Abstract
Blood neutrophils recruited to cystic fibrosis (CF) airways are believed to be rapidly killed by resident bacteria and to passively release elastase and other toxic by-products that promote disease progression. By single-cell analysis, we demonstrate that profound functional and signaling changes readily occur within viable neutrophils recruited to CF airways, compared with their blood counterparts. Airway neutrophils have undergone conventional activation, as shown by decreased intracellular glutathione, increased lipid raft assembly, surface mobilization of CD11b+ and CD66b+ granules, and increased levels of the cytoskeleton-associated phospho-Syk kinase. Unexpectedly, they also mobilize to the surface CD63+ elastase-rich granules, usually confined intracellularly, and lose surface expression of CD16 and CD14, both key receptors in phagocytosis. Furthermore, they express CD80, major histocompatibility complex type II, and the prostaglandin D2 receptor CD294, all normally associated with other lineages, which reflects functional reprogramming. This notion is reinforced by their decreased total phosphotyrosine levels, mirroring a postactivated stage, and increased levels of the phospho-S6 ribosomal protein, a key anabolic switch. Thus, we identified a subset of neutrophils within CF airways with a viable but dysfunctional phenotype. This subset provides a possible therapeutic target and indicates a need to revisit current paradigms of CF airway disease.
Keywords: cystic fibrosis transmembrane conductance regulator, flow cytometry, inflammation, lung disease, phosphoepitope
Cystic fibrosis (CF) is the most common recessive disease among Caucasians, affecting ≈1 in 2,500 live births. CF patients suffer from multiple dysfunctions of exocrine organs, caused by mutations in the CF transmembrane conductance regulator (CFTR) channel (1). Airway disease, the main cause of morbidity and mortality among CF patients, is dominated by massive recruitment of blood neutrophils to lungs (2) that fail to clear CF-tropic opportunistic bacteria, such as mucoid Pseudomonas aeruginosa (3).
CF lung fluid is rich in undigested DNA and actin, as well as extracellular neutrophil elastase (NE) (4), originating from a set of neutrophil granules (primary or azurophil granules) that generally are not actively exocytosed from neutrophils (5). To date, it is commonly assumed that neutrophil death, possibly accelerated by resident bacteria, occurs rapidly upon recruitment to CF airways (6, 7). Passive release of NE and other toxic by-products (myeloperoxidase, DNA, actin) is thought to ensue (8), perpetuating inflammation (3), infection, and obstruction (9). Proteolytic destruction of the airway fabric eventually leads to parenchymal scarring and respiratory failure (1, 2).
Here, we describe a systematic effort to characterize novel mechanisms that may underlie neutrophil demise in CF airways. We compared neutrophils from induced sputum, a well standardized procedure that yields cells from conducting airways (10), where CF symptoms are the most manifest, to their blood counterparts. We combined multiparametric profiling (11) of functional properties (lipid raft and degranulation markers, immune receptors) and intracellular phosphoepitopes (total phosphotyrosine levels, intermediate kinases, and final effectors that reflect key signaling pathways). Our results establish that CF airways contain a significant fraction of nonapoptotic viable neutrophils that bear extensive functional and signaling changes, suggesting far more complex neutrophil immunomodulation than previously thought.
Results
Direct ex Vivo Analysis of CF Neutrophils.
We performed simultaneous blood and induced sputum collection from 33 CF patients with stable disease, spanning a large range of disease status [for demographic details, see supporting information (SI) Table 2]. Fifteen of 33 subjects yielded samples on several occasions, providing cross-sectional and longitudinal validation to our assays. To limit artifactual activation of cells, circulating neutrophils were stained and analyzed directly from whole blood (11). Moreover, in contrast to the protocol used for most CF studies, sputum was not treated with dithiothreitol at 37°C for 30 min (10), because this combination of a potent redox effector and high temperature alters intracellular glutathione (GSH) levels and cell activation status (ref. 12 and SI Fig. 4). Instead, sputum neutrophils were obtained by gentle dissociation in buffer (see SI Methods).
Detection of Viable Nonapoptotic Neutrophils in Blood and Airway Samples.
Viable nonapoptotic neutrophils were defined by stepwise exclusion of doublets and cell clumps, apoptotic and compromized cells, nongranulocytes, and nonneutrophils by using flow cytometric analysis (SI Fig. 5). We used the positivity for GSH-bimane adducts to gate on viable nonapoptotic cells and exclude compromised cells that would appear viable using less stringent criteria (e.g., annexin V and size alone). Although viable airway neutrophils maintained significant levels of intracellular GSH (Fig. 1), these levels were significantly lower (P < 10−3) than in their blood counterparts, consistent with increased activation and exposure to high redox stress in the airway environment (13). The number of viable airway neutrophils correlated well (in negative fashion) with functional expiratory volume in 1 s, percent predicted, a well established measure of CF lung function (Spearman ρ = −0.5, P = 0.003), thus underlining the importance of these cells in CF airway disease.
