Abstract
HIV-2 is distinguished clinically and immunologically from HIV-1 infection by delayed disease progression and maintenance of HIV-specific CD4+ T cell help in most infected subjects. Thus, HIV-2 provides a unique natural human model in which to investigate correlates of immune protection against HIV disease progression. Here, we report a detailed assessment of the HIV-2-specific CD4+ and CD8+ T cell response compared to HIV-1, using polychromatic flow cytometry to assess the quality of the HIV-specific T cell response by measuring IFN-γ, IL-2, TNF-α, MIP-1β, and CD107a mobilization (degranulation) simultaneously following Gag peptide stimulation. We find that HIV-2-specific CD4+ and CD8+ T cells are more polyfunctional that those specific for HIV-1 and that polyfunctional HIV-2-specific T cells produce more IFN-γ and TNF-α on a per-cell basis than monofunctional T cells. Polyfunctional HIV-2-specific CD4+ T cells were generally more differentiated and expressed CD57, while there was no association between function and phenotype in the CD8+ Tcell fraction. Polyfunctional HIV-specific T cell responses are a hallmark of non-progressive HIV-2 infection and may be related to good clinical outcome in this setting.
Keywords: Chemokines, Cytokines, HIV, T cells
Introduction
The clinical course of disease with HIV-2 infection is attenuated when compared to HIV-1. The vast majority of HIV-2-infected individuals experience non-progressive disease [1] and the mortality rate of this subset is not significantly higher than the uninfected population [1, 2]. HIV-2 can cause AIDS in a minority of infected individuals [3], however, most HIV-2-infected individuals behave as long-term non-progressors (LTNP). Most HIV-2-infected individuals have low to undetectable viral loads [4], yet levels of proviral DNA in peripheral blood CD4+ T cells is equivalent to that found in HIV-1-infected individuals at the same stage of disease [5–7]. These observations are consistent with control of HIV-2 viral replication by host immune responses leading to the lack of subsequent CD4+ T cell decline in typical HIV-2 infection [8].
HIV-2-specific cellular and humoral immune responses have been documented in small numbers of infected individuals. HIV-2-specific CD8+ T cell immune responses have been shown to be present [9, 10], but not different from HIV-1-specific responses in terms of IFN-γ production [11–13], proliferative capacity [14], or cytotoxicity [12], suggesting that these aspects of the adaptive immune response to HIV-2 are not the key features contributing to enhanced viral control and better clinical outcome. One of the characteristic immune defects in HIV-1 infection is the early loss of HIV-specific CD4+ T cells that have proliferative capacity and the ability to secrete IL-2 [15, 16]. The HIV-specific CD4+ T cell response in chronic infection is thus either entirely absent, severely impaired, or skewed towards a population that produces IFN-γ only [17–19]. In contrast, HIV-specific CD4+ T cells are more frequent in chronic HIV-2 infection [13, 20–22]. Recently, we assessed the CD4+ T cell response to HIV-2 in terms of cytokine production and proliferative capacity [14]. We found that maintenance of HIV-2-specific CD4+ T cell help was a hallmark of asymptomatic, non-progressive HIV-2 infection. The CD4+ T cell response to HIV-2 was both stronger (higher frequency) and better in quality than the HIV-1-specific CD4+ T cell response; HIV-2-specific CD4+ T cells could produce IFN-γ, IL-2, or both and retained proliferative capacity.
Measuring only a few functions of antigen-specific T cells is likely to give an incomplete and inadequate assessment of functionality, as T cells are capable of exerting many functions simultaneously. Polyfunctional T cell responses have been documented in HIV-1 LTNP [23], Hepatitis B virus vaccine- [24], HIV vaccine- [24, 25], and vaccinia-induced responses [26]. Technological advances in flow cytometry now allow for the detection of up to 18 functional, phenotypic, and lineage markers on T cells, greatly enhancing the information one can obtain about a cell simultaneously [27]. In this study, we sought to further characterize the functional quality of the HIV-2-specific CD4+ and CD8+ T cell responses in comparison to HIV-1 and to describe the surface phenotype of T cells responsive to HIV-2 in a cohort of 18 HIV-2+ and 16 HIV-1+ Gambians. We employed 12-color flow cytometry to measure five functions of CD4+ and CD8+ T cells at the single-cell level: cytokine production (IFN-γ, IL-2, and TNF-α), chemokine production (MIP-1β), and degranulation (surface mobilization of CD107a). Additionally, we assessed the memory phenotype of functional populations of HIV-2-specific T cells. For this type of in-depth analysis of multiple functions of T cells it was necessary to ensure that results were not biased by very few cells in each functional population. For this reason, we chose to study individuals who had large magnitude of HIV-specific CD4+ T cell responses in our first study [14], predominantly comprising individuals at asymptomatic or non-progressive stages of disease. Our findings indicated that HIV-2-infected individuals mount a functionally superior HIV-specific T cell response characterized by highly polyfunctional HIV-specific CD4+ and CD8+ T cells.
Results
Measuring multiple functional parameters of HIV-1 and HIV-2-specific CD4+ T cells
Fig. 1A shows the gating scheme and response pattern in a single HIV-2-infected individual. It demonstrates that stimulation of PBMC from HIV-2-infected individuals with overlapping HIV-2 Gag peptides can stimulate CD4+ T cell responses characterized functionally by any of the five parameters we measured. Consistent with their being chosen based upon having similar magnitudes of CD4+ T cell responses to HIV, medians 0.21 and 0.23% of total memory CD4+ T cells were specific for HIV in our HIV-1 and HIV-2 cohort, respectively (Fig. 1C). However, there were differences in the character of the CD4+ T cell responses to HIV-1 and HIV-2. IFN-γ and TNF-α dominate the HIV-2-specific CD4+ T cell response with over 50% of responding cells producing one of these cytokines (Fig. 1B). Interestingly, the HIV-2-specific CD4+ T cell response is also characterized by 25% of responding cells producing the CCR5-binding chemokine MIP-1β. Specifically, the HIV-1-specific CD4+ T cell response is characterized by a significantly lower proportion of cells producing IFN-γ, TNF-α, and MIP-1β, compared to HIV-2-specific CD4+ T cells. In addition, CD107a is increased in the HIV-1-specific response with nearly half of all CD4+ T cells specific for HIV-1 mobilizing CD107a in response to Gag peptide stimulus. The proportion of CD4+ T cells producing IL-2 did not differ between HIV-1 and HIV-2 infected individuals.
Figure 1.
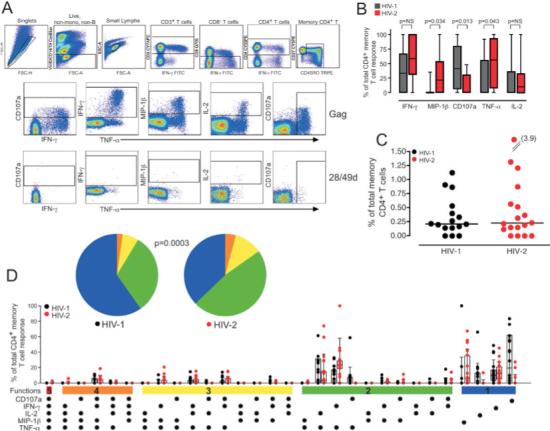
Polyfunctional HIV-specific CD4+ T cells are prominent in HIV-2. (A) Representative gating scheme for identification of HIV Gag-specific CD4+ T cell responses is shown for single HIV-2-infected individual. Gates for each of the five functions were set based on the negative control (28/49d sample) for each individual. (B) The proportion of the total HIV-specific memory CD4+ T cell response contributed by each function is shown with boxes representing medians and interquartile ranges in 16 HIV-1 (grey) and 18 HIV-2 (red) subjects. Differences were assessed by Mann-Whitney U test and p values are shown. NS = not significant. (C) The magnitude of the total HIV Gag-specific memory CD4+ T cell response was calculated based on the summation of all individual functional response patterns. The median line is shown. (D) The functional complexity of the HIV-specific CD4+ T cells response was assessed by analyzing the individual response patterns. The x-axis displays each response pattern, whose composition is denoted with a dot for the presence of CD107a, IFN-γ, IL-2, MIP-1β, and TNF-α. The proportion of the total memory CD4+ T cell response contributed by each response pattern for each individual and the median and interquartile ranges are shown. The response patterns are grouped and color-coded by number of positive functions and summarized in pie chart form where each pie slice represents the mean proportion of the total CD4+ T cell response contributed by response patterns that have all five (red) or any combination of four (orange), three (yellow), two (green), or one (blue) of the measured functions.
