Abstract
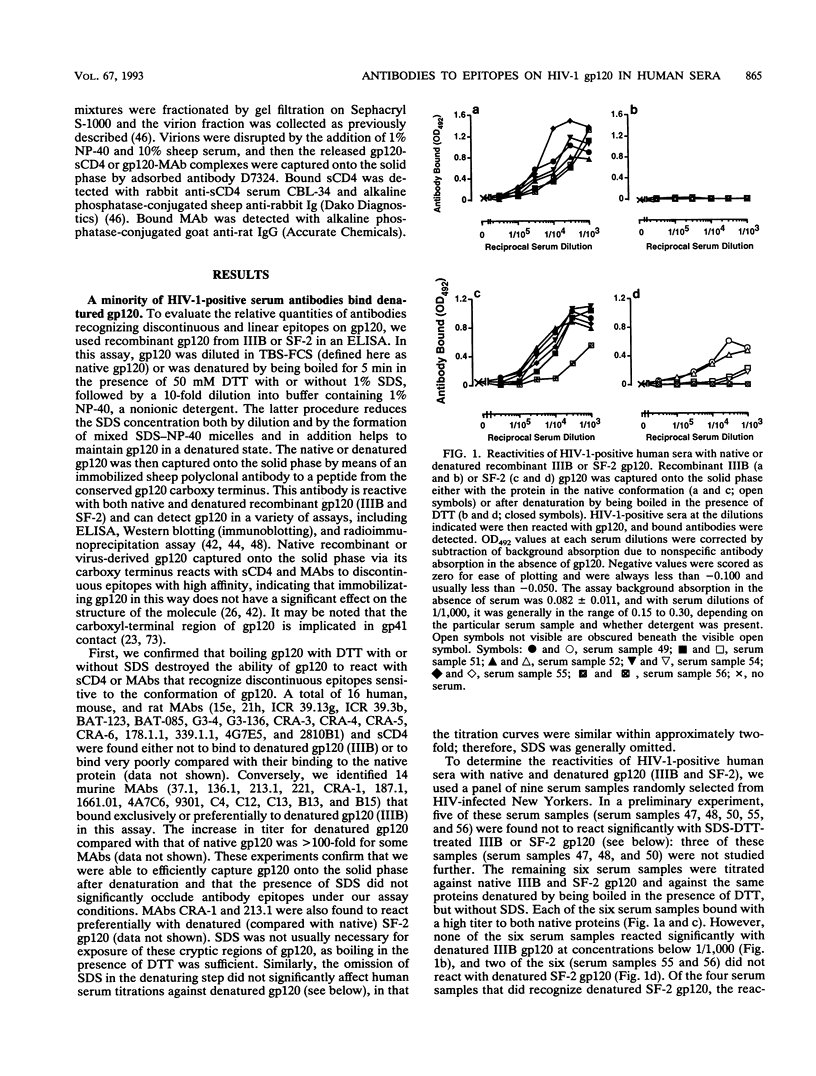
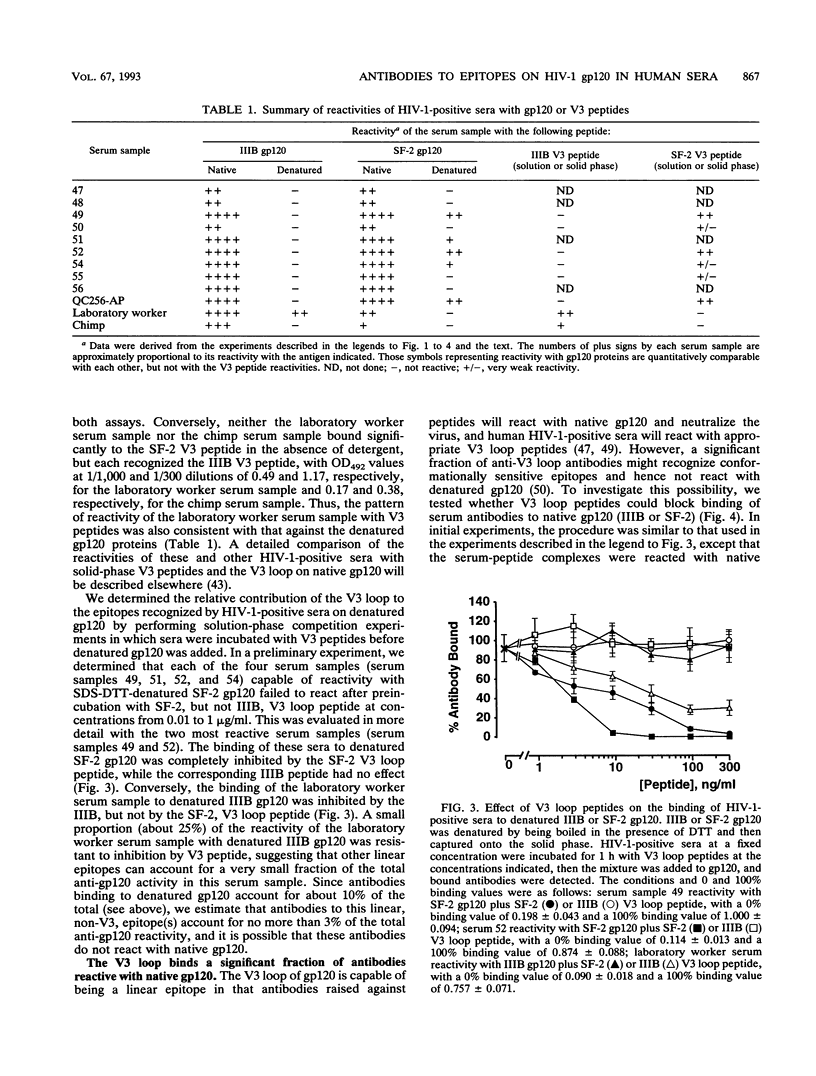
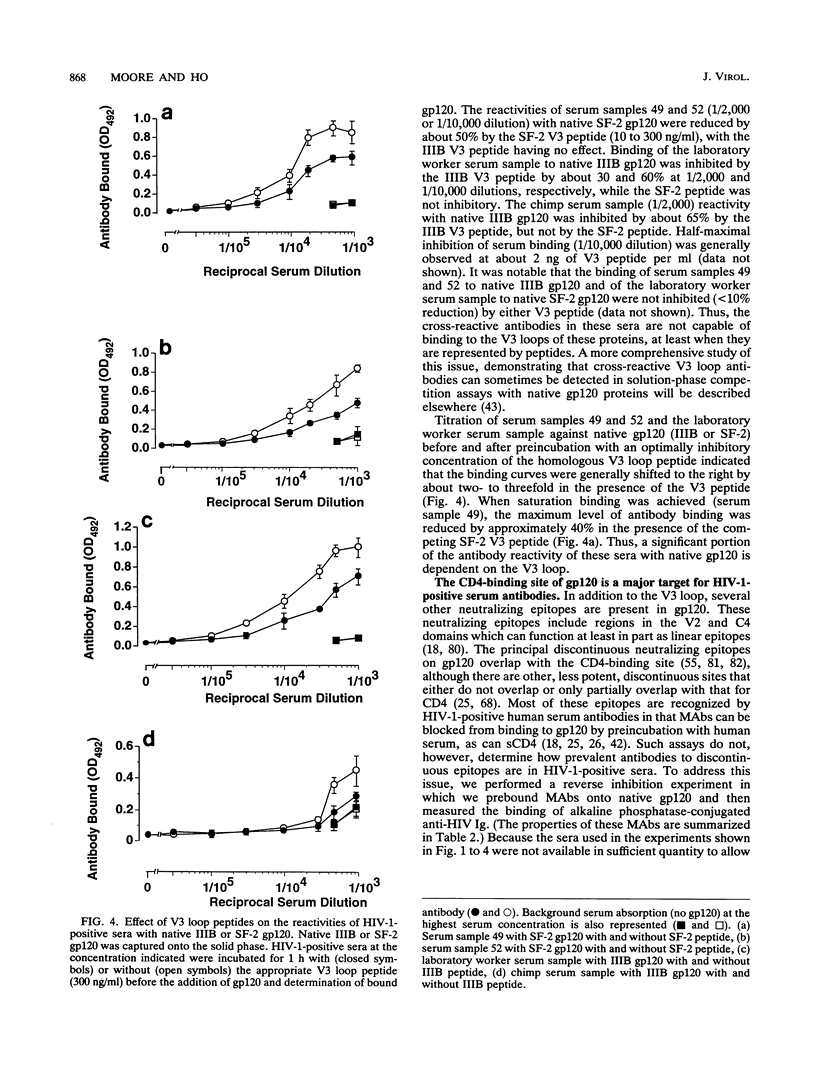
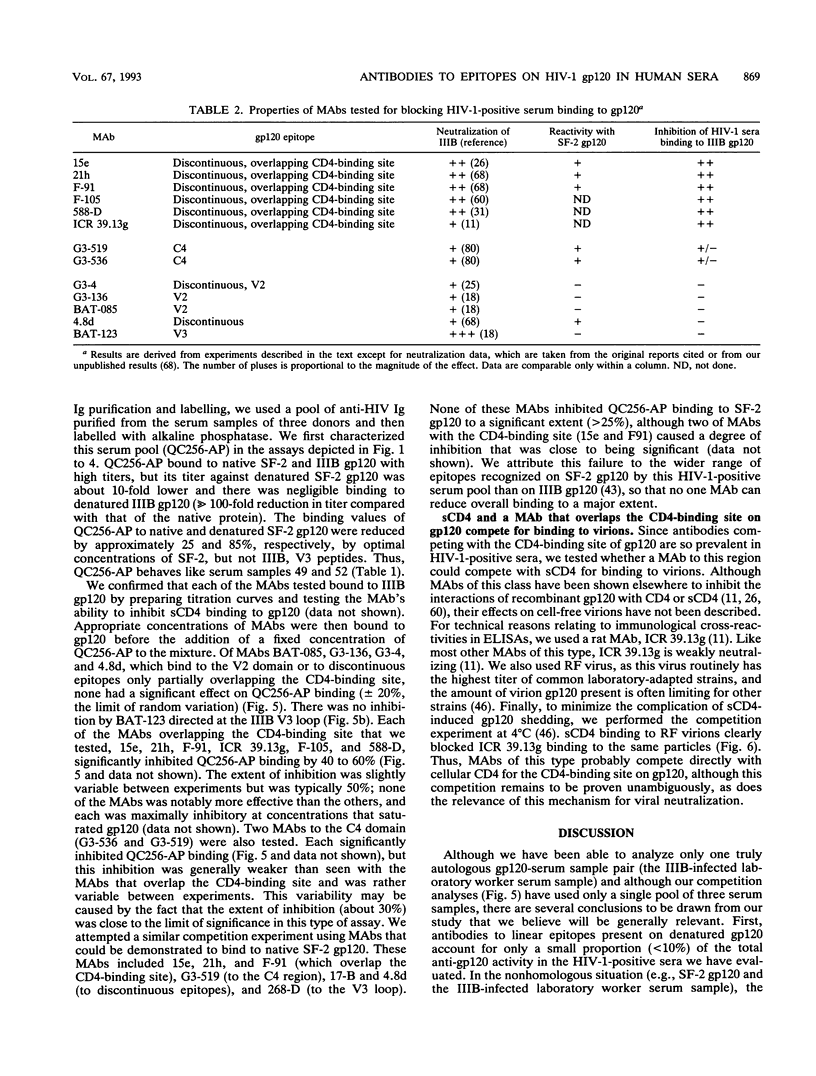
We have used an indirect-capture enzyme-linked immunosorbent assay to quantitate the reactivity of sera from human immunodeficiency virus type 1 (HIV-1)-infected humans with native recombinant gp120 (HIV-1 IIIB or SF-2) or with the gp120 molecule (IIIB or SF-2) denatured by being boiled in the presence of dithiothreitol with or without sodium dodecyl sulfate. Denaturation of IIIB gp120 reduced the titers of sera from randomly selected donors by at least 100-fold, suggesting that the majority of cross-reactive anti-gp120 antibodies present are directed against discontinuous or otherwise conformationally sensitive epitopes. When SF-2 gp120 was used, four of eight serum samples reacted significantly with the denatured protein, albeit with ca. 3- to 50-fold reductions in titer. Only those sera reacting with denatured SF-2 gp120 bound significantly to solid-phase-adsorbed SF-2 V3 loop peptide, and none bound to IIIB V3 loop peptide. Almost all antibody binding to reduced SF-2 gp120 was blocked by preincubation with the SF-2 V3 loop peptide, as was about 50% of the binding to native SF-2 gp120. When sera from a laboratory worker or a chimpanzee infected with IIIB were tested, the pattern of reactivity was reversed, i.e., there was significant binding to reduced IIIB gp120, but not to reduced SF-2 gp120. Binding of these sera to reduced IIIB gp120 was 1 to 10% that to native IIIB gp120 and was substantially decreased by preincubation with IIIB (but not SF-2) V3 loop peptide. To analyze which discontinuous or conformational epitopes were predominant in HIV-1-positive sera, we prebound monoclonal antibodies (MAbs) to IIIB gp120 and then added alkaline phosphatase-labelled HIV-1-positive sera. MAbs (such as 15e) that recognize discontinuous epitopes and compete directly with CD4 reduced HIV-1-positive sera binding by about 50%, whereas neutralizing MAbs to the C4, V2, and V3 domains of gp120 were either not inhibitory or only weakly so. Thus, antibodies to the discontinuous CD4-binding site on gp120 are prevalent in HIV-1-positive sera, antibodies to linear epitopes are less common, most of the antibodies to linear epitopes are directed against the V3 region, and most cross-reactive antibodies are directed against discontinuous epitopes, including regions involved in CD4 binding.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J. S., Coligan J. E., Barin F., McLane M. F., Sodroski J. G., Rosen C. A., Haseltine W. A., Lee T. H., Essex M. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science. 1985 May 31;228(4703):1091–1094. doi: 10.1126/science.2986290. [DOI] [PubMed] [Google Scholar]
- Amadori A., Zamarchi R., Ciminale V., Del Mistro A., Siervo S., Alberti A., Colombatti M., Chieco-Bianchi L. HIV-1-specific B cell activation. A major constituent of spontaneous B cell activation during HIV-1 infection. J Immunol. 1989 Oct 1;143(7):2146–2152. [PubMed] [Google Scholar]
