Abstract
The tight junction forms a barrier that limits paracellular movement of water, ions, and macromolecules. The permeability properties of this barrier are regulated in response to both physiological and pathophysiological stimuli, and this regulation has been modeled by pharmacological agents. Although it is now known that vesicular traffic plays important roles in tight junction assembly, the molecular mechanisms by which vesicular traffic contributes to tight junction regulation remain to be defined. This review summarizes recent progress in understanding mechanisms and pathways of tight junction protein internalization and the relevance of these to tight junction regulation.
Keywords: tight junction, tumor necrosis factor, endocytosis, cytoskeleton, myosin, inflammatory bowel disease
Introduction
Complex organisms are composed of multiple organs that serve unique functions. This requires a relatively constant internal milieu as well as the presence of some compartments in which fluid composition is markedly different from the overall milieu. The maintenance of these distinct tissue compartments with defined fluid composition requires barriers to free diffusion. Plasma membranes of individual cells provide some barrier function, but intercellular junctions are needed to seal the paracellular space between cells. Such junctions are present in epithelial and endothelial cells that line surfaces in many tissues within the gastrointestinal, respiratory, and urinary tracts as well as the integument. In each of these tissues, tight junctions join adjacent epithelial or endothelial cells to restrict diffusion, thereby limiting paracellular movement [1]. However, many physiological and pathophysiological situations require exchange of solutes and water between compartments. This demands that the tight junction be able to rapidly regulate barrier function in response to appropriate stimuli [2–4]. While great progress has been made in understanding these processes, the molecular mechanisms by which the tight junction is regulated remains a fundamental question in cell biology, physiology, and medicine.
Tight junction structure and molecular composition
The tight junction is the most luminal component of the apical junctional complex, which also includes the adherens junction [5]. The latter is composed of cadherin proteins, E-cadherin in epithelia, that form calcium-dependent intercellular bonds [6, 7]. Tight junction assembly and maintenance typically requires the presence of intact adherens junctions, though the converse is not true [7–9]. In addition, the structural and functional integrity of the tight junction depends on the presence of a perijunctional ring of actin and myosin which can also contribute to the regulation of paracellular permeability [3, 10].
As initially defined by transmission electron microscopy, the tight junction is a site where membranes of adjacent cells appear to nearly fuse [11]. Freeze-fracture electron microscopy shows the tight junction as multiple continuous, anastomosing strands composed of intramembranous particles [12]. Two models have been proposed to explain the chemical nature of the tight junction strands: the lipid model and the protein model. The lipid model proposes that individual tight junction strands represent pairs of inverted cylindrical micelles with the polar head groups of the lipids directed inward and the hydrophobic tails immersed in the lipid matrix of the plasma membranes of both contacting cells [13]. While this model has been generally discarded, the recognition that tight junction membrane domains are enriched in cholesterol and sphingolipids provides some support for the concept that tight junctions are formed, in part, by specialized lipid-based structures. The protein model of tight junction structure (Figure 1) proposes that strands are formed by interactions between integral membrane proteins and peripheral membrane proteins [5, 14]. Identification of tight junction integral membrane proteins, particularly the observation that several of these can form strands of intramembranous particles, provides strong support for the protein model [15–18].
Figure 1.
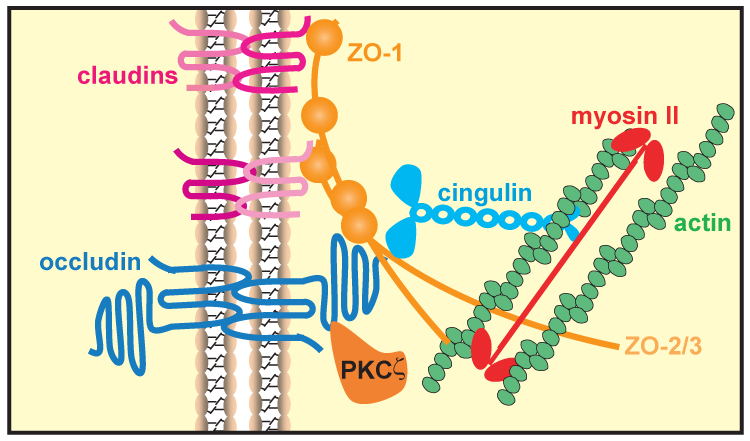
Molecular composition of the tight junction. Tight junctions consist of three main groups of proteins. They are transmembrane proteins, e.g. claudins and occludin, cytoplasmic plaque proteins, e.g. ZO-1, ZO-2, ZO-3, and cingulin, and cytoskeletal and signaling proteins, e.g. actin, myosin II, and PKCζ. These proteins are interact to maintain tight junction structure.
Occludin was the first transmembrane tight junction protein to be discovered [19]. Freeze-fracture immunoelectron microscopy showed occludin to be a component of the tight junction strands, and, in fibroblasts, occludin expression induced formation of tight junction-like strands [18, 20, 21]. Moreover, expression of occludin in fibroblasts induced cell adhesion [20]. Despite this and additional in vitro functional data and in vivo correlative data, the role of occludin in tight junction function has fallen into question because occludin-deficient embryonic stem cells have the capability to develop tight-junction strands indistinguishable from normal strands [22] and occludin-null mice are viable and have normal-appearing intestinal tight junctions [23]. This, however, remains an area in need of further study, as a plethora of in vitro studies do suggest that occludin has important functional roles.