HIV-2-specific CD4+ T cells are more polyfunctional than those specific for HIV-1
The true complexity of the response is greater than is defined by the proportion of HIV-2-specific CD4+ T cells that express any one function. For example, measuring five functional parameters allows for the identification of 25 unique response patterns. Boolean gating analysis identifies these 32 response patterns by creating every possible combination of the five individual functional parameters (IFN-γ, IL-2, TNF-α, MIP-1β, and CD107a). The frequency of each of these 32 functional subpopulations can then be assessed and analyzed for its contribution to the total HIV-specific CD4+ T cell response. Using this technique, we found that the HIV-2-specific CD4+ T cell response was more poly-functional than the response to HIV-1 (Fig. 1D). Cells simultaneously producing two or more of the five functions comprised approximately 60% of the HIV-2-specific CD4+ T cell response as compared to less than half of the HIV-1-specific CD4+ T cell response.
It is important to note that not all CD4+ T cell response patterns are elicited after Gag peptide stimulus. Rather, only particular combinations of simultaneous functions are detected. Cells expressing all five functional parameters (five-functional cells) are rare, but are detectable in HIV-2-infected individuals. The four-functional response to Gag is confined to a particular combination of functional parameters (production of IFN-γ, TNF-α, MIP-1β, and mobilization of CD107a in the absence of IL-2 production) while other combinations of four functions are not elicited at all. The three-functional Gag response is heterogeneous, but all elicited response combinations include the production of both IFN-γ and TNF-α. Finally, the two-functional response is composed mainly of cells producing TNF-α and either IFN-γ or IL-2. By examining each subject's total HIV-specific CD4+ T cell response as a combination of cells with five, four, three, two, or one function, the difference in the quality between HIV-1 and HIV-2-specific CD4+ T cell responses is appreciable (pie charts, Fig. 1D). Within each individual subject's response, cells with three or more functions are prominent in HIV-2-infected individuals, while the response is mainly monofunctional in most HIV-1-infected subjects.
Phenotype of HIV-2-specific CD4+ T cells
Combining functional and phenotypic markers in 12-color flow cytometry allows the identification of phenotypic subsets of virus-specific CD4+ T cells and assessment of their functional properties. Surface phenotypes of antigen-specific cells can be defined descriptively (as in Fig. 2A) by overlaying antigen-specific CD4+ T cells on a background of total CD4+ T cells and examining expression of CD27 and CD45RO, which we have used to assign designations of “central” and “effector” memory cells. We use these two markers because the more traditional markers are either notoriously poorly expressed (CCR7) or absent (CD62L) on cryopreserved PBMC. In Fig. 2A it can be appreciated that HIV-2-specific CD4+ T cells in general are CD27low and CD45RO+. However, some functional populations of cells, such as the 4+ population, are phenotypically heterogeneous with a proportion of cells that lack CD45RO expression.
Figure 2.
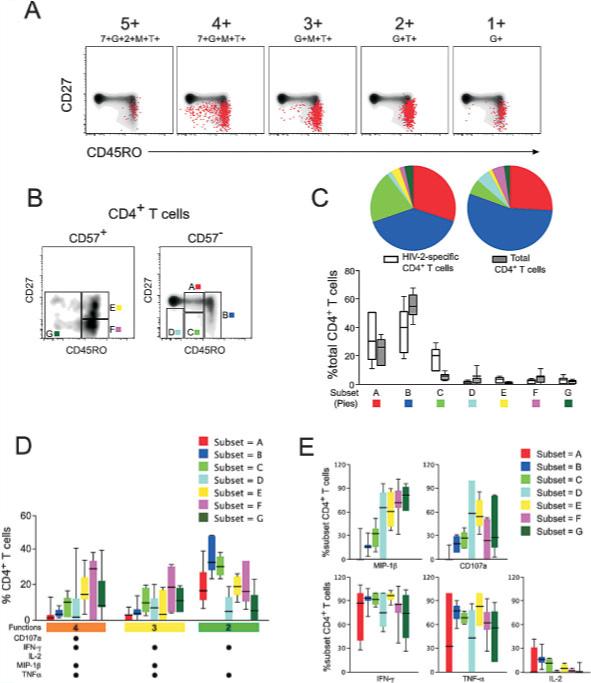
Association between function and phenotype in HIV-2-specific CD4+ T cells. (A) Surface phenotype of HIV-2-specific CD4+ T cells in shown for representative functional populations (5+:7+G+2+M+T+; 4+:7+G+M+T+; 3+:G+M+T+; 2+:G+T+; 1+:G+) in a single HIV-2 subject by overlaying responding cells (red dots) on a density plot of total CD4+ T cells, showing CD27 and CD45RO expression. (B) Further phenotype analysis incorporated CD57 expression and responding cells were divided into seven phenotypic subsets (A–G). (C) The distribution of total peripheral CD4+ T cells (grey bars) or HIV-2-specific CD4+ T cells (white bars) among the seven phenotypic subsets was examined in six HIV-2+ individuals. The proportion of CD4+ T cells that fell within each phenotypic subset is shown with boxes representing medians and interquartile ranges. These data are summarized in color-coded pie chart form where each pie slice represents the mean proportional contribution of each subset to the total. (D) The phenotypic distribution of cells with a given functional profile was examined. The relative contribution to a 4+,3+, and 2+ functional population was examined for each of the seven phenotypic subsets. Median and interquartile ranges are shown. (E) The functional profile of each phenotypic subset is shown. For each phenotype, the proportion of cells producing a given function is shown with median and interquartile ranges; 7 = CD107a; G = IFN-γ; 2 = IL-2; M = MIP-1β; T = TNF-α.
Using surface staining for CD27, CD45RO, and CD57, we arbitrarily divided the HIV-2-specific CD4+ T cell response into seven distinct subsets of memory CD4+ T cells (Fig. 2B, subsets A–G). Eighty percent of total peripheral blood memory CD4+ T cells from HIV-2-infected individuals were found to have a surface phenotype that fell within subsets A and B and are principally central memory (Fig. 2C) [28]. These cells lacked expression of CD57 and were CD27+ or CD27intermediate and expressed intermediate to high levels of CD45RO. HIV-2-specific CD4+ T cells also predominantly had the characteristic “central memory” phenotype. However, 20% of HIV-2-specific CD4+ T cells were distinguished phenotypically by intermediate expression of both CD27 and CD45RO, and lack of surface CD57 expression (subset C), a phenotype not prominent among total peripheral blood CD4+ T cells. An additional 10% of HIV-2-specific CD4+ T cells were terminally differentiated, as assessed by expression of CD57 (subsets E–G).
We observed an association between polyfunctional HIV-2-specific CD4+ T cells and stage of phenotypic differentiation. Among the polyfunctional 4+ population, a median 60% of cells fell within phenotypic subsets E–G (Fig. 2D). A similar trend was seen in the 3+ population, where a greater proportion of these cells were at more advanced stages of differentiation. In contrast, we observed the opposite trend for 2+ cells: The majority of these cells were found among the less differentiated phenotypic subsets A–C.
We also observed that MIP-1β and CD107a were over-represented functions among cells at later stages of differentiation. Greater than 50% of HIV-2-specific CD4+ T cells in phenotypic subsets E–G produced MIP-1β on stimulation with overlapping Gag peptides. In contrast, only a small proportion of less differentiated cells produced MIP-1β or CD107a (Fig. 2E). There appeared to be an inverse trend among IL-2-producing cells, which were proportionally more represented among phenotypically less differentiated cells. There was no apparent association between function and phenotype among IFN-γ or TNF-α-producing CD4+ T cells.
