Abstract
Reproducible visualization of neurons and glia in human brain is essential for quantitative studies of the cellular changes in neurological disease. However, immunohistochemistry in human brain specimens is often compromised because of prolonged fixation. To select cell lineage–specific antibodies for quantitative studies of neurons and the major types of glia, we used 29 different antibodies, different epitope retrieval methods, and different detection systems to stain tissue arrays of formalin-fixed human brain. The screening pointed at CD45/leukocyte common antigen (LCA), CD68(KP1), 2′,3′ cyclic nucleotide phosphatase (CNPase), glial fibrillary acidic protein (GFAP), HLA-DR, Ki67, neuronal nuclei (NeuN), p25α-antigen, and S100β as candidates for future cell counting purposes, because these markers visualized specific neuronal and glial cell bodies. However, significant negative correlation between staining result and formalin fixation was observed by blinded scoring of staining for CD45/LCA, CNPase, GFAP, and NeuN in brain specimens fixed by immersion and stored up to 10 years in 4% formalin solution at room temperature, independent of donor sex and postmortem interval. In contrast, improved preservation of NeuN and CNPase staining, and full preservation of GFAP and CD45/LCA staining in tissue fixed by perfusion and stored for up to 3 years in 0.1% paraformaldehyde solution at 4C, indicated that immunohistochemistry can be performed in well-preserved biobank material. (J Histochem Cytochem 56:201–221, 2008)
Keywords: tissue array, heat-induced epitope retrieval, stereology, astrocyte, oligodendrocyte, microglia
Studies of the human postmortem brain may lead to generation of new hypotheses on the etiology and pathophysiology of neurological diseases. Archival brain tissue is routinely used in quantitative studies based on classical histochemical methods (Cameron and Rakic 1991; Kostovic and Judas 2002; Marner et al. 2003) and in qualitative immunohistochemical studies (Honig et al. 1996; Delalle et al. 1997; Back et al. 2001; Tosic et al. 2002; Rakic and Zecevic 2003; Tiu et al. 2003; Rezaie et al. 2005). Since the introduction of stereological techniques in neuroscience in the 1980s, quantitative studies of the neuronal and glial cell populations in the human brain have generated significant new knowledge about the histoarchitectonic basis for brain function (Pakkenberg and Gundersen 1997; Pelvig et al. 2003; Pelvig et al. in press; Samuelsen et al. 2003; Abitz et al. 2007) and changes related to pathological conditions (West et al. 1994; Stark et al. 2004; Dorph-Petersen et al. 2007; Kreczmanski et al. 2007). Most stereological studies of neurons and glia are based on identification of cells using morphological criteria (West et al. 1994; Pakkenberg and Gundersen 1997; Pelvig et al. 2003; Pelvig et al. in press; Stark et al. 2004). Despite the widespread use of immunohistochemistry in experimental neuroscience and neuropathology, no studies published in refereed journals have successfully combined stereology and immunohistochemistry in the human brain. To count cells, the cell body and nucleus must be visualized in a way that allows for unequivocal identification of the counting item (Sterio 1984; Gundersen 1986) Furthermore, reproducible staining of collections of brain specimens is required, because quantitative studies inherently are performed in groups of individuals (Lewis 2002; Schmitz and Hof 2005). Difficulties in meeting these basic demands have compromised the combined use of stereology and cell lineage specific immunohistochemical markers in the study of the human brain.
Large collections of human brain material in universities and hospital research departments are available for neurodevelopmental and neuropathological studies (Kostovic et al. 1991; Schmitt et al. 2007; see also www.brainnet-europe.org and www.intbbn.org). To preserve tissue for future analyses, brain banks often store the tissue in fixative, primarily buffered formaldehyde solutions—in some cases for decades (Evers and Uylings 1994,1997; Perl et al. 2000; Pelvig et al. 2003; Pelvig et al. in press; Samuelsen et al. 2003; Schmitt et al. 2007). It is well known that prolonged fixation with formaldehyde leads to covalent alterations and cross-binding of the tissue molecules, giving rise to structural and electrostatic changes of the antigens (Puchtler and Meloan 1985; Mason and O'Leary 1991; Boenisch 2002,2005). Masking of antigens can to some extent be reversed using epitope retrieval techniques. Heat-induced epitope retrieval (HIER), in which the tissue section is heated in a buffer (Shi et al. 1991; Pileri et al. 1997; Evers and Uylings 2000; Boenisch 2001; Kahveci et al. 2003), and treatment with proteolytic enzymes (PrER), which leads to degradation of cross-bound protein in the tissue (Pileri et al. 1997; Frost et al. 2000; Kahveci et al. 2003), are the most widely used epitope retrieval techniques and have been successfully applied to a broad range of antibodies and used in standardized protocols for research and diagnostic purposes (www.nordiqc.org and www.ukneqasicc.ucl.ac.uk).