The observation that occludin is not absolutely required for development of polarized epithelial cells bearing normal tight junctions led to a search for additional tight junction proteins. This resulted in identification of the claudin family of proteins [15]. Over 20 claudin family members have been identified in humans where they are localized to tight junction strands and along lateral membranes of epithelial cells [16]. Either individually or in combination with other family members claudin proteins define the charge selective permeability of the tight junction [24]. For example, mutations in claudin-16 prevent paracellular Mg2+ reabsorption in the renal tubule, resulting in the Mg2+-wasting disease of familial hypomagnesemia. Interestingly, most claudin-16 mutations result in trafficking defects that limit incorporation of this protein into tight junctions [25]. General trafficking of claudin proteins to the tight junction also requires ZO-1 and ZO-2, which may serve to form a complex linking claudins, occludin, actin, and other tight junction proteins to one another [26, 27].
ZO-1 is the prototype of many peripheral membrane proteins localized to the cytoplasmic face of the tight junction [28, 29]. Many of these proteins are described as “scaffold” proteins because they interact with both transmembrane and cytoskeletal proteins. Thus, they are thought to provide some essential part of the regulatory control the perijunctional actomyosin ring exerts on the tight junction, although the details of this are not well understood. In addition to ZO-1, which includes three PDZ domains and an actin binding region, tight junction scaffold proteins include ZO-2, ZO-3, cingulin, and MUPP1 [28, 30–33]. A number of kinases, most prominently PKCζ, have also been localized to the tight junction [34–36].
Role of vesicular traffic in tight junction assembly and regulation
Early studies using the calcium switch model of accelerated tight junction assembly showed that apical membrane components accumulated in intracellular vesicles termed the vacuolar apical compartment (VAC) [37]. When extracellular Ca2+ concentration was increased to a level sufficient to allow adherens junction assembly, tight junction assembly also occurred and VACs fused with the newly-developed apical membrane. Identification of individual tight junction proteins and preparation of antisera to these led to the conclusion that VACs also contain some tight junction proteins [38, 39]. Thus, regulated membrane traffic, which depends on the prior assembly of adherens junctions, appears to be required for tight junction assembly. One exception to this may be the partial polarization of solitary epithelial cells that occurs after activation of STRAD, which activates the serine-threonine kinase mutated in Peutz-Jegher’s syndrome, LKB1 [40]. It is notable that STRAD activation only causes incomplete targeting of actin and the cytoplasmic proteins ZO-1 and villin to appropriate locations, while some membrane proteins, such as E-cadherin, are not targeted at al. Data on the effect of STRAD activation on occludin and claudin localization have not been reported.
Endocytosis of tight junction proteins occurs commonly
Given the critical role of membrane traffic in tight junction assembly, it is not surprising that endocytosis of tight junction components also occurs. For over 30 years, electron micrographs have documented the presence of tight junction remnants in cytosolic vesicles, both in cultured cells and in tissues, suggesting that vesicular traffic might be a common mechanism for modification of tight junctions in response to physiological and pathological stimuli [12, 41–44]. More recently, occludin internalization has been shown in vitro and in vivo in response to cytokines, bacteria, toxins, and calcium depletion [45–51] (Table 1). We recently visualized the process of occludin internalization after TNF treatment in real time in vivo using mice expressing an EGFP-occludin fusion protein [52]. In addition to these pathological conditions, endocytosis is also seen in physiological conditions and with other type of integral proteins. For example, claudin-3 internalization occurs during movement of epithelial cells within a monolayer [42].
Table 1.
Mechanisms of stimulus-induced regulation of tight junction structure.
| stimulus | cell line/tissue | endocytic pathway | signaling event | cytoskeletal event | vesicle content | reference |
|---|---|---|---|---|---|---|
| E. coli CNF-1 toxin | T84 | caveolae | rhoA, rac1, and cdc42 activation, MLC dephosphorylation | occludin, claudin-1, JAM-A, ZO-1 | [49] | |
| Enteropathogenic E. coli infection | T84 | ezrin phosphorylation | MLC phosphorylation | occludin, claudin-1 | [47, 48, 58, 83] | |
| Enteropathogenic E. coli infection | mouse jejunum and colon | occludin | [46] | |||
| Clostridium difficile toxins | T84 | caveolae | PKC a/β activation | rho GTPase inactivation | occludin, ZO-1 | [84–86] |
| Calcium depletion | T84 | clathrin | adherens junction disruption | myosin ATPase activation | occludin, JAM-A, claudin-1, ZO-1 | [50, 54] |
| IFN-γ | T84 | macropinocytosis | rho kinase | myosin ATPase activation | occludin, JAM-A, claudin-1 | [55] |
| Helicobacter pylori CagA | MDCK | c-Src inactivation | cortactin dephosphorylation | ZO-1, JAM | [87] [88] | |
| latrunculin A | MDCK | caveolae | actin depolymerization | occludin | [53] | |
| TNF | Caco-2 | MLCK activation | MLC phosphorylation | occludin, claudin-1 | [45] | |
| LIGHT | Caco-2 | caveolae | MLCK activation | MLC phosphorylation | occludin, claudin-1 | [74] |
| T cell activation, TNF, or LIGHT | mouse jejunum | MLCK activation | MLC phosphorylation | occludin, JAM-A | [75, 76] |
Mechanisms of tight junction protein internalization
The mechanisms that regulate tight junction protein internalization have only been studied in detail over the last decade as transmembrane tight junction proteins were identified and means of detecting individual tight junction proteins have become available. The latter include a wide array of commercial antisera and well-characterized fluorescent fusion constructs of representative tight junction proteins [53]. These tools and growing interest in the role of tight junction disruption in pathophysiology, particularly in the gastrointestinal tract, have spawned a large number of studies evaluating tight junction protein trafficking in response to physiological, pharmacological, and pathophysiological stimuli. Remarkably, all three classic pathways of endocytosis have been reported to be involved in tight junction protein internalization, depending on the stimulus used (Figure 2).