- Arthur L. O., Bess J. W., Jr, Waters D. J., Pyle S. W., Kelliher J. C., Nara P. L., Krohn K., Robey W. G., Langlois A. J., Gallo R. C. Challenge of chimpanzees (Pan troglodytes) immunized with human immunodeficiency virus envelope glycoprotein gp120. J Virol. 1989 Dec;63(12):5046–5053. doi: 10.1128/jvi.63.12.5046-5053.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman P. W., Gregory T. J., Riddle L., Nakamura G. R., Champe M. A., Porter J. P., Wurm F. M., Hershberg R. D., Cobb E. K., Eichberg J. W. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990 Jun 14;345(6276):622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- Berman P. W., Matthews T. J., Riddle L., Champe M., Hobbs M. R., Nakamura G. R., Mercer J., Eastman D. J., Lucas C., Langlois A. J. Neutralization of multiple laboratory and clinical isolates of human immunodeficiency virus type 1 (HIV-1) by antisera raised against gp120 from the MN isolate of HIV-1. J Virol. 1992 Jul;66(7):4464–4469. doi: 10.1128/jvi.66.7.4464-4469.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamat S., Nara P., Berquist L., Whalley A., Morrow W. J., Köhler H., Kang C. Y. Two major groups of neutralizing anti-gp120 antibodies exist in HIV-infected individuals. Evidence for epitope diversity around the CD4 attachment site. J Immunol. 1992 Jul 15;149(2):649–654. [PubMed] [Google Scholar]
- Chanh T. C., Dreesman G. R., Kanda P., Linette G. P., Sparrow J. T., Ho D. D., Kennedy R. C. Induction of anti-HIV neutralizing antibodies by synthetic peptides. EMBO J. 1986 Nov;5(11):3065–3071. doi: 10.1002/j.1460-2075.1986.tb04607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell J., Moore J. P., Dean C. J., Klasse P. J., Weiss R. A., McKeating J. A. Rat monoclonal antibodies to nonoverlapping epitopes of human immunodeficiency virus type 1 gp120 block CD4 binding in vitro. Virology. 1991 Nov;185(1):72–79. doi: 10.1016/0042-6822(91)90755-z. [DOI] [PubMed] [Google Scholar]
- D'Souza M. P., Durda P., Hanson C. V., Milman G. Evaluation of monoclonal antibodies to HIV-1 by neutralization and serological assays: an international collaboration. Collaborating Investigators. AIDS. 1991 Sep;5(9):1061–1070. doi: 10.1097/00002030-199109000-00001. [DOI] [PubMed] [Google Scholar]
- Deen K. C., McDougal J. S., Inacker R., Folena-Wasserman G., Arthos J., Rosenberg J., Maddon P. J., Axel R., Sweet R. W. A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature. 1988 Jan 7;331(6151):82–84. doi: 10.1038/331082a0. [DOI] [PubMed] [Google Scholar]
- Devash Y., Matthews T. J., Drummond J. E., Javaherian K., Waters D. J., Arthur L. O., Blattner W. A., Rusche J. R. C-terminal fragments of gp120 and synthetic peptides from five HTLV-III strains: prevalence of antibodies to the HTLV-III-MN isolate in infected individuals. AIDS Res Hum Retroviruses. 1990 Mar;6(3):307–316. doi: 10.1089/aid.1990.6.307. [DOI] [PubMed] [Google Scholar]
- Dolin R., Graham B. S., Greenberg S. B., Tacket C. O., Belshe R. B., Midthun K., Clements M. L., Gorse G. J., Horgan B. W., Atmar R. L. The safety and immunogenicity of a human immunodeficiency virus type 1 (HIV-1) recombinant gp160 candidate vaccine in humans. NIAID AIDS Vaccine Clinical Trials Network. Ann Intern Med. 1991 Jan 15;114(2):119–127. doi: 10.7326/0003-4819-114-2-119. [DOI] [PubMed] [Google Scholar]
- Evans D. J., McKeating J., Meredith J. M., Burke K. L., Katrak K., John A., Ferguson M., Minor P. D., Weiss R. A., Almond J. W. An engineered poliovirus chimaera elicits broadly reactive HIV-1 neutralizing antibodies. Nature. 1989 Jun 1;339(6223):385-8, 340. doi: 10.1038/339385a0. [DOI] [PubMed] [Google Scholar]
