing small portions of chromosome 8.
Structural aberrations are considered clonal if two or more cells contain the same aberration[15]. With respect to numerical aberrations, gains of chromosomes are considered clonal if two or more cells contain the same extra chromosome, and losses are classified as clonal if they are observed in three or more cells[15].
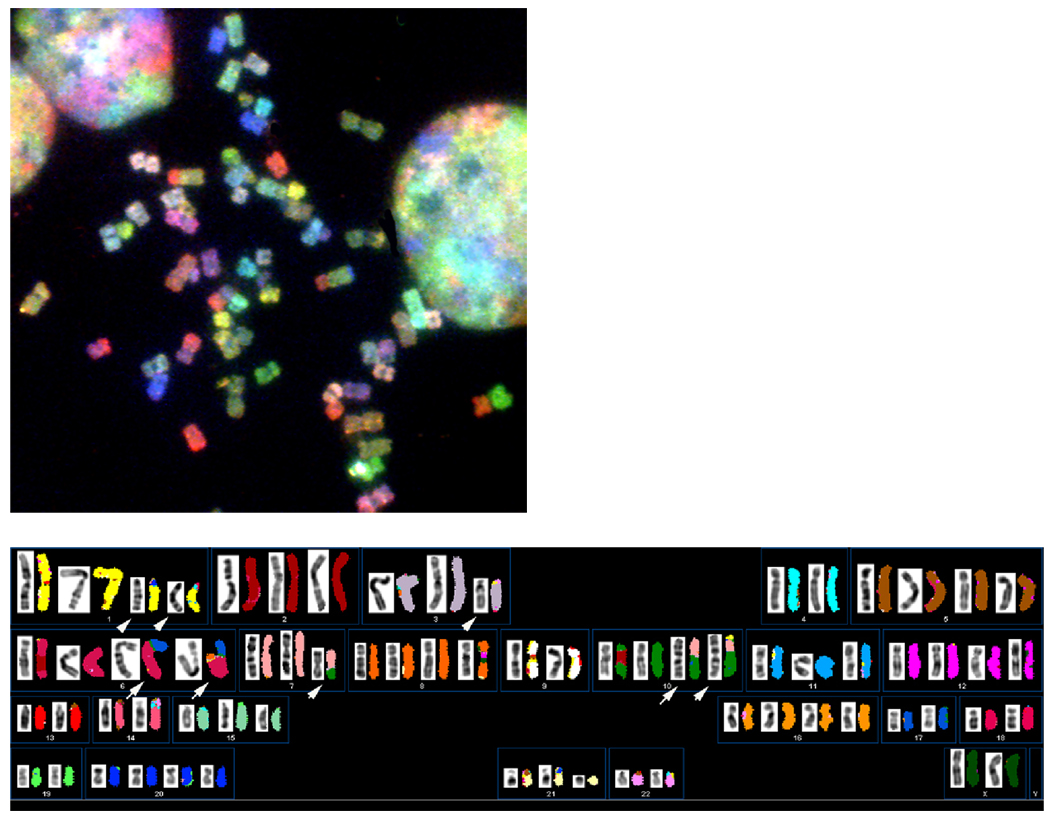
The karyotype for UOK 257 is shown in Figure 4 and [has been entered into the NCBI, SKY/CGH interactive online database; http://www.ncbi.nlm.nih.gov/sky/skyweb.cgi (submitter: Padilla-Nash)(public access pending publication). In this database, the patient’s karyotypes are depicted as a colored human ideogram, called a SKYGRAM. Each SKYGRAM presents the complement of normal and abnormal chromosomes with the position of their breakpoints linked to the NCBI DNA sequence database.]
Fig. 4.
A. A representative metaphase spread from cell line UOK 257 at passage18. (B). Composite karyotype of UOK 257 is near triploid, displaying multiple unbalanced translocations and deletions of chromosomes (white arrows).Presented to the left are inverted-DAPI stained chromosomes. The pseudo-colored chromosomes to the right were hybridized with SKY probes.
2.8 Image Acquisition
The UOK 257 cell morphology was examined with an inverted microscope with a digital camera [Olympus 1X71, Olympus Optical Co. LTD. Japan]. Images were acquired with a 10x objective from 6–8 random fields per culture. The cell counting and mean cell size (micron) were analyzed with Cellometer Auto T4 image-based cell counter. (Nexcelom Bioscience, Lawrence, MA). The ultrastructural images on thin section were taken on a ZEISS EM109 electronic microscope (at x6500, x11,000 and high magnification x21,000). FISH fluorescence images were acquired by Leica DMR XA [Leica Microsystems, GmbH], with loaded IP lab version 3.7 software, plus customized scripts. Image acquisition of SKY-labeled metaphase chromosome spreads were carried out by using a SpectraCube™ (Applied Spectral Imaging, Vista, CA) and a charge-coupled device camera on top of an epifluorescence microscope equipped with custom-designed-triple band pass optical SKY filter (Chroma Technology, Rockingham, VT). Analysis of the metaphase spread karyotypes was performed using SkyView™ software (ASI)[12].
