Abstract
Although there exists compelling genetic evidence for a homologous recombination-independent pathway for repair of interstrand cross-links (ICLs) involving translesion synthesis (TLS), biochemical support for this model is lacking. To identify DNA polymerases that may function in TLS past ICLs, oligodeoxynucleotides were synthesized containing site-specific ICLs in which the linkage was between N2-guanines, similar to cross-links formed by mitomycin C and enals. Here, data are presented that mammalian cell replication of DNAs containing these lesions was ∼97% accurate. Using a series of oligodeoxynucleotides that mimic potential intermediates in ICL repair, we demonstrate that human polymerase (pol) κ not only catalyzed accurate incorporation opposite the cross-linked guanine but also replicated beyond the lesion, thus providing the first biochemical evidence for TLS past an ICL. The efficiency of TLS was greatly enhanced by truncation of both the 5 ′ and 3 ′ ends of the nontemplating strand. Further analyses showed that although yeast Rev1 could incorporate a dCTP opposite the cross-linked guanine, no evidence was found for TLS by pol ζ or a pol ζ/Rev1 combination. Because pol κ was able to bypass these ICLs, biological evidence for a role for pol κ in tolerating the N2-N2-guanine ICLs was sought; both cell survival and chromosomal stability were adversely affected in pol κ-depleted cells following mitomycin C exposure. Thus, biochemical data and cellular studies both suggest a role for pol κ in the processing of N2-N2-guanine ICLs.
The biological efficacy of ICL4-inducing agents resides in their ability to prevent transient strand separation that is integral to DNA replication, RNA transcription, and recombination, making these bifunctional compounds effective antimicrobial, antiviral, and chemotherapeutic agents (1, 2). In addition to the classes of exogenous chemicals listed above, various endogenously generated bis-electrophiles, for example products of lipid peroxidation, are also capable of forming ICLs (3).
The processing and repair of ICLs in eukaryotic cells are extremely complex, potentially involving multiple DNA repair and damage tolerance pathways, including homologous recombination, nucleotide excision repair, translesion DNA synthesis, transcription-coupled repair, nonhomologous end joining, mismatch repair, cell cycle checkpoints, and ubiquitination/deubiquitination pathways (1, 2). The complexity of ICL repair and tolerance is further evidenced by data demonstrating that different organisms may preferentially use alternative pathways that are dependent on the stage of the cell cycle in which the ICL is encountered (1, 2).
Although many models for ICL repair require the involvement of homologous recombination, an alternative, recombination-independent pathway exists that utilizes endonucleases for strand incision surrounding the ICL on one of the two DNA strands and TLS polymerases for gap-filling replication past the ICL site on the other strand (4–9). In these repair models, the dually incised strand possesses sufficient mobility that a DNA polymerase can strand displace the nucleotide patch that is 5′ to the lesion, then replicate past the ICL site to complete the repair gap-filling synthesis.
Insights into the essential genes for ICL repair and mutagenesis in Saccharomyces cerevisiae demonstrate a role for the product of rev3, the catalytic subunit of pol ζ (10, 11). Further support for involvement of pol ζ and another TLS polymerase, Rev1, in tolerance to ICL damage and their contribution to ICL-associated mutagenesis was obtained in both yeast (7, 12) and vertebrate cells (6, 8, 13).
TLS past a variety of base lesions is achieved in cells by the action of several DNA polymerases with distinct substrate specificities (14). Thus, it is highly unlikely that a single polymerase will be responsible for replication bypass of every ICL, because the chemical linkages of ICLs can differ significantly.
A common site of ICL formation is between exocyclic amino groups of guanines in a CpG sequence context; this can be mediated by mitomycin C and bifunctional enals, such as acrolein, crotonaldehyde, and 4-hydroxynonenal (1, 3, 15). When plasmids containing MMC-induced ICLs were used to measure the fidelity of recombination-independent repair in mammalian cells, the majority of recovered plasmids did not acquire sequence alterations (5). Additionally, replication of plasmids containing crotonaldehyde-mediated N2-N2-guanine ICLs in human cells revealed that in the absence of homologous recombination, these lesions were primarily repaired in an error-free manner (9).
Previous investigations have suggested that replication bypass of N2-guanine monoadducts in eukaryotic cells can be performed by either pol κ (16–20) or sequential action of Rev1 and pol ζ (21). In the latter case, Rev1 inserts the correct nucleotide, C, opposite the lesion, whereas pol ζ extends the primer from such a C. Here we hypothesized that pol κ, Rev1, and pol ζ may also function in replication bypass of N2-N2-guanine ICLs. With synthetic chemical strategies for the construction of oligodeoxynucleotides containing model acrolein-derived site-specific N2-N2-guanine ICLs, we examined the ability of human pol κ, yeast Rev1, and pol ζ to replicate past these lesions (Fig. 1A).
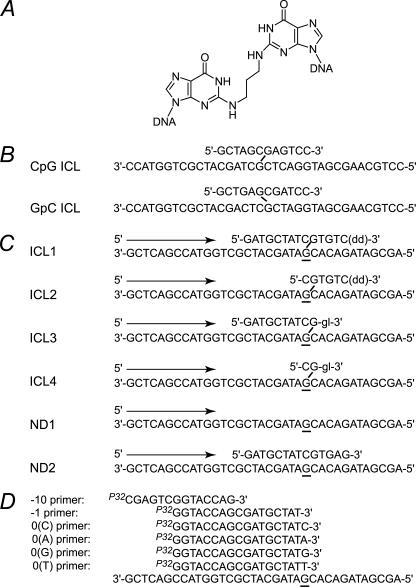
FIGURE 1.