HIV-2-specific CD8+ T cells are more polyfunctional than those specific for HIV-1
In addition to measuring CD4+ T cell responses to overlapping Gag peptides, this 12-color panel allowed us to assess CD8+ T cell responses. Fig. 3A shows the CD8+ T cell response pattern in a single HIV-2-infected individual. Notably, there are strong IFN-γ, TNF-α, CD107a, and MIP-1β responses, but a paucity of IL-2 produced by HIV-2-specific CD8+ T cells. MIP-1β, CD107a, and IFN-γ dominate the HIV-2-specific CD8+ T cell response with over 60% of responding cells exhibiting these functions (Fig. 3B). TNF-α and IL-2 represent a much smaller proportion of the HIV-2-specific CD8+ T cell response. The HIV-1-specific CD8+ T cell response is very similar in composition, with CD107a, MIP-1β, and IFN-γ dominating, though the proportion of cells producing MIP-1β and TNF-γ is significantly lower than among HIV-2-specific CD8+ T cells. The frequency of CD8+ T cells specific for HIV did not differ in these two cohorts, with a median 1.77 and 1.41% of total memory CD8+ T cells specific for HIV in our HIV-1 and HIV-2 cohort, respectively (Fig. 3C).
Figure 3.
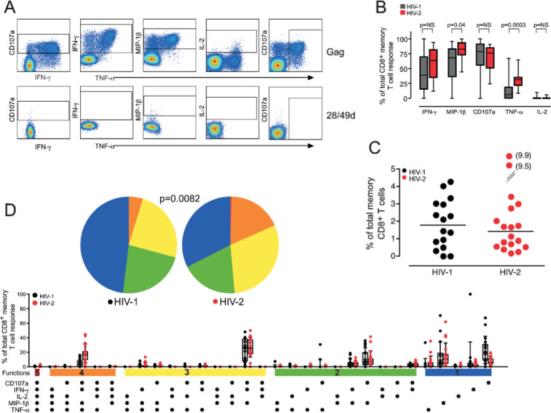
HIV-2-specific CD8+ T cells express multiple effector functions. (A) HIV-specific CD8+ T cell responses were assessed using the same five functions as for CD4+ T cell responses (CD107a, IFN-γ, MIP-1β, IL-2, and TNF-α). Gates for each function were set based on the negative control sample for each individual and are shown for a single HIV-2+ subject. (B) The proportion of the total HIV-specific CD8+ T cell response contributed by each of the five functions was assessed in 16 HIV-1+ and 18 HIV-2+ subjects and is reported as single function frequency divided by the frequency of the total response. Medians and interquartile ranges are shown. Differences were assessed by Mann-Whitney U test and p values are shown. NS = not significant. (C) The magnitude of the total memory CD8+ T cell response was calculated by summing all individual response patterns (median line is shown). (D) All possible response patterns were analyzed for proportional contribution to the total Gag-specific CD8+ T cell response and data are displayed as in Fig. 1D.
We found that polyfunctional CD8+ T cells comprised a much greater proportion of the total HIV-specific response in HIV-2 infection compared with HIV-1 infection (Fig. 3D). Nearly half of the HIV-2-specific CD8+ T cell response is composed of cells with three or more functions. In contrast, polyfunctional CD8+ T cells were less frequent among responses in HIV-1 infected individuals; particularly striking was the scarcity of 4+ cells. Again, monofunctional cells were dominant in the HIV-1-specific CD8+ T cell response, particularly those producing MIP-1β or mobilizing CD107a. These findings are particularly evident when one examines individual subjects' responses. Cells with four of the five measured functions are detectable in the vast majority of HIV-2-specific CD8+ T cell responses, but are detectable only in a minority of HIV-1-infected individuals, whose responses are dominated by monofunctional cells (data not shown).
Phenotype of HIV-2-specific CD8+ T cells
Phenotypically, HIV-2-specific CD8+ T cells are principally “central memory”, CD27+CD45RO+ (Fig. 4A). Interestingly, some functional populations of cells are more heterogeneous in surface phenotype. For example, functional populations that make MIP-1β tend to have a greater proportion of cells with an effector phenotype (i.e. CD27−CD45RO−). This is quite prominent for cells producing MIP-1β only and is in contrast to the CD4+ T cell responses where 4+, rather than monofunctional cells, were more likely to exhibit greater differentiation (Fig. 2A). Cells that exert all five functions, including production of IL-2, are almost entirely CD27+CD45RO+ even though these cells produce MIP-1β, indicating that IL-2 production, even in the presence of MIP-1β functionality, defines CD8+ T cells with a central memory-like phenotype.
Figure 4.
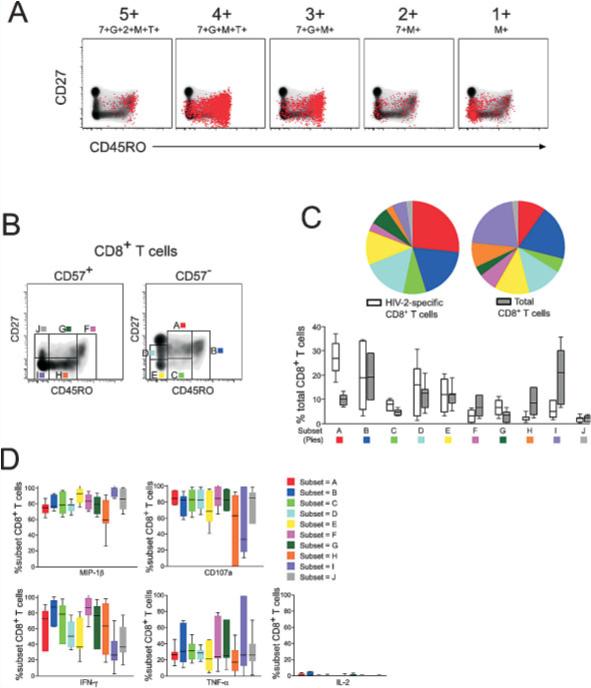
No association between function and phenotype in HIV-2-specific CD8+ T cells. (A) Surface phenotype of HIV-2-specific CD8+ T cells in shown for representative functional populations (5+:7+G+2+M+T+; 4+:7+G+M+T+; 3+:7+G+M+; 2+:7+M+; 1+:M+)in a single HIV-2 subject by overlaying responding cells (red dots) on a density plot of total CD8+ T cells, showing CD27 and CD45RO expression. (B) Further phenotype analysis incorporated CD57 expression and responding cells were divided into ten phenotypic subsets (A–J). (C) The distribution of total peripheral CD8+ T cells or HIV-2-specific CD8+ T cells among the ten phenotypic subsets was examined in six HIV-2+ individuals. These data are displayed as in Fig. 2C. (D) No association between functionality and stage of phenotypic differentiation was observed in the CD8+ T cell fraction. The functional profile of each of the ten phenotypic subsets was similar. For each phenotype, the proportion of cells producing a given function is shown with median and interquartile ranges; 7 = CD107a; G = IFN-γ; 2 = IL-2; M = MIP-1β; T = TNF-α.
Using surface staining for CD27, CD45RO, and CD57, we divided the responding CD8+ T cells into 10 distinct subsets of memory CD8+ T cells (Fig. 4B, subsets A–J). Both total peripheral blood CD8+ T cells and HIV-2-specific CD8+ T cells were predominantly non-terminally differentiated (subsets A–E; Fig. 4C). With the exception of subsets A, H, and I, there were no major differences between total and HIV-2-specific CD8+ T cells in terms of the proportion of cells in each subset. A greater proportion of HIV-2-specific CD8+ T cells fell within phenotypic subset A compared to total CD8+ T cells. In contrast, a greater proportion of total memory CD8+ T cells were phenotypically CD57+CD27− and CD45ROintermediate or CD45ROnegative (subsets H and I). This might be attributed to the presence of chronic memory CD8+ T cells specific for another pathogen, such as CMV, in the peripheral blood. It is known that CMV seropositivity correlates with immunosenescence of T cells as measured by CD57 expression [29, 30]. Recent data from neonates in the Gambia indicates that 85% of Gambian individuals are CMV+ by 12 months of life and that CD8+ T cells in these CMV+ infants have reached a fully differentiated memory phenotype [31].