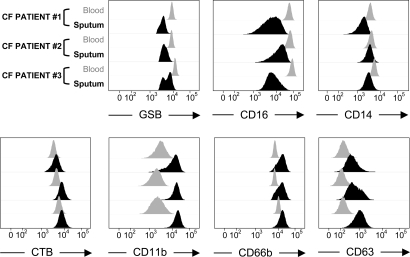
Fig. 1.
Activation pattern of viable CF airway neutrophils. Viable neutrophils from induced sputum are compared with their blood counterparts (three representative CF patients) for levels of activation markers. Intracellular GSH levels, as measured by GSH-bimane adducts (see SI Methods), are decreased in sputum neutrophils (Upper Left). CD16 and CD14 are also decreased in sputum neutrophils (Upper Middle and Right, respectively). By contrast, surface lipid rafts (labeled by cholera toxin B, or CTB), as well as CD11b, CD66b, and CD63 (specific for NE-rich granules) are all increased in sputum neutrophils (Lower, from left to right). Differences in median fluorescence intensities between sputum and blood neutrophils were highly significant among the 33 patients included in the study (P < 10−3 for all).
Lipid Raft Assembly and Mobilization of CD11b+ and CD66+ Vesicles and Granules.
Compared with their blood counterparts, viable airway neutrophils showed increased surface levels of cholera toxin B-labeled lipid rafts (Fig. 1, P < 10−3), which work as surface signaling platforms (14, 15). CD11b and CD66b also displayed highly increased surface expression on airway compared with blood neutrophils (Fig. 1, P < 10−3 for both), reflecting release of NE-poor secretory vesicles and tertiary granules (CD11+) and secondary granules (CD66b+), all easily mobilizable to the surface. Increased surface lipid rafts, CD11b and CD66b, and decreased intracellular GSH also were seen in viable neutrophils from healthy airways (n = 18, SI Fig. 6) and thus represent normal activation patterns related to migration from blood to airways (16, 17).
Mobilization of CD63+ NE-Rich Granules and Loss of the Phagocytic Receptors CD16 and CD14.
Unexpectedly, most CF airway neutrophils showed strong mobilization of CD63+ NE-rich primary granules (Fig. 1, P < 10−3), usually confined intracellularly (18). Moreover, CF airway neutrophils showed decreased expression of the phagocytic receptors CD14 and CD16 (Fig. 1, P < 10−3 for both). At the single-cell level, increased expression of CD63 and decreased expression of CD16 [a known target for NE-mediated cleavage (19)], were associated (SI Fig. 7). No such association was seen between CD63 and CD14 (our unpublished data). Importantly, sputum neutrophil fractions obtained from the entire cohort of 33 CF patients with stable disease all demonstrated active NE release and loss of CD14 and CD16, regardless of common CF disease predictors (1) including age, gender, genotype, pancreatic status, neutrophil count, or infection with P. aeruginosa, whether mucoid or non mucoid (P > 0.1 for all). In healthy controls (SI Fig. 6), increased CD63 expression was observed only in a minor fraction of airway neutrophils, and surface levels of both CD16 and CD14 were increased, potentiating phagocytosis (16, 17).
Expression by Viable CF Airway Neutrophils of Unconventional Immunoregulatory Receptors.
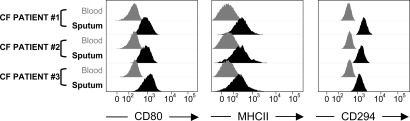
Previous studies have documented de novo expression by peripheral tissue-activated neutrophils of immunoregulatory receptors excluded from or minimally expressed in blood neutrophils (5). Here, we show significant expression by viable CF airway, but not blood, neutrophils, of CD80 and major histocompatibility class II (Fig. 2), both linked to antigen-presenting cell function (20). Moreover, based on reports that CF patients display signs of T helper type 2 (Th2) immune polarization (21), we tested for the presence of the receptor to prostaglandin D2 CD294, generally expressed on Th2-polarized T cells, eosinophils, and basophils (22), on CF neutrophils. CF airway, but not blood, neutrophils expressed CD294 (Fig. 2). We ensured that results obtained with CD80, major histocompatibility class II, and CD294 (all up-regulated with P < 10−3 compared with blood), as with conventional neutrophil markers (CD11b, CD14, CD16, CD66b, and CD63) detailed above, held true in repeated measurements of paired sputum/blood samples obtained 4 wk or more apart (our unpublished data). Of note, we did not obtain sufficient material to test these three markers on healthy airway cells.
