Abstract
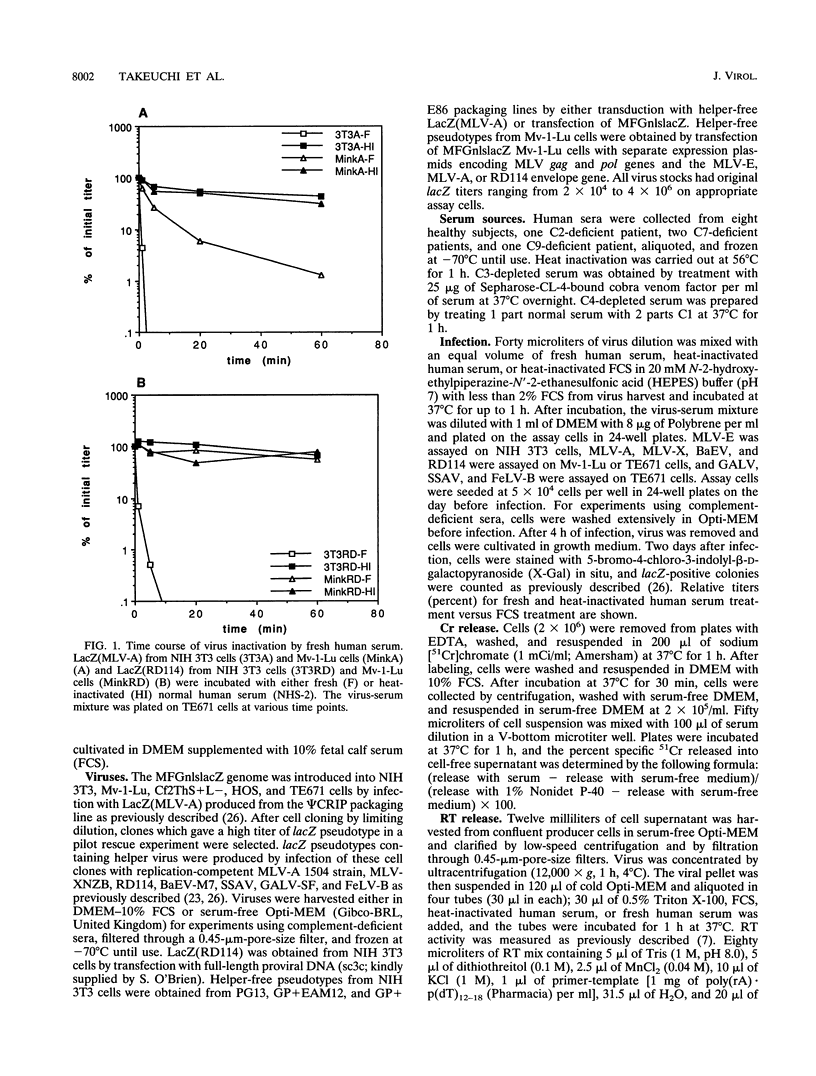
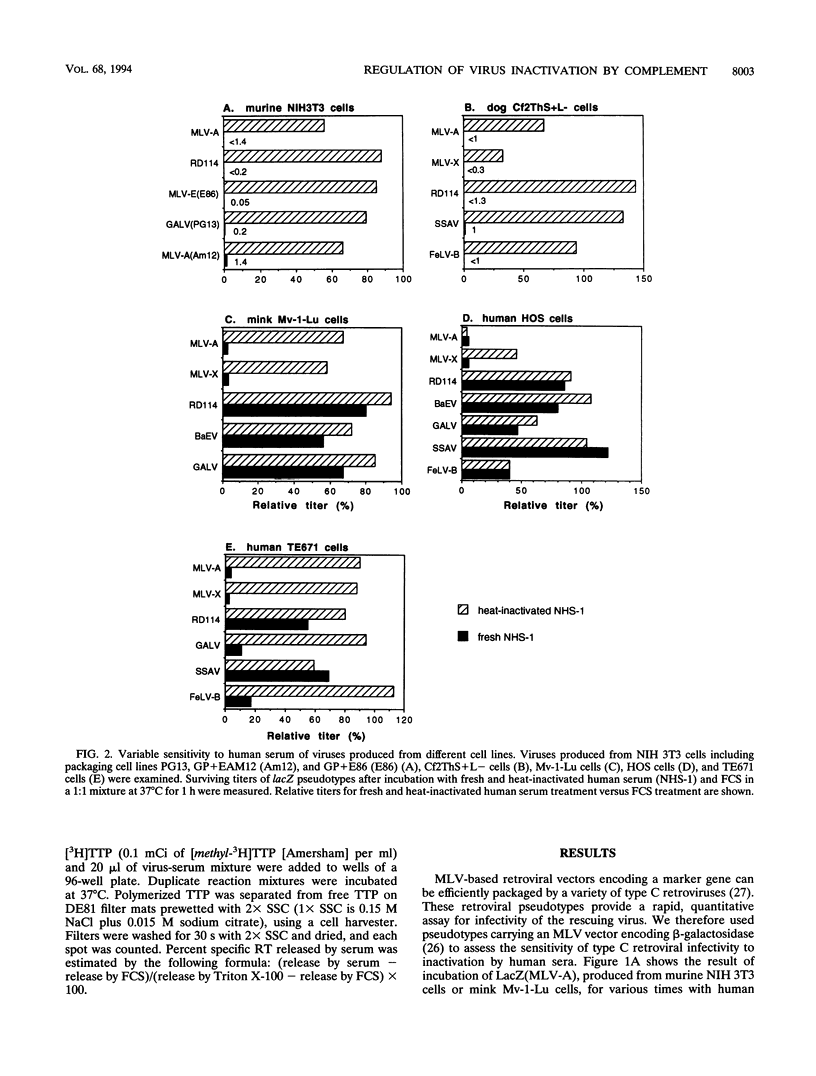
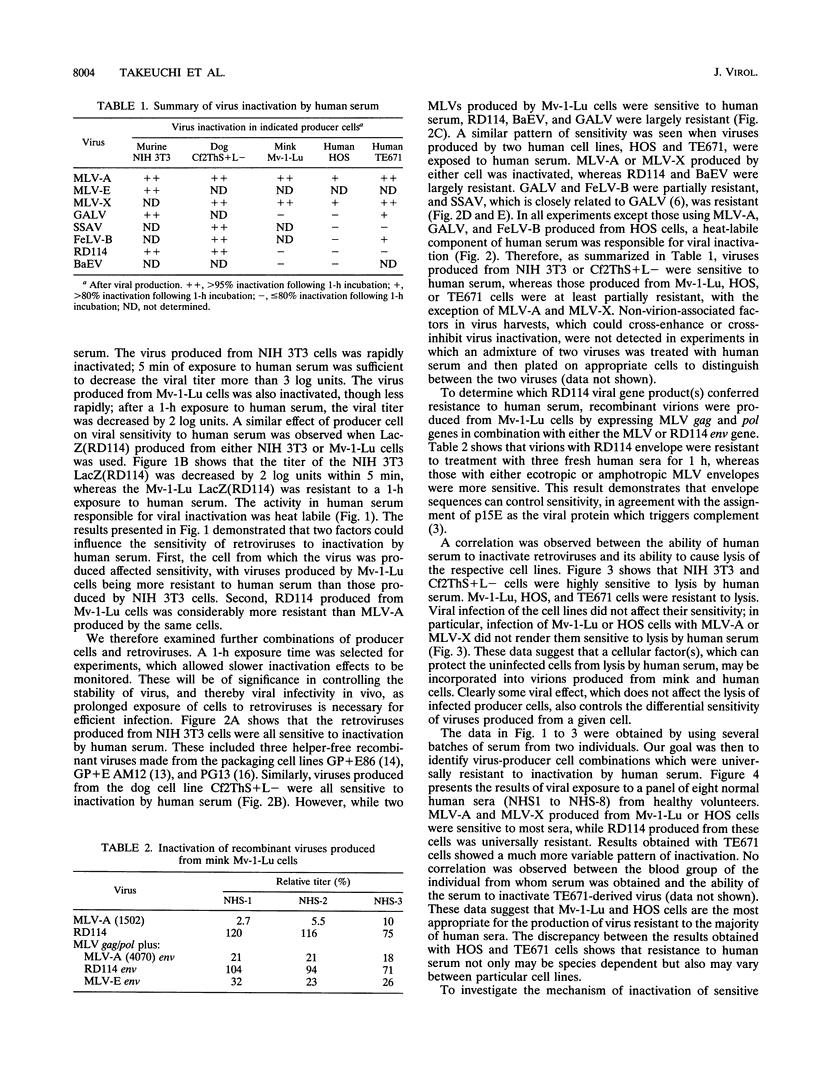
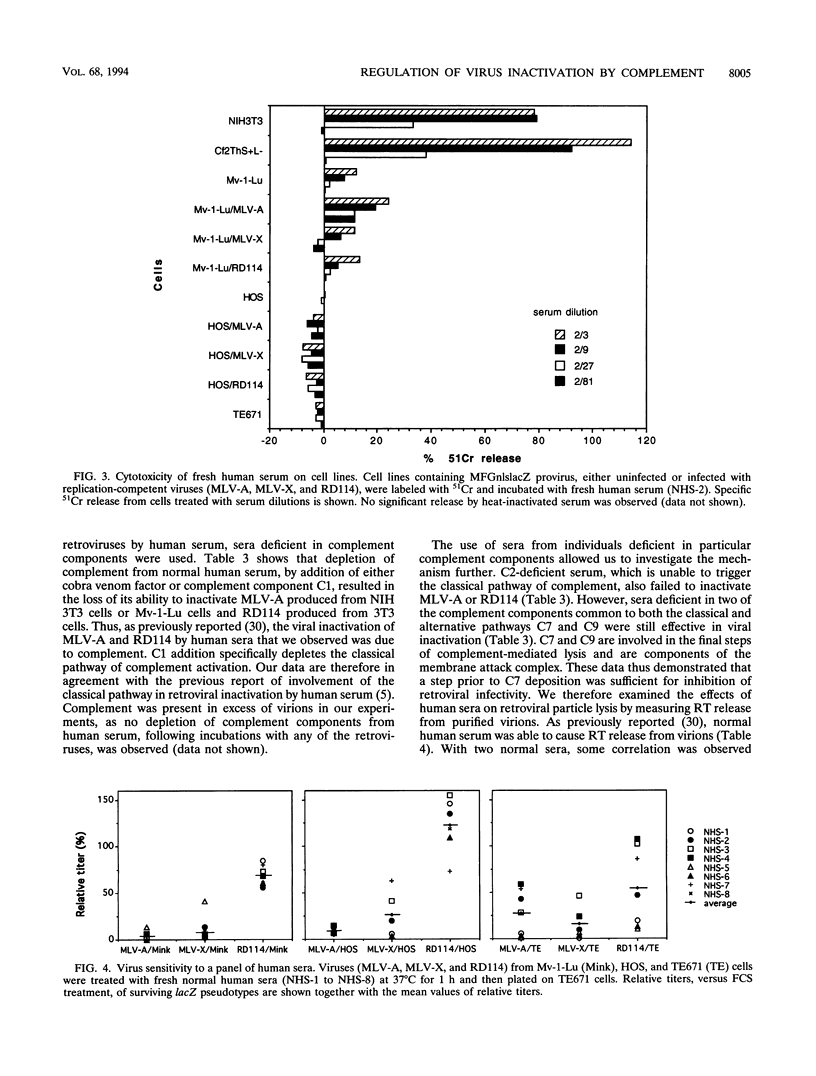
The inactivation of type C retroviruses by human serum may be a considerable impediment to the use of retroviral vectors in vivo for gene therapy. Here we show that virus inactivation is dependent both on the virus and on the cell line used to produce the virus. All viruses produced from murine NIH 3T3 or dog Cf2ThS+L- cells are sensitive to human serum. In contrast, those produced from mink Mv-1-Lu and human HOS or TE671 cells are at least partially resistant, with the exception of murine leukemia viruses. In particular, the feline endogenous virus RD114 is completely resistant to a panel of eight human sera when produced from Mv-1-Lu or HOS cells. This differential resistance is controlled by the viral envelope proteins. Virus inactivation can be correlated with the ability of the producer cells to be lysed by human serum. Inactivation of sensitive viruses requires the classical pathway of complement but does not require virion lysis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur L. O., Bess J. W., Jr, Sowder R. C., 2nd, Benveniste R. E., Mann D. L., Chermann J. C., Henderson L. E. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science. 1992 Dec 18;258(5090):1935–1938. doi: 10.1126/science.1470916. [DOI] [PubMed] [Google Scholar]
- Banapour B., Sernatinger J., Levy J. A. The AIDS-associated retrovirus is not sensitive to lysis or inactivation by human serum. Virology. 1986 Jul 15;152(1):268–271. doi: 10.1016/0042-6822(86)90392-2. [DOI] [PubMed] [Google Scholar]