- Fung M. S., Sun C. R., Gordon W. L., Liou R. S., Chang T. W., Sun W. N., Daar E. S., Ho D. D. Identification and characterization of a neutralization site within the second variable region of human immunodeficiency virus type 1 gp120. J Virol. 1992 Feb;66(2):848–856. doi: 10.1128/jvi.66.2.848-856.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny M. K., Xu J. Y., Gianakakos V., Karwowska S., Williams C., Sheppard H. W., Hanson C. V., Zolla-Pazner S. Production of site-selected neutralizing human monoclonal antibodies against the third variable domain of the human immunodeficiency virus type 1 envelope glycoprotein. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3238–3242. doi: 10.1073/pnas.88.8.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsmit J., Boucher C. A., Meloen R. H., Epstein L. G., Smit L., van der Hoek L., Bakker M. Human antibody response to a strain-specific HIV-1 gp120 epitope associated with cell fusion inhibition. AIDS. 1988 Jun;2(3):157–164. [PubMed] [Google Scholar]
- Haigwood N. L., Nara P. L., Brooks E., Van Nest G. A., Ott G., Higgins K. W., Dunlop N., Scandella C. J., Eichberg J. W., Steimer K. S. Native but not denatured recombinant human immunodeficiency virus type 1 gp120 generates broad-spectrum neutralizing antibodies in baboons. J Virol. 1992 Jan;66(1):172–182. doi: 10.1128/jvi.66.1.172-182.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helseth E., Olshevsky U., Furman C., Sodroski J. Human immunodeficiency virus type 1 gp120 envelope glycoprotein regions important for association with the gp41 transmembrane glycoprotein. J Virol. 1991 Apr;65(4):2119–2123. doi: 10.1128/jvi.65.4.2119-2123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins P. J., Paradis T., Potts B. J., White-Scharf M. E., Rusche J. R., Scott C. F. In vitro inhibition of a variety of human immunodeficiency virus isolates by a broadly reactive, V3-directed heteroconjugate antibody. J Infect Dis. 1992 Jul;166(1):198–202. doi: 10.1093/infdis/166.1.198. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Fung M. S., Cao Y. Z., Li X. L., Sun C., Chang T. W., Sun N. C. Another discontinuous epitope on glycoprotein gp120 that is important in human immunodeficiency virus type 1 neutralization is identified by a monoclonal antibody. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8949–8952. doi: 10.1073/pnas.88.20.8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D. D., McKeating J. A., Li X. L., Moudgil T., Daar E. S., Sun N. C., Robinson J. E. Conformational epitope on gp120 important in CD4 binding and human immunodeficiency virus type 1 neutralization identified by a human monoclonal antibody. J Virol. 1991 Jan;65(1):489–493. doi: 10.1128/jvi.65.1.489-493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D. D., Sarngadharan M. G., Hirsch M. S., Schooley R. T., Rota T. R., Kennedy R. C., Chanh T. C., Sato V. L. Human immunodeficiency virus neutralizing antibodies recognize several conserved domains on the envelope glycoproteins. J Virol. 1987 Jun;61(6):2024–2028. doi: 10.1128/jvi.61.6.2024-2028.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., LaRosa G. J., Profy A. T., Bolognesi D. P., Herlihy W. C., Putney S. D., Matthews T. J. Broadly neutralizing antibodies elicited by the hypervariable neutralizing determinant of HIV-1. Science. 1990 Dec 14;250(4987):1590–1593. doi: 10.1126/science.1703322. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., McDanal C., Ross K. L., Eckler L. I., Jellis C. L., Profy A. T., Rusche J. R., Bolognesi D. P., Putney S. D. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6768–6772. doi: 10.1073/pnas.86.17.