3. Results
3.1 Histopathology
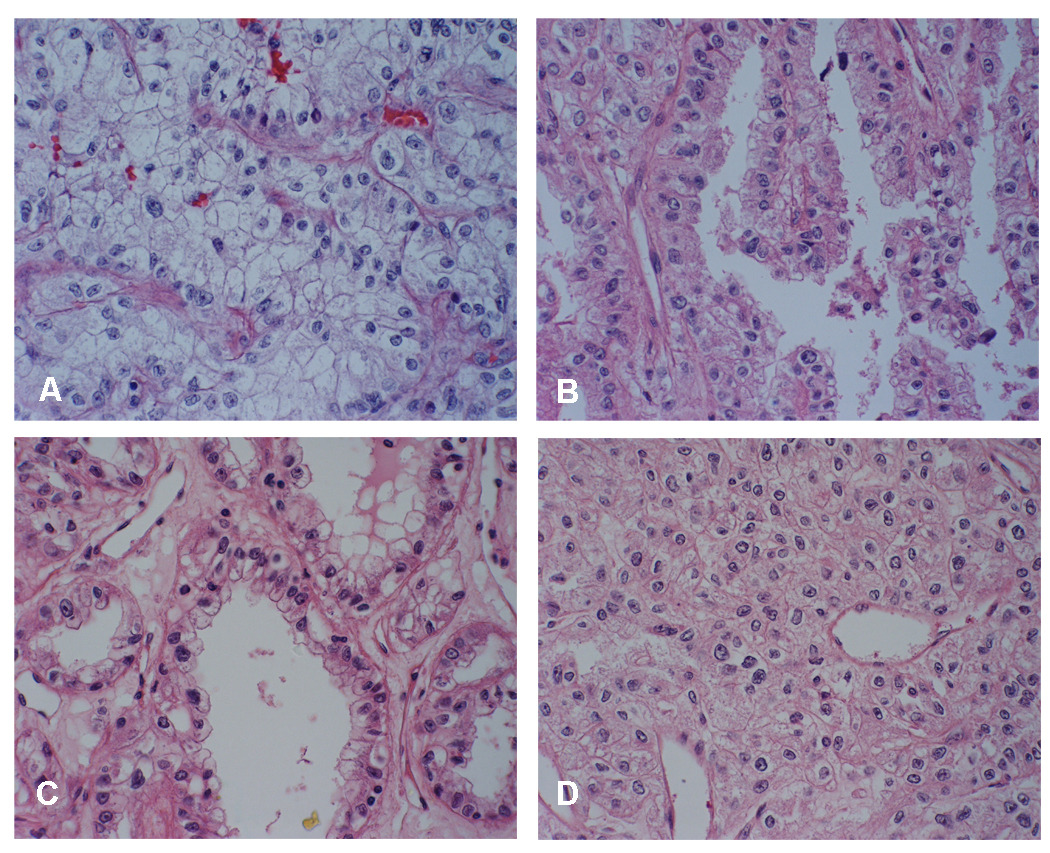
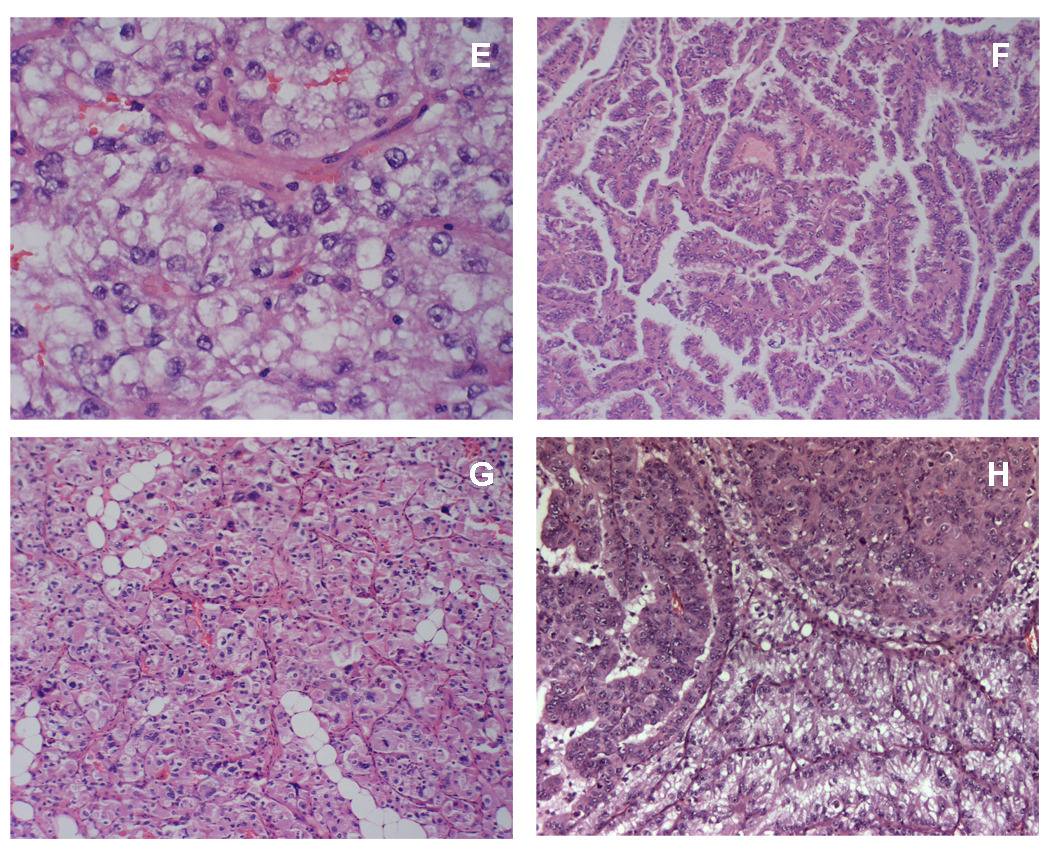
Fig. 1 (A–D) shows the light microscope appearance of an aggressive renal cell carcinoma derived from this individual surgical specimen. The tumor was characterized as being predominantly atypical clear epithelial cell type (Fig. 1A), with enlarged, round to irregular nuclei, prominent nucleoli and moderate to abundant vacuolated cytoplasm seen as single cells, clusters, and sheets. The tumor also demonstrated a variety of histologies with detailed junctional complexity including tubular papillaries (Fig. 1B), solid areas (Fig. 1C), and notably, foci reminiscent of chromophobe renal cell carcinoma (Fig. 1D). Ten SKID/BEIG mice injected with the UOK 257 primary cell line showed outgrowth within three weeks post injection, and palpable tumors were formed in 10 out of 10 mice. Twenty-two weeks after injection, tumor size was measured at 1.5 cm in diameter. H & E slides were processed from tumor xenografts, revealing pathologic features similar to that of the case report. Notably, in some of the slower growing tumors aged 28 weeks in mice by the time of euthanization, pathologic analysis showed features not only of the predominantly clear cell type (Fig. 2E), but also complex junctions with papillary architecture, solid areas of cells with eosinophilic cytoplasm