Abstract
The effects of solution conditions on protein collapse were studied by measuring the hydrodynamic radii of two unfolded proteins, α-synuclein and acid-denatured ferricytochrome c, in dilute solution and in 1 M glucose. The radius of α-synuclein in dilute solution is less than that predicted for a highly denatured state, and adding 1 M glucose causes further collapse. Circular dichroic data show that α-synuclein lacks organized structure in both dilute solution and 1 M glucose. On the other hand, the radius of acid-denatured cytochrome c in dilute solution is consistent with that of a highly denatured state, and 1 M glucose induces collapse to the size and structure of native cytochrome c. Taken together, these data show that α-synuclein, a natively unfolded protein, is collapsed even in dilute solution, but lacks structure.
Keywords: α-Synuclein, crowding, cytochrome c, protein folding, protein structure
The cell's interior is crowded with small and large molecules (Fulton 1982; Yancey et al. 1982), yet most studies of biological reactions are conducted in dilute solutions. A number of biologically relevant proteins appear to be fully or partially unfolded in dilute solution (Weinreb et al. 1996; Uversky et al. 2000), but in several instances it has been shown that crowding can induce collapse to more compact states (Shearwin and Winzor 1990; Baskakov and Bolen 1998). Crowding-induced collapse is important because it may lead to aggregation (van den Berg et al. 1999), which is associated with several neurodegenerative disorders, including Alzheimer's, Parkinson's, prion diseases, and dementia with Lewy bodies (Wanker 2000). Most studies that probe the effect of crowding focus on the stability of native globular proteins. Here we focus on the collapse of unfolded states. The motivation for this work is twofold: to understand the effect of molecular crowding by sugars, and to apply this knowledge to two unfolded proteins, one of medical importance.
Crowding-induced collapse and structure formation have only been studied in a few proteins, including RNase A (Qu et al. 1998), RNase T1, a staphylococcal nuclease variant (Baskakov and Bolen 1998), and glucocorticoid receptor fragments (Baskakov et al. 1999). For this study we chose two unfolded proteins: acid-denatured cytochrome c and α-synuclein. Cytochrome c is one of the most studied proteins (Moore and Pettigrew 1990). Its primary function lies in the mitochondrial eukaryotic respiratory chain, but its role in apoptosis (Kluck et al. 1997) and Parkinson's disease (Hashimoto et al. 1999) is under intense investigation. Native cytochrome c has a hydrated radius of 18 Å, but at pH 2 it unfolds, causing the radius to increase to 30 Å (Wilkins et al. 1999). α-Synuclein is associated with Parkinson's disease, the second most common neurodegenerative disease and the most common age-related movement disorder. The cause of Parkinson's disease is unknown, but a possible clue is the presence in some nigral neurons of cytoplasmic inclusions, called Lewy bodies (Galvin et al. 1999). The major component of Lewy bodies is the presynaptic protein α-synuclein (Spillantini et al. 1997). α-Synuclein is a 140-amino acid protein of unknown function that is highly expressed in the human brain (Iwai et al. 1995). Human α-synuclein is unstructured in aqueous solution (Weinreb et al. 1996). Understanding its physiological structure and function is an important step in elucidating the cause of Parkinson's disease and other neurodegenerative disorders.
Here we investigate the solvent-induced collapse of α-synuclein and acid-denatured ferricytochrome c in crowded and dilute solutions by using sugars as crowding agents, pulsed-field gradient NMR to probe protein radius, and circular dichroism to probe secondary structure.
Results and Discussion
The mean effective hydrodynamic radius, RH, is the radius of a sphere with the same diffusion coefficient as the species being studied. For many globular proteins, RH can be reliably calculated by using Equations 1 and 2, which are derived from pulsed-field gradient NMR studies:
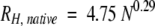 |
(1) |
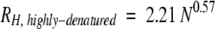 |
(2) |
where N represents the number of residues and the term "highly denatured" refers to the protein under strongly denaturing conditions such as high urea or guanidinium chloride concentrations (Wilkins et al. 1999). For example, the calculated values for native and denatured lysozyme (19.4 and 38.9 Å), which has about the same molecular weight as α-synuclein, agree with those from experiment (19.5 and 35.1 Å) (Uversky 1993). However, the denatured state radius is overestimated, suggesting that even highly denatured proteins are not random coils. Studies on reduced bovine pancreatic trypsin inhibitor (Pan et al. 1997) and staphylococcal nuclease (Shortle and Ackerman 2001) confirm this conclusion. Equations similar to Equations 1 and 2 have been derived from gel-exclusion chromatography data (Uversky 1993).