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Nara P., Chamat S., Caralli V., Ryskamp T., Haigwood N., Newman R., Köhler H. Evidence for non-V3-specific neutralizing antibodies that interfere with gp120/CD4 binding in human immunodeficiency virus 1-infected humans. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6171–6175. doi: 10.1073/pnas.88.14.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwowska S., Gorny M. K., Buchbinder A., Gianakakos V., Williams C., Fuerst T., Zolla-Pazner S. Production of human monoclonal antibodies specific for conformational and linear non-V3 epitopes of gp120. AIDS Res Hum Retroviruses. 1992 Jun;8(6):1099–1106. doi: 10.1089/aid.1992.8.1099. [DOI] [PubMed] [Google Scholar]
- Kennedy R. C., Dreesman G. R., Chanh T. C., Boswell R. N., Allan J. S., Lee T. H., Essex M., Sparrow J. T., Ho D. D., Kanda P. Use of a resin-bound synthetic peptide for identifying a neutralizing antigenic determinant associated with the human immunodeficiency virus envelope. J Biol Chem. 1987 Apr 25;262(12):5769–5774. [PubMed] [Google Scholar]
- Klinman D. M., Steimer K. S., Conover J. Comparative immunogenicity of gp120-derived proteins and their induction of anti-V3 loop region antibodies. J Acquir Immune Defic Syndr. 1992 Oct;5(10):1005–1008. [PubMed] [Google Scholar]
- Krowka J. F., Singh B., Stites D. P., Maino V. C., Narindray D., Hollander H., Jain S., Chen H., Blackwood L., Steimer K. S. Epitopes of human immunodeficiency virus type 1 (HIV-1) envelope glycoproteins recognized by antibodies in the sera of HIV-1-infected individuals. Clin Immunol Immunopathol. 1991 Apr;59(1):53–64. doi: 10.1016/0090-1229(91)90081-k. [DOI] [PubMed] [Google Scholar]
- LaRosa G. J., Davide J. P., Weinhold K., Waterbury J. A., Profy A. T., Lewis J. A., Langlois A. J., Dreesman G. R., Boswell R. N., Shadduck P. Conserved sequence and structural elements in the HIV-1 principal neutralizing determinant. Science. 1990 Aug 24;249(4971):932–935. doi: 10.1126/science.2392685. [DOI] [PubMed] [Google Scholar]
- Laman J. D., Schellekens M. M., Abacioglu Y. H., Lewis G. K., Tersmette M., Fouchier R. A., Langedijk J. P., Claassen E., Boersma W. J. Variant-specific monoclonal and group-specific polyclonal human immunodeficiency virus type 1 neutralizing antibodies raised with synthetic peptides from the gp120 third variable domain. J Virol. 1992 Mar;66(3):1823–1831. doi: 10.1128/jvi.66.3.1823-1831.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Webster R. G., Smith-Gill S. J. Epitopes on protein antigens: misconceptions and realities. Cell. 1990 May 18;61(4):553–556. doi: 10.1016/0092-8674(90)90464-p. [DOI] [PubMed] [Google Scholar]
- Leonard C. K., Spellman M. W., Riddle L., Harris R. J., Thomas J. N., Gregory T. J. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem. 1990 Jun 25;265(18):10373–10382. [PubMed] [Google Scholar]
- Matthews T. J., Langlois A. J., Robey W. G., Chang N. T., Gallo R. C., Fischinger P. J., Bolognesi D. P. Restricted neutralization of divergent human T-lymphotropic virus type III isolates by antibodies to the major envelope glycoprotein. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9709–9713. doi: 10.1073/pnas.83.24.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrow S., Hahn B. H., Shaw G. M., Gallo R. C., Wong-Staal F., Wolf H. Computer-assisted analysis of envelope protein sequences of seven human immunodeficiency virus isolates: prediction of antigenic epitopes in conserved and variable regions. J Virol. 