Abstract
From previous studies on the induction of DNA synthesis in quiescent primary baby rat kidney cells by adenovirus type 5 (Ad5) E1A deletion mutants, we concluded that induction is prevented only when cellular proteins p300 and pRb are both uncomplexed with E1A (J.A. Howe, J.S. Mymryk, C. Egan, P.E. Branton, and S.T. Bayley, Proc. Natl. Acad. Sci. USA 87:5883-5887, 1990). We have now examined induction by these same mutants in virus lacking the E1B region, so that cellular p53 was no longer complexed to the E1B 55-kDa protein. E1A mutants that fail to bind pRb induced DNA synthesis at a significantly lower level in Ad5 lacking E1B than in Ad5 containing E1B. Apparently, therefore, uncomplexed p53 can partially replace p300 in cooperating with pRb to suppress DNA synthesis in baby rat kidney cells.
Full text
PDF
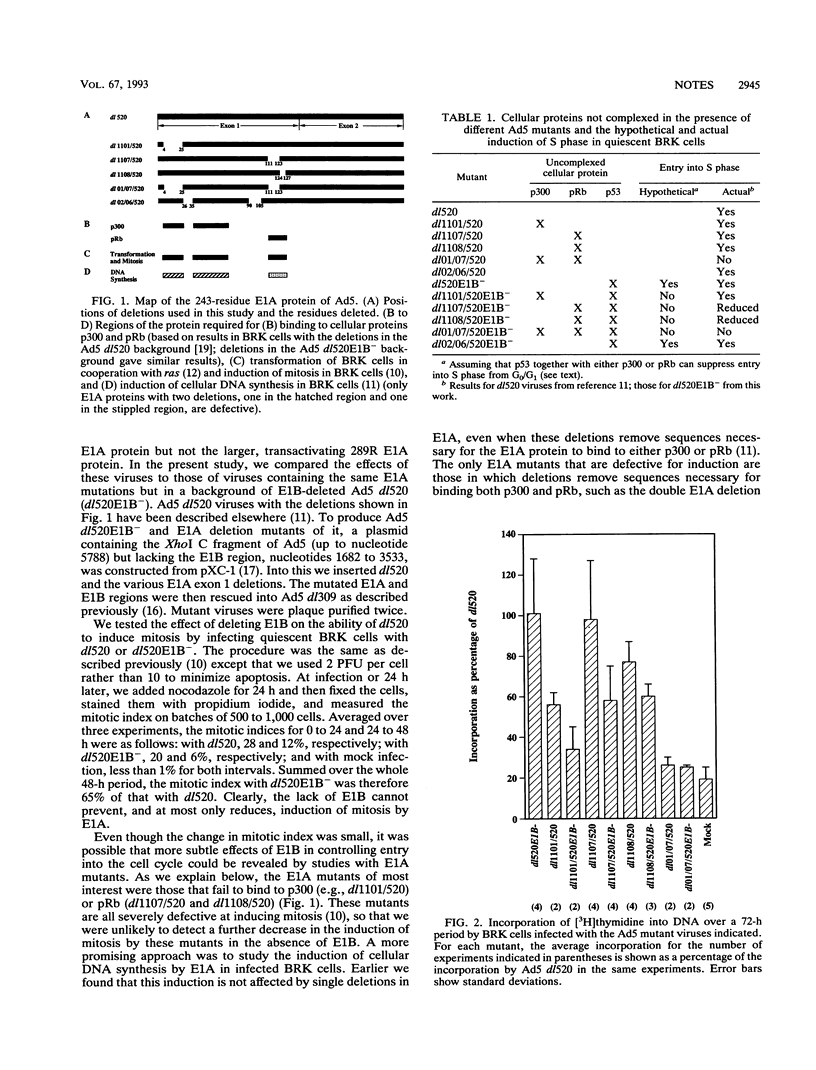
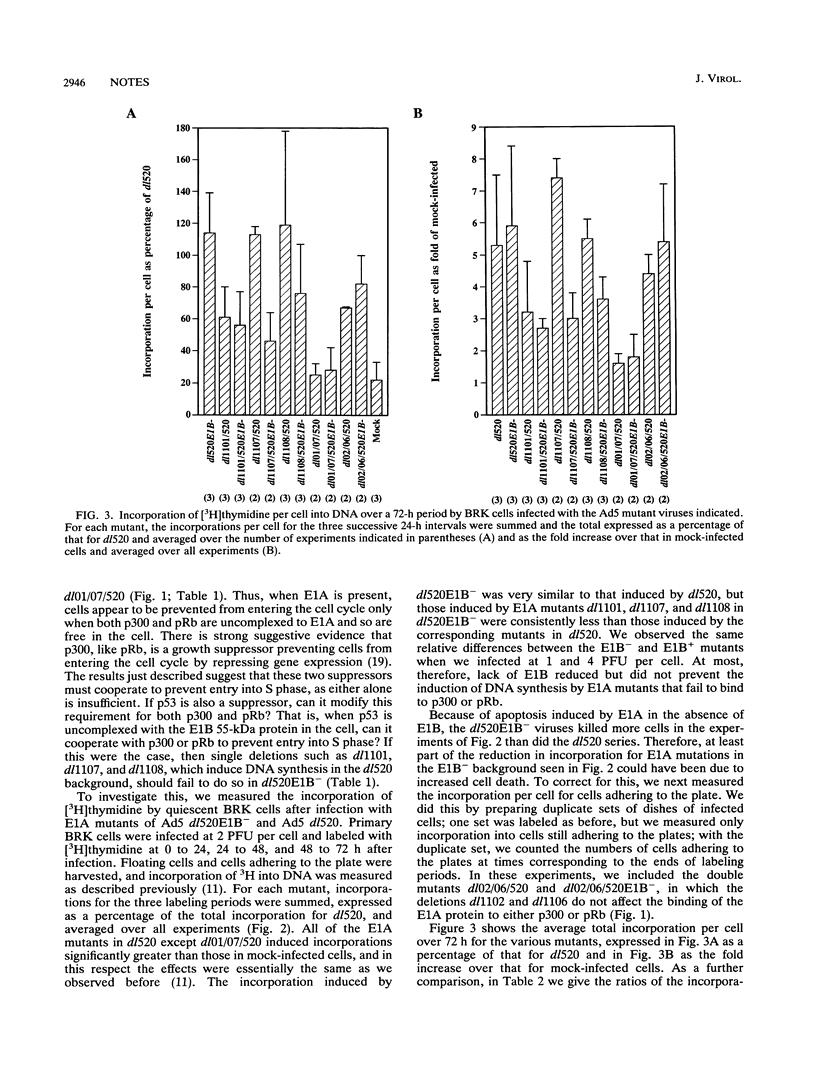
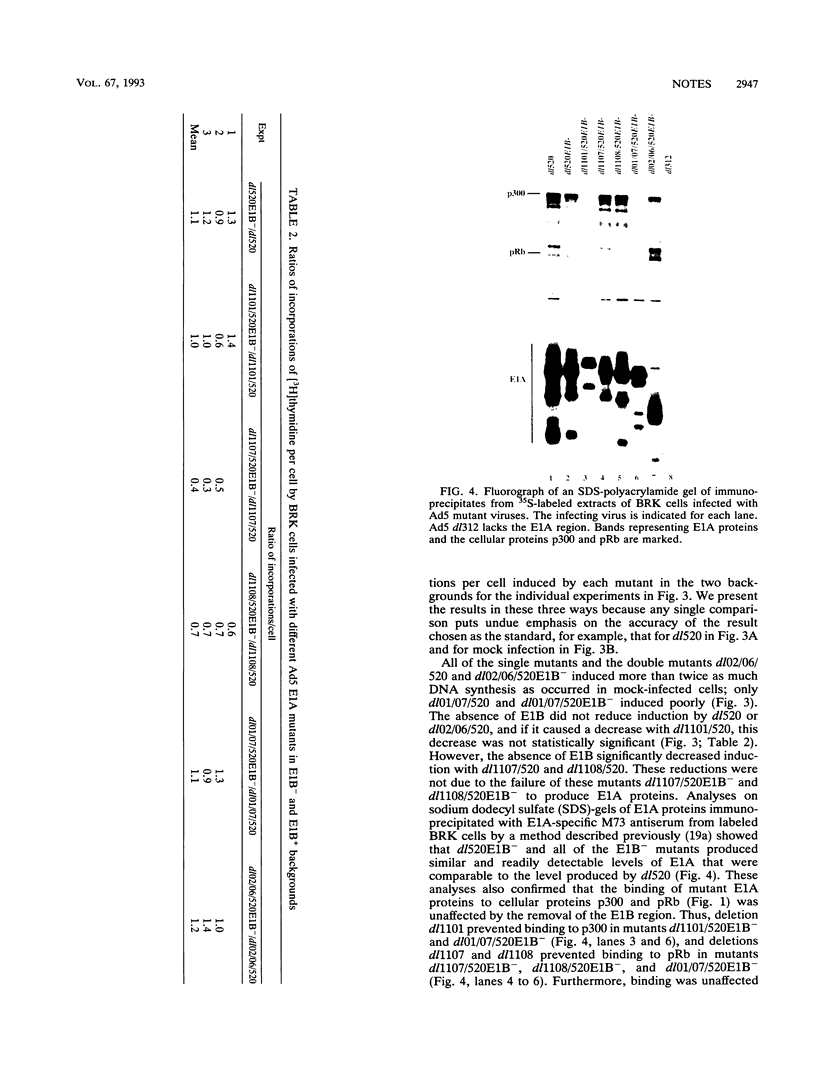


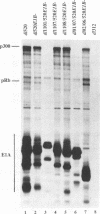
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellett A. J., Li P., David E. T., Mackey E. J., Braithwaite A. W., Cutt J. R. Control functions of adenovirus transformation region E1A gene products in rat and human cells. Mol Cell Biol. 1985 Aug;5(8):1933–1939. doi: 10.1128/mcb.5.8.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J. Functions of adenovirus E1A. Cancer Surv. 1986;5(2):367–387. [PubMed] [Google Scholar]
- Braithwaite A. W., Cheetham B. F., Li P., Parish C. R., Waldron-Stevens L. K., Bellett A. J. Adenovirus-induced alterations of the cell growth cycle: a requirement for expression of E1A but not of E1B. J Virol. 1983 Jan;45(1):192–199. doi: 10.1128/jvi.45.1.192-199.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton P. E., Bayley S. T., Graham F. L. Transformation by human adenoviruses. Biochim Biophys Acta. 1985;780(1):67–94. doi: 10.1016/0304-419x(84)90007-6. [DOI] [PubMed] [Google Scholar]