Fig. 2.
Unconventional surface markers found on viable CF airway neutrophils. Viable neutrophils from induced sputum are compared with their blood counterparts (three representative CF patients) for levels of CD80, major histocompatibility class II (MHCII), and the prostaglandin D2 receptor CD294 (from left to right). All three markers appear significantly up-regulated on sputum versus blood neutrophils (P < 10−3 for all).
CF Airway Neutrophils Show Limited Yet Key Changes in Intracellular Signaling.
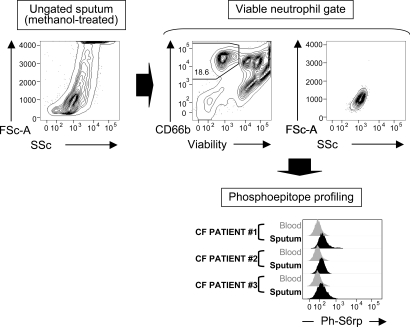
Next, we characterized the intracellular phosphosignaling profile of CF neutrophils via direct ex vivo analysis, as we established before (23). Generally, phosphoepitope profiling requires methanol-based permeabilization, which is incompatible with conventional methods for viable cell detection. In Fig. 3, we illustrate a generalizable method for gating viable from apoptotic/necrotic neutrophils, which withstands methanol treatment. Similar gating was applied on blood neutrophils and allowed us to compare the baseline phosphoepitope profile of viable airway neutrophils to that of their blood counterparts, focusing on nine specific targets (Table 1). Viable CF airway neutrophils showed increased levels of the membrane-associated kinase phospho-Syk (24), consistent with ongoing surface rearrangements, as shown above. We measured decreased phosphotyrosine levels (25), reflecting an overall decrease in tyrosine kinase activity, and unchanged levels of phospho-Akt, JNK, p38 MAPK, p44/42, NF-κB p65, and STAT5 (Table 1), suggesting that viable CF airway neutrophils were in a postactivated stage. Moreover, this stage may be associated with cellular reprogramming, because, unexpectedly, phospho-S6rp, a key anabolic switch (26), also was significantly increased in viable CF airway neutrophils (Table 1 and Fig. 3). Here again, material collected from healthy airways was insufficient to profile phosphoepitopes robustly.
Fig. 3.
Methanol-compatible method for phosphoprofiling of viable airway neutrophils. From a complex mixture of viable, apoptotic, and necrotic cells (Upper Left), viable neutrophils are gated (Upper Middle) by using a combination of a fixable viability dye and a methanol-resistant CD66b-quantum dot conjugate (see SI Methods), yielding a clean subset as visualized by forward scatter and side scatter (Upper Right). This subset is then compared with the equivalent subset in blood for phosphoepitope expression (Lower, showing ph-S6rp expression, as an example, in three representative CF patients).
Table 1.
Phosphoepitope profile of viable blood and airway CF neutrophils (gated as detailed in text and illustrated in Fig. 3)
| Phosphoepitope |
MFI* in blood neutrophils, Median [IR*] | MFI in airway neutrophils, Median [IR] | Difference, P value | |
|---|---|---|---|---|
| Type | Name | |||
| Total phosphorylation | ph-Tyrosine | 362 [282; 549] | 163 [81; 214] | <10−3 |
| Intermediate kinases | ph-Akt | 267 [98; 342] | 300 [220; 353] | 0.12 |
| ph-JNK* | 73.0 [55.0; 233] | 100 [45.0; 161] | 0.24 | |
| ph-p38 MAPK* | 54.3 [15.8; 64.9] | 37.5 [1.25; 106] | 0.37 | |
| ph-p44/42* | 38.0 [20.0; 49.6] | 37.1 [14.5; 86.8] | 0.37 | |
| ph-Syk | 15.5 [7.5; 35.5] | 24.0 [8.50; 110] | 0.003 | |
| Final effectors | ph-NFkBp65* | 42.8 [19.7; 111] | 22.5 [6.00; 61.5] | 0.06 |
| ph-S6rp* | 66.9 [28.8; 118] | 83.7 [28.8; 166] | 0.03 | |
| ph-STAT5* | 81.5 [67.3; 107] | 65.4 [44.3; 162] | 0.23 | |
*IR, interquartile range defined by encompassing 25th and 75th percentile; JNK, cJun N-terminal kinase; MFI, median fluorescence intensity; NFkBp65, nuclear factor κ B p65 subunit; p38 MAPK, p38 mitogen-activated protein kinase; ph-, phospho-; S6rp, S6 ribosomal protein; STAT5, signal transducer and activator of transcription 5.