Diffusion is sensitive to the time-averaged dimensions of a particle (Haner and Schleich 1989). The translational diffusion coefficient, D, is inversely related to RH. Thus, D provides a measure of molecular size. D can be measured by using pulsed-field gradient NMR. Such studies have been used to provide important information about protein association (Altieri et al. 1995), aggregation (Pan et al. 1997), and unfolding (Jones et al. 1997).
We used pulsed-field gradient NMR to compare RH values for α-synuclein and for native and acid-denatured cytochrome c in the presence and absence of 1 M perdeuterated glucose (Fig. 1 ▶). Dioxane was used as an internal radius standard (2.12 Å) and viscosity probe (Wilkins et al. 1999). This approach requires neither the direct determination of the diffusion coefficients nor knowledge of the solution viscosity. The decay rates, d, which are proportional to diffusion coefficients, were calculated from the dependence of resonance intensities, S(g), on gradient amplitude, g (Fig. 2 ▶), according to the equation:
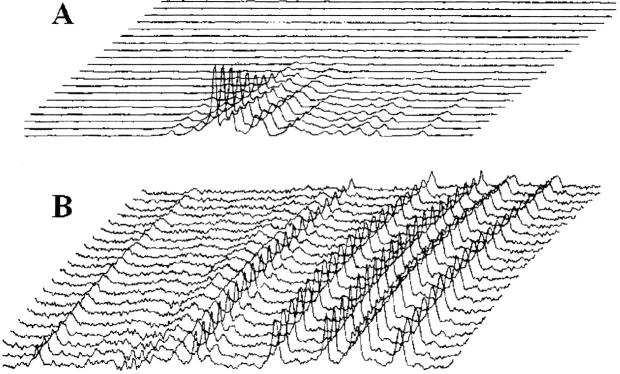
Fig. 1.
Stack plots of signal decay of the spectral regions corresponding to (A) the dioxane (3.1–4.2 ppm) and (B) the aromatic protons (6.3–8.6 ppm) of acid-denatured cytochrome c in 1 M perdeuterated glucose. A series of 1H NMR spectra were recorded as a function of gradient strength. The diffusion gradients were varied between 6 and 94% of their maximum value. The decrease in protein signal intensity is 72%. The decrease in dioxane intensity is 99%.
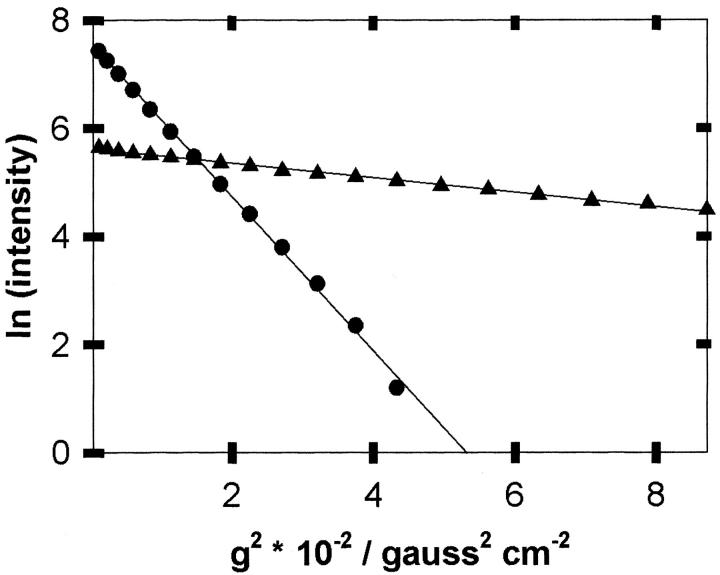
Fig. 2.