Figure 2.
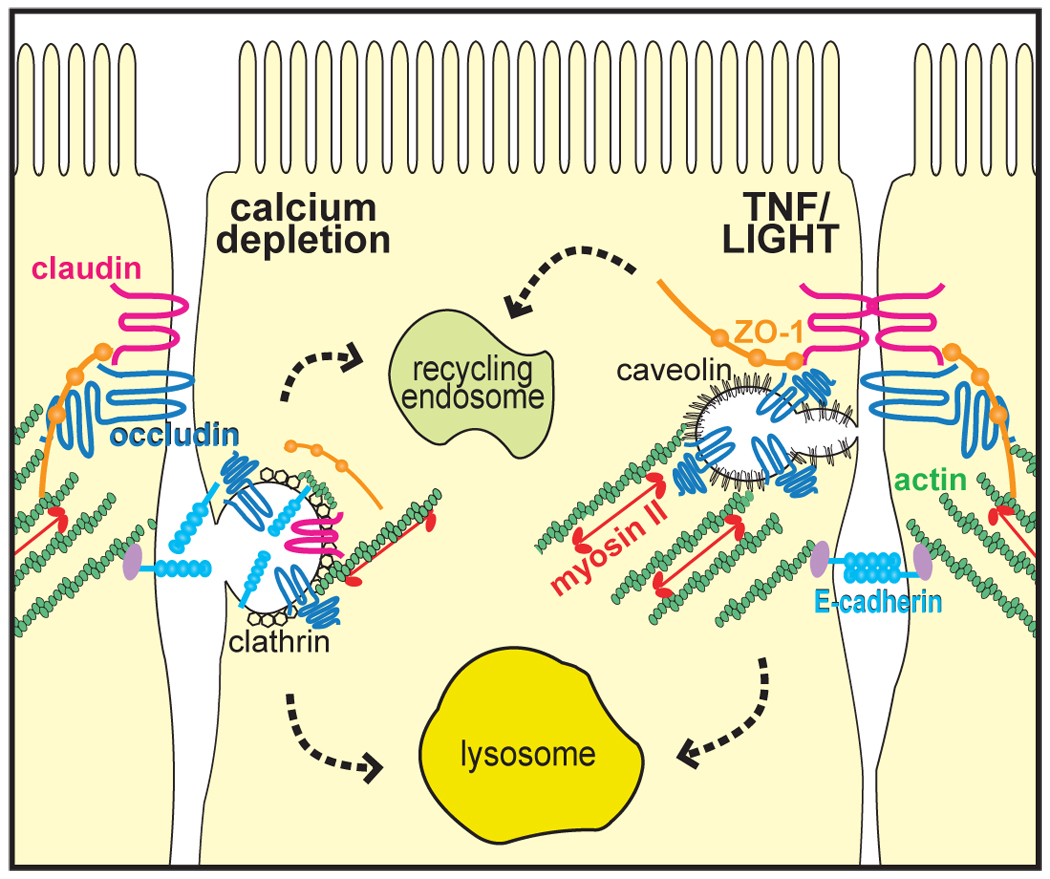
Stimulus-induced endocytosis of tight junction proteins. Calcium depletion causes clathrin-mediated endocytosis of adherens junction (E-cadherin) and tight junction (claudin, occludin) proteins. TNF core family members caused caveolae-mediated endocytosis of occludin, but not other proteins. Both pathways involve actomyosin-dependent processes. The fate of these vesicles, including whether they fuse with lysosomes, resulting in protein degradation, or with recycling endosomes, with protein trafficking back to the tight junction, remains to be defined.
For example, calcium depletion, a non-physiological stimulus that may trigger tight junction disassembly by releasing intercellular cadherin interactions, causes internalization of both adherens and tight junction proteins [50, 54]. Immunofluorescent studies suggest that several proteins, including E-cadherin, β-catenin, occludin, and junctional adhesion molecule-A (JAM-A). These proteins co-localize with one another as well as α-adaptin, a marker of clathrin-mediated endocytosis [50], suggesting that all are internalized via clathrin-coated vesicles. The adherens and tight junction proteins do not appear to remain in clathrin-coated vesicales, as co-localization with markers of early endosomes, such as rab5 and EEA1, is also seen. The colocalization in these early endocytic compartments suggests that the intact complex may be internalized. E-cadherin, β-catenin, occludin, and JAM-A also co-localized with the basolateral marker syntaxin-4, but only E-cadherin and β-catenin co-localized with the Na+K+-ATPase. This implies that, once internalized, there is some sorting of junction proteins as well as mixing of basolaterally-derived and junction-derived endosomes, but that this may be differentially-regulated for tight junction and adherens junction proteins [50]. In support of this hypothesis, tight junction and adherens junction proteins appear to segregate from one another in subapical endosomes one hour after calcium depletion. Consistent with the proposed role of clathrin-mediated endocytosis, agents that grossly perturb endocytosis, including acidic and hyperosmotic media, were each able to prevent occludin and E-cadherin endocytosis after calcium depletion. Further support of this interpretation is provided by the failure to detect co-localization of occludin or E-cadherin with caveolin-1 or internalized dextran, or to prevent occludin or E-cadherin internalization with cholesterol depletion or PI3-kinase inhibition, which interfere with caveolar endocytosis and macropinocytosis, respectively. Thus, it appears clear that clathrin-mediated endocytosis of tight junction and adherens junction components occurs after calcium depletion [50]. While it remains to be determined if blockade of clathrin-mediated endocytosis can prevent the loss of tight junction barrier function after calcium depletion, inhibition of myosin II ATPase with blebbistatin, which also prevents occludin and E-cadherin internalization, prevents barrier loss to a limited degree [54]. However, as might be expected of a drug that interferes with actomyosin function, blebbistatin also disrupts the tight junction barrier, even in the presence of extracellular calcium, thereby complicating interpretation of these data.