1987 Feb;61(2):570–578. doi: 10.1128/jvi.61.2.570-578.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., Jarrett R. F. Sensitive ELISA for the gp120 and gp160 surface glycoproteins of HIV-1. AIDS Res Hum Retroviruses. 1988 Oct;4(5):369–379. doi: 10.1089/aid.1988.4.369. [DOI] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Jones I. M., Stephens P. E., Clements G., Thomson S., Weiss R. A. Characterization of recombinant gp120 and gp160 from HIV-1: binding to monoclonal antibodies and soluble CD4. AIDS. 1990 Apr;4(4):307–315. doi: 10.1097/00002030-199004000-00004. [DOI] [PubMed] [Google Scholar]
- Moore J. P., Nara P. L. The role of the V3 loop of gp120 in HIV infection. AIDS. 1991;5 (Suppl 2):S21–S33. doi: 10.1097/00002030-199101001-00004. [DOI] [PubMed] [Google Scholar]
- Moore J. P. Simple methods for monitoring HIV-1 and HIV-2 gp120 binding to soluble CD4 by enzyme-linked immunosorbent assay: HIV-2 has a 25-fold lower affinity than HIV-1 for soluble CD4. AIDS. 1990 Apr;4(4):297–305. doi: 10.1097/00002030-199004000-00003. [DOI] [PubMed] [Google Scholar]
- Moore J. P., Wallace L. A., Follett E. A., McKeating J. A. An enzyme-linked immunosorbent assay for antibodies to the envelope glycoproteins of divergent strains of HIV-1. AIDS. 1989 Mar;3(3):155–163. doi: 10.1097/00002030-198903000-00006. [DOI] [PubMed] [Google Scholar]
- Nara P. L., Garrity R. R., Goudsmit J. Neutralization of HIV-1: a paradox of humoral proportions. FASEB J. 1991 Jul;5(10):2437–2455. doi: 10.1096/fasebj.5.10.1712328. [DOI] [PubMed] [Google Scholar]
- Nara P. L., Robey W. G., Pyle S. W., Hatch W. C., Dunlop N. M., Bess J. W., Jr, Kelliher J. C., Arthur L. O., Fischinger P. J. Purified envelope glycoproteins from human immunodeficiency virus type 1 variants induce individual, type-specific neutralizing antibodies. J Virol. 1988 Aug;62(8):2622–2628. doi: 10.1128/jvi.62.8.2622-2628.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath A. R., Strick N., Lee E. S. B cell epitope mapping of human immunodeficiency virus envelope glycoproteins with long (19- to 36-residue) synthetic peptides. J Gen Virol. 1990 Jan;71(Pt 1):85–95. doi: 10.1099/0022-1317-71-1-85. [DOI] [PubMed] [Google Scholar]
- Niedrig M., Bröker M., Walter G., Stüber W., Harthus H. P., Mehdi S., Gelderblom H. R., Pauli G. Murine monoclonal antibodies directed against the transmembrane protein gp41 of human immunodeficiency virus type 1 enhance its infectivity. J Gen Virol. 1992 Apr;73(Pt 4):951–954. doi: 10.1099/0022-1317-73-4-951. [DOI] [PubMed] [Google Scholar]
- Norrby E., Putkonen P., Böttiger B., Utter G., Biberfeld G. Comparison of linear antigenic sites in the envelope proteins of human immunodeficiency virus (HIV) type 2 and type 1. AIDS Res Hum Retroviruses. 1991 Mar;7(3):279–285. doi: 10.1089/aid.1991.7.279. [DOI] [PubMed] [Google Scholar]
- Olshevsky U., Helseth E., Furman C., Li J., Haseltine W., Sodroski J. Identification of individual human immunodeficiency virus type 1 gp120 amino acids important for CD4 receptor binding. J Virol. 1990 Dec;64(12):5701–5707. doi: 10.1128/jvi.64.12.5701-5707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M., Vella C., Corcoran T., Dilger P., Ling C., Heath A., Thorpe R. Restriction of serum antibody reactivity to the V3 neutralizing domain of HIV gp120 with progression to AIDS. AIDS. 1992 May;6(5):441–446. doi: 10.1097/00002030-199205000-00001. [DOI] [PubMed] [Google Scholar]