Plots of the natural logarithm of signal intensities (filled triangle, aromatic region [6.5–8.5 ppm]; filled circle, dioxane peak) versus the gradient strength squared for 0.1 mM α-synuclein in 1 M perdeuterated glucose, 1.2 mM dioxane in D2O (pH 7, 298 K).
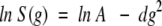 |
(3) |
The decay data were also fit directly to a single exponential decay. Both methods gave values that are the same to within the experimental uncertainties. Values of d were then used to calculate the protein radii according to the equation:
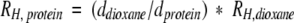 |
(4) |
Table 1 summarizes the calculated and measured values for cytochrome c. In Tables 1 and 2, a range is given for the calculated values. The range comes from using both the equations based on pulsed-field gradient NMR data (Wilkins et al. 1999) and gel-exclusion data (Uversky 1993). The two calculated values are in good agreement. Inspection of the Table 1 shows that acid-denatured cytochrome c approximates a highly denatured state, and that the RH for native protein is not affected by glucose. However, RH for acid-denatured cytochrome c decreases to the native state value upon adding 1 M glucose. This observation agrees with our circular dichroism and 1H/2H exchange studies, which show that sugars induce native structure (Davis-Searles et al. 1998; Saunders et al. 2000). These data show that pulsed-field gradient NMR is useful for studying crowding-induced collapse.
Table 1.
Calculated and observed hydrodynamic radii of native and acid-denatured cytochrome c in the presence and absence of 1 M perdeuterated glucose at 298 K
| Observeda | ||||||
| Calculated for a 104 residue globular protein | pH 7.0 | pH 2.0 | ||||
| Native | Highly denatured | Dilute | 1 M glucose | Dilute | 1 M glucose | |
| RH (Å) | 17.7–18.3 | 29.8–31.6 | 18.8 ± 0.78 | 17.7 ± 1.21 | 30.6 ± 1.31 | 17.7 ± 1.36 |
a The uncertainties are from linear least-squares fitting of Equation 3 and propagation of the uncertainties through Equation 4.
Table 2.
Calculated and observed hydrodynamic radii of α-synuclein in the presence and absence of 1 M perdeuterated glucose at pH 7 and 298 K
| Calculated for a 140 residue globular protein | Observeda | |||
| Native | Highly denatured | Dilute | 1 M glucose | |
| RH (Å) | 17.8–19.9 | 33.3–36.9 | 26.6 ± 0.5 | 22.5 ± 0.6 |
a The uncertainties are from linear least-squares fitting of Equation 3 and propagation of the uncertainties through Equation 4.
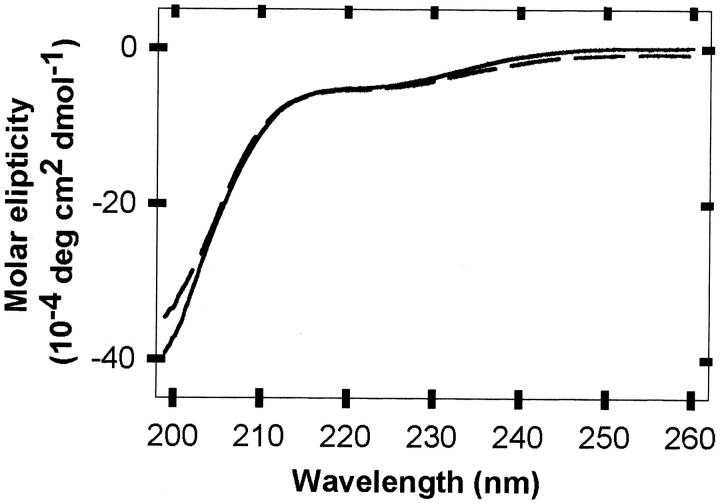
Similarly, Table 2 summarizes the calculated and measured RH values for α-synuclein. The measured value in dilute solution is between the value calculated for a native globular protein and the value calculated for a highly denatured state, so even in dilute solution α-synuclein is not a random coil. This observation is consistent with other data showing that α-synuclein is only ∼74% random coil (Davidson et al. 1998). Glucose (1 M) decreases RH by another 4 Å, consistent with the idea that crowding stabilizes more compact states (Davis-Searles et al. 2001). However, circular dichroism data in the presence of 1 M glucose (Fig. 3 ▶) provide no evidence for crowding-induced secondary structure. In both dilute and crowded solutions α-synuclein has a far-UV circular dichroic spectrum of a typical unfolded polypeptide chain. Although a partly folded intermediate of α-synuclein with some β-structure was reported recently at low pH or high temperature (Uversky et al. 2001), we found no evidence of β-structure. Preliminary studies also show that the dye 1-anilino-naphtalene-8-sulfonate (Semisotnov et al. 1991) does not interact strongly with α-synuclein in 1 M glucose under the conditions used here (A. Olteanu and G.J. Pielak, unpubl.). This result suggests that the species we observe in sugar is not the intermediate observed in dilute solution at low pH (Uversky et al. 2001). The absence of a crowding-induced structure has also been reported for the C-terminal activation domain of c-Fos and the kinase-inhibition domain of p27Kip1 (Flaugh and Lumb 2001).