Interestingly, blebbistatin has also been reported to prevent the occludin internalization induced by interferon-γ (IFN-γ) in cultured intestinal epithelial monolayers [55]. Unlike the endocytosis of tight junction and adherens proteins seen after calcium depletion, IFN-γ induces macropinocytosis-like endocytosis of tight junction proteins [56]. These co-localize with the early endosomes marker EEA1 as well as markers of recycling endosomes, rab4 and rab11, but not lysosomal markers such as LAMP1. It thus appears that, while the general mechanisms of tight junction protein internalization induced by calcium depletion or IFN-γ in cultured intestinal epithelia differ, both involve actomyosin-dependent processes.
Unlike calcium depletion and IFN-γ treatment, the Escherichia coli toxin cytotoxic necrotizing factor-1 (CNF-1), which activates Rho GTPases, seems to induce occludin internalization into caveolin-1-positive structures [49]. This suggests that such endocytosis occurs via caveolae, which are thought to form from lipid rafts and concentrate cargo by nature of the specialized lip[id composition of these domains. CNF-1 also induces redistribution of claudin-1, JAM-A, and ZO-1 though, unlike calcium depletion, these tight junction proteins were not co-localized with occludin and adherens junctions were not disrupted. While internalized occludin did not co-localize with LAMP1, some occludin did co-localize with EEA1 and rab11. The quantitative significance of this was not evaluated, but it does raise the possibility that occludin, and perhaps other tight junction proteins, could be recycled to the tight junction after stimulus-induced internalization. CNF-1 also induced actin reorganization and loss of the pool of phosphorylated myosin II regulatory light chain (MLC) normally present at the tight junction [49, 57]. Thus, although intermediates that trigger occludin internalization or barrier loss have not been defined, it appears that the cytoskeleton is also involved in this caveolae-mediated tight junction protein endocytosis. Although the pathway of endocytosis has not been characterized, the noninvasive diarrheal pathogen enteropathogenic E. coli, also causes occludin endocytosis in vitro and in vivo [46–48]. A large part of this barrier loss is also regulated by cytoskeletal mechanisms, at least in vitro [58, 59].
Direct microfilament perturbation causes tight junction protein internalization
Consistent with a central role of actomyosin in regulating barrier function, direct perturbation of filamentous actin with the depolymerizing drug latrunculin A causes tight junction disruption [53, 60–63]. Epithelial monolayers expressing fluorescent fusion constructs of occludin, claudin-1, ZO-1, and β-actin and real time fluorescence microscopy with simultaneous measurement of barrier function demonstrated that only occludin internalization correlated with latrunculin A-induced barrier loss [53]. While claudin-1 and ZO-1 were redistributed after latrunculin A treatment, these changes occurred after the bulk of barrier loss, suggesting a secondary event not directly related to barrier loss. Further real time imaging using monolayers expressing red fluorescent-tagged occludin and either green fluorescent-tagged caveolin-1 or yellow fluorescent-tagged clathrin light chain showed that latrunculin A-induced occludin internalization occurred through caveolin-1-positive structures, but not occludin-positive structures. Morphometric and kinetic analyses showed that while occludin trafficked through caveolin-1-positive compartments, relatively little internalized occludin trafficked to EEA1-positive endosomes or LAMP1-positive lysosomes [53]. Moreover, inhibition of endocytosis by cooling to 14°C, hyperosmotic media prevented occludin internalization and barrier loss. Disruption of lipid rafts by cholesterol depletion, which interferes with endocytosis via caveolae, also prevented occludin internalization, but inhibition of Na+H+-exchange, PI3-kinase, or clathrin assembly were all without effect [53]. Thus, pharmacological actin depolymerization induces occludin internalization via caveolae and interruption of this process prevents barrier loss. However, these data do not show that occludin is functionally important, only that occludin is a sensitive marker of the endocytic events that accompany actin depolymerization-induced barrier loss. Nonetheless, common themes in tight junction disruption by disparate stimuli appear to include modification of the perijunctional actomyosin ring and occludin internalization by one of several different endocytic pathways.
Relationship of tight junction protein internalization to pathophysiological barrier loss
In recent years, intestinal epithelial barrier loss induced by TNF has received a great deal of attention [35, 41, 64]. This is, in part, due to the fact that intestinal barrier function is disrupted in Crohn’s disease and that TNF neutralization is able to induce disease remission and restore barrier function in patients [65–68]. Thus, it appears that TNF-induced barrier dysfunction may be a critical contributor to human disease [41, 67]. Although it had been known for several years that tumor necrosis factor could also induce barrier loss in cultured epithelial monolayers [69, 70], the discovery that MLC phosphorylation was markedly increased by TNF represented a major breakthrough in understanding the mechanisms of this process [59]. In addition to a correlation between MLC phosphorylation and barrier loss, specific inhibition of MLC kinase reversed in vitro barrier loss induced by TNF [59]. This TNF-induced barrier loss was associated with enzymatic as well as transcriptional activation of MLC kinase [45, 71–73]. Moreover, occludin and claudin-1 internalization were induced by TNF.[45]
Although the mechanisms of in vitro TNF-induced occludin internalization have not been studied in detail, a TNF core family member, LIGHT (lymphotoxin-like inducible protein that competes with glycoprotein D for herpes virus entry on T cells), also induces MLC kinase-dependent occludin and claudin-1 internalization [74]. Detailed morphometric analysis showed that the progressive decreases in tight junction barrier function correlate with increased numbers of intracellular occludin-containing vesicles [74]. These occludin vesicles also contained caveolin-1, but not clathrin heavy chain. More importantly, inhibition of caveolar endocytosis, but not clathrin-mediated endocytosis or macropinocytosis, prevented occludin internalization and barrier loss [74]. Since inhibition of caevolar endocytosis did not prevent MLC phosphorylation, but MLC kinase inhibition prevented occludin endocytosis and barrier loss, these data also show that MLC phosphorylation precedes and is necessary for occludin internalization and barrier dysfunction, but that caveolar endocytosis is not required for MLC phosphorylation. Thus, like the pharmacological stimulus latrunculin A, caveolar endocytosis seems to be critical to this cytokine-induced barrier dysfunction.