In contrast to HIV-2-specific CD4+ T cells, we did not observe a clear association between functional capacity of HIV-2-specific CD8+ T cells and stage of phenotypic differentiation. Seventy to eighty percent of cells in most phenotypic subsets produced MIP-1β and CD107a in response to overlapping Gag peptides (Fig. 4D), though a smaller proportion of subsets H and I produced these functions. Additionally, 20% of cells in every subset produced TNF-α but few cells in any subset produced IL-2. The trends for IFN-γ were less clear. There was no association with CD57 expression and functional capacity, but phenotypic subsets that lacked CD45RO expression tended to have a lower proportion of cells that produced IFN-γ. These results indicate that, in contrast to CD4+ T cells, there is no clear association between surface phenotype and functional capacity for HIV-2-specific CD8+ T cells.
Polyfunctional HIV-2-specific CD4+ and CD8+ T cells produce more cytokine on a per-cell basis
Having determined increased functionality in HIV-2-specific T cells, it was important to determine if these antigen-specific cells also produced increased amounts of cytokines in response to peptide stimulation. We calculated the median fluorescence intensity (MFI) of each of our five functional parameters for each of the 32 response patterns. We found that HIV-2-specific CD4+ and CD8+ T cells expressing multiple functions had a much higher MFI for IFN-γ (Fig. 5) and TNF-α (data not shown) than cells that were less polyfunctional in nature. Presence or absence of surface CD107a expression did not affect the MFI of IFN-γ or TNF-α, and was disregarded for this analysis. As shown for a single HIV-2-infected individual in Fig. 5A and C, monofunctional HIV-2-specific CD4+ and CD8+ T cells have a very low MFI for IFN-γ. In cells with two, three, or four functions, the MFI for IFN-γ gradually increases as the number of functions expressed by these cells increases. Most striking is the difference between the most polyfunctional cells and the cells with the capacity to exert only a single function, where the MFI for IFN-γ is 20-fold higher among the polyfunctional HIV-2-specific CD4+ T cells (Fig. 5A) and 15-fold higher among polyfunctional HIV-2-specific CD8+ T cells (Fig. 5C). We observed this trend in all 18 HIV-2-infected individuals we studied, where the MFI for IFN-γ was highest among the most polyfunctional cells and decreased to very low levels among the least polyfunctional cells (Fig. 5B and D). This trend was also seen for TNF-α in CD4+ and CD8+ T cells, but there was no association between polyfunctionality and MFI for IL-2, MIP-1β, or CD107a (data not shown).
Figure 5.
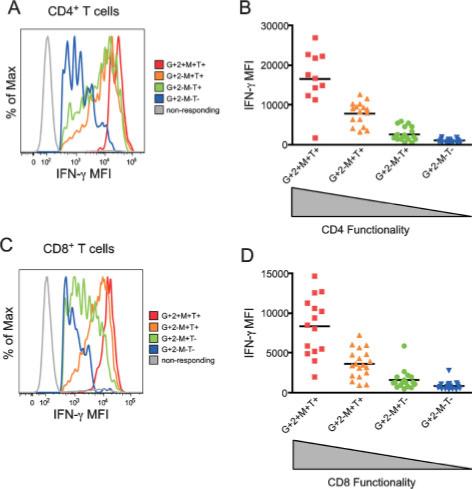
Polyfunctional T cells produce more cytokine than monofunctional cells. (A) Median fluorescent intensity (MFI) for each function was calculated for the dominant HIV-2-specific CD4+ T cell populations. IFN-γ MFI for each functional population is shown as a histogram and color-coded according to the number of positive functions. Data from a single HIV-2-infected individual are shown and is representative of 17 additional HIV-2+ subjects. (B) IFN-γ MFI data was compiled for 18 HIV-2+ individuals for each functional population. IFN-γ MFI was highest among the most polyfunctional populations, and lowest among monofunctional cells. Median IFN-γ MFI for each population is shown. (C) IFN-γ MFI for dominant HIV-2-specific CD8+ T cell responses are shown as histograms in a single HIV-2+ subject as in (A). (D) IFN-γ MFI data was compiled for 18 HIV-2+ subjects. Median IFN-γ MFI for each population is shown; 7 = CD107a; G = IFN-γ; 2 = IL-2; M = MIP-1β; T = TNF-α.
Discussion
The clinical phenotype of HIV-2 infection is quite disparate from typical HIV-1 infection. For most infected individuals HIV-2 is a lifelong and relatively benign infection characterized by non-progression even in the absence of antiretroviral therapy. As such, HIV-2-infected individuals are a unique and under-studied population in which to characterize and define correlates of protective immunity against HIV disease progression. Here, we describe a detailed functional and phenotypic assessment of the HIV-2-specific T cell response in comparison to HIV-1.
HIV-1-specific CD4+ T cells are preferentially targeted by the virus and deleted from the repertoire [32], leaving an HIV-specific CD4+ T cell response that is not only quantitatively, but also qualitatively impaired. CD4+ T cells specific for HIV-1 are skewed towards a sub-population of cells that is generally proliferation-incompetent and capable of producing only IFN-γ in the absence of IL-2 [15–19]. We have previously shown that in typical HIV-2 infection there is maintenance of HIV-specific CD4+ T cell help [14]. Here, controlling for the maintenance of HIV-specific CD4+ T cell numbers by selecting HIV-1 and HIV-2 infected individuals with a large magnitude of HIV-specific CD4+ T cell response, we further show that the HIV-2-specific T cells exhibit greater functionality. Both CD4+ and CD8+ T cells specific for HIV-2 are more polyfunctional than those specific for HIV-1 and exhibit the ability to simultaneously exert multiple functions.
Examining only a few selected functions of virus-specific T cells may generate a distorted and incomplete interpretation of the function and phenotype of these cells. For example, previously we examined IFN-γ and IL-2 production by HIV-1 and HIV-2 specific CD4+ T cells and observed that HIV-2-specific CD4+ T cells were capable of producing IFN-γ, IL-2, or both, while HIV-1-specific CD4+ T cells were capable of producing only IFN-γ [14]. In the current study, however, we assessed three additional functional parameters and generated a more complex interpretation of phenotype and functionality of these cells. Though IFN-γ and IL-2 captured the bulk of the HIV-2-specific CD4+ T cell response, a significant 12.9% (range 0−62%) of the HIV-2-specific response would have been missed if TNF-α, MIP-1β, and CD107a had not been examined (data not shown). MIP-1β contributes a small but substantial proportion of the HIV-2-specific CD4+ T cell response, but is almost entirely absent in the HIV-1-specific CD4+ T cell response.It is conceivable that MIP-1β produced by HIV-2-specific CD4+ T cells competes with HIV-2 for the CCR5 receptor, and may be particularly advantageous function, controlling HIV-2 replication by blocking infection of susceptible cells and contributing to better clinical outcome.
Cytotoxic T cells induce death of target cells through release of perforin and granzymes from cytolytic granules, a process termed degranulation [33, 34]. Cytotoxicity is primarily a function attributed to CD8+ T cells and is directly linked to the ability of these cells to degranulate [35, 36]. Cytotoxic CD4+ T cells are rare, but have been described in chronic viral infections [37–39]. A subset of HIV-1-specific CD4+ T cells have been shown to express perforin and these perforin+CD4+ CTL represented highly differentiated, end-stage memory CD4+ T cells [38]. We find that HIV-1-specific CD4+ T cells appear to degranulate (express CD107a), a function that is consistent with perforin expression. However, degranulation is not a dominant function exhibited by HIV-2-specific CD4+ T cells (Fig. 1). Expansions of CD4+ CTL have also been documented in the setting of other chronic viral infections, such as EBV and CMV, and in some rheumatoid arthritis patients. Chronic inflammation may drive the progressive differentiation of CD4+ T cells, as has been observed for CD8+ T cells, initially evidenced by the progressive loss of costimulatory molecules CD28 and CD27 and subsequent acquisition of cytotoxic granules and perforin. In the absence of generalized hyperimmune activation and chronic antigen stimulation, CD4+ T cells in HIV-2-infected individuals may not be driven towards this particular pathway of differentiation, as evidenced by their lack of propensity to degranulate (Fig. 1).