- Bartholomew R. M., Esser A. F., Müller-Eberhard H. J. Lysis of oncornaviruses by human serum. Isolation of the viral complement (C1) receptor and identification as p15E. J Exp Med. 1978 Mar 1;147(3):844–853. doi: 10.1084/jem.147.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubbers J. E., Lilly F. Selective incorporation of H-2 antigenic determinants into Friend virus particles. Nature. 1977 Mar 31;266(5601):458–459. doi: 10.1038/266458a0. [DOI] [PubMed] [Google Scholar]
- Cooper N. R., Jensen F. C., Welsh R. M., Jr, Oldstone M. B. Lysis of RNA tumor viruses by human serum: direct antibody-independent triggering of the classical complement pathway. J Exp Med. 1976 Oct 1;144(4):970–984. doi: 10.1084/jem.144.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delassus S., Sonigo P., Wain-Hobson S. Genetic organization of gibbon ape leukemia virus. Virology. 1989 Nov;173(1):205–213. doi: 10.1016/0042-6822(89)90236-5. [DOI] [PubMed] [Google Scholar]
- Goff S., Traktman P., Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981 Apr;38(1):239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch R. L., Griffin D. E., Winkelstein J. A. Host modification of Sindbis virus sialic acid content influences alternative complement pathway activation and virus clearance. J Immunol. 1981 Nov;127(5):1740–1743. [PubMed] [Google Scholar]