lined by clear cells (Fig. 2H), and chromophobe foci surrounding the clear cell tumor area (Fig. 2G). Concurrently, xenograft tumor specimens were reimplanted into new mice for several generations. They were also brought back to lab for proliferation cultures, and retained the ability to grow in culture. A subline derived from the xenograft, designated UOK 257 XRP1., when injected back into SKID/BEIG mice, resulted in a more rapid outgrowth than the parent line.
Fig. 1.
(A–D). Light microscopic appearance with different histologies of kidney tumor mass from the patient with Birt-Hogg-Dubé ( BHD ) syndrome. (A) Large areas composed of nests of clear cells with enlarged, round to irregular nuclei with prominent nucleoli and abundant vacuolated cytoplasm, scattered atypical “naked” nuclei (x400) , (B) papillae lined by atypical epithelial cells with granular eosinophilic cytoplasm (x400) , ( C) atypical lining of the tubular structures (x400) , (D) histologically reminiscent foci of chromophobe renal cell carcinoma (x400) .
(E–H) Tumor xenografts revealed similar histologic features compare with the case pathologic report. (E) Clear cell areas throughout the tumor (x400). (F) Papillary architecture (x200). (G) Areas with histologic resemblance to chromophobe (x200). (H) Solid areas of cells with eosinophilic cytoplasm, junction with complex of different histologies. (x200).