HIV-2-specific CD8+ T cell responses have been shown to be present [9, 10], but not different from HIV-1-specific responses in terms of IFN-γ production [11–13], proliferative capacity [14], or cytotoxicity [12], suggesting that these aspects of the adaptive immune response to HIV-2 are not defining features contributing to enhanced viral control and better clinical outcome. As has been reported previously in other virus-specific CD8+ T cell responses [23, 24], we found that MIP-1β was even more prominent than IFN-γ in the HIV-specific CD8+ T cell response, in both HIV-1 and HIV-2 infected individuals. Though these two functions are often expressed simultaneously, there is a substantial fraction of the total HIV-specific CD8+ T cell response that is represented by cells expressing MIP-1β in the absence of IFN-γ expression, a functional profile that would be missed by excluding MIP-1β from analysis. Similarly, CD107a expression among HIV-specific CD8+ T cells is dominant; 75% of both the HIV-1 and HIV-2-specific CD8+ T cell response consists of cells with the capacity to degranulate and many of these CD107a expressing cells do not produce IFN-γ. This is an additional major function of the HIV-specific CD8+ T cell response that is not fully captured by analyzing only IFN-γ expression. For the first time, we have documented differences in the HIV-1- and HIV-2-specific CD8+ T cell response. HIV-2-specific CD8+ T cells are highly polyfunctional with nearly half of the entire response composed of cells that express at least three functional parameters simultaneously, including MIP-1β and CD107a expression. However, these differences in the HIV-1 and HIV-2-specific CD8+ T cell response would not have been apparent by studying only IFN-γ and IL-2 expression. Measuring IFN-γ production alone may not be a sufficient tool for measuring or describing virus-specific T cell responses, as smaller but significant functional populations may not produce IFN-γ.
We have previously reported that the difference in functionality between HIV-1 and HIV-2 specific CD4+ T cells is among non-terminally differentiated (CD57−) cells [14]. However, analysis of additional functional parameters extends this interpretation. Here, we have observed that there is an association between function and phenotype of HIV-2-specific CD4+ T cells. In particular, production of MIP-1β and CD107a define cells at a later stage of differentiation. The phenotype of IFN-γ or IL-2 producing cells is predominantly CD57−CD27+CD45RO+, a phenotype associated with central memory characteristics. HIV-2-specific CD4+ T cells that produce MIP-1β, CD107a, or TNF-α in the absence of IFN-γ and IL-2 are more heterogeneous in phenotype. A proportion of these cells do express CD57 and are intermediate or negative for CD27 expression and lack CD45RO, an advanced maturational phenotype stage that would not have been appreciated fully without assessing these additional parameters (data not shown).
Virus-specific CD4+ T cells which produce multiple effector functions simultaneously, such as IL-2 and IFN-γ, have been shown to be a hallmark of protective immunity in controlled viral infections, such as CMV and EBV [39, 40]. Recent data suggest that potent prime-boost vaccine strategies can generate polyfunctional memory CD4+ T cell responses against other pathogens such as Mycobacterium tuberculosis [41], resulting in long-lasting immunity against this infection. CD4+ T cells that produce both IFN-γ and IL-2 have also been described in HIV-1 infected individuals [42, 43] and the presence of these 'polyfunctional' HIV-specific CD4+ T cells has been shown to cluster among populations of HIV-1+ individuals with non-progressive disease and relative virologic control (low to undetectable viral load) [42–44]. However, as many as half of HIV-1 elite controllers or LTNP lack these polyfunctional HIV-specific CD4+ T cells [18, 45]. Studies assessing multiple functional parameters of virus-specific CD8+ T cells are limited. HIV-1 LTNP have been shown to have a greater proportion of polyfunctional HIV-specific CD8+ T cells producing several effector molecules (IFN-γ, IL-2, TNF-α, MIP-1β, and CD107a) than HIV-1 progressors [23, 46], and polyfunctional T cells have been documented in HIV and Hepatitis B vaccine recipients [24] as well as individuals vaccinated with vaccinia virus [26]. Here, we have documented a predominance of polyfunctional CD4+ and CD8+ T cells specific for HIV-2 in individuals with virologic control. HIV-2-infected individuals with low or undetectable viral load and preserved CD4+ T cell counts maintain both a CD4+ and CD8+ HIV-specific T cell response that is highly polyfunctional in nature.
Not only are these polyfunctional T cells more frequent in HIV-2 than in HIV-1-infected individuals, accounting for a larger proportion of the response, but also they are capable of producing much more cytokine than is predicted based on their frequency. Each cell produces 15−20 times more IFN-γ and TNF-α than cells that have the capacity to produce only one function. Therefore, one polyfunctional cell producing multiple types of effector molecules generates a greater quantity of effector molecules than do five monofunctional cells each producing one type of effector molecule. This suggests that these polyfunctional antigen-specific T cells are highly potent effectors, as they are individually capable of producing increased quantities of multiple effector molecules. Additional support for this phenomenon has been described in human and mouse vaccine studies where vaccine-induced multifunctional T cells specific for vaccinia virus [26] and Leishmania major [47] produce higher amounts of IFN-γ than monofunctional cells.
Here, we documented the presence of HIV-2-specific CD4+ and CD8+ T cells capable of producing multiple antiviral effector functions in response to HIV peptide stimulus in a cohort of asymptomatic HIV-2-infected individuals. Indeed, aside from four individuals with very low plasma viral load, all individuals had undetectable virus in plasma. It is certainly plausible that these polyfunctional CD4+ and CD8+ Tcells specific for HIV-2 play a contributory role in maintaining HIV-2 viral loads at undetectable levels for many years. Certainly, polyfunctional CD8+ T cells specific for HIV-1 have also been shown to be elevated in rare LTNP who are also distinguished by undetectable plasma viral loads [23]. Alternatively, in the absence of extreme viral pressure in the form of high levels of plasma viremia, the development of highly polyfunctional Tcells specific for HIV-2 may be preferentially selected. Either as a cause or consequence of low viral burden, polyfunctional CD4+ and CD8+ T cells specific for HIV-2 endowed with the capacity to secrete multiple effector cytokines simultaneously and at high concentrations per cell are a hallmark of this generally well-controlled infection. Recent evidence suggests that polyfunctional T cells are operative in control of infection by some pathogens. Specifically, mice immunized against Leishmania major that develop polyfunctional CD4+ T cell responses are protected against subsequent challenge with L. major while the same is not true of mice that develop a similar magnitude of CD4+ T cell responses that are not polyfunctional [47]. The antigen-specific CD4+ T cells that simultaneously produce IFN-γ, IL-2, and TNF-α appear to mediate protection against infection and produce much more IFN-γ per cell than monofunctional T cells generated by the vaccine [47]. These data offer support that polyfunctional T cells are more than just a correlate of protection; they are more efficient effectors and are causally linked to protection from disease. It is clear that measuring multiple antiviral effector cytokines, chemokines, and other molecules simultaneously is essential for generating a complete picture of antiviral T cell immunity, and will be important in the proper and full evaluation of vaccine-induced T cell responses against numerous pathogens.
Materials and methods
Study subjects
Eighteen HIV-2+ and 16 HIV-1+ subjects were recruited from the clinical HIV cohort attending the Genitourinary Medicine Clinic based at the Medical Research Council Unit in Fajara, The Gambia. These subjects had participated in our previous study [14]. In order to focus on differences in the quality, rather than the magnitude, of the CD4+ T cell responses to HIV-1 and HIV-2, we chose subjects with similar and high CD4+ T cell responses to HIV. All subjects were antiretroviral therapy naive. Study participants were screened for HIV-1 and HIV-2 using the Murex ICE HIV-1.2.0 capture enzyme immunoassay (Murex Diagnostics, Kent, UK). All reactive samples were confirmed as HIV-1- or HIV-2-positive using two type-specific competitive ELISA (Murex). HIV-1 and HIV-2 plasma viral loads were measured by an in-house reverse-transcriptase PCR assay using specific long terminal repeat primers [48]. Lower limit of detection for viral load assays was 100 RNA copies/mL. Percentages of CD4+ T lymphocytes and absolute CD4+ T cell counts were determined by flow cytometry using BD MultiTest reagents and MultiSet software. Ethical approval was obtained from the Gambian Government/ MRC Ethics Committee and each study participant gave informed consent prior to entry into the study. CD4 counts and viral load data are shown in Table 1. HIV-2-infected individuals had lower viral loads and higher CD4+ T cell counts than the HIV-1-infected cohort did. Of 18 HIV-2+ individuals, 14 had undetectable viral load, and among those with detectable viral load, none was above 5000 RNA copies/mL. Median CD4 percentage was 34% with a range of 21−54%. Mean viral load among HIV-1 infected individuals was 146 040 (range 10 555−478 962) and CD4 percentage was substantially lower than the HIV-2 cohort with a median 19% (range 6−39%). PBMC were isolated via Hypaque-Ficoll (Pharmacia) density centrifugation and cryopreserved at −80[notdef]C in 90% FCS and 10% DMSO (Sigma) until use.