In vivo analysis of stimulus-induced barrier loss and tight junction reorganization
Until recently, analysis of tight junction protein endocytosis in response to defined stimuli had only been studied in vitro. However, recent work has shown that either T cell activation or direct administration of recombinant TNF or LIGHT induces occludin internalization and barrier dysfunction in mice [75, 76]. As predicted by some of the in vitro data, this was associated with epithelial MLC phosphorylation and either genetic or pharmacological inhibition of intestinal epithelial MLC kinase prevented both occludin internalization and barrier dysfunction [75]. In an observation only possible in an intact in vivo system, barrier dysfunction induced by T cell activation or TNF administration resulted in net intestinal water secretion, i.e. diarrhea, which was also prevented by either genetic or pharmacological MLC kinase inhibition [75, 76]. In contrast to TNF, LIGHT, which induced similar MLC kinase-dependent barrier dysfunction, actually enhanced water absorption [76]. This difference was due to the second effect of TNF, downregulation of epithelial Na+ absorption, which was not induced by LIGHT. Thus, in addition to demonstrating that MLC kinase-dependent barrier dysfunction is necessary for cytokine-driven acute diarrhea to occur in vivo, these data also demonstrate that the barrier dysfunction is only part of the story. Water can flow in either direction through tight junctions in which cytokines have caused increased permeability. The determination of the direction of water flow depends on osmotic gradients generated by active transepithelial transport [76, 77].
The in vivo studies also show that, despite MLC kinase-dependent occludin internalization (Figure 3), endocytosis of ZO-1, claudins, and adherens junction proteins did not occur [75]. Occludin endocytosis without claudin endocytosis is similar to that induced by latrunculin A in vitro, but contrasts sharply with the other in vitro studies which generally showed internalization of occludin along with claudin, JAM-A, or other tight junction proteins. While it remains to be characterized, these disparate results may well reflect the difference between acute or transient stimuli and chronic stimuli. In the case of latrunculin A, claudin-1 redistribution was seen at later time points, after most barrier function losses had occurred. Thus, if only the endpoint, rather than many intermediate time points, were assessed, the results might have been mistakenly interpreted to imply that claudin redistribution coincides with barrier loss. In the in vivo example given a pulse of cytokine release occurs and then rapidly diminishes. Thus, it may be that prolonged cytokine simulation, as occurs in chronic disease, may induce internalization of claudin proteins as well as occludin. This, in fact, has recently been observed in epithelia from patients with Crohn’s disease [78–80].
Figure 3.
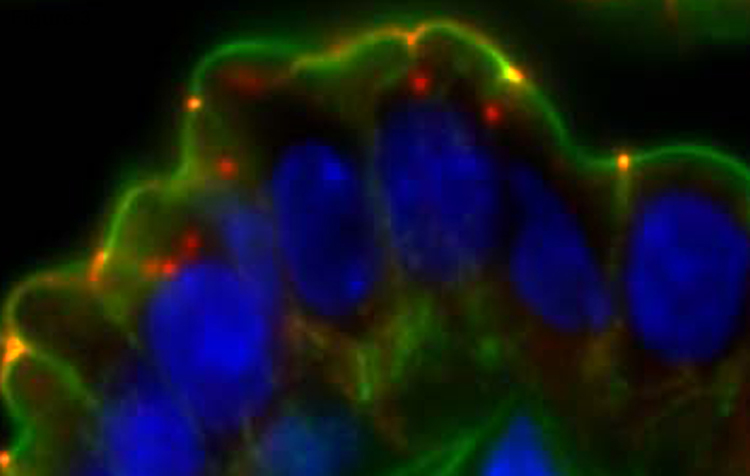
Occludin is internalized in vivo after immune activation. T cells were activated in vivo by intraperitoneal injection of anti-CD3 antibody. This resulted in TNF-dependent occludin (red) internalization. F-actin (green) and nuclei (blue) are shown for reference.
The temporal correlation of occludin internalization with initial barrier loss also suggests that occludin may be a critically important functional component of the tight junction. However, reports that occludin knockout mice have normal intestinal barrier function have been interpreted to imply that occludin does not play an important role in tight junction structure or regulation [23, 81]. While no in vivo data are available to refute this interpretation, the fact remains that abundant in vitro data clearly support a functional regulatory role for occludin. These include data from epithelial cells in which occludin expression was suppressed by stably expressing short interfering RNA [82]. Thus, it may be that the few studies of intestinal function in occludin knockout mice have failed to show a functional deficit because the mice have not been subjected to stress or, alternatively, because there are compensatory changes in expression of another, as yet unidentified, protein. Clearly, this is an area in which more work is needed.