- Pahwa S., Chirmule N., Leombruno C., Lim W., Harper R., Bhalla R., Pahwa R., Nelson R. P., Good R. A. In vitro synthesis of human immunodeficiency virus-specific antibodies in peripheral blood lymphocytes of infants. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7532–7536. doi: 10.1073/pnas.86.19.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palker T. J., Clark M. E., Langlois A. J., Matthews T. J., Weinhold K. J., Randall R. R., Bolognesi D. P., Haynes B. F. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palker T. J., Matthews T. J., Clark M. E., Cianciolo G. J., Randall R. R., Langlois A. J., White G. C., Safai B., Snyderman R., Bolognesi D. P. A conserved region at the COOH terminus of human immunodeficiency virus gp120 envelope protein contains an immunodominant epitope. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2479–2483. doi: 10.1073/pnas.84.8.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M. R., Hideshima T., Cannon T., Mukherjee M., Mayer K. H., Byrn R. A. An IgG human monoclonal antibody that reacts with HIV-1/GP120, inhibits virus binding to cells, and neutralizes infection. J Immunol. 1991 Jun 15;146(12):4325–4332. [PubMed] [Google Scholar]
- Profy A. T., Salinas P. A., Eckler L. I., Dunlop N. M., Nara P. L., Putney S. D. Epitopes recognized by the neutralizing antibodies of an HIV-1-infected individual. J Immunol. 1990 Jun 15;144(12):4641–4647. [PubMed] [Google Scholar]
- Putney S. D., Matthews T. J., Robey W. G., Lynn D. L., Robert-Guroff M., Mueller W. T., Langlois A. J., Ghrayeb J., Petteway S. R., Jr, Weinhold K. J. HTLV-III/LAV-neutralizing antibodies to an E. coli-produced fragment of the virus envelope. Science. 1986 Dec 12;234(4782):1392–1395. doi: 10.1126/science.2431482. [DOI] [PubMed] [Google Scholar]
- Redfield R. R., Birx D. L. HIV-specific vaccine therapy: concepts, status, and future directions. AIDS Res Hum Retroviruses. 1992 Jun;8(6):1051–1058. doi: 10.1089/aid.1992.8.1051. [DOI] [PubMed] [Google Scholar]
- Redfield R. R., Birx D. L., Ketter N., Tramont E., Polonis V., Davis C., Brundage J. F., Smith G., Johnson S., Fowler A. A phase I evaluation of the safety and immunogenicity of vaccination with recombinant gp160 in patients with early human immunodeficiency virus infection. Military Medical Consortium for Applied Retroviral Research. N Engl J Med. 1991 Jun 13;324(24):1677–1684. doi: 10.1056/NEJM199106133242401. [DOI] [PubMed] [Google Scholar]
- Robert-Guroff M., Brown M., Gallo R. C. HTLV-III-neutralizing antibodies in patients with AIDS and AIDS-related complex. Nature. 1985 Jul 4;316(6023):72–74. doi: 10.1038/316072a0. [DOI] [PubMed] [Google Scholar]
- Robey W. G., Arthur L. O., Matthews T. J., Langlois A., Copeland T. D., Lerche N. W., Oroszlan S., Bolognesi D. P., Gilden R. V., Fischinger P. J. Prospect for prevention of human immunodeficiency virus infection: purified 120-kDa envelope glycoprotein induces neutralizing antibody. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7023–7027. doi: 10.1073/pnas.83.18.7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey W. G., Arthur L. O., Matthews T. J., Langlois A., Copeland T. D., Lerche N. W., Oroszlan S., Bolognesi D. P., Gilden R. V., Fischinger P. J. Prospect for prevention of human immunodeficiency virus infection: purified 120-kDa envelope glycoprotein induces neutralizing antibody. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7023–7027. doi: 10.1073/pnas.83.18.7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey W. G., Safai B., Oroszlan S., Arthur L. O., Gonda M. A., Gallo R. C., Fischinger P. J. Characterization of envelope and core structural gene products of HTLV-III with sera from AIDS patients. Science. 1985 May 3;228(4699):593–595. doi: 10.1126/science.2984774. [DOI] [PubMed] [Google Scholar]