Table 1.
Characteristics of study cohort
| Subject | Viral load (RNA copies/mL) | CD4% | Absolute CD4 (cells/μL) |
|---|---|---|---|
| HIV-2−1 | <100 | 54 | 1080 |
| HIV-2−2 | <100 | 30 | 690 |
| HIV-2−3 | 685 | 49 | 640 |
| HIV-2−4 | <100 | 42 | 560 |
| HIV-2−5 | 3842 | 46 | 810 |
| HIV-2−6 | <100 | 42 | 780 |
| HIV-2−7 | <100 | 30 | 700 |
| HIV-2−8 | <100 | 33 | 1050 |
| HIV-2−9 | <100 | 32 | 670 |
| HIV-2−10 | <100 | 36 | 1270 |
| HIV-2−11 | <100 | 29 | 1090 |
| HIV-2−12 | <100 | 35 | 450 |
| HIV-2−13 | 4810 | 23 | 520 |
| HIV-2−14 | <100 | 39 | 1160 |
| HIV-2−15 | <100 | 26 | 640 |
| HIV-2−16 | <100 | 28 | 450 |
| HIV-2−17 | 1927 | 24 | 270 |
| HIV-2−18 | <100 | 21 | 360 |
| HIV-1−1 | 97 059 | 21 | 450 |
| HIV-1−2 | 194 809 | 19 | 490 |
| HIV-1−3 | 149 461 | 17 | 220 |
| HIV-1−4 | 36 689 | 29 | 850 |
| HIV-1−5 | 191 082 | 11 | 120 |
| HIV-1−6 | 10 555 | 39 | 420 |
| HIV-1−7 | 43 742 | 14 | 170 |
| HIV-1−8 | 96 984 | 12 | 90 |
| HIV-1−9 | 105 951 | 31 | 980 |
| HIV-1−10 | 320 430 | 7 | 240 |
| HIV-1−11 | 206 553 | 10 | 550 |
| HIV-1−12 | 273 340 | 13 | 320 |
| HIV-1−13 | 88 910 | 8 | 250 |
| HIV-1−14 | 478 962 | 6 | 190 |
| HIV-1−15 | 26 138 | 31 | 650 |
| HIV-1−16 | 15 969 | 31 | 690 |
Synthetic peptides
Fifteen-mer peptides overlapping by 11 amino acids corresponding to the full-length Gag consensus sequence from five Gambian HIV-2 isolates were synthesized as free amino acids and were greater than 85% pure by HPLC. HIV-1 Clade A full-length Gag consensus sequence was used to create 15-mers overlapping by 11 (NIH AIDS Research and Reference Reagent Program) as most HIV-1 infections in the Gambia are CRF-02, an A/G recombinant strain of which gag is subtype A [49, 50]. Lyophylized peptides were reconstituted in DMSO at 50 mg/mL. Overlapping peptides were grouped into an HIV-2 Gag pool (128 peptides) and HIV-1 Gag pool (105 peptides) such that the concentration of each peptide within the pool was 400 μg/mL. Peptide pools were used at a final concentration of 2 μg/mL/peptide within the pool such that each peptide was at a final concentration of 2 μg/mL in all experiments.
Polychromatic flow cytometry mAb
The following 12-color flow cytometric panel was used to analyze five T cell functions and describe maturation phenotype. Directly conjugated mAb were obtained from BD PharMingen (San Diego, CA): IFN-γ-FITC, MIP-1β-PE, IL-2-APC, TNF-α-Cy7PE, CD3-Cy7APC, Caltag (Burlingame, CA): CD4-Cy5.5PE, and from Beckman Coulter (Miami, FL): CD45RO-ECD. The following antibodies were conjugated in our laboratory according to standard protocols (http://drmr.com/abcon/index.html): CD107a-Alexa 680, CD27-Cy5PE, CD14-Cascade blue, CD19-Cascade blue. The following antibodies were conjugated in our laboratory as described [27]: CD8-QD705, CD57-QD545. Unconjugated antibodies were obtained from BD Biosciences. Quantum Dots were obtained from Quantum Dot Corporation (Hayward, CA), Alexa 680 and Cascade blue were obtained from Molecular Probes (Eugene, OR), and Cy5 was obtained from Amersham Biosciences (Pittsburgh, PA). A violet fluorescent reactive dye (Molecular Probes) was used as a viability marker to exclude dead cells from analysis, as described [51].
Stimulation and staining
Freshly thawed, cryopreserved PBMC were resuspended at 1−5 × 106 cells/mL in complete RPMI media (RPMI 1640 supplemented with 10% heat inactivated FCS, 100 U/mL penicillin G, 100 μg/mL streptomycin sulfate and 1.7 mM sodium glutamate) containing 10 U/mL DNaseI (Roche Diagnostics, Indianapolis, IN) and rested overnight at 37°C. Costimulatory antibodies (anti-CD28 and anti-CD49d; 1 μg/ mL each; BD Biosciences), monensin (Golgistop, 0.7 μL/mL; BD Biosciences), brefeldin A (10 μg/mL; Sigma-Aldrich), and anti-CD107a-Alexa 680 were then added to all tubes. Overlapping Gag peptide pools (final concentration 2 μg/mL/ peptide) were used to stimulate HIV-specific responses. HIV-1 or HIV-2 Gag peptide pools were utilized according to the status of the individual. For each individual, a negative control containing only anti-CD28 and anti-CD49d was used to measure antigen-independent stimulation and a positive control Staphylococcus Enterotoxin B (SEB; final concentration 2 μg/mL; Sigma-Aldrich) was used to ensure that cells were responsive during each experiment. Cells were incubated for 5.5 h at 37°C. Following incubation, cells were washed using wash buffer (PBS containing 1% serum bovine albumin and 0.1% sodium azide) and stained with pre-titered surface antibodies. Cells were then washed and fix/permeabilized with Cytofix/Cytoperm according to the manufacturer's instructions (BD PharMingen). Following permeabilization, cells were washed twice with Cytofix/Cytoperm wash buffer and stained intracellularly with pre-titered antibodies specific for CD3, cytokines, and chemokines. Following staining, cells were washed, fixed in PBS containing 1% paraformaldehyde, and analyzed immediately by flow cytometry.
Flow cytometry
Cells were analyzed on a FACSAria cell sorter modified to detect 18 fluorophores. Between 1 × 106 and 2.5 × 106 events were collected per sample for analysis. Data analysis was performed using FlowJo version 8.1.1 (TreeStar, San Carlos, CA). Forward scatter (FSC)-area versus FSC-height parameters were used to exclude cell doublets followed by exclusion of dead cells, monocytes, and B cells. Small lymphocytes were identified using forward and side scatter properties. T cells were identified by gating on cells positive for CD3. CD4+ T cells were identified by first excluding any CD3+CD8+ cells and then gating on cells positive for CD4. Similarly, CD8+ T cells were identified by initial exclusion of CD3+CD4+ cells, and subsequent gating on cells positive for CD8. These gates were designed to include stimulated cells that may have down-regulated surface expression of CD3, CD4, or CD8 by plotting versus IFN-γ (for example, see Fig. 1A). For functional analysis, memory T cells were then isolated by excluding naive cells (CD27+CD45RO−). After identifying memory CD4+ or CD8+ T cells, functional analysis included creation of gates for each of our measured five functions (IFN-γ, IL-2, TNF-α, MIP-1β, CD107a). Boolean gate arrays were then created using the FlowJo platform. This analysis determined the frequency of each of 32 possible response patterns based on all possible combinations of our five functions of interest. Nonspecific background responses detected in the 28/49d control tubes were then subtracted from responses in stimulated samples for each of the 32 response patterns individually. We detected a significant amount of TNF-α background in our unstimulated samples from Gambian HIV-1 and HIV-2 infected donors. These were live, memory CD4+ T cells producing TNF-α alone (in the absence of any of the other four functions) ex vivo. This TNF-α single-positive background was specific to CD4+ T cells, was not detected among HIV-specific CD8+ T cells, and was found exclusively in PBMC samples isolated from Gambians. We did not detect this background in normal healthy volunteers or PBMC from HIV-infected donors in the United States. We hypothesize that these responses come from real, activated memory CD4+ T cells in the peripheral blood of these HIV-infected Gambians, possibly a population of cells that were activated in vivo in response to another antigen. This population of cells was excluded from further analysis for the CD4+ T cell population, resulting in an analysis of 30 distinct functional populations of CD4+ T cells as opposed to 31 populations of CD8+ T cells.