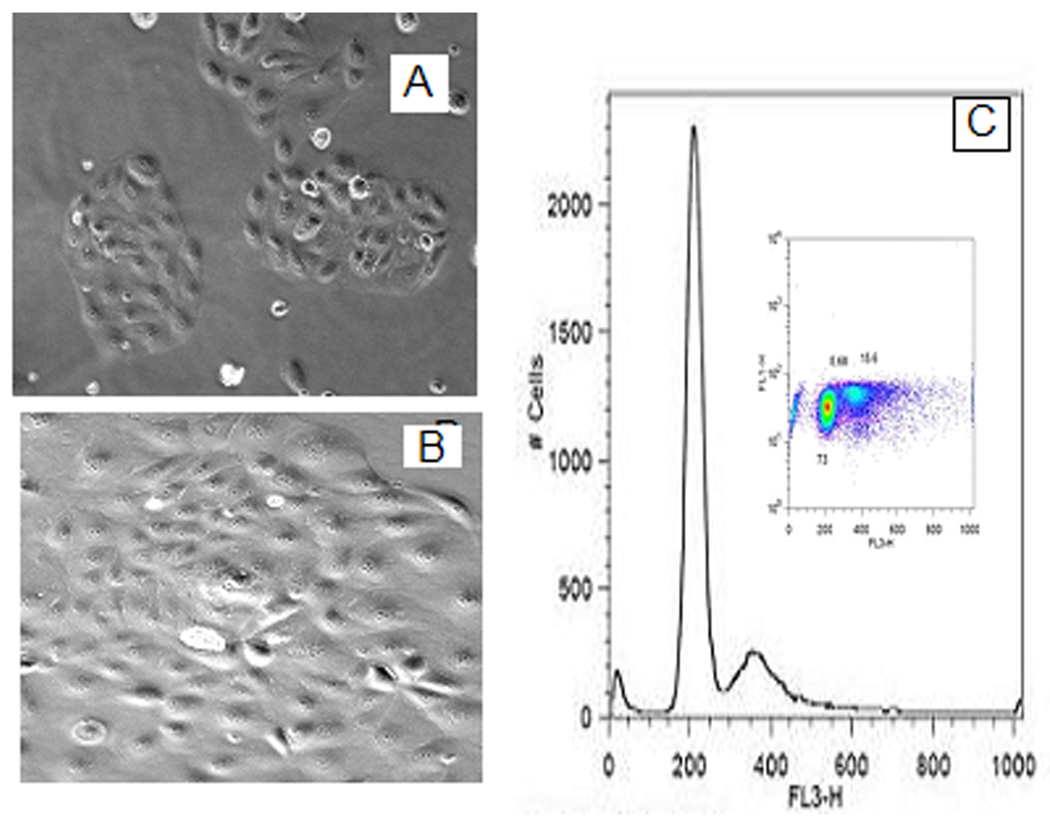
Fig. 2.
Morphology of UOK 257 at 50% confluence (A) appearance small patched islands, at 90% (B) confluence, loss of contact inhibition is evident. From flow cytometry, the scatterplot (C) and its one-dimensional histogram projection depict the cell cycle distribution of the tumor cell population.
3.2 Cell proliferation, cell cycle distribution and ploidy pattern
Morphological aspects of primary human hereditary renal carcinoma cultures are shown in Fig. 2 B. The UOK 257 cells cultured in vitro show an adherent monolayer cultured epithelial cell phenotype and display a loss of contact inhibition. Unlike other renal cell lines, the UOK 257 cells form into small patches of cellular islands in culture at ~50% confluence. Cell cycle and ploidy pattern of UOK 257 cells were evaluated by flow cytometric DNA histogram of 7-AAD-stained tumor cells, which were cultured three days to reach around 90% confluence. The distribution of the tumor cell population at each phase in the cell cycle is given in Fig. 2C.