Fig. 3.
Far-UV circular dichroic spectra of recombinant human α-synuclein (20 μM) in dilute solution (dashed line) and 1 M glucose (solid line), measured in H2O (pH 7, 298 K).
In summary, our data show that crowding does not affect the size of native cytochrome c but does cause the collapse of acid-denatured cytochrome c, which is random coil in dilute solution, to a species with the same size and structure as the native protein. Our data also show that α-synuclein is smaller than a random coil in dilute solution, and crowding causes further collapse.
These results have important implications for understanding protein folding and stability in vivo, especially for understanding biologically active proteins that have little or no structure in dilute solution (Uversky et al. 2000). Studies of these natively unfolded proteins will enhance our knowledge of folding, transport across membranes and human diseases associated with protein aggregation. Contrary to the idea that natively unfolded proteins are random coils, our data show that these proteins are collapsed, but not necessarily structured, under conditions that may mimic the crowded cellular environment. Given the susceptibility of unfolded proteins to proteolysis, our observations concerning collapse could explain how natively unfolded proteins avoid being consumed by intracellular proteases.
Materials and methods
Expression and purification of α-synuclein
Luria Broth cultures of Escherichia coli containing the plasmid pT7-7, which expresses α-synuclein, were induced with IPTG, incubated at 37°C for 4 h, and pelleted by centrifugation at 6,000 rpm (Sorvall RC-3B, H-6000 rotor). The cell pellet was resuspended in 10 mM Tris (hydroxymethylaminomethane), pH 8, containing 1 mM phenylmethylsulfonyl fluoride (Sigma), and lysed by sonication. The clarified lysate was collected after centrifugation at 10,000 rpm for 30 min. (NH4)2SO4 (0.166 g/mL) was added to the supernatant, and the mixture was centrifuged again at 13,500 rpm for 30 min. The protein pellet was resuspended in 10 mM Tris, pH 8, and boiled for 15 min. The solution was centrifuged at 10,000 rpm for 10 min. α-Synuclein was ideied in the supernatant by SDS polyacrylamide gel electrophoresis, and its concentration was determined by measuring the absorbance at 276 nm by using a molar absorptivity of 8.0 mM−1cm−1 (R. Nowak, pers. commun.). The final yield was 17 mg of pure protein per liter of culture.
Pulsed-field gradient NMR
α-Synuclein samples were dialyzed against D2O for 48 h. Horse heart cytochrome c samples were prepared by dissolving lyophilized protein (Sigma) in D2O solutions containing 1.2 mM 1,4-dioxane. Cytochrome c concentration was determined at 410 nm by using a molar absorptivity of 106 mM−1cm−1 (Margoliash et al. 1959). Perdeuterated glucose was purchased from ISOTEC, Inc. The pH was adjusted to ∼7 or ∼2 with NaOD and DCl. Samples were sterile filtered (0.2 μm acrodisc), and 300 μL were transferred to a Shigemi tube. The NMR spectra were acquired at 298 K on a Varian 500-MHz spectrometer with a 5 mm triple-resonance pulsed-field gradient probe. Sixteen K complex points were acquired with a spectral width of 16 kHz. Each spectrum comprised 128 scans plus 16 steady-state scans. The HOD resonance was suppressed by presaturation. A series of 20 spectra were collected as a function of gradient amplitude. The maximum gradient strength was 31.72 Gauss cm−1, and the total acquisition time was ∼2 h. Diffusion gradients were applied along the x-axis. Gradient linearity was confirmed by measuring the width of the profile of a 1% H2O signal at various gradient strengths. For all experiments, the diffusion delay was 100 ms. The results are independent of protein concentration between 0.1 and 1 mM. Spectra were processed with VNMR 6.1C.