Data analysis and statistics
SPICE (Simplified Presentation of Incredibly Complex Evaluations, Version 2.9, Mario Roederer, Vaccine Research Center, NIAID, NIH) was used to analyze polychromatic flow cytometry data and to generate graphical representations of T cell responses. Since all Gag-stimulated samples were background subtracted, those response patterns where the response frequency in the 28/49d sample was greater than the peptide-stimulated sample had negative values. These values were set to zero. However, in order to avoid a systematic bias in setting to zero only these negative values, we set a threshold of twice the 90th percentile (which approximates the 95th percentile), such that frequencies below this value were also set to zero. This value was chosen based on the distribution of negative values in the background-subtracted dataset for CD4+ and CD8+ T cell responses individually. This threshold was 0.012% for CD4+ T cell responses and 0.008% for CD8+ T cell responses in this dataset. Statistical tests were performed using Mann Whitney U test (GraphPad Prism version 4.0) and permutation test (SPICE, version 2.9). Differences were considered statistically significant when p <0.05.
Acknowledgements
We thank the medical, nursing, field, and laboratory staff of the MRC Unit in The Gambia for help in obtaining blood samples for this study, in particular Kevin Peterson, Akum Aveika, Ramu Sarge-Njie, and Bakary Sanneh, for patient recruitment and laboratory assistance. We also acknowledge the generosity of the patients from the clinic who donated these blood samples. We thank Joanne Yu and Pratip Chattopadhyay for custom antibody conjugation and Stephen Perfetto and Richard Nguyen for flow cytometry assistance. Finally, we thank Danny Douek and Jason Brenchley for thoughtful discussions and critical review of this manuscript. This work was funded by the National Institutes of Health, USA, and the Medical Research Council, UK.
Abbreviation
LTNP:
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Poulsen AG, Aaby P, Larsen O, Jensen H, Naucler A, Lisse IM, Christiansen CB, et al. 9-year HIV-2-associated mortality in an urban community in Bissau, West Africa. Lancet. 1997;349:911–914. doi: 10.1016/S0140-6736(96)04402-9. [DOI] [PubMed] [Google Scholar]
- 2.Schim van der Loeff MF, Jaffar S, Aveika AA, Sabally S, Corrah T, Harding E, Alabi A, et al. Mortality of HIV-1, HIV-2 and HIV-1/HIV-2 dually infected patients in a clinic-based cohort in The Gambia. Aids. 2002;16:1775–1783. doi: 10.1097/00002030-200209060-00010. [DOI] [PubMed] [Google Scholar]
- 3.Ariyoshi K, Jaffar S, Alabi AS, Berry N, Schim van der Loeff M, Sabally S, N'Gom PT, et al. Plasma RNA viral load predicts the rate of CD4 T cell decline and death in HIV-2-infected patients in West Africa. Aids. 2000;14:339–344. doi: 10.1097/00002030-200003100-00006. [DOI] [PubMed] [Google Scholar]
- 4.Berry N, Jaffar S, Schim van der Loeff M, Ariyoshi K, Harding E, N'Gom PT, Dias F, et al. Low level viremia and high CD4% predict normal survival in a cohort of HIV type-2-infected villagers. AIDS Res. Hum. Retroviruses. 2002;18:1167–1173. doi: 10.1089/08892220260387904. [DOI] [PubMed] [Google Scholar]
- 5.Ariyoshi K, Berry N, Wilkins A, Ricard D, Aaby P, Naucler A, Ngom PT, et al. A community-based study of human immunodeficiency virus type 2 provirus load in rural village in West Africa. J. Infect. Dis. 1996;173:245–248. doi: 10.1093/infdis/173.1.245. [DOI] [PubMed] [Google Scholar]
- 6.Berry N, Ariyoshi K, Jobe O, Ngum PT, Corrah T, Wilkins A, Whittle H, Tedder R. HIV type 2 proviral load measured by quantitative polymerase chain reaction correlates with CD4+ lymphopenia in HIV type 2-infected individuals. AIDS Res. Hum. Retroviruses. 1994;10:1031–1037. doi: 10.1089/aid.1994.10.1031. [DOI] [PubMed] [Google Scholar]
- 7.Sarr AD, Popper S, Thior I, Hamel DJ, Sankale JL, Siby T, Marlink R, et al. Relation between HIV-2 proviral load and CD4+ lymphocyte count differs in monotypic and dual HIV infections. J. Hum. Virol. 1999;2:45–51. [PubMed] [Google Scholar]
- 8.Whittle HC, Ariyoshi K, Rowland-Jones S. HIV-2 and T cell recognition. Curr. Opin. Immunol. 1998;10:382–387. doi: 10.1016/s0952-7915(98)80108-8. [DOI] [PubMed] [Google Scholar]
- 9.Gotch F, McAdam SN, Allsopp CE, Gallimore A, Elvin J, Kieny MP, Hill AV, et al. Cytotoxic T cells in HIV2 seropositive Gambians. Identification of a virus-specific MHC-restricted peptide epitope. J. Immunol. 1993;151:3361–3369. [PubMed] [Google Scholar]
- 10.Bertoletti A, Cham F, McAdam S, Rostron T, Rowland-Jones S, Sabally S, Corrah T, et al. Cytotoxic T cells from human immunodeficiency virus type 2-infected patients frequently cross-react with different human immunodeficiency virus type 1 clades. J. Virol. 1998;72:2439–2448. doi: 10.1128/jvi.72.3.2439-2448.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillespie GM, Pinheiro S, Sayeid-Al-Jamee M, Alabi A, Kaye S, Sabally S, Sarge-Njie R, et al. CD8(+) T cell responses to human immunodeficiency viruses type 2 (HIV-2) and type 1 (HIV-1) gag proteins are distinguishable by magnitude and breadth but not cellular phenotype. Eur. J. Immunol. 2005;35:1445–1453. doi: 10.1002/eji.200526007. [DOI] [PubMed] [Google Scholar]
- 12.Jaye A, Sarge-Njie R, Schim van der Loeff M, Todd J, Alabi A, Sabally S, Corrah T, Whittle H. No differences in cellular immune responses between asymptomatic HIV type 1- and type 2-infected Gambian patients. J. Infect. Dis. 2004;189:498–505. doi: 10.1086/381185. [DOI] [PubMed] [Google Scholar]
- 13.Zheng NN, Kiviat NB, Sow PS, Hawes SE, Wilson A, Diallo-Agne H, Critchlow CW, et al. Comparison of human immunodeficiency virus (HIV)-specific T-cell responses in HIV-1- and HIV-2-infected individuals in Senegal. J. Virol. 2004;78:13934–13942. doi: 10.1128/JVI.78.24.13934-13942.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duvall MG, Jaye A, Dong T, Brenchley JM, Alabi AS, Jeffries DJ, van der Sande M, et al. Maintenance of HIV-specific CD4+ T cell help distinguishes HIV-2 from HIV-1 infection. J. Immunol. 2006;176:6973–6981. doi: 10.4049/jimmunol.176.11.6973. [DOI] [PubMed] [Google Scholar]
- 15.Clerici M, Stocks NI, Zajac RA, Boswell RN, Lucey DR, Via CS, Shearer GM. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J. Clin. Invest. 1989;84:1892–1899. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musey LK, Krieger JN, Hughes JP, Schacker TW, Corey L, McElrath MJ. Early and persistent human immunodeficiency virus type 1 (HIV-1)-specific T helper dysfunction in blood and lymph nodes following acute HIV-1 infection. J. Infect. Dis. 1999;180:278–284. doi: 10.1086/314868. [DOI] [PubMed] [Google Scholar]