Oligodeoxynucleotide structures. A, structure of model acrolein-derived N2-N2-guanine cross-link. B, schematic of the cross-linked oligodeoxynucleotides used for mutagenesis assays. C, schematic of the cross-linked and nondamaged template oligodeoxynucleotides. Template deoxyguanosines associated with cross-link and the corresponding unmodified deoxyguanosines are underlined. Arrows indicate the direction of DNA synthesis. To prevent DNA synthesis off of the shorter strand of the duplex oligodeoxynucleotide, either a 3′-glycerol unit (gl) or a dideoxycytidine (dd) were incorporated. To inhibit DNA synthesis off of the shorter strand in ND2, a double mismatch was placed at the end of the duplex region. D, primer oligodeoxynucleotides.
EXPERIMENTAL PROCEDURES
Preparation of Oligodeoxynucleotides—Unmodified oligodeoxynucleotides were synthesized by the Molecular Microbiology and Immunology Research Core Facility, Oregon Health & Science University. Modified oligodeoxynucleotides were synthesized as reported previously (22, 23). The experimental procedure that summarizes the sequential synthesis steps for ICL formation is shown in supplemental Fig. 1. Detailed descriptions of this procedure, the characterization of ICL-containing products, and the preparation of ICL2 and ICL4 from uracil-containing precursor oligodeoxynucleotides are given in supplemental Methods.
Cell Lines—COS-7 cells were purchased from the American Type Culture Collection. GM639 human fibroblasts were obtained from NIGMS Human Genetic Cell Repository.
Mutagenesis Assays—Mutagenesis assays were performed using a previously developed pMS2 shuttle vector/COS-7 system (24). The pMS2 shuttle vector was a gift from Dr. M. Moriya (State University of New York, Stony Brook). In its single-stranded form, the pMS2 vector contains a hairpin loop with an internal EcoRV restriction site. Incorporation of control nondamaged oligodeoxynucleotides into this site was done in the presence of a scaffold DNA according to a published method (25). To create a single-stranded pMS2 containing site-specific interstrand cross-link, the procedure was modified as follows. Oligodeoxynucleotides containing a model acrolein-derived site-specific interstrand cross-link (Fig. 1, A and B) were designed in such a way that peripheral regions of the longer strands (36-mer) were complementary to the vector sequences immediately adjacent to the EcoRV site. A circular single-stranded pMS2 vector (∼15 pmol) was digested with 40 units of EcoRV (New England Biolabs) for 3 h at 37°C to generate a linear DNA. 5′-Phosphorylated cross-linked inserts (60 pmol) were added to the linear single-stranded pMS2 (15 pmol) and ligated using 60 units of T4 DNA ligase (New England Biolabs) for 48 h at 4 °C. To obtain double-stranded pMS2 vectors, the 36-mer strand of the cross-link or the scaffold oligodeoxynucleotide in control DNA was extended using 5 units of T4 DNA polymerase (New England Biolabs) and 1 mm dNTPs at 37 °C for 1 h. A newly synthesized strand was concomitantly sealed using 5 units of T4 DNA ligase (New England Biolabs). The creation of double-stranded pMS2 was verified by PstI (New England Biolabs) digestion. Transfection of pMS2 vector into COS-7 cells, isolation of progeny DNA, selection of individual clones by Escherichia coli transformation, and differential hybridization analyses were done as described previously (24, 25).
DNA Polymerase Bypass Assays—Preparations of primer-template DNA substrates were performed as described previously (26). Human recombinant pol κ and S. cerevisiae recombinant Rev1 and pol ζ were obtained from Enzymax. Prior to polymerase bypass assays, polymerase preparations were tested for contaminating exonucleolytic activities. Primer-template nondamaged DNA substrate (5 nm) was incubated with each individual polymerase (2 nm) under conditions identical to polymerase reactions but in the absence of dNTPs. No primer degradation was observed after 30 min of incubation at 37 °C. Polymerase bypass assays were performed using 5 nm primer-template DNA substrates in the presence of 25 mm sodium phosphate (pH 7.5), 5 mm MgCl2, 10% glycerol, 10 mm NaCl, 0.1 mg/ml bovine serum albumin, and 5 mm dithiothreitol at 37 °C. Primer extensions were conducted with 100 μm dNTPs. Single nucleotide incorporations were performed with 20 μm of an individual dNTP. Protein concentrations and incubation times are indicated on figures or given in the figure legends. Prior to reactions using a combination of pol ζ and Rev1 proteins (Fig. 5D), pol ζ alone, or in a mixture with Rev1 was preincubated overnight on ice in the presence of 25 mm sodium phosphate (pH 7.5), 10% glycerol, 0.1 mg/ml bovine serum albumin, and 5 mm dithiothreitol. Polymerase reactions were terminated by the addition of an equal volume of a solution consisting of 95% formamide, 20 mm EDTA, 0.2% (w/v) bromphenol blue, and 0.2% xylene cyanol. Products were resolved through a 15% denaturing polyacrylamide gel in the presence of 8 m urea and visualized using a PhosphorImager screen (GE Healthcare).