- Caporossi D., Bacchetti S. Definition of adenovirus type 5 functions involved in the induction of chromosomal aberrations in human cells. J Gen Virol. 1990 Apr;71(Pt 4):801–808. doi: 10.1099/0022-1317-71-4-801. [DOI] [PubMed] [Google Scholar]
- Dobbelstein M., Arthur A. K., Dehde S., van Zee K., Dickmanns A., Fanning E. Intracistronic complementation reveals a new function of SV40 T antigen that co-operates with Rb and p53 binding to stimulate DNA synthesis in quiescent cells. Oncogene. 1992 May;7(5):837–847. [PubMed] [Google Scholar]
- Egan C., Jelsma T. N., Howe J. A., Bayley S. T., Ferguson B., Branton P. E. Mapping of cellular protein-binding sites on the products of early-region 1A of human adenovirus type 5. Mol Cell Biol. 1988 Sep;8(9):3955–3959. doi: 10.1128/mcb.8.9.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E. Retinoblastoma. For our eyes only. Nature. 1992 Sep 24;359(6393):270–271. doi: 10.1038/359270a0. [DOI] [PubMed] [Google Scholar]
- Herrmann C. H., Mathews M. B. The adenovirus E1B 19-kilodalton protein stimulates gene expression by increasing DNA levels. Mol Cell Biol. 1989 Dec;9(12):5412–5423. doi: 10.1128/mcb.9.12.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. A., Bayley S. T. Effects of Ad5 E1A mutant viruses on the cell cycle in relation to the binding of cellular proteins including the retinoblastoma protein and cyclin A. Virology. 1992 Jan;186(1):15–24. doi: 10.1016/0042-6822(92)90057-v. [DOI] [PubMed] [Google Scholar]
- Howe J. A., Mymryk J. S., Egan C., Branton P. E., Bayley S. T. Retinoblastoma growth suppressor and a 300-kDa protein appear to regulate cellular DNA synthesis. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5883–5887. doi: 10.1073/pnas.87.15.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelsma T. N., Howe J. A., Mymryk J. S., Evelegh C. M., Cunniff N. F., Bayley S. T. Sequences in E1A proteins of human adenovirus 5 required for cell transformation, repression of a transcriptional enhancer, and induction of proliferating cell nuclear antigen. Virology. 1989 Jul;171(1):120–130. doi: 10.1016/0042-6822(89)90518-7. [DOI] [PubMed] [Google Scholar]