- Rusche J. R., Lynn D. L., Robert-Guroff M., Langlois A. J., Lyerly H. K., Carson H., Krohn K., Ranki A., Gallo R. C., Bolognesi D. P. Humoral immune response to the entire human immunodeficiency virus envelope glycoprotein made in insect cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6924–6928. doi: 10.1073/pnas.84.19.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattentau Q. J., Moore J. P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991 Aug 1;174(2):407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattentau Q. J., Weiss R. A. The CD4 antigen: physiological ligand and HIV receptor. Cell. 1988 Mar 11;52(5):631–633. doi: 10.1016/0092-8674(88)90397-2. [DOI] [PubMed] [Google Scholar]
- Schulz T. F., Jameson B. A., Lopalco L., Siccardi A. G., Weiss R. A., Moore J. P. Conserved structural features in the interaction between retroviral surface and transmembrane glycoproteins? AIDS Res Hum Retroviruses. 1992 Sep;8(9):1571–1580. doi: 10.1089/aid.1992.8.1571. [DOI] [PubMed] [Google Scholar]
- Scott C. F., Jr, Silver S., Profy A. T., Putney S. D., Langlois A., Weinhold K., Robinson J. E. Human monoclonal antibody that recognizes the V3 region of human immunodeficiency virus gp120 and neutralizes the human T-lymphotropic virus type IIIMN strain. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8597–8601. doi: 10.1073/pnas.87.21.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafferman A., Lennox J., Grosfeld H., Sadoff J., Redfield R. R., Burke D. S. Patterns of antibody recognition of selected conserved amino acid sequences from the HIV envelope in sera from different stages of HIV infection. AIDS Res Hum Retroviruses. 1989 Feb;5(1):33–39. doi: 10.1089/aid.1989.5.33. [DOI] [PubMed] [Google Scholar]
- Skinner M. A., Langlois A. J., McDanal C. B., McDougal J. S., Bolognesi D. P., Matthews T. J. Neutralizing antibodies to an immunodominant envelope sequence do not prevent gp120 binding to CD4. J Virol. 1988 Nov;62(11):4195–4200. doi: 10.1128/jvi.62.11.4195-4200.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcich B. R., Hahn B. H., Shaw G. M., McNeely P. D., Modrow S., Wolf H., Parks E. S., Parks W. P., Josephs S. F., Gallo R. C. Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell. 1986 Jun 6;45(5):637–648. doi: 10.1016/0092-8674(86)90778-6. [DOI] [PubMed] [Google Scholar]
- Steimer K. S., Klasse P. J., McKeating J. A. HIV-1 neutralization directed to epitopes other than linear V3 determinants. AIDS. 1991;5 (Suppl 2):S135–S143. doi: 10.1097/00002030-199101001-00019. [DOI] [PubMed] [Google Scholar]
- Steimer K. S., Scandella C. J., Skiles P. V., Haigwood N. L. Neutralization of divergent HIV-1 isolates by conformation-dependent human antibodies to Gp120. Science. 1991 Oct 4;254(5028):105–108. doi: 10.1126/science.1718036. [DOI] [PubMed] [Google Scholar]