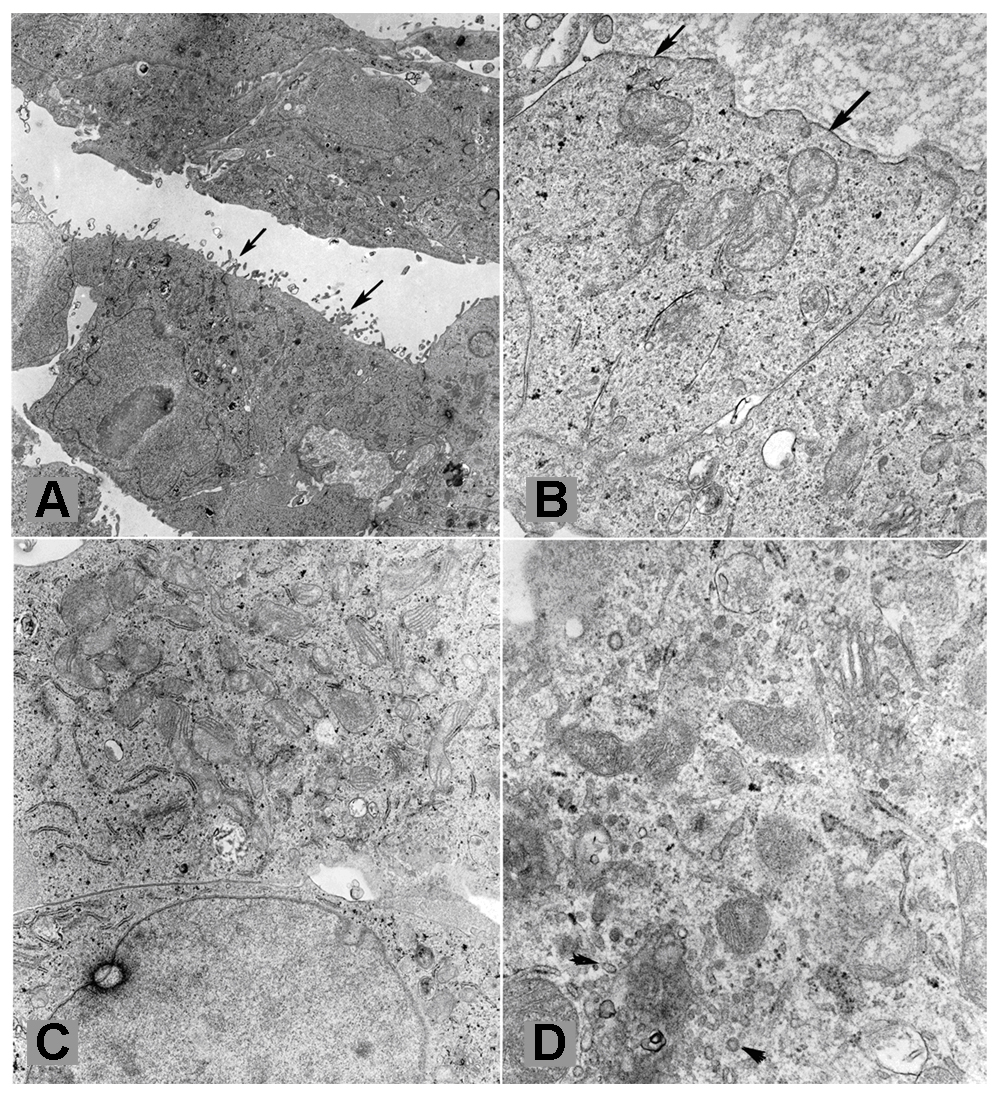
3.3 Ultrastructural analysis
The cells exhibited ultrastructural features of tubular epithelial cells, such as microvilli on one (apical) surface and basal lamina on the opposite surface (Fig. 3A). Basal lamina-like material was focally abundant in-between tumor cells (Fig. 3B). Desmosomes and tight junctions were present. Variable numbers of mitochondria were seen in the tumor cells. In some of them the mitochondria were abundant consistent with oncocytic change (Fig. 3C). Cytoplasmic microvesicles similar to those present in chromophobe renal cell carcinoma were present in rare tumor cells (Fig. 3D). Some tumor cells contained a significant amount of glycogen, but lipid droplets were not common. The tumor cells were ultra-structurally comparable to those of the original tumor, which had also been examined ultra-structurally, and which had shown areas reminiscent of oncocytoma and chromophobe cell carcinoma.
Fig. 3.
(A–D) Ultrastructural features of findings from cultured UOK 257 tumor cells (Thin section (90nm) from fixed cells pellet) (X 6,500). (A): The arrows showed microvilli on one (apical) surface and basal lamina on the opposite surface. (B): Basal lamina –like material was focally abundant in between tumor cells. (C): Abundant mitochondria have appeared, consistent with oncocytic change. (D): Cytoplasmic microvesicles (arrowheads) were present in chromophobe renal cell carcinoma and rare tumor cells.