- Kuerbitz S. J., Plunkett B. S., Walsh W. V., Kastan M. B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- McGrory W. J., Bautista D. S., Graham F. L. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988 Apr;163(2):614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- McKinnon R. D., Bacchetti S., Graham F. L. Tn5 mutagenesis of the transforming genes of human adenovirus type 5. Gene. 1982 Jul-Aug;19(1):33–42. doi: 10.1016/0378-1119(82)90186-x. [DOI] [PubMed] [Google Scholar]
- Moran B., Zerler B. Interactions between cell growth-regulating domains in the products of the adenovirus E1A oncogene. Mol Cell Biol. 1988 Apr;8(4):1756–1764. doi: 10.1128/mcb.8.4.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mymryk J. S., Lee R. W., Bayley S. T. Ability of adenovirus 5 E1A proteins to suppress differentiation of BC3H1 myoblasts correlates with their binding to a 300 kDa cellular protein. Mol Biol Cell. 1992 Oct;3(10):1107–1115. doi: 10.1091/mbc.3.10.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao L., Debbas M., Sabbatini P., Hockenbery D., Korsmeyer S., White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich S. J., Anderson C. W., Mercer W. E., Appella E. The p53 tumor suppressor protein, a modulator of cell proliferation. J Biol Chem. 1992 Aug 5;267(22):15259–15262. [PubMed] [Google Scholar]
- White E., Cipriani R. Specific disruption of intermediate filaments and the nuclear lamina by the 19-kDa product of the adenovirus E1B oncogene. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9886–9890. doi: 10.1073/pnas.86.24.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew P. R., Berk A. J. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature. 1992 May 7;357(6373):82–85. doi: 10.1038/357082a0. [DOI] [PubMed] [Google Scholar]
- Zerler B., Roberts R. J., Mathews M. B., Moran E. Different functional domains of the adenovirus E1A gene are involved in regulation of host cell cycle products. Mol Cell Biol. 1987 Feb;7(2):821–829. doi: 10.1128/mcb.7.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]