- 17.Younes SA, Yassine-Diab B, Dumont AR, Boulassel MR, Grossman Z, Routy JP, Sekaly RP. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ Tcells endowed with proliferative capacity. J. Exp. Med. 2003;198:1909–1922. doi: 10.1084/jem.20031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pitcher CJ, Quittner C, Peterson DM, Connors M, Koup RA, Maino VC, Picker LJ. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 19.Iyasere C, Tilton JC, Johnson AJ, Younes S, Yassine-Diab B, Sekaly RP, Kwok WW, et al. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J. Virol. 2003;77:10900–10909. doi: 10.1128/JVI.77.20.10900-10909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinto LA, Covas MJ, Victorino RM. T-helper cross reactivity to viral recombinant proteins in HIV-2-infected patients. Aids. 1993;7:1389–1391. doi: 10.1097/00002030-199310000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Pinto LA, Covas MJ, Victorino RM. T-helper reactivity to simian immunodeficiency virus gag synthetic peptides in human immunodeficiency virus type 2 infected individuals. J. Med. Virol. 1995;47:139–144. doi: 10.1002/jmv.1890470206. [DOI] [PubMed] [Google Scholar]
- 22.Sousa AE, Chaves AF, Loureiro A, Victorino RM. Comparison of the frequency of interleukin (IL)-2-, interferon-gamma-, and IL-4-producing T cells in 2 diseases, human immunodeficiency virus types 1 and 2, with distinct clinical outcomes. J. Infect. Dis. 2001;184:552–559. doi: 10.1086/322804. [DOI] [PubMed] [Google Scholar]
- 23.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Rosa SC, Lu FX, Yu J, Perfetto SP, Falloon J, Moser S, Evans TG, et al. Vaccination in humans generates broad T cell cytokine responses. J. Immunol. 2004;173:5372–5380. doi: 10.4049/jimmunol.173.9.5372. [DOI] [PubMed] [Google Scholar]
- 25.Betts MR, Exley B, Price DA, Bansal A, Camacho ZT, Teaberry V, West SM, et al. Characterization of functional and phenotypic changes in anti-Gag vaccine-induced Tcell responses and their role in protection after HIV-1 infection. Proc. Natl. Acad. Sci. USA. 2005;102:4512–4517. doi: 10.1073/pnas.0408773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, Asher TE, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8+ T Cell responses. J. Exp. Med. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chattopadhyay PK, Price DA, Harper TF, Betts MR, Yu J, Gostick E, Perfetto SP, et al. Quantum dot semiconductor nanocrystals for immunophenotyping by polychromatic flow cytometry. Nat. Med. 2006;12:972–977. doi: 10.1038/nm1371. [DOI] [PubMed] [Google Scholar]
- 28.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 29.Wang EC, Moss PA, Frodsham P, Bell JI, Borysiewicz LK. CD8highCD57+ T lymphocytes in normal healthy individuals are oligoclonal and respond to human cytomegalovirus. J. Immunol. 1995;155:5046–5056. [PubMed] [Google Scholar]
- 30.Wang EC, Taylor-Wiedeman J, Perera P, Fisher J, Borysiewicz LK. Subsets of CD8+, CD57+ cells in normal healthy individuals: correlations with human cytomegalovirus (HCMV) carrier status, phenotypic and functional analyses. Clin. Exp. Immunol. 1993;94:297–305. doi: 10.1111/j.1365-2249.1993.tb03447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miles DJ, van der Sande M, Jeffries D, Kaye S, Ismaili J, Ojuola O, Sanneh M, et al. Cytomegalovirus infection in Gambian infants leads to profound CD8 T-cell differentiation. J. Virol. 2007;81:5766–5776. doi: 10.1128/JVI.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 33.Henkart P, Henkart M, Millard P, Frederikse P, Bluestone J, Blumenthal R, Yue C, Reynolds C. The role of cytoplasmic granules in cytotoxicity by large granular lymphocytes and cytotoxic T lymphocytes. Adv. Exp. Med. Biol. 1985;184:121–138. doi: 10.1007/978-1-4684-8326-0_9. [DOI] [PubMed] [Google Scholar]
- 34.Henkart PA. Mechanism of lymphocyte-mediated cytotoxicity. Annu. Rev. Immunol. 1985;3:31–58. doi: 10.1146/annurev.iy.03.040185.000335. [DOI] [PubMed] [Google Scholar]
- 35.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 36.Rubio V, Stuge TB, Singh N, Betts MR, Weber JS, Roederer M, Lee PP. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat. Med. 2003;9:1377–1382. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 37.Zaunders JJ, Dyer WB, Wang B, Munier ML, Miranda-Saksena M, Newton R, Moore J, et al. Identification of circulating antigen-specific CD4+ T lymphocytes with a CCR5+, cytotoxic phenotype in an HIV-1 long-term nonprogressor and in CMV infection. Blood. 2004;103:2238–2247. doi: 10.1182/blood-2003-08-2765. [DOI] [PubMed] [Google Scholar]
- 38.Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, Easterbrook P, et al. Characterization of CD4(+) CTLs ex vivo. J. Immunol. 2002;168:5954–5958. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- 39.Casazza JP, Betts MR, Price DA, Precopio ML, Ruff LE, Brenchley JM, Hill BJ, et al. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J. Exp. Med. 2006;203:2865–2877. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harari A, Vallelian F, Meylan PR, Pantaleo G. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J. Immunol. 2005;174:1037–1045. doi: 10.4049/jimmunol.174.2.1037. [DOI] [PubMed] [Google Scholar]
- 41.Beveridge NE, Price DA, Casazza JP, Pathan AA, Sander CR, Asher TE, Ambrozak DR, et al. Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4(+) memory T lymphocyte populations. Eur. J. Immunol. 2007;37:3089–3100. doi: 10.1002/eji.200737504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harari A, Petitpierre S, Vallelian F, Pantaleo G. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood. 2004;103:966–972. doi: 10.1182/blood-2003-04-1203. [DOI] [PubMed] [Google Scholar]
- 43.Boaz MJ, Waters A, Murad S, Easterbrook PJ, Vyakarnam A. Presence of HIV-1 Gag-specific IFN-gamma+IL-2+ and CD28+IL-2+ CD4 T cell responses is associated with nonprogression in HIV-1 infection. J. Immunol. 2002;169:6376–6385. doi: 10.4049/jimmunol.169.11.6376. [DOI] [PubMed] [Google Scholar]
- 44.Emu B, Sinclair E, Favre D, Moretto WJ, Hsue P, Hoh R, Martin JN, et al. Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J. Virol. 2005;79:14169–14178. doi: 10.1128/JVI.79.22.14169-14178.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 46.Zimmerli SC, Harari A, Cellerai C, Vallelian F, Bart PA, Pantaleo G. HIV-1-specific IFN-{gamma}/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. USA. 2005;102:7239–7244. doi: 10.1073/pnas.0502393102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 48.Berry N, Ariyoshi K, Jaffar S, Sabally S, Corrah T, Tedder R, Whittle H. Low peripheral blood viral HIV-2 RNA in individuals with high CD4 percentage differentiates HIV-2 from HIV-1 infection. J. Hum. Virol. 1998;1:457–468. [PubMed] [Google Scholar]
- 49.Cham F, Heyndrickx L, Janssens W, Van der Auwera G, Vereecken K, De Houwer K, Coppens S, et al. Study of HIV type 1 gag/env variability in The Gambia, using a multiplex DNA polymerase chain reaction. AIDS Res. Hum. Retroviruses. 2000;16:1915–1919. doi: 10.1089/08892220050195874. [DOI] [PubMed] [Google Scholar]
- 50.Andersson S, Norrgren H, Dias F, Biberfeld G, Albert J. Molecular characterization of human immunodeficiency virus (HIV)-1 and –2 in individuals from guinea-bissau with single or dual infections: predominance of a distinct HIV-1 subtype A/G recombinant in West Africa. Virology. 1999;262:312–320. doi: 10.1006/viro.1999.9867. [DOI] [PubMed] [Google Scholar]
- 51.Perfetto SP, Chattopadhyay PK, Lamoreaux L, Nguyen R, Ambrozak D, Koup RA, Roederer M. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J. Immunol. Methods. 2006;313:199–208. doi: 10.1016/j.jim.2006.04.007. [DOI] [PubMed] [Google Scholar]