Discussion
In CF, chronic neutrophilic inflammation of the airways plays a major pathological role, yet remains largely unexplained (2). The prevailing view is that blood-to-airway migration of neutrophils is enhanced in CF and that neutrophils homing to CF airways subsequently undergo rapid apoptosis and necrosis, leading to passive spillage into the lumen of toxic by-products, such as NE (8). In stark contrast, we provide clear evidence that profound changes readily occur in viable, nonapoptotic airway neutrophils, pointing to a grossly underappreciated role for these cells in CF pathophysiology.
Because of the sensitivity of neutrophils to a wide range of stimuli, experimental studies using preparatory steps such as gradient centrifugation or chemical treatments often are marred by artifacts (12, 27, 28). Here, we coupled minimal sample preparation with direct functional and signaling analysis to bring insight into the properties of these cells. Two methodological advances were used: (i) dithiothreitol was omitted during sputum homogeneization steps, which enabled us to measure airway cell properties in their native state; and (ii) a methanol-compatible method was developed for viable neutrophil gating in induced sputum, which enabled subsequent phosphoepitope profiling. Both methods are generalizable to other disease contexts in which such ex vivo data are lacking.
Functional analysis of viable CF airway neutrophils revealed decreased GSH levels, increased assembly of lipid rafts, and surface mobilization of CD11b+ and CD66b+ NE-poor vesicles and granules, as also seen in neutrophils from healthy airways. Unexpectedly, however, CD63+ NE-rich granules also were massively mobilized to the surface of viable CF airway neutrophils. Recent studies established that NE and other cationic effectors require active desorption from the anionic primary granule matrix to become operative (29). This is a carefully orchestrated process that is unlikely to occur in apoptotic/necrotic cells. Our results are consistent with this notion and suggest that free NE activity in CF airways stems, at least partly, from viable neutrophils.
Active NE-rich granule release was detected regardless of the disease state of subjects, which suggests that this process is intrinsically triggered in CF airways although infection may further exacerbate it. This finding is consistent with the spontaneous inflammatory syndrome observed within uninfected human CF lung xenografts in mice (30). Conditions that cause this aberrant behavior may include CFTR-dependent alteration of ion transport and/or pH, processes that affect granule mobilization (18). Clinically, increased extracellular NE activity is the best correlate to declining CF lung function (4). Our results suggest that inhibitors of NE-rich granule mobilization, targeting viable airway neutrophils, may benefit patients. Such inhibitors include lipoxin A4, which reported lack in CF (31) remains debated (32).
The proteolytic burden that characterizes CF airways (9) has been found to disarm various immune and scavenging receptors on T cells (33), macrophages (34), dendritic cells (35), and neutrophils themselves (36). Here, we demonstrate that CF airway neutrophils lose surface expression of CD14 and CD16, otherwise up-regulated in healthy airway neutrophils (16, 17). Single-cell data suggest that this loss may occur in autocrine fashion for CD16, via NE release and surface proteolysis (37). Both CD14 and CD16 are essential for phagocytosis and their loss, along with that of CXCR1 (36), could account, at least in part, for the inability of CF airway neutrophils to kill bacteria (3).
Another interesting set of findings lies in the expression by viable CF airway neutrophils of immunomodulating receptors that are generally associated with other lineages. Presence of CD80, major histocompatibility class II, and the Th2-associated receptor CD294 on the surface of CF airway neutrophils suggests that these cells are undergoing a significant switch toward cooperative functions with T cells, as described in other chronic inflammatory conditions (20, 22). T cells migrate to the CF airway mucosa (38) and could play a nonnegligible role in neutrophil immunomodulation (2). Further studies are needed to determine whether de novo receptor expression by neutrophils plays a role in mitigating or accelerating CF airway inflammation.
We also report single-cell phosphoepitope profiling of CF neutrophils. Total phospho-tyrosine levels (25) were decreased compared with blood neutrophils, and levels of the main intermediate kinases phospho-Akt, JNK, p38 MAPK, and p44/42 were not changed. These findings are consistent with CF airway neutrophils being in a postactivated stage. Additionally, conventional transcription factors of activated neutrophils phospho-STAT5 (39) and phospho-p65 of NF-κB (3) were not significantly increased in airway neutrophils. We recognize that transient changes in the above phosphoepitopes (notably intermediate kinases, which are characterized by rapid on/off rates) may have occurred at some point during neutrophil recruitment to CF airways, which would not be discernable in the context of this direct endpoint analysis, ex vivo.