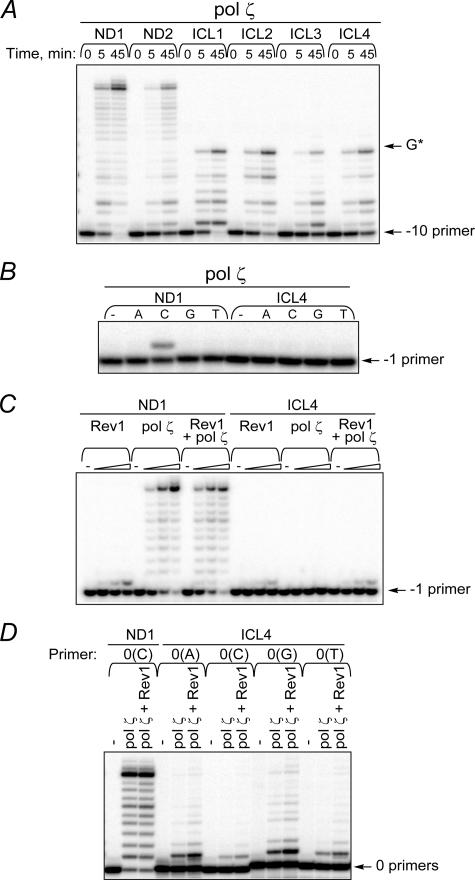
FIGURE 5.
Replication bypass of ICLs by yeast polζ. A, primer extensions by pol ζ (5 nm) were conducted for a period of time as indicated. B, single nucleotide incorporations by pol ζ (5 nm) were carried out for 30 min. C, primer extensions were carried out for 30 min at increasing concentrations of pol ζ (5, 10, or 20 nm) and Rev1 (5, 10, or 20 nm). Polymerases were present in reactions either individually or in combination. D, primer extensions in the presence of pol ζ (10 nm) or a combination of pol ζ (10 nm) and Rev1 (10 nm) were carried out for 45 min.
Steady-state kinetic analyses were performed according to a standard procedure (27). Briefly, reactions were conducted under the same conditions as single nucleotide incorporation assays except that the primer-template DNA concentration was 10 nm; dCTP or dGTP was used at various concentrations; reactions were conducted at 22 °C, and polymerase concentrations and incubation times were adjusted not to exceed 25% of the product formation. Quantitative analyses were performed using ImageQuant 5.2 software (GE Healthcare). Rates of dNTP incorporation were plotted as a function of dNTP concentration, and the data were analyzed using Kaleidagraph 3.6 software (Synergy Software). The kcat and Km parameters with their error values were obtained from the best fit of the data to the following Michaelis-Menten equation: vobs = kcat[E][dNTP]/(Km + [dNTP]). Relative efficiencies were calculated as a ratio of the efficiency of reaction using ICL template to the efficiency measured for undamaged template.
Transfection of GM639 Cells—siRNA transfections were performed as described previously (28). Briefly, cells were transfected with SMARTpool siRNA (Dharmacon) specific to pol κ diluted to 0.30 μm or mock-transfected. The final transfection volumes were 1.0 ml for a T25 flask and 3.2 ml for a 100-mm dish.
Cell Survival Assays—Twenty-four hours after siRNA transfection, cells were plated on 100-mm dishes to a density of 300 cells per dish in α-minimum Eagle's medium, treated with MMC (Sigma), and allowed to grow for 10 days. Cells were fixed in a solution of 50% MeOH and 1% new methylene blue (Sigma), and surviving colonies were counted.
Chromosome Stability—Twenty-four hours after siRNA transfection, cells were treated with MMC (40 ng/ml), incubated for 48 h, and harvested as described (28). Slides were stained with Wright's stain, and 50 metaphases from each culture were scored for radial formation.
Quantification of pol κ mRNA—Cells were harvested 24 h after siRNA transfection. RNA was stabilized using RNAlater (Ambion), extracted using the RNeasy mini kit (Qiagen), and quantified. Reverse transcription was performed using the high capacity cDNA reverse transcription kit (Applied Biosystems) using 1 μg of starting RNA material. Real time quantitative PCR was performed using the iCycler iQ detection system (Bio-Rad) with 10 ng of starting cDNA material using the TaqMan gene expression assay specific to pol κ (Applied Biosystems). Primers specific to the housekeeping gene β-actin (Applied Biosystems) were used for internal control. Each sample was tested in triplicate. An amplification plot was created for each sample. Threshold values were calculated from the amplification plots correlating to the cycle number where fluorescence was detected above a calculated threshold. The mRNA concentrations for each sample were calculated with the Ct value and were normalized against β-actin expression. Negative controls for each primer were included in each experiment.
Statistical Analysis—The means, means ± S.E., and p values from the Student's t tests were obtained using Kaleidagraph 3.6 software (Synergy Software).
RESULTS
Mutagenic Properties of Model Acrolein-derived N2-N2-Guanine ICLs in Mammalian (COS-7) Cells—Previous studies have established that in human cells crotonaldehyde-mediated N2-N2-guanine ICLs were repaired mostly (>94%) in an error-free manner (9). Because acrolein-mediated ICLs are structurally similar to crotonaldehyde-mediated ICLs, we suggested that our model acrolein-derived N2-N2-guanine ICL (Fig. 1A) would not be highly mutagenic when plasmid vectors containing this lesion were replicated in mammalian cells. To test this hypothesis, oligodeoxynucleotides were constructed to contain a site-specific ICL in either a CpG or GpC sequence context (Fig. 1B) and engineered into a double-stranded DNA shuttle vector (pMS2) (24). The vector was replicated in COS-7 cells, and progeny DNAs were analyzed to determine frequency and types of mutations. In both the CpG and GpC sequence contexts, the mutation frequencies were very low, 3.0 and 3.2%, respectively (Table 1). Mutations were single base substitutions as well as deletions that generally began at, or one nucleotide downstream from the ICL and extended <15 nucleotides. Thus, during replication of plasmids containing model acrolein-mediated N2-N2-guanine ICLs, most bypass events were error-free in COS-7 cells. In the CpG sequence context, the base substitutions were G to T transversions, a result that is in agreement with previously reported data on mutagenic properties of crotonaldehyde-(9) and MMC-mediated N2-N2-guanine ICLs (5); in both earlier studies the most frequently observed single base substitutions were G to T transversions.