Concluding comments
It is now clear that vesicular traffic plays an important role in regulating tight junction structure and function in response to a wide variety of stimuli. The demonstration that this process also occurs in vivo, and is specifically-related to intestinal disease, makes it essential that these observations be defined in greater detail. For example, although various pathways of endocytosis have been described morphologically, few studies have shown that these events are absolutely required for regulation of barrier function. The molecular events that trigger endocytosis and how these relate to tight junction structure are also in need of further characterization. Nonetheless, the available data argue strongly that the tight junction is a far more dynamic and complex structure than previously recognized. Enhanced understanding of these processes may provide means to selectively and specifically regulate the tight junction and allow development of targeted therapies for diverse diseases of barrier function.
Acknowledgements
We thank W. Vallen Graham, Amanda M. Marchiando, Le Shen, Liping Su, and Erika A. Sullivan for their insightful comments. This work was supported by National Institutes of Health grants (DK61931 and DK68271).
Footnotes
No conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cereijido M, Meza I, Martinez-Palomo A. Occluding junctions in cultured epithelial monolayers. Am J Physiol. 1981;240:C96–C102. doi: 10.1152/ajpcell.1981.240.3.C96. [DOI] [PubMed] [Google Scholar]
- 2.Schulzke JD, Fromm M, Zeitz M, Menge H, Riecken EO, Bentzel CJ. Tight junction regulation during impaired ion transport in blind loops of rat jejunum. Res Exp Med. 1990;190:59–68. doi: 10.1007/pl00020007. [DOI] [PubMed] [Google Scholar]
- 3.Bentzel CJ, Hainau B, Ho S, Hui SW, Edelman A, Anagnostopoulos T, Benedetti EL. Cytoplasmic regulation of tight-junction permeability: effect of plant cytokinins. Am J Physiol. 1980;239:C75–C89. doi: 10.1152/ajpcell.1980.239.3.C75. [DOI] [PubMed] [Google Scholar]
- 4.Atisook K, Carlson S, Madara JL. Effects of phlorizin and sodium on glucose-elicited alterations of cell junctions in intestinal epithelia. Am J Physiol. 1990;258:C77–C85. doi: 10.1152/ajpcell.1990.258.1.C77. [DOI] [PubMed] [Google Scholar]
- 5.Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- 6.Hyafil F, Babinet C, Jacob F. Cell-cell interactions in early embryogenesis: a molecular approach to the role of calcium. Cell. 1981;26:447–454. doi: 10.1016/0092-8674(81)90214-2. [DOI] [PubMed] [Google Scholar]
- 7.Gumbiner B, Simons K. The role of uvomorulin in the formation of epithelial occluding junctions. Ciba Foundation symposium. 1987;125:168–186. doi: 10.1002/9780470513408.ch11. [DOI] [PubMed] [Google Scholar]
- 8.Hermiston ML, Wong MH, Gordon JI. Forced expression of E-cadherin in the mouse intestinal epithelium slows cell migration and provides evidence for nonautonomous regulation of cell fate in a self-renewing system. Genes Dev. 1996;10:985–996. doi: 10.1101/gad.10.8.985. [DOI] [PubMed] [Google Scholar]
- 9.Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270:1203–1207. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- 10.Sheff DR, Kroschewski R, Mellman I. Actin dependence of polarized receptor recycling in Madin-Darby canine kidney cell endosomes. Mol Biol Cell. 2002;13:262–275. doi: 10.1091/mbc.01-07-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hull BE, Staehelin LA. The terminal web. A reevaluation of its structure and function. J Cell Biol. 1979;81:67–82. doi: 10.1083/jcb.81.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staehelin LA. Further observations on the fine structure of freeze-cleaved tight junctions. J Cell Sci. 1973;13:763–786. doi: 10.1242/jcs.13.3.763. [DOI] [PubMed] [Google Scholar]
- 13.Kachar B, Reese TS. Evidence for the lipidic nature of tight junction strands. Nature. 1982;296:464–466. doi: 10.1038/296464a0. [DOI] [PubMed] [Google Scholar]
- 14.Tsukita S, Furuse M. Pores in the wall: claudins constitute tight junction strands containing aqueous pores. J Cell Biol. 2000;149:13–16. doi: 10.1083/jcb.149.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci U S A. 1999;96:511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuse M, Fujimoto K, Sato N, Hirase T, Tsukita S. Overexpression of occludin, a tight junction-associated integral membrane protein, induces the formation of intracellular multilamellar bodies bearing tight junction-like structures. J Cell Sci. 1996;109:429–435. doi: 10.1242/jcs.109.2.429. [DOI] [PubMed] [Google Scholar]
- 19.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Itallie CM, Anderson JM. Occludin confers adhesiveness when expressed in fibroblasts. J Cell Sci. 1997;110:1113–1121. doi: 10.1242/jcs.110.9.1113. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109(Pt 9):2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- 22.Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, Takano H, Noda T, Tsukita S. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heiskala M, Peterson PA, Yang Y. The roles of claudin superfamily proteins in paracellular transport. Traffic. 2001;2:93–98. doi: 10.1034/j.1600-0854.2001.020203.x. [DOI] [PubMed] [Google Scholar]
- 25.Kausalya PJ, Amasheh S, Gunzel D, Wurps H, Muller D, Fromm M, Hunziker W. Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of Claudin-16. J Clin Invest. 2006;116:878–891. doi: 10.1172/JCI26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 28.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (Zonula Occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol. 2003;81:1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- 31.Jesaitis LA, Goodenough DA. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J Cell Biol. 1994;124:949–961. doi: 10.1083/jcb.124.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature. 1988;333:272–275. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- 33.Stevenson BR, Heintzelman MB, Anderson JM, Citi S, Mooseker MS. ZO-1 and cingulin: tight junction proteins with distinct identities and localizations. Am J Physiol. 1989;257:C621–C628. doi: 10.1152/ajpcell.1989.257.4.C621. [DOI] [PubMed] [Google Scholar]