This notion, however, is challenged by the fact that, in contrast with other intermediate kinases, levels of membrane-associated phospho-Syk were significantly increased in airway neutrophils, compared with blood. This increase is consistent with the presence of profound membrane rearrangements (24), as reflected by increased lipid raft assembly and massive degranulation measured on CF airway neutrophils. Increased Syk phosphorylation also is linked to CD16 signaling (24) and phagocytosis (40) in normal neutrophil activation, and loss of surface CD16 observed on CF airway neutrophils may therefore evoke paradoxical cues, rendering them dysfunctional.
Importantly, we also observed that phosphorylated S6rp levels in viable CF airway neutrophils were significantly higher than in their blood counterparts. Because of the crucial role of this anabolic factor in the induction of cell survival and gene neotranscription (26), this result further supports the concept that neutrophils, upon recruitment to CF airways, modify their default program to survive and undergo potential functional changes, likely caused by conditioning by CF epithelial cells (30, 41). The maintenance of high levels of phosphorylated Akt, which has strong anti-apoptotic and anabolic activities (42), is consistent with an increased survival of CF airway neutrophils.
S6rp phosphorylation is controlled by nutrient availability, notably amino acids (43). Thus, the well recognized abundance of free amino acids in CF airway fluid (2), in part because of abnormal reabsorption caused by CFTR dysfunction (44), may in itself trigger an increase in S6rp phosphorylation. Alternatively, increased phospho-S6rp may be linked to other anabolic growth factors enriched in CF airway fluid (45). Further in vitro studies using purified CF airway fluid fractions and modulators of S6rp phosphorylation are needed to explore the exact role of this pathway in controlling neutrophil behavior.
Together, our results emphasize the power of direct ex vivo analysis combined with single-cell analytical methods to probe functional and signaling changes in inflammatory samples from CF patients. Most importantly, they argue for a paradigm change in our understanding of CF airway pathology. Central to this paradigm change is the notion that viable neutrophils are important contributors to CF airway disease and thus bona fide targets for novel anti-inflammatory/immunomodulatory approaches (e.g., blockers of NE-rich granule release, modulators of S6rp signaling). Arguably, this does not diminish the fact that apoptotic and necrotic neutrophils play a major role in the disease, notably through release of DNA and actin (enhancing mucus viscosity), but rather suggests additional, upstream routes for therapy, i.e., before damage is exerted.
Beyond their functional and signaling properties, further investigation of the genomic and proteomic characteristics of viable CF airway neutrophils, upon cell sorting, now is warranted. Although this study focused on providing comparative analysis of blood and airway neutrophils in the sole context of stable CF disease, it will be interesting to extend the analytical framework pioneered here to study the pathophysiology of acute CF exacerbations (2). This framework also may apply to other diseases of chronic/acute neutrophilic inflammation, e.g., severe asthma and non-CF bronchiectasis. In these contexts, renewed single-cell analysis of functional and signaling pathways should help better identify dysfunctional patterns within viable inflammatory neutrophils.
Materials and Methods
Human Subjects.
This study received the approval of the Stanford Administrative Panel on Human Subjects in Medical Research. All subjects included in this study signed informed consent forms before undergoing study procedures. Diagnosis of CF was by documented sweat chloride >60 mequiv/liter by quantitative iontophoresis test and/or one or more clinical features consistent with CF and/or preexisting documentation of two identifiable mutations. Healthy controls, presented for comparison in some figures, were nonsmokers. CFTR genotype was confirmed on all enrolled subjects using the Elucigene CF29 assay (Tepnel Diagnostics). CF samples were obtained when patients were in stable clinical condition. The presence of P. aeruginosa in the lungs of CF patients was tested by routine sputum culture. Lung function was tested by spirometry using American Thoracic Society criteria.
Collection and Processing of Samples.
Blood and lung fluid were simultaneously collected from the subjects by venipuncture and standardized sputum induction, respectively. Sputum induction is a minimally invasive procedure was shown by several independent groups to faithfully reflect CF inflammatory lung disease (reviewed in ref. 10). Because our study focused on characterizing baseline functional and signaling profiles of CF neutrophils, we sought to prevent any artefactual ex vivo activation of these cells, as detailed in the online supplement, and excluded any culture, expansion, or stimulation. In brief, cells were isolated from sputum by repeated pipetting through a 18-gauge needle in saline without the addition of the reducing agent dithiothreitol, as commonly used.