TABLE 1.
Mutations generated during replication of site-specifically modified pMS2 vector in COS-7 cells
|
DNA modification
|
Colonies scored
|
Single base substitutions, number
|
Deletions
|
Frequency of mutations
|
||
|---|---|---|---|---|---|---|
| G to A | G to C | G to T | ||||
| % | ||||||
| Undamaged | 315 | 0 | 0 | 0 | 0 | 0 |
| ICL in GpC | 342 | 4 | 1 | 1 | 5 | 3.2 |
| ICL in CpG | 66 | 0 | 0 | 2 | 0 | 3.0 |
Design and Construction of DNA Substrates for Replication Bypass Assays—To investigate DNA polymerase bypass of ICLs, DNAs containing acrolein-derived N2-N2 guanine cross-links (Fig. 1A) were designed to mimic potential intermediates in the processing of ICLs (Fig. 1C). Cross-linked DNA 1 (ICL1) represents a model of the DNA product following dual incision around the ICL site, whereas ICL2, ICL3, and ICL4 represent the products of either exonucleolytic or endonucleolytic processing of the 5′ or 3′ end or both, respectively, up to but not including the cross-link; justification for the specific design of these substrates is discussed below. The nondamaged ND2 substrate was designed to mimic a structure encountered by a polymerase in ICL1 and ICL3 but contains no damage.
Endonucleolytic processing of ICL-containing DNAs has been addressed in a number of biochemical studies using either preparations of chromatin-associated proteins (29) or the purified XPF-ERCC1 complex (30, 31), a structure-specific endonuclease that is essential for ICL repair (12, 32). In these investigations, a variety of structurally diverse substrates was utilized, including splayed, Y-shaped, and fully duplex DNAs. Incisions were commonly observed both 5′ and 3′ to the ICL sites, suggesting generation of excision products, i.e. the DNA fragments that presumably remain connected to the opposite strand. The estimated sizes of these DNA fragments were in a range from 4 to 17 deoxynucleotides (29–31). Thus, our ICL1 substrate, in which a 12-mer oligodeoxynucleotide is covalently attached to the longer strand, represents a reasonable model for a dually incised ICL-containing DNA.
Recently, significant progress has been made in structural characterization of TLS polymerases. Although TLS polymerases appear to have relatively spacious active sites (14, 33–36), it is hard to imagine that a dually incised ICL-containing DNA could be accommodated there, properly positioned, and utilized as template. Thus, remodeling of the overall DNA structure and/or processing of the incised DNA fragment is thought to be required prior to TLS. In this regard, it is significant that the XPF-ERCC1 complex and its yeast counterpart, Rad1-Rad10, both possess the 3′ to 5′ exonucleolytic activity (37, 38). Specifically, it has been shown that Rad1–Rad10 degrades DNA from the 3′ end, releasing products 3–6 nucleotides in length (37). Thus, it is possible that following the endonucleolytic cleavage 3′ to the ICL site, incised DNA will be further processed exonucleolytically by the XPF-ERCC1 (Rad1–Rad10) complex. Such an action could create the structure as in our ICL3. ICL2 represents the product of exonucleolytic processing of the 5′ of the excised DNA fragment. The potential candidate to perform this reaction would be Snm1, which has a demonstrated 5′- to 3′-exonuclease activity (39, 40) and in yeast is critical for ICL repair (10–12). However, alternative enzymes could also play a role in the processing of ICL-containing DNA to yield structures that would favor replication bypass. These may include alternative exonucleases, replicative and repair helicases, flap endonucleases, and exonuclease activities associated with helicases and polymerases. Primer oligodeoxynucleotides (Fig. 1D) were designed to perform DNA syntheses under running start (–10 primer) or standing start (–1 primer) conditions or to test for extension from a nucleotide that is opposite the cross-linked G (0 primers).
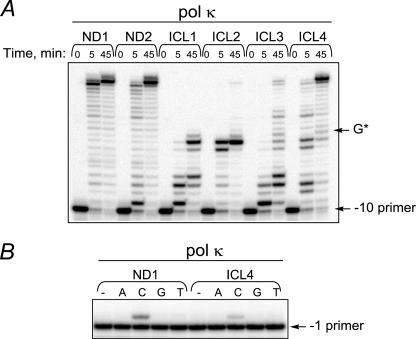
Replication Bypass of Model Acrolein-derived N2-N2-Guanine ICLs by Human pol κ—Prior findings demonstrated that pol κ could carry out TLS past various bulky N2-guanine adducts and was critical in limiting mutagenesis from these lesions (16–20). Because in our model ICL the linkage is mediated through the exocyclic amino group of guanines, we hypothesized that pol κ may catalyze replication bypass of this lesion. A 32P-labeled oligodeoxynucleotide primer (–10 primer, Fig. 1D) was hybridized with template DNAs, and primer extension reactions were conducted using human pol κ (Fig. 2A). A processive DNA synthesis by pol κ was observed on both undamaged substrates with a partial inhibition of replication at initiation of strand displacement synthesis using ND2. Analyses of primer extension reactions on cross-linked substrates, in which the four nucleotides that were 3′ to the cross-link had been removed (ICL3 and ICL4), revealed that pol κ could also catalyze TLS past the cross-linked site; in 45-min reactions, ∼10 and 74% of primers were extended beyond the cross-linked guanine in ICL3 and ICL4, respectively, versus ∼79% of primers extended beyond the corresponding guanine in ND1. Additionally, pol κ was able to catalyze limited synthesis past the cross-link in ICL2 with ∼2% of primers extended beyond the lesion.
FIGURE 2.
Replication bypass of ICLs by human pol κ. A, primer extensions by pol κ (2 nm) were conducted for a period of time as indicated. B, single nucleotide incorporations by pol κ (1 nm) were carried out for 30 min.