- Hoshino H., Tanaka H., Miwa M., Okada H. Human T-cell leukaemia virus is not lysed by human serum. 1984 Jul 26-Aug 1Nature. 310(5975):324–325. doi: 10.1038/310324a0. [DOI] [PubMed] [Google Scholar]
- Jones J. S., Risser R. Cell fusion induced by the murine leukemia virus envelope glycoprotein. J Virol. 1993 Jan;67(1):67–74. doi: 10.1128/jvi.67.1.67-74.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando Z., Sarin P., Megson M., Greene W. C., Waldman T. A., Gallo R. C., Broder S. Association of human T-cell leukaemia/lymphoma virus with the Tac antigen marker for the human T-cell growth factor receptor. Nature. 1983 Oct 20;305(5936):733–736. doi: 10.1038/305733a0. [DOI] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. Construction and use of a safe and efficient amphotropic packaging cell line. Virology. 1988 Dec;167(2):400–406. [PubMed] [Google Scholar]
- Miller A. D., Garcia J. V., von Suhr N., Lynch C. M., Wilson C., Eiden M. V. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J Virol. 1991 May;65(5):2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D. Human gene therapy comes of age. Nature. 1992 Jun 11;357(6378):455–460. doi: 10.1038/357455a0. [DOI] [PubMed] [Google Scholar]
- Oldfield E. H., Ram Z., Culver K. W., Blaese R. M., DeVroom H. L., Anderson W. F. Gene therapy for the treatment of brain tumors using intra-tumoral transduction with the thymidine kinase gene and intravenous ganciclovir. Hum Gene Ther. 1993 Feb;4(1):39–69. doi: 10.1089/hum.1993.4.1-39. [DOI] [PubMed] [Google Scholar]