3.4 SKY analysis
Figure 4 displays the composite karyotype of UOK 257 (passage 18). The cell line is hypotriploid, with chromosome numbers ranging from 57–68 and a modal chromosome number of 65. The karyotype for UOK 257 is: 57~68<3n->,XX,−Y,+1,del(1)(p12)X2,del(3)(p12)X2,−4,+5,+6,der(6)t(6;17)(p11.2;q11.2)inv(6)(p25p11.2), der(6)t(6;17)(p11.2;q11.2)inv(6)(p25p11.2)ish t(6;8)(q27;q21)(MYC+), der(7)t(7;10)(q11.2;p12), ish+8(MYC+),−9,+10,der(10)t(7;10)(q11.2;p11.2),der(10)t(1;7)(?;q32)t(7;10)(q11.2;p11.2),+12,−13,−14,+16,der(11;16)(p10;p10)[2],−18,−19,+20,−22[cp15]. SKY analysis reveals whole chromosome gains for 1, 5, 6, 8, 10, 12, 16, and 20, and whole chromosome losses for Y, 4, 9, 13, 14, 18, 19, and 22. SKY detected partial gains of 1q, 8q21->8qter (MYC+), and 10q, and partial losses for 1p, and 3p. In addition, UOK 257 displayed an overall gain for 17q (four copies) and loss of 17p (two copies) due to the presence of two normal copies of chromosome 6 plus two unbalanced translocations; der(6)t(6;17) and der(6)t(6;8;17).
3.5 FISH with specific gene probes
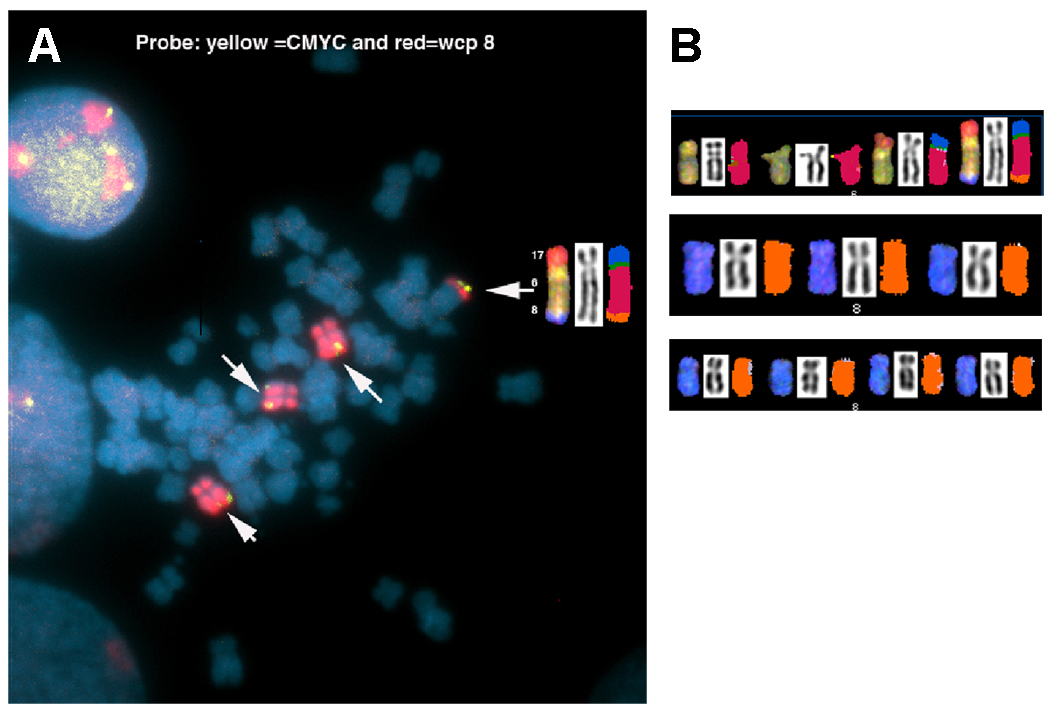
Figure 5 depicts a metaphase spread analyzed by FISH, using a probe for the MYC oncogene on chromosome 8. The copy number for MYC in UOK 257 is heterogeneous, with some metaphase spreads containing three, four, and up to five copies, including the unbalanced translocation der(6)t(6;8;17).
Fig. 5.
(A) Two clones of UOK 257 are revealed by SKY and FISH, one with three copies of chromosome 8 and the other with four copies of chromosome 8 found in 50% of cells. (B) Fluorescence in situ hybridization using a locus-specific probe for the MYV oncogene (yellow signal) and whole chromosome paint for chromosome 8 (red). In addition, SKY analysis revealed that 8q21->8q24 (white arrows-MYC) is fused to the distal region of the unbalanced translocation, der(6)t(6;17)t(6;8).