To identify nucleotide(s) that pol κ inserts opposite the cross-linked G, single nucleotide incorporation assays were conducted using the –1 primer (Fig. 2B). Similar to results obtained for the ND1 substrate, using the ICL4 template, extension products were detected in reactions supplemented with dCTP but not the other dNTPs. The catalytic efficiency (kcat/ Km) of dCTP incorporation by pol κ opposite control G versus the cross-linked G revealed a 35-fold decrease, whereas efficiency of extension from a C opposite the lesion was reduced ∼7-fold (Table 2). Overall, these data indicate that pol κ can accurately bypass N2-N2 guanine ICLs, representing the first biochemical evidence for TLS by any DNA polymerase past any ICL.
TABLE 2.
Steady-state kinetic parameters for ICL bypass by pol κ
| Primer | DNA substrate | dNTP | kcat | Km | kcat/Km | Relative efficiency |
|---|---|---|---|---|---|---|
| min-1 | μm | μm-1 min-1 | ||||
| -1 | ND1 | dCTP | 0.19 ± 0.01 | 22 ± 2 | 8.6 × 10-3 | 1 |
| -1 | ICL4 | dCTP | 0.026 ± 0.001 | 110 ± 11 | 0.23 × 10-3 | 0.03 |
| 0(C) | ND1 | dGTP | 0.10 ± 0.01 | 1.2 ± 0.2 | 85 × 10-3 | 1 |
| 0(C) | ICL4 | dGTP | 0.022 ± 0.001 | 1.8 ± 0.4 | 12 × 10-3 | 0.14 |
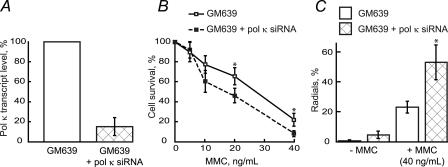
Cellular Responses to MMC Exposure in pol κ-depleted Cells—To determine whether pol κ functions intracellularly in the processing of N2-N2-guanine ICLs, the cytotoxicity of MMC in pol κ-depleted human cells was assessed. GM639 cells were treated with a specific siRNA that was shown to reduce pol κ transcript level by ∼85% (Fig. 3A). When subsequently challenged with MMC, the pol κ-depleted cells showed decreased survival versus control, mock-transfected cells (Fig. 3B). To seek additional biological evidence for a role for pol κ in the repair of these ICLs, mock-transfected and pol κ-depleted cells were examined for chromosomal damage in the form of radial formation. An increased frequency of radial formation is known to correspond with defective ICL repair in Fanconi anemia cells (28, 41). Following MMC treatment, the percent of cells containing radial structures was significantly increased in pol κ-depleted versus control cells (Fig. 3C). Combined, these data are strongly consistent with a biologically relevant role for pol κ in TLS-assisted repair of N2-N2-guanine ICLs in human cells.
FIGURE 3.
Cellular responses to MMC exposure in pol κ-depleted GM639 cells. A, pol κ transcript levels following treatment by specific siRNA. B, relative colony-forming ability. C, radial formation. Error bars represent standard errors for at least four independent experiments, and data points are marked by asterisk with p < 0.05 (relative to corresponding control).
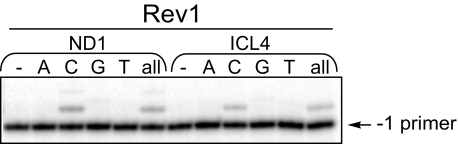
Replication Bypass of Model Acrolein-derived N2-N2-Guanine ICLs by Yeast Rev1—Previous studies have shown that recombination-independent repair of MMC-induced ICLs was less efficient in Rev1-deficient mutant cells relative to the wild type (8), whereas in vitro, Rev1 strongly favored dCTP incorporation opposite N2-adducted guanines (21). Thus, the ability of Rev1 to insert nucleotides opposite the ICL4 was examined (Fig. 4). These data revealed that on both nondamaged (ND1) and ICL4 templates, Rev1 inserted a nucleotide opposite G but could not extend the primer further. In reactions supplemented with individual dNTPs, insertion products were detected in the presence of dCTP exclusively (Fig. 4). As measured from three independent experiments, the rate of incorporation opposite the undamaged versus cross-linked G was (1.84 ± 0.31) × 10–3 min–1 to (0.70 ± 0.03) × 10–3 min–1. Thus, for an ICL containing a cross-linked G in a CpG local sequence context, Rev1 can accurately incorporate the first nucleotide.
FIGURE 4.
Replication bypass of ICLs by yeast Rev1. Primer extensions (marked all) and single nucleotide incorporations by Rev1 (10 nm) were carried out for 30 min.
Inability of Yeast pol ζ to Catalyze Replication Bypass of Model Acrolein-derived N2-N2-Guanine ICLs—Cumulative genetic data support an involvement of pol ζ in the tolerance to MMC-induced damage and suggest a role for its contribution to MMC-associated mutagenesis (8, 13). However, a diploid yeast mutant that is deficient in rev3 does not show significantly elevated cytotoxic sensitivity following exposure to MMC (12). In addition, pol ζ has been proposed to play a role in TLS past N2-guanine adducts by efficiently extending a C that had been inserted opposite the lesion by Rev1 (21). Here we examined the ability of yeast pol ζ to bypass N2-N2-guanine ICLs. As shown in Fig. 5A, using undamaged templates, pol ζ efficiently extended primers to yield products up to full-length DNA. In contrast, replication of all four ICL templates was completely blocked one nucleotide prior to the cross-linked guanine. To verify the inability of pol ζ to catalyze nucleotide incorporation opposite the template ICL, a –1 primer was hybridized to the unadducted control template (ND1) and the ICL4, and reactions were conducted in the presence of each individual dNTP. Error-free insertion of dCTP by polζ was observed using ND1 with ∼25% of primers being extended; however, no incorporation of any dNTP could be detected using the ICL4 template (Fig. 5B).