- Reeves R. H., O'Brien S. J. Molecular genetic characterization of the RD-114 gene family of endogenous feline retroviral sequences. J Virol. 1984 Oct;52(1):164–171. doi: 10.1128/jvi.52.1.164-171.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. E., Jr, Montefiori D. C., Mitchell W. M. Antibody-dependent enhancement of human immunodeficiency virus type 1 infection. Lancet. 1988 Apr 9;1(8589):790–794. doi: 10.1016/s0140-6736(88)91657-1. [DOI] [PubMed] [Google Scholar]
- Schols D., Pauwels R., Desmyter J., De Clercq E. Presence of class II histocompatibility DR proteins on the envelope of human immunodeficiency virus demonstrated by FACS analysis. Virology. 1992 Jul;189(1):374–376. doi: 10.1016/0042-6822(92)90719-6. [DOI] [PubMed] [Google Scholar]
- Sherwin S. A., Benveniste R. E., Todaro G. J. Complement-mediated lysis of type-C virus: effect of primate and human sera on various retroviruses. Int J Cancer. 1978 Jan 15;21(1):6–11. doi: 10.1002/ijc.2910210103. [DOI] [PubMed] [Google Scholar]
- Sommerfelt M. A., Weiss R. A. Receptor interference groups of 20 retroviruses plating on human cells. Virology. 1990 May;176(1):58–69. doi: 10.1016/0042-6822(90)90230-o. [DOI] [PubMed] [Google Scholar]
- Sommerfelt M. A., Williams B. P., McKnight A., Goodfellow P. N., Weiss R. A. Localization of the receptor gene for type D simian retroviruses on human chromosome 19. J Virol. 1990 Dec;64(12):6214–6220. doi: 10.1128/jvi.64.12.6214-6220.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott E. J. Anti-cell antibody in macaques. Nature. 1991 Oct 3;353(6343):393–393. doi: 10.1038/353393a0. [DOI] [PubMed] [Google Scholar]