3.6 Sequencing Analysis
We have confirmed the germline FLCN gene mutation from the primary UOK 257 cell line and in the single subcloned cell lines. The tumor lines showed the insertion of a “C” in nucleotide 1733 as shown in a sequencing chromatogram of DNA as previously described by us[7]. This is the same mutation that had been found in the patient’s germline. Notably, in the tumor lines, only the germline mutant copy is retained; the wild type copy is lost (data not shown). Furthermore, the patient’s germline mutation was present in the DNA from the implanted xenografts, confirming that the tumors were human in origin (data not shown). Since the UOK 257 cell line was established from a predominant clear cell type of tissue, we have examined VHL gene mutation status, and it demonstrated wild type VHL gene sequences retained in this cell line.
4. Discussion
Here we report for the first time the establishment of a novel renal tumor cell line, UOK 257, derived from a patient with the hereditary cancer syndrome, Birt-Hogg-Dubé. The line exhibits the same characteristics that are present in tumor cells representative of the BHD phenotype, and therefore serves as an extremely valuable resource for studying the mechanism of disease. In addition, UOK 257 is tumorigenic in SKID/BEIG mice, allowing for a xenograft model system in conjunction with an in vitro cell culture model.
Examination of the UOK 257 cell line reveals that it exhibits the same morphological and ultrastructural features that are present in the tumor from which it was derived. Namely, the cells exhibit features of tubular epithelial cells, growing as an adherent monolayer and displaying loss of contact inhibition. Abundant mitochondria and cytoplasmic microvesicles were present, consistent with the features of the tumor. Notably, the histological features of the original tumor are recapitulated in the tumors that arise in SKID/BEIG mice when UOK 257 is injected subcutaneously: predominantly clear cell tumors with areas of papillary architecture, eosinophilic cells and chromophobic cells.
UOK 257 cells contain a mutant copy of the FLCN gene, folliculin, but have lost the wild type copy, as revealed by DNA sequencing. The mutant copy contains an insertion of a C at nucleotide 1733 of FLCN, resulting in a frameshift mutation that is assumed to destroy the function of folliculin. This is a region that normally contains a tract of 8 Cs and is considered a “hot spot” for FLCN mutations, as roughly 44% of known germline mutations consist of either an insertion or deletion of a C in this poly-C tract. Therefore, the line is useful for studying not only mutant FLCN function, but also wild type function in reconstituted lines.
The ploidy pattern of UOK 257 is consistent with those previously demonstrated for clear and papillary RCC, i.e. predominantly diploidy with certain percentage of aneuploidy[13]. This line is hypotriploid with a modal chromosome number of 65. Notable chromosome losses include 3p, where the kidney cancer tumor suppressor gene VHL is located, and 17p, where FLCN is located. Interestingly, there is a gain of chromosome 8, and an increase in copies of the MYC gene (between 3 and 5 copies) as determined by FISH. Therefore, it is possible that molecular pathways of VHL, MYC and other genes may be contributing to the phenotype of this line, in addition to the loss of function of FLCN.
Due to the distinctive histological, morphological and genomic characteristics of UOK 257 that correlate with tumor cells derived from Birt-Hogg- Dubé patients, as well as the potential to grow this line in vitro as well as in xenograft models, this line serves as an extremely valuable resource to be used in studying the mechanism of this disease. In addition, UOK 257 shares many characteristics with sporadic chromophobe renal carcinomas and renal oncocytomas and may serve as an excellent model to examine these diseases as well. Furthermore, this line will facilitate the ability to characterize molecular markers for diagnosis and prognosis of FLCN, as well as potential drug therapies for the disease.
Acknowledgments
This research was supported in part by the National Research Program of the U.S. National Institutes of Health, National Cancer Institute, Center for Cancer Research. The authors are grateful to Linda Stapleton-Barenboim for preparation of the SKY probes and to Jianping Huang and Zhiyu Li for assisting in flow cytometric facilities.