Multiparametric Flow Cytometry.
A full account of cell staining, analytical and standardization procedures for multiparametric flow cytometric assays for surface, and phosphoepitope single-cell profiling is presented in SI Methods. Key fluorescent reagents included Live/Dead fixable violet dye, a custom Qdot 605 anti-CD66b conjugate, and monochlorobimane, all from Invitrogen, as well as monoclonal antibodies against surface receptors and intracellular phosphoepitopes from BD Biosciences and Cell Signaling Technologies.
Analysis and Presentation of Data.
Flow cytometry data were exported to the FlowJo software (Treestar) and compensated by using single-stained beads or cells, as detailed in ref. 11. Median fluorescence values were calculated and compared with the appropriate background controls. Statistical analysis was performed by using JMP5 software (SAS Institute). Distributions were further compared between blood and sputum neutrophils by using the paired Wilcoxon signed-rank test. Correlations between continuous variables were tested by using the Spearman ρ test. Differences in flow cytometry measures following nominal categories (e.g., gender, pancreatic status, or mucoid P. aeruginosa infection) were assessed by using the unpaired Wilcoxon rank-sum test. Differences or correlations were considered significant with P < 0.05.
Supplementary Material
Acknowledgments.
We thank M. Anderson, B. Aram, K. Atkuri, D. Diaz, D. Parks, and J. Tung for help with the experiments and advice. This study was funded in part by grants from the Cystic Fibrosis Foundation (to R.T. and C.K.C.), the Food and Drug Administration Office for Orphan Product Development (to R.T. and C.K.C.), Vaincre la Mucoviscidose (to R.T. and Y.G.), the France–Stanford Development Project Fund (to R.T. and Y.G.), and by fellowships from Association pour le Développement des Recherches Biomédicales au Centre Hospitalier de Marseille (ADEREM), Association Régionale d'Assistance Respiratoire à Domicile (ARARD) (to Y.G.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712386105/DC1.
References
- 1.Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173:475–482. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- 2.Tirouvanziam R. Neutrophilic inflammation as a major determinant in the progression of cystic fibrosis. Drug News Perspect. 2006;19:609–614. doi: 10.1358/dnp.2006.19.10.1068008. [DOI] [PubMed] [Google Scholar]
- 3.Chmiel JF, Davis PB. State of the art: Why do the lungs of patients with cystic fibrosis become infected and why can't they clear the infection? Respir Res. 2003;4:8. doi: 10.1186/1465-9921-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer KC, Zimmerman J. Neutrophil mediators, Pseudomonas, and pulmonary dysfunction in cystic fibrosis. J Lab Clin Med. 1993;121:654–661. [PubMed] [Google Scholar]
- 5.Borregaard N, Sorensen OE, Theilgaard-Monch K. Neutrophil granules: A library of innate immunity proteins. Trends Immunol. 2007;28:340–345. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Jensen PO, et al. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology. 2007;153:1329–1338. doi: 10.1099/mic.0.2006/003863-0. [DOI] [PubMed] [Google Scholar]
- 7.Watt AP, Courtney J, Moore J, Ennis M, Elborn JS. Neutrophil cell death, activation and bacterial infection in cystic fibrosis. Thorax. 2005;60:659–664. doi: 10.1136/thx.2004.038240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabroe I, Whyte MK. Incapacitating the immune system in cystic fibrosis. Nat Med. 2007;13:1417–1418. doi: 10.1038/nm1207-1417. [DOI] [PubMed] [Google Scholar]
- 9.Birrer P. Proteases and antiproteases in cystic fibrosis: Pathogenetic considerations and therapeutic strategies. Respiration. 62 Suppl. 1995;1:25–28. doi: 10.1159/000196490. [DOI] [PubMed] [Google Scholar]