- Tailor C. S., Takeuchi Y., O'Hara B., Johann S. V., Weiss R. A., Collins M. K. Mutation of amino acids within the gibbon ape leukemia virus (GALV) receptor differentially affects feline leukemia virus subgroup B, simian sarcoma-associated virus, and GALV infections. J Virol. 1993 Nov;67(11):6737–6741. doi: 10.1128/jvi.67.11.6737-6741.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Simpson G., Vile R. G., Weiss R. A., Collins M. K. Retroviral pseudotypes produced by rescue of a Moloney murine leukemia virus vector by C-type, but not D-type, retroviruses. Virology. 1992 Feb;186(2):792–794. doi: 10.1016/0042-6822(92)90049-u. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y., Vile R. G., Simpson G., O'Hara B., Collins M. K., Weiss R. A. Feline leukemia virus subgroup B uses the same cell surface receptor as gibbon ape leukemia virus. J Virol. 1992 Feb;66(2):1219–1222. doi: 10.1128/jvi.66.2.1219-1222.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M. Host cell modification of lymphocytic choriomeningitis virus and Newcastle disease virus altering viral inactivation by human complement. J Immunol. 1977 Jan;118(1):348–354. [PubMed] [Google Scholar]
- Welsh R. M., Jr, Cooper N. R., Jensen F. C., Oldstone M. B. Human serum lyses RNA tumour viruses. Nature. 1975 Oct 16;257(5527):612–614. doi: 10.1038/257612a0. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Jensen F. C., Cooper N. R., Oldstone M. B. Inactivation of lysis of oncornaviruses by human serum. Virology. 1976 Oct 15;74(2):432–440. doi: 10.1016/0042-6822(76)90349-4. [DOI] [PubMed] [Google Scholar]