Footnotes
Lead article
Reference List
- 1.Khoo SK, Bradley M, Wong FK, Hedblad MA, Nordenskjold M, Teh BT. Birt-Hogg-Dube syndrome: mapping of a novel hereditary neoplasia gene to chromosome 17p12-q11.2. Oncogene. 2001;20:5239–5242. doi: 10.1038/sj.onc.1204703. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt LS, Warren MB, Nickerson ML, Weirich G, Matrosova V, Toro JR, Turner ML, Duray P, Merino MJ, Hewitt S, Pavlovich CP, Glenn GM, Greenberg CR, Linehan WM, Zbar B. Birt-Hogg-Dube syndrome, a genodermatosis associated with spontaneous pneumothorax and kidney neoplasia, maps to chromosome 17p11.2. Am J Hum Genet. 2001;69:876–882. doi: 10.1086/323744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nickerson ML, Warren MB, Toro JR, Matrosova V, Glenn GM, Turner ML, Duray P, Merino MJ, Choyke P, Pavlovich CP, Sharma N, Walther MM, Munroe D, Hill R, Maher E, Greenberg C, Lerman MI, Linehan WM, Zbar B, Schmidt LS. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dube syndrome. Cancer Cell. 2002;2:157–164. doi: 10.1016/s1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 4.Pavlovich CP, Walther MM, Eyler RA, Hewitt SM, Zbar B, Linehan WM, Merino MJ. Renal tumors in the Birt-Hogg-Dube syndrome. Am J Surg Pathol. 2002;26:1542–1552. doi: 10.1097/00000478-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Tickoo SK, Reuter VE, Amin MB, Srigley JR, Epstein JI, Min KW. Renal Oncocytosis: A Morphologic Study of Fourteen Cases. Am J Surg Pathol. 1999;23:1101. doi: 10.1097/00000478-199909000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Adley BP, Smith ND, Nayar R, Yang XJ. Birt-Hogg-Dube syndrome: clinicopathologic findings and genetic alterations. Arch Pathol Lab Med. 2006;130:1865–1870. doi: 10.5858/2006-130-1865-BSCFAG. [DOI] [PubMed] [Google Scholar]
- 7.Vocke CD, Yang Y, Pavlovich CP, Schmidt LS, Nickerson ML, Torres-Cabala CA, Merino MJ, Walther MM, Zbar B, Linehan WM. High Frequency of Somatic Frameshift BHD Gene Mutations in Birt-Hogg-Dube-Associated Renal Tumors. J Natl Cancer Inst. 2005;97:931–935. doi: 10.1093/jnci/dji154. [DOI] [PubMed] [Google Scholar]
- 8.Gad S, Lefevre SH, Khoo SK, Giraud S, Vieillefond A, Vasiliu V, Ferlicot S, Molinie V, Denoux Y, Thiounn N, Chretien Y, Mejean A, Zerbib M, Benoit G, Herve JM, Allegre G, Bressac-de PB, Teh BT, Richard S. Mutations in BHD and TP53 genes, but not in HNF1beta gene, in a large series of sporadic chromophobe renal cell carcinoma. Br J Cancer. 2007;96:336–340. doi: 10.1038/sj.bjc.6603492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baba M, Hong SB, Sharma N, Warren MB, Nickerson ML, Iwamatsu A, Esposito D, Gillette WK, Hopkins RF, III, Hartley JL, Furihata M, Oishi S, Zhen W, Burke TR, Jr, Linehan WM, Schmidt LS, Zbar B. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proceedings of the National Academy of Sciences USA. 2006;103:15552–15557. doi: 10.1073/pnas.0603781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garvin AJ, Re GG, Tarnowski BI, Hazen-Martin DJ, Sens DA. The G401 cell line, utilized for studies of chromosomal changes in Wilms' tumor, is derived from a rhabdoid tumor of the kidney. Am J Pathol. 1993;142:375–380. [PMC free article] [PubMed] [Google Scholar]
- 11.Williams RD, Elliott AY, Stein N, Fraley EE. In vitro cultivation of human renal cell cancer. I. Establishment of cells in culture. In Vitro. 1976;12:623–627. doi: 10.1007/BF02797460. [DOI] [PubMed] [Google Scholar]
- 12.Schrock E, du MS, Veldman T, Schoell B, Wienberg J, Ferguson-Smith MA, Ning Y, Ledbetter DH, Bar-Am I, Soenksen D, Garini Y, Ried T. Multicolor spectral karyotyping of human chromosomes. Science. 1996;273:494–497. doi: 10.1126/science.273.5274.494. [DOI] [PubMed] [Google Scholar]
- 13.Li G, Cottier M, Sabido O, Gentil-Perret A, Lambert C, Genin C, Tostain J. Different DNA ploidy patterns for the differentiation of common subtypes of renal tumors. Cell Oncol. 2005;27:51–56. doi: 10.1155/2005/575769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padilla-Nash HM, Barenboim-Stapleton L, Difilippantonio MJ, Ried T. Spectral karyotyping analysis of human and mouse chromosomes. Nat Protoc. 2006;1:3129–3142. doi: 10.1038/nprot.2006.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ISCN. An International System for Human Cytogenetic Nomenclature. Basel: Karger, S.; 2005. [Google Scholar]
- 16.Kuroda N, Toi M, Hiroi M, Shuin T, Enzan H. Review of renal oncocytoma with focus on clinical and pathobiological aspects. Histol Histopathol. 2003;18:935–942. doi: 10.14670/HH-18.935. [DOI] [PubMed] [Google Scholar]