Next, the possibility was considered that bypass of the cross-link could be accomplished by sequential action of Rev1 and pol ζ. In this model, pol ζ would extend from a C that had been inserted opposite the cross-linked G by Rev1. Primer extension experiments were conducted in the presence of all four dNTPs and both Rev1 and pol ζ using a –1 primer that was annealed to either undamaged or ICL4 template. Under conditions in which ∼84% of primers were utilized in the presence of these proteins with undamaged template, no primer extension was observed beyond an ICL site (Fig. 5C).
Additional reactions were carried out with polζ using a 0 primer that contained a C at its 3′ end. Because Rev1 can stimulate the activity of pol ζ (42), primer extensions were also performed using a combination of these proteins. As shown in Fig. 5D, pol ζ alone efficiently extended primers annealed to undamaged template (∼89% of primer utilization) and showed similar efficiency in the presence of Rev1 (∼91% of primer utilization). In contrast, extension by pol ζ from a C opposite the cross-linked G (ICL4 template) appeared to be very inefficient with only ∼7% of primers being extended, and little stimulation of polymerization by Rev1 was observed. In parallel reactions, a series of mismatched primers were tested. These primers were extended by pol ζ better than the correctly paired primer; ∼19, 21, and 10% of primers were extended when A, G, and T were placed opposite the cross-linked guanine, respectively. This error-prone DNA synthesis was enhanced in the presence of Rev1 (Fig. 5D) with ∼29, 33, and 16% of the corresponding primers being extended. Thus, replication bypass of N2-N2-guanine ICLs by pol ζ was highly inefficient and error-prone.
DISCUSSION
Previous models for ICL repair have postulated the following sequential steps leading to restoration of an intact DNA strand: 1) DNA strand incision by components of the nucleotide excision repair; 2) failure of pol δ to catalyze gap filling; 3) monoubiquitination of proliferating cell nuclear antigen; and 4) recruitment of a TLS polymerase to replicate past the lesion and fill the gap (7). Our model is consistent with these speculations and adds important specificity to the biochemical processing of ICLs.
First, we addressed the question of the identity of DNA polymerases that are involved in recombination-independent repair of ICLs, anticipating that, in vivo, the choice of polymerase to be utilized in this process will be dictated by the chemical structure of a particular ICL. N2-N2-Guanine ICLs are the focus of this study. We hypothesized that pol κ, Rev1, and pol ζ may play a role in replication bypass of N2-N2-guanine ICLs, and we demonstrated an in vitro ability of pol κ to catalyze accurate TLS past these lesions. Our data represent the first biochemical evidence for replication bypass of any ICL and suggest that pol κ could be involved in TLS-assisted repair of N2-N2-guanine ICLs in vivo. Consistent with this suggestion, both cell survival and chromosomal stability were adversely affected in pol κ-depleted cells following MMC exposure.
An earlier study demonstrated that pol κ-deficient chicken DT40 mutants are no more sensitive to cytotoxic effects of cisplatin than the wild type cells (13). Because the vast majority of cisplatin adducts are intrastrand cross-links and the minor portion of cross-links that have an interstrand linkage are structurally different from those that are formed by MMC (1), there is no contradiction between these published data and the results presented here. Instead, collectively these observations substantiate our suggestion that the specificity of the DNA polymerase involved in ICL repair is linkage-dependent.
Our proposed model is in agreement with findings that suggest a specific role for polκ in the bypass of N2-guanine lesions (16–20) (i.e. the cross-linked strand may be regarded as a bulky N2-guanine adduct). Germane to these investigations, both the crystal (33) and cocrystal structures (35) of pol κ catalytic core have been solved. The structure of the ternary complex of the pol κ catalytic core with DNA and the incoming nucleotide revealed that the DNA was completely encircled by polymerase, with an N-clasp augmenting the thermodynamic stability of the catalytic complex. It is hypothesized that such a locked structure leads to an increased time of polymerase residence on DNA substrate, thus allowing the efficient extension of the distorted primer termini. However, it is not immediately apparent from these structural data how pol κ is able to both incorporate nucleotides opposite the bulky N2-guanine lesions, as well as extend the primer to full-length DNAs.
The ability to replicate past N2-N2-guanine ICLs has been shown for E. coli pol IV,5 the bacterial orthologue of pol κ (43, 44). In addition, a pol IV-deficient mutant appeared to be more sensitive to MMC exposure relative to wild type, thus providing analogous support for a role of pol κ in the bypass of such ICLs.
Previous investigations demonstrated a biological role for Rev1 and pol ζ in tolerance to various ICL-inducing agents and the involvement of these activities in recombination-independent ICL repair (7, 8, 10–13). Regarding N2-N2-guanine ICLs, deficiency in Rev3 causes extreme sensitivity to MMC in chicken DT40 cells (13), and recombination-independent repair of MMC-induced ICLs is significantly reduced in both REV1–/– and REV3–/– strains (8). However, a rev3–/– yeast mutant is only moderately sensitive to MMC exposure (12). Furthermore, Rev1 has been shown to efficiently incorporate a C opposite an N2-guanine adduct, from which pol ζ could subsequently extend (21). In this study, we tested whether bypass of N2-N2-guanine ICLs may also be achieved by sequential action of Rev1 and pol ζ and showed that Rev1 was able to efficiently insert the correct nucleotide opposite the cross-linked guanine. However, it is not apparent from our data what contribution pol ζ might have in the bypass of such lesions. We cannot completely rule out the possibility that in vivo pol ζ might be capable of extending a primer positioned opposite the cross-linked guanine when stimulated by proliferating cell nuclear antigen or other auxiliary factors. In addition, pol ζ may function in recruitment of other polymerases to the ICL sites and/or assembly of the replication bypass complex. However, to tolerate MMC-induced DNA damage, Rev1 and pol ζ do not fully compensate for pol κ deficiency in human cells, as demonstrated here.