- 34.Nusrat A, Chen JA, Foley CS, Liang TW, Tom J, Cromwell M, Quan C, Mrsny RJ. The coiled-coil domain of occludin can act to organize structural and functional elements of the epithelial tight junction. J Biol Chem. 2000;275:29816–29822. doi: 10.1074/jbc.M002450200. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki A, Yamanaka T, Hirose T, Manabe N, Mizuno K, Shimizu M, Akimoto K, Izumi Y, Ohnishi T, Ohno S. Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J Cell Biol. 2001;152:1183–1196. doi: 10.1083/jcb.152.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Izumi Y, Hirose T, Tamai Y, Hirai S, Nagashima Y, Fujimoto T, Tabuse Y, Kemphues KJ, Ohno S. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J Cell Biol. 1998;143:95–106. doi: 10.1083/jcb.143.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vega-Salas DE, Salas PJ, Rodriguez-Boulan E. Exocytosis of vacuolar apical compartment (VAC): a cell-cell contact controlled mechanism for the establishment of the apical plasma membrane domain in epithelial cells. J Cell Biol. 1988;107:1717–1728. doi: 10.1083/jcb.107.5.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denisenko N, Burighel P, Citi S. Different effects of protein kinase inhibitors on the localization of junctional proteins at cell-cell contact sites. J Cell Sci. 1994;107:969–981. doi: 10.1242/jcs.107.4.969. [DOI] [PubMed] [Google Scholar]
- 39.Siliciano JD, Goodenough DA. Localization of the tight junction protein, ZO-1, is modulated by extracellular calcium and cell-cell contact in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1988;107:2389–2399. doi: 10.1083/jcb.107.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116:457–466. doi: 10.1016/s0092-8674(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 41.Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282–291. doi: 10.1038/labinvest.3700050. [DOI] [PubMed] [Google Scholar]
- 42.Matsuda M, Kubo A, Furuse M, Tsukita S. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J Cell Sci. 2004;117:1247–1257. doi: 10.1242/jcs.00972. [DOI] [PubMed] [Google Scholar]
- 43.Polak-Charcon S, Ben-Shaul Y. Degradation of tight junctions in HT29, a human colon adenocarcinoma cell line. J Cell Sci. 1979;35:393–402. doi: 10.1242/jcs.35.1.393. [DOI] [PubMed] [Google Scholar]
- 44.Madara JL. Maintenance of the macromolecular barrier at cell extrusion sites in intestinal epithelium: physiological rearrangement of tight junctions. J Membr Biol. 1990;116:177–184. doi: 10.1007/BF01868675. [DOI] [PubMed] [Google Scholar]
- 45.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shifflett DE, Clayburgh DR, Koutsouris A, Turner JR, Hecht GA. Enteropathogenic E. coli disrupts tight junction barrier function and structure in vivo. Lab Invest. 2005;85:1308–1324. doi: 10.1038/labinvest.3700330. [DOI] [PubMed] [Google Scholar]
- 47.Muza-Moons MM, Schneeberger EE, Hecht GA. Enteropathogenic Escherichia coli infection leads to appearance of aberrant tight junctions strands in the lateral membrane of intestinal epithelial cells. Cell Microbiol. 2004;6:783–793. doi: 10.1111/j.1462-5822.2004.00404.x. [DOI] [PubMed] [Google Scholar]
- 48.Simonovic I, Rosenberg J, Koutsouris A, Hecht G. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell Microbiol. 2000;2:305–315. doi: 10.1046/j.1462-5822.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- 49.Hopkins AM, Walsh SV, Verkade P, Boquet P, Nusrat A. Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J Cell Sci. 2003;116:725–742. doi: 10.1242/jcs.00300. [DOI] [PubMed] [Google Scholar]
- 50.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell. 2004;15:176–188. doi: 10.1091/mbc.E03-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheth P, Basuroy S, Li C, Naren AP, Rao RK. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J Biol Chem. 2003;278:49239–49245. doi: 10.1074/jbc.M305654200. [DOI] [PubMed] [Google Scholar]
- 52.Marchiando AM, Shen L, Guan Y, Watson AJM, Montrose MH, Turner JR. Real time analysis of TNF-induced occludin internalization within jejnal epithelia of living mice. Faseb J. 2007;21:A663. [Google Scholar]
- 53.Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell. 2005;16:3919–3936. doi: 10.1091/mbc.E04-12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ivanov AI, McCall IC, Parkos CA, Nusrat A. Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol Biol Cell. 2004;15:2639–2651. doi: 10.1091/mbc.E04-02-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. 2005;16:5040–5052. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. Faseb J. 2005;19:923–933. doi: 10.1096/fj.04-3260com. [DOI] [PubMed] [Google Scholar]
- 57.Berglund JJ, Riegler M, Zolotarevsky Y, Wenzl E, Turner JR. Regulation of human jejunal transmucosal resistance and MLC phosphorylation by Na+-glucose cotransport. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1487–G1493. doi: 10.1152/ajpgi.2001.281.6.G1487. [DOI] [PubMed] [Google Scholar]
- 58.Yuhan R, Koutsouris A, Savkovic SD, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113:1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]
- 59.Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002;123:163–172. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- 60.Matthews JB, Smith JA, Hrnjez BJ. Effects of F-actin stabilization or disassembly on epithelial Cl- secretion and Na-K-2Cl cotransport. Am J Physiol. 1997;272:C254–C262. doi: 10.1152/ajpcell.1997.272.1.C254. [DOI] [PubMed] [Google Scholar]