- Sun N. C., Ho D. D., Sun C. R., Liou R. S., Gordon W., Fung M. S., Li X. L., Ting R. C., Lee T. H., Chang N. T. Generation and characterization of monoclonal antibodies to the putative CD4-binding domain of human immunodeficiency virus type 1 gp120. J Virol. 1989 Sep;63(9):3579–3585. doi: 10.1128/jvi.63.9.3579-3585.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thali M., Furman C., Ho D. D., Robinson J., Tilley S., Pinter A., Sodroski J. Discontinuous, conserved neutralization epitopes overlapping the CD4-binding region of human immunodeficiency virus type 1 gp120 envelope glycoprotein. J Virol. 1992 Sep;66(9):5635–5641. doi: 10.1128/jvi.66.9.5635-5641.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thali M., Olshevsky U., Furman C., Gabuzda D., Posner M., Sodroski J. Characterization of a discontinuous human immunodeficiency virus type 1 gp120 epitope recognized by a broadly reactive neutralizing human monoclonal antibody. J Virol. 1991 Nov;65(11):6188–6193. doi: 10.1128/jvi.65.11.6188-6193.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiriart C., Francotte M., Cohen J., Collignon C., Delers A., Kummert S., Molitor C., Gilles D., Roelants P., van Wijnendaele F. Several antigenic determinants exposed on the gp120 moiety of HIV-1 gp160 are hidden on the mature gp120. J Immunol. 1989 Sep 15;143(6):1832–1836. [PubMed] [Google Scholar]
- Tilley S. A., Honnen W. J., Racho M. E., Hilgartner M., Pinter A. A human monoclonal antibody against the CD4-binding site of HIV1 gp120 exhibits potent, broadly neutralizing activity. Res Virol. 1991 Jul-Aug;142(4):247–259. doi: 10.1016/0923-2516(91)90010-z. [DOI] [PubMed] [Google Scholar]
- VanCott T. C., Loomis L. D., Redfield R. R., Birx D. L. Real-time biospecific interaction analysis of antibody reactivity to peptides from the envelope glycoprotein, gp160, of HIV-1. J Immunol Methods. 1992 Feb 5;146(2):163–176. doi: 10.1016/0022-1759(92)90225-i. [DOI] [PubMed] [Google Scholar]
- Warren R. Q., Wolf H., Shuler K. R., Eichberg J. W., Zajac R. A., Boswell R. N., Kanda P., Kennedy R. C. Synthetic peptides define the fine specificity of the human immunodeficiency virus (HIV) gp160 humoral immune response in HIV type 1-infected chimpanzees. J Virol. 1990 Feb;64(2):486–492. doi: 10.1128/jvi.64.2.486-492.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A., Clapham P. R., Cheingsong-Popov R., Dalgleish A. G., Carne C. A., Weller I. V., Tedder R. S. Neutralization of human T-lymphotropic virus type III by sera of AIDS and AIDS-risk patients. Nature. 1985 Jul 4;316(6023):69–72. doi: 10.1038/316069a0. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Clapham P. R., Weber J. N., Dalgleish A. G., Lasky L. A., Berman P. W. Variable and conserved neutralization antigens of human immunodeficiency virus. Nature. 1986 Dec 11;324(6097):572–575. doi: 10.1038/324572a0. [DOI] [PubMed] [Google Scholar]
- Weiss S. H., Goedert J. J., Gartner S., Popovic M., Waters D., Markham P., di Marzo Veronese F., Gail M. H., Barkley W. E., Gibbons J. Risk of human immunodeficiency virus (HIV-1) infection among laboratory workers. Science. 1988 Jan 1;239(4835):68–71. doi: 10.1126/science.3336776. [DOI] [PubMed] [Google Scholar]
- Zwart G., de Jong J. J., Wolfs T., van der Hoek L., Smit L., de Ronde A., Tersmette M., Nara P., Goudsmit J. Predominance of HIV-1 serotype distinct from LAV-1/HTLV-IIIB. Lancet. 1990 Feb 24;335(8687):474–474. doi: 10.1016/0140-6736(90)90707-c. [DOI] [PubMed] [Google Scholar]
- di Marzo Veronese F., Rahman R., Pal R., Boyer C., Romano J., Kalyanaraman V. S., Nair B. C., Gallo R. C., Sarngadharan M. G. Delineation of immunoreactive, conserved regions in the external glycoprotein of the human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1992 Jun;8(6):1125–1132. doi: 10.1089/aid.1992.8.1125. [DOI] [PubMed] [Google Scholar]