- 10.Sagel SD, Chmiel JF, Konstan MW. Sputum biomarkers of inflammation in cystic fibrosis lung disease. Proc Am Thorac Soc. 2007;4:406–417. doi: 10.1513/pats.200703-044BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tung JW, et al. Modern flow cytometry: A practical approach. Clin Lab Med. 2007;27:453–468. doi: 10.1016/j.cll.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woolhouse IS, Bayley DL, Stockley RA. Effect of sputum processing with dithiothreitol on the detection of inflammatory mediators in chronic bronchitis and bronchiectasis. Thorax. 2002;57:667–671. doi: 10.1136/thorax.57.8.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tirouvanziam R, Conrad CK, Bottiglieri T, Herzenberg LA, Moss RB, Herzenberg LA. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proc Natl Acad Sci USA. 2006;103:4628–4633. doi: 10.1073/pnas.0511304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheriff A, et al. Loss of GM1 surface expression precedes annexin V-phycoerythrin binding of neutrophils undergoing spontaneous apoptosis during in vitro aging. Cytometry A. 2004;62:75–80. doi: 10.1002/cyto.a.20090. [DOI] [PubMed] [Google Scholar]
- 15.Shao D, Segal AW, Dekker LV. Lipid rafts determine efficiency of NADPH oxidase activation in neutrophils. FEBS Lett. 2003;550:101–106. doi: 10.1016/s0014-5793(03)00845-7. [DOI] [PubMed] [Google Scholar]
- 16.Alexis N, Soukup J, Ghio A, Becker S. Sputum phagocytes from healthy individuals are functional and activated: A flow cytometric comparison with cells in bronchoalveolar lavage and peripheral blood. Clin Immunol. 2000;97:21–32. doi: 10.1006/clim.2000.4911. [DOI] [PubMed] [Google Scholar]
- 17.Kinhult J, Egesten A, Benson M, Uddman R, Cardell LO. Increased expression of surface activation markers on neutrophils following migration into the nasal lumen. Clin Exp Allergy. 2003;33:1141–1146. doi: 10.1046/j.1365-2222.2003.01682.x. [DOI] [PubMed] [Google Scholar]
- 18.Sengelov H, Follin P, Kjeldsen L, Lollike K, Dahlgren C, Borregaard N. Mobilization of granules and secretory vesicles during in vivo exudation of human neutrophils. J Immunol. 1995;154:4157–4165. [PubMed] [Google Scholar]
- 19.Middelhoven PJ, Van Buul JD, Hordijk PL, Roos D. Different proteolytic mechanisms involved in Fc gamma RIIIb shedding from human neutrophils. Clin Exp Immunol. 2001;125:169–175. doi: 10.1046/j.1365-2249.2001.01548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandilands GP, McCrae J, Hill K, Perry M, Baxter D. Major histocompatibility complex class II (DR) antigen and costimulatory molecules on in vitro and in vivo activated human polymorphonuclear neutrophils. Immunology. 2006;119:562–571. doi: 10.1111/j.1365-2567.2006.02471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moser C, Kjaergaard S, Pressler T, Kharazmi A, Koch C, Hoiby N. The immune response to chronic Pseudomonas aeruginosa lung infection in cystic fibrosis patients is predominantly of the Th2 type. Apmis. 2000;108:329–335. doi: 10.1034/j.1600-0463.2000.d01-64.x. [DOI] [PubMed] [Google Scholar]
- 22.Takeshita K, et al. CRTH2 is a prominent effector in contact hypersensitivity-induced neutrophil inflammation. Int Immunol. 2004;16:947–959. doi: 10.1093/intimm/dxh096. [DOI] [PubMed] [Google Scholar]
- 23.Gernez Y, Tirouvanziam R, Nguyen KD, Herzenberg LA, Krensky AM, Nadeau KC. Altered phosphorylated signal transducer and activator of transcription profile of CD4+CD161+ T cells in asthma: Modulation by allergic status and oral corticosteroids. J Allergy Clin Immunol. 2007;120:1441–1448. doi: 10.1016/j.jaci.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes MJ, et al. CD16b associates with high-density, detergent-resistant membranes in human neutrophils. Biochem J. 2006;393:351–359. doi: 10.1042/BJ20050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spitzer JA, Zhang P. Protein tyrosine kinase activity and the influence of gender in phagocytosis and tumor necrosis factor secretion in alveolar macrophages and lung-recruited neutrophils. Shock. 1996;6:426–433. doi: 10.1097/00024382-199612000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci. 2006;31:342–348. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Watson F, Robinson JJ, Edwards SW. Neutrophil function in whole blood and after purification: Changes in receptor expression, oxidase activity and responsiveness to cytokines. Biosci Rep. 1992;12:123–133. doi: 10.1007/BF02351217. [DOI] [PubMed] [Google Scholar]