Additionally, we have discovered that the efficiency of TLS was greatly facilitated by truncation of the 5′ and 3′ ends of the incised strand. Even though pol κ was able to catalyze strand displacement synthesis and replicate past the ICL3, a significant stimulation of bypass was achieved by resection of the 5′ end of the nonreplicated strand. Thus, utilization of the 5′ to 3′ exonucleolytic activity of an enzyme such as Snm1 (39, 40) or the combined activity of a 3′- to 5′-helicase with DNA flap incision by FEN1 would enhance cell survival in response to ICL damage. Facilitation of the TLS was even more significant when the 3′ end of the incised strand was truncated; only very modest bypass of ICL2 was observed. Currently, there is no genetic evidence that a 3′- to 5′-exonuclease is required for ICL repair. However, both the human XPF-ERCC1 complex (38) and the yeast homologue, Rad1-Rad10 complex (37), have a 3′- to 5′-exonuclease activity. In addition, it has been suggested that pol δ may provide such activity during the course of ICL removal (45). Importantly, most proteins employed by this proposed mechanism are associated with the nucleotide excision repair machinery, including pol κ that was shown to function in this pathway (46).
Supplementary Material
Acknowledgments
We thank A. K. McCullough, J. L. Johnson, M. P. Stone, and F. P. Guengerich for discussion and comments. We are grateful to M. Moriya (State University of New York, Stony Brook) for providing the pMS2 vector.
This work was supported, in whole or in part, by National Institutes of Health Grant ES 05355 and Center Grant ES 000267. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Methods, additional references, Table 1, and Fig. 1.
Footnotes
The abbreviations used are: ICL, interstrand cross-link; TLS, translesion DNA synthesis; pol, polymerase; MMC, mitomycin C; siRNA, short interfering RNA.
A. Kumari, I. G. Minko, M. B. Harbut, S. E. Finkel, M. F. Goodman, and R. S. Lloyd, unpublished data.
References
- 1.Noll, D. M., Mason, T. M., and Miller, P. S. (2006) Chem. Rev. 106 277–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehoczky, P., McHugh, P. J., and Chovanec, M. (2007) FEMS Microbiol. Rev. 31 109–133 [DOI] [PubMed] [Google Scholar]
- 3.Kozekov, I. D., Nechev, L. V., Moseley, M. S., Harris, C. M., Rizzo, C. J., Stone, M. P., and Harris, T. M. (2003) J. Am. Chem. Soc. 125 50–61 [DOI] [PubMed] [Google Scholar]
- 4.Wang, X., Peterson, C. A., Zheng, H., Nairn, R. S., Legerski, R. J., and Li, L. (2001) Mol. Cell. Biol. 21 713–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng, H., Wang, X., Warren, A. J., Legerski, R. J., Nairn, R. S., Hamilton, J. W., and Li, L. (2003) Mol. Cell. Biol. 23 754–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richards, S., Liu, S. T., Majumdar, A., Liu, J. L., Nairn, R. S., Bernier, M., Maher, V., and Seidman, M. M. (2005) Nucleic Acids Res. 33 5382–5393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarkar, S., Davies, A. A., Ulrich, H. D., and McHugh, P. J. (2006) EMBO J. 25 1285–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen, X., Jun, S., O'Neal, L. E., Sonoda, E., Bemark, M., Sale, J. E., and Li, L. (2006) J. Biol. Chem. 281 13869–13872 [DOI] [PubMed] [Google Scholar]
- 9.Liu, X., Lao, Y., Yang, I. Y., Hecht, S. S., and Moriya, M. (2006) Biochemistry 45 12898–12905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henriques, J. A., and Moustacchi, E. (1980) Genetics 95 273–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grossmann, K. F., Ward, A. M., Matkovic, M. E., Folias, A. E., and Moses, R. E. (2001) Mutat. Res. 487 73–83 [DOI] [PubMed] [Google Scholar]
- 12.Wu, H. I., Brown, J. A., Dorie, M. J., Lazzeroni, L., and Brown, J. M. (2004) Cancer Res. 64 3940–3948 [DOI] [PubMed] [Google Scholar]
- 13.Nojima, K., Hochegger, H., Saberi, A., Fukushima, T., Kikuchi, K., Yoshimura, M., Orelli, B. J., Bishop, D. K., Hirano, S., Ohzeki, M., Ishiai, M., Yamamoto, K., Takata, M., Arakawa, H., Buerstedde, J. M., Yamazoe, M., Kawamoto, T., Araki, K., Takahashi, J. A., Hashimoto, N., Takeda, S., and Sonoda, E. (2005) Cancer Res. 65 11704–11711 [DOI] [PubMed] [Google Scholar]
- 14.Prakash, S., Johnson, R. E., and Prakash, L. (2005) Annu. Rev. Biochem. 74 317–353 [DOI] [PubMed] [Google Scholar]
- 15.Tomasz, M. (1995) Chem. Biol. 2 575–579 [DOI] [PubMed] [Google Scholar]
- 16.Zhang, Y., Wu, X., Guo, D., Rechkoblit, O., and Wang, Z. (2002) DNA Repair 1 559–569 [DOI] [PubMed] [Google Scholar]