Circular dichroism spectropolarimetry
Far-UV spectra of 20 μM α-synuclein in water and in 1 M glucose were acquired on an Aviv spectropolarimeter at 298 K, with 1 nm resolution, an averaging time of 3 sec per point, in 0.1-cm quartz cuvettes.
Acknowledgments
We thank Richard Nowak for the T7-7 plasmid and the purification protocol of α-synuclein. This work was supported by the NIH (R21 ES10774) and by the Petroleum Research Fund (35338-AC4). We thank Nancy Thompson and Jennifer Mitchel for help with fluorescence studies.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
The first two authors contributed equally to this work.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1101/ps.24301.
References
- Altieri, A.A., Hinton, D.P., Byrd, R.A. 1995. Association of biomolecular systems via pulsed field gradient NMR self-diffusion measurements. J. Am. Chem. Soc. 117 7566–7567. [Google Scholar]
- Baskakov, I., Bolen, D.W. 1998. Forcing thermodynamically unfolded proteins to fold. J. Biol. Chem. 2734831–4834. [DOI] [PubMed] [Google Scholar]
- Baskakov, I.V., Kumar, R., Srinivasan, G., Ji, Y.S., Bolen, D.W., Thompson, E.B. 1999. Trimethylamine N-oxide-induced cooperative folding of an intrinsically unfolded transcription-activating fragment of human glucocorticoid receptor. J. Biol. Chem. 274 10693–10696. [DOI] [PubMed] [Google Scholar]
- Davidson, W.S., Jonas, A., Clayton, D.F., George, J.M. 1998. Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 2739443–9449. [DOI] [PubMed] [Google Scholar]
- Davis-Searles, P.R., Morar, A.S., Saunders, A.J., Erie, D.A., Pielak, G.J. 1998. A sugar-induced molten globule model. Biochemistry 3717048–17053. [DOI] [PubMed] [Google Scholar]
- Davis-Searles, P.R., Saunders, A.J., Erie, D.A., Winzor, D.J., Pielak, G.J. 2001. Interpreting the effects of small uncharged solutes on protein-folding equilibria. Annu. Rev. Biophys. Biomol. Struct. 30 271–306. [DOI] [PubMed] [Google Scholar]
- Flaugh, S.L., Lumb, K.J. 2001. Effects of macromolecular crowding on the intrinsically disordered proteins c-Fos and p27Kip1. Biomacromolecules 2 538–540. [DOI] [PubMed] [Google Scholar]
- Fulton, A.B. 1982. How crowded is the cytoplasm? Cell 30 345–347. [DOI] [PubMed] [Google Scholar]
- Galvin, J.E., Lee, V.M., Schmidt, M.L., Tu, P.H., Iwatsubo, T., Trojanowski, J.Q. 1999. Pathobiology of the Lewy body. Adv. Neurol. 80 313–324. [PubMed] [Google Scholar]
- Haner, R.L., Schleich, T. 1989. Measurement of translational motion by pulse-gradient spin-echo nuclear magnetic resonance. Methods Enzymol. 176 418–446. [DOI] [PubMed] [Google Scholar]
- Hashimoto, M., Takeda, A., Hsu, L.J., Takenouchi, T., Masliah, E. 1999. Role of cytochrome c as a stimulator of α-synuclein aggregation in Lewy body disease. J. Biol. Chem. 27428849–28852. [DOI] [PubMed] [Google Scholar]
- Iwai, A., Masliah, E., Yoshimoto, M., Ge, N., Flanagan, L., de Silva, H.A., Kittel, A., Saitoh, T. 1995. The precursor protein of non-Aβ component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron 14 467–475. [DOI] [PubMed] [Google Scholar]
- Jones, J.A., Wilkins, D.K., Smith, L.J., Dobson, C.M. 1997. Characterization of protein unfolding by NMR diffusion measurements. J. Biomolec. NMR 10 199–203. [Google Scholar]
- Kluck, R.M., Bossy-Wetzel, E., Green, D.R., Newmeyer, D.D. 1997. The release of cytochrome c from mitochondria: A primary role for Bcl-2 regulation of apoptosis. Science 237 1132–1136. [DOI] [PubMed] [Google Scholar]
- Margoliash, E., Frohwirt, N., Weiner, E. 1959. A study of the cytochrome c heamochromogen. Biochem. J. 71 550–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, G.R., Pettigrew, G.W. 1990. Cytochromes c evolutionary, structural and physicochemical aspects. Springer, Berlin.