- 61.Madara JL, Barenberg D, Carlson S. Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J Cell Biol. 1986;102:2125–2136. doi: 10.1083/jcb.102.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meza I, Sabanero M, Stefani E, Cereijido M. Occluding junctions in MDCK cells: modulation of transepithelial permeability by the cytoskeleton. J Cell Biochem. 1982;18:407–421. doi: 10.1002/jcb.1982.240180403. [DOI] [PubMed] [Google Scholar]
- 63.Stevenson BR, Begg DA. Concentration-dependent effects of cytochalasin D on tight junctions and actin filaments in MDCK epithelial cells. J Cell Sci. 1994;107:367–375. doi: 10.1242/jcs.107.3.367. [DOI] [PubMed] [Google Scholar]
- 64.Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suenaert P, Bulteel V, Lemmens L, Noman M, Geypens B, Van Assche G, Geboes K, Ceuppens JL, Rutgeerts P. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn's disease. Am J Gastroenterol. 2002;97:2000–2004. doi: 10.1111/j.1572-0241.2002.05914.x. [DOI] [PubMed] [Google Scholar]
- 66.D'Haens G, Van Deventer S, Van Hogezand R, Chalmers D, Kothe C, Baert F, Braakman T, Schaible T, Geboes K, Rutgeerts P. Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn's disease: A European multicenter trial. Gastroenterology. 1999;116:1029–1034. doi: 10.1016/s0016-5085(99)70005-3. [DOI] [PubMed] [Google Scholar]
- 67.Hollander D. The intestinal permeability barrier. A hypothesis as to its regulation and involvement in Crohn's disease. Scand J Gastroenterol. 1992;27:721–726. doi: 10.3109/00365529209011172. [DOI] [PubMed] [Google Scholar]
- 68.Marin ML, Greenstein AJ, Geller SA, Gordon RE, Aufses AH., Jr A freeze fracture study of Crohn's disease of the terminal ileum: changes in epithelial tight junction organization. Am J Gastroenterol. 1983;78:537–547. [PubMed] [Google Scholar]
- 69.Mullin JM, Snock KV. Effect of tumor necrosis factor on epithelial tight junctions and transepithelial permeability. Cancer. Res. 1990;50:2172–2176. [PubMed] [Google Scholar]
- 70.Taylor CT, Dzus AL, Colgan SP. Autocrine regulation of epithelial permeability by hypoxia: role for polarized release of tumor necrosis factor alpha. Gastroenterology. 1998;114:657–668. doi: 10.1016/s0016-5085(98)70579-7. [DOI] [PubMed] [Google Scholar]
- 71.Graham WV, Wang F, Clayburgh DR, Cheng JX, Yoon B, Wang Y, Lin A, Turner JR. Tumor necrosis factor-induced long myosin light chain kinase transcription is regulated by differentiation-dependent signaling events. Characterization of the human long myosin light chain kinase promoter. J Biol Chem. 2006;281:26205–26215. doi: 10.1074/jbc.M602164200. [DOI] [PubMed] [Google Scholar]
- 72.Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288:G422–G430. doi: 10.1152/ajpgi.00412.2004. [DOI] [PubMed] [Google Scholar]
- 73.Blair SA, Kane SV, Clayburgh DR, Turner JR. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest. 2006;86:191–201. doi: 10.1038/labinvest.3700373. [DOI] [PubMed] [Google Scholar]
- 74.Schwarz BT, Wang F, Shen L, Clayburgh DR, Su L, Wang Y, Fu YX, Turner JR. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology. 2007 doi: 10.1053/j.gastro.2007.02.052. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest. 2006;116:2682–2694. doi: 10.1172/JCI29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turner JR, Clayburgh DR, Musch MW, Leitges M, Fu YX. Response to field. J Clin Invest. 2006;116:3088–3089. doi: 10.1172/JCI29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE. Inflammatory processes have differential effects on Claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest. 2005;85:1139–1162. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- 79.Weber CR, Turner JR. Inflammatory bowel disease: is it really just another break in the wall? Gut. 2007;56:6–8. doi: 10.1136/gut.2006.104182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schulzke JD, Gitter AH, Mankertz J, Spiegel S, Seidler U, Amasheh S, Saitou M, Tsukita S, Fromm M. Epithelial transport and barrier function in occludin-deficient mice. Biochim Biophys Acta. 2005;1669:34–42. doi: 10.1016/j.bbamem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 82.Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol. 2005;288:C1231–C1241. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]
- 83.Simonovic I, Arpin M, Koutsouris A, Falk-Krzesinski HJ, Hecht G. Enteropathogenic Escherichia coli activates ezrin, which participates in disruption of tight junction barrier function. Infect Immun. 2001;69:5679–5688. doi: 10.1128/IAI.69.9.5679-5688.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hecht G, Pothoulakis C, LaMont JT, Madara JL. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J. Clin. Invest. 1988;82:1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nusrat A, von Eichel-Streiber C, Turner JR, Verkade P, Madara JL, Parkos CA. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun. 2001;69:1329–1336. doi: 10.1128/IAI.69.3.1329-1336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen ML, Pothoulakis C, LaMont JT. Protein kinase C signaling regulates ZO-1 translocation and increased paracellular flux of T84 colonocytes exposed to Clostridium difficile toxin A. J Biol Chem. 2002;277:4247–4254. doi: 10.1074/jbc.M109254200. [DOI] [PubMed] [Google Scholar]
- 87.Selbach M, Moese S, Hurwitz R, Hauck CR, Meyer TF, Backert S. The Helicobacter pylori CagA protein induces cortactin dephosphorylation and actin rearrangement by c-Src inactivation. Embo J. 2003;22:515–528. doi: 10.1093/emboj/cdg050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430–1434. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]