- 17.Ogi, T., Shinkai, Y., Tanaka, K., and Ohmori, H. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 15548–15553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avkin, S., Goldsmith, M., Velasco-Miguel, S., Geacintov, N., Friedberg, E. C., and Livneh, Z. (2004) J. Biol. Chem. 279 53298–53305 [DOI] [PubMed] [Google Scholar]
- 19.Choi, J. Y., Angel, K. C., and Guengerich, F. P. (2006) J. Biol. Chem. 281 21062–21072 [DOI] [PubMed] [Google Scholar]
- 20.Jarosz, D. F., Godoy, V. G., Delaney, J. C., Essigmann, J. M., and Walker, G. C. (2006) Nature 439 225–228 [DOI] [PubMed] [Google Scholar]
- 21.Washington, M. T., Minko, I. G., Johnson, R. E., Haracska, L., Harris, T. M., Lloyd, R. S., Prakash, S., and Prakash, L. (2004) Mol. Cell. Biol. 24 6900–6906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dooley, P. A., Tsarouhtsis, D., Korbel, G. A., Nechev, L. V., Shearer, J., Zegar, I. S., Harris, C. M., Stone, M. P., and Harris, T. M. (2001) J. Am. Chem. Soc. 123 1730–1739 [DOI] [PubMed] [Google Scholar]
- 23.Dooley, P. A., Zhang, M., Korbel, G. A., Nechev, L. V., Harris, C. M., Stone, M. P., and Harris, T. M. (2003) J. Am. Chem. Soc. 125 62–72 [DOI] [PubMed] [Google Scholar]
- 24.Moriya, M. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 1122–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanuri, M., Minko, I. G., Nechev, L. V., Harris, T. M., Harris, C. M., and Lloyd, R. S. (2002) J. Biol. Chem. 277 18257–18265 [DOI] [PubMed] [Google Scholar]
- 26.Minko, I. G., Washington, M. T., Kanuri, M., Prakash, L., Prakash, S., and Lloyd, R. S. (2003) J. Biol. Chem. 278 784–790 [DOI] [PubMed] [Google Scholar]
- 27.Creighton, S., Bloom, L. B., and Goodman, M. F. (1995) Methods Enzymol. 262 232–256 [DOI] [PubMed] [Google Scholar]
- 28.Bruun, D., Folias, A., Akkari, Y., Cox, Y., Olson, S., and Moses, R. (2003) DNA Repair 2 1007–1013 [DOI] [PubMed] [Google Scholar]
- 29.Kumaresan, K. R., Hang, B., and Lambert, M. W. (1995) J. Biol. Chem. 270 30709–30716 [DOI] [PubMed] [Google Scholar]
- 30.Kuraoka, I., Kobertz, W. R., Ariza, R. R., Biggerstaff, M., Essigmann, J. M., and Wood, R. D. (2000) J. Biol. Chem. 275 26632–26636 [DOI] [PubMed] [Google Scholar]
- 31.Fisher, L. A., Bessho, M., and Bessho, T. (2008) J. Biol. Chem. 283 1275–1281 [DOI] [PubMed] [Google Scholar]
- 32.De Silva, I. U., McHugh, P. J., Clingen, P. H., and Hartley, J. A. (2000) Mol. Cell. Biol. 20 7980–7990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uljon, S. N., Johnson, R. E., Edwards, T. A., Prakash, S., Prakash, L., and Aggarwal, A. K. (2004) Structure (Lond.) 12 1395–1404 [DOI] [PubMed] [Google Scholar]
- 34.Nair, D. T., Johnson, R. E., Prakash, L., Prakash, S., and Aggarwal, A. K. (2005) Science 309 2219–2222 [DOI] [PubMed] [Google Scholar]
- 35.Lone, S., Townson, S. A., Uljon, S. N., Johnson, R. E., Brahma, A., Nair, D. T., Prakash, S., Prakash, L., and Aggarwal, A. K. (2007) Mol. Cell 25 601–614 [DOI] [PubMed] [Google Scholar]
- 36.Broyde, S., Wang, L., Zhang, L., Rechkoblit, O., Geacintov, N. E., and Patel, D. J. (2008) Chem. Res. Toxicol. 21 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guzder, S. N., Torres-Ramos, C., Johnson, R. E., Haracska, L., Prakash, L., and Prakash, S. (2004) Genes Dev. 18 2283–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mu, D., Bessho, T., Nechev, L. V., Chen, D. J., Harris, T. M., Hearst, J. E., and Sancar, A. (2000) Mol. Cell. Biol. 20 2446–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li, X., Hejna, J., and Moses, R. E. (2005) DNA Repair 4 163–170 [DOI] [PubMed] [Google Scholar]
- 40.Hejna, J., Philip, S., Ott, J., Faulkner, C., and Moses, R. (2007) Nucleic Acids Res. 35 6115–6123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sasaki, M. S. (1975) Nature 257 501–503 [DOI] [PubMed] [Google Scholar]
- 42.Acharya, N., Johnson, R. E., Prakash, S., and Prakash, L. (2006) Mol. Cell. Biol. 26 9555–9563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerlach, V. L., Feaver, W. J., Fischhaber, P. L., and Friedberg, E. C. (2001) J. Biol. Chem. 276 92–98 [DOI] [PubMed] [Google Scholar]
- 44.Lee, C. H., Chandani, S., and Loechler, E. L. (2006) J. Mol. Graph. Model 25 87–102 [DOI] [PubMed] [Google Scholar]
- 45.McElhinny, S. A., Pavlov, Y. I., and Kunkel, T. A. (2006) Cell Cycle 5 958–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogi, T., and Lehmann, A. R. (2006) Nat. Cell Biol. 8 640–642 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.