- Pan, H., Barany, G., Woodward, C. 1997. Reduced BPTI is collapsed. A pulsed field gradient NMR study of unfolded and partially folded bovine pancreatic trypsin inhibitor. Protein Sci. 6 1985–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, Y., Bolen, C.L., Bolen, D.W. 1998. Osmolyte-driven contraction of a random coil protein. Proc. Natl. Acad. Sci. 95 9268–9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders, A.J., Davis-Searles, P.R., Allen, D.L., Pielak, G.J., Erie, D.A. 2000. Osmolyte-induced changes in protein conformational equilibria. Biopolymers 53 293–307. [DOI] [PubMed] [Google Scholar]
- Semisotnov, G.V., Rodionova, N.A., Razgulyaev, O.I., Uversky, V.N. 1991. Study of the "molten globule" intermediate state in protein folding by a hydrophobic fluorescent probe. Biopolymers 31 119–128. [DOI] [PubMed] [Google Scholar]
- Shearwin, K.E., Winzor, D.J. 1990. Thermodynamic nonideality as a probe of reversible protein unfolding effected by variations in pH and temperature: Studies of ribonuclease. Arch. Biochem. Biophys. 282297–301. [DOI] [PubMed] [Google Scholar]
- Shortle, D., Ackerman, M.S. 2001. Persistence of native-like topology in a denatured protein in 8 M urea. Science 293 487–489. [DOI] [PubMed] [Google Scholar]
- Spillantini, M.G., Schmidt, M.L., Lee, V.M., Trojanowski, J.Q., Jakes, R., Goedert, M. 1997. α-Synuclein in Lewy bodies. Nature 388 839–840. [DOI] [PubMed] [Google Scholar]
- Uversky, V. 1993. Use of fast protein size-exclusion liquid chromatography to study the unfolding of proteins which denature through the molten globule. Biochemistry 32 13288–13298. [DOI] [PubMed] [Google Scholar]
- Uversky, V.N., Gillespie, J.R., Fink, A.L. 2000. Why are "natively unfolded" proteins unstructured under physiologic conditions? Proteins 41 415–427. [DOI] [PubMed] [Google Scholar]
- Uversky, V.N., Li, J., Fink, A.L. 2001. Evidence for a partially folded intermediate in α-synuclein fibril formation. J. Biol. Chem. 276 10737–10744. [DOI] [PubMed] [Google Scholar]
- van den Berg, B., Ellis, R.J., Dobson, C.M. 1999. Effects of macromolecular crowding on protein folding and aggregation. EMBO J. 186927–6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanker, E.E. 2000. Protein aggregation in Huntington's and Parkinson's disease: Implications for therapy. Mol. Med. Today 6 387–391. [DOI] [PubMed] [Google Scholar]
- Weinreb, P.H., Zhen, W., Poon, A.W., Conway, K.A., Lansbury, P.T.J. 1996. NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry 35 13709–13715. [DOI] [PubMed] [Google Scholar]
- Wilkins, D.K., Grimshaw, S.B., Receveur, V., Dobson, C.M., Jones, J.A., Smith, L.J. 1999. Hydrodynamic radii of native and denatured proteins measured by pulse field gradient NMR techniques. Biochemistry 38 16424–16431. [DOI] [PubMed] [Google Scholar]
- Yancey, P.H., Clark, M.E., Hand, S.C., Bowlus, R.D., Somero, G.N. 1982. Living with water stress: Evolution of osmolyte systems. Science 217 1214–1222. [DOI] [PubMed] [Google Scholar]