- 28.Zahler S, Kowalski C, Brosig A, Kupatt C, Becker BF, Gerlach E. The function of neutrophils isolated by a magnetic antibody cell separation technique is not altered in comparison to a density gradient centrifugation method. J Immunol. Methods. 1997;200:173–179. doi: 10.1016/s0022-1759(96)00206-2. [DOI] [PubMed] [Google Scholar]
- 29.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tirouvanziam R, Khazaal I, Peault B. Primary inflammation in human cystic fibrosis small airways. Am J Physiol Lung Cell Mol Physiol. 2002;283:L445–L451. doi: 10.1152/ajplung.00419.2001. [DOI] [PubMed] [Google Scholar]
- 31.Karp CL, Flick LM, Yang R, Uddin J, Petasis NA. Cystic fibrosis and lipoxins. Prostaglandins Leukot Essent Fatty Acids. 2005;73:263–270. doi: 10.1016/j.plefa.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Starosta V, Ratjen F, Rietschel E, Paul K, Griese M. Anti-inflammatory cytokines in cystic fibrosis lung disease. Eur Respir J. 2006;28:581–587. doi: 10.1183/09031936.06.00071405. [DOI] [PubMed] [Google Scholar]
- 33.Doring G, Frank F, Boudier C, Herbert S, Fleischer B, Bellon G. Cleavage of lymphocyte surface antigens CD2, CD4, and CD8 by polymorphonuclear leukocyte elastase and cathepsin G in patients with cystic fibrosis. J Immunol. 1995;154:4842–4850. [PubMed] [Google Scholar]
- 34.Vandivier RW, et al. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest. 2002;109:661–670. doi: 10.1172/JCI13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roghanian A, Drost EM, MacNee W, Howie SE, Sallenave JM. Inflammatory lung secretions inhibit dendritic cell maturation and function via neutrophil elastase. Am J Respir Crit Care Med. 2006;174:1189–1198. doi: 10.1164/rccm.200605-632OC. [DOI] [PubMed] [Google Scholar]
- 36.Hartl D, et al. Cleavage of CXCR1 on neutrophils disables bacterial killing in cystic fibrosis lung disease. Nat Med. 2007;13:1423–1430. doi: 10.1038/nm1690. [DOI] [PubMed] [Google Scholar]
- 37.Owen CA, Campbell MA, Sannes PL, Boukedes SS, Campbell EJ. Cell surface-bound elastase and cathepsin G on human neutrophils: a novel, non-oxidative mechanism by which neutrophils focus and preserve catalytic activity of serine proteinases. J Cell Biol. 1995;131:775–789. doi: 10.1083/jcb.131.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azzawi M, Johnston PW, Majumdar S, Kay AB, Jeffery PK. T lymphocytes and activated eosinophils in airway mucosa in fatal asthma and cystic fibrosis. Am Rev Respir Dis. 1992;145:1477–1482. doi: 10.1164/ajrccm/145.6.1477. [DOI] [PubMed] [Google Scholar]
- 39.Fievez L, et al. STAT5 is an ambivalent regulator of neutrophil homeostasis. PLoS ONE. 2007;2:e727. doi: 10.1371/journal.pone.0000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raeder EM, Mansfield PJ, Hinkovska-Galcheva V, Shayman JA, Boxer LA. Syk activation initiates downstream signaling events during human polymorphonuclear leukocyte phagocytosis. J Immunol. 1999;163:6785–6793. [PubMed] [Google Scholar]
- 41.Wiszniewski L, et al. Long-term cultures of polarized airway epithelial cells from patients with cystic fibrosis. Am J Respir Cell Mol Biol. 2006;34:39–48. doi: 10.1165/rcmb.2005-0161OC. [DOI] [PubMed] [Google Scholar]
- 42.Zhu D, et al. Deactivation of phosphatidylinositol 3,4,5-trisphosphate/Akt signaling mediates neutrophil spontaneous death. Proc Natl Acad Sci USA. 2006;103:14836–14841. doi: 10.1073/pnas.0605722103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meijer AJ, Dubbelhuis PF. Amino acid signalling and the integration of metabolism. Biochem Biophys Res Commun. 2004;313:397–403. doi: 10.1016/j.bbrc.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 44.Mager S, Sloan J. Possible role of amino acids, peptides, and sugar transporter in protein removal and innate lung defense. Eur J Pharmacol. 2003;479:263–267. doi: 10.1016/j.ejphar.2003.08.075. [DOI] [PubMed] [Google Scholar]
- 45.Gomez-Cambronero J, Horn J, Paul CC, Baumann MA. Granulocyte–macrophage colony-stimulating factor is a chemoattractant cytokine for human neutrophils: involvement of the ribosomal p70 S6 kinase signaling pathway. J Immunol. 2003;171:6846–6855. doi: 10.4049/jimmunol.171.12.6846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.