This study was initiated to identify antibodies and protocols that could visualize neurons and glia in formalin-fixed human brain sufficient to meet the requirements for quantitative stereological studies and to investigate the effect of prolonged fixation on staining quality. In the first part of the study, we used 29 different antibodies, directed against the major types of brain cells and immature neurons and glia, in combination with different epitope retrieval methods and different detection systems to stain tissue arrays containing human brain specimens that had been fixed for 24 hr and up to 10 years. On this basis, we identified protocols for candidate antibodies for future stereological applications, visualizing cell bodies of target cells. In the second part of the study, we applied staining protocols for detection of four candidate antibody combinations—neuronal nuclei (NeuN), 2′,3′ cyclic nucleotide phosphatase (CNPase), glial fibrillary acidic protein (GFAP), and leukocyte common antigen (CD45/LCA)—on a tissue array of parietal cortex from human brains fixed from 8 days to 10 years. The staining quality was scored, and the data were analyzed for correlation between the staining result and fixation, using stratification for sex and postmortem interval (PMI).
Materials and Methods
Human Tissue
The use of archival human brain tissue for this study was approved by the Danish Biomedical Research Ethical Committee for the Region of Southern Denmark (permission number S-20070065). All brains included in the study had been subjected to routine neuropathological examination, and no neuropathological changes had been observed.
For screening of antibodies, two tissue arrays were made from identical samples (tissue array A; Table 1): one as paraffin embedded material and one as frozen material. Both contained 16 specimens from the neocortex, white matter, or pons from normal adult, perinatal, and fetal brains. Samples from Donors 2, 3, 5, 8, 9, and 10 were kindly provided by Dr. Bente Pakkenberg, The Research Laboratory for Stereology and Neuroscience, Bispebjerg Hospital, Copenhagen, Denmark (Danish Biomedical Research Ethical Committee for the Region of Sealand, permission number KF 01-068/98 and KF 01-328/98). Samples from Donors 1, 4, 6, and 7 were from the biobank at the Department of Pathology, Odense University Hospital (OUH), Odense, Denmark.
Table 1.
Human brain material in tissue array A
| Donor
|
Fixation
|
|||||
|---|---|---|---|---|---|---|
| Number | Age | Sex | PMI (days) | Time | Fixative | Diagnosis |
| 1 | 20 G.W. | NA | 3 | 2 days | 4% Lillies | Spontaneous abortion, acute infection |
| 2 | 16 G.W. | NA | NA | 10 years | Picric acid | Provoked abortion, causa socialis |
| 3 | 20 G.W. | NA | NA | 10 years | 4% Lillies | Provoked abortion, suspected syndrome |
| 4 | 40 G.W. | M | 1 | 2½ months | 4% Lillies | Stillborn, ischemia during delivery |
| 5 | 1 month | M | NA | 10 years | 9% Lillies | DiGeorge syndrome |
| 6 | 82 years | M | 2 | 1 day | 4% Lillies | Ruptured abdominal aneurism of aorta |
| 7 | 75 years | M | <1 | 1 day | 4% PFA | NA |
| 8 | 62 years | F | 4 | 3 months | 4% Lillies | NA |
| 9 | 88 years | F | 3 | 4 months | 4% Lillies | Heart failure |
| 10 | 93 years | F | NA | 10 years | 9% Lillies | Pulmonary emboli |
From the fetal brain material (Donors 1–3), specimens were sampled from the lateral telencephalic wall including the cortical plate, intermediate zone, and subventricular zone. From the brains from newborns (Donors 4 and 5), specimens from the neocortex and subcortical white matter were sampled from the frontal region, and in material from adults (Donors 6–10), specimens were sampled from the neocortex and subcortical white matter from the frontal or parietal lobes. PMI, postmortem interval; G.W., gestational week; NA, not available; M, male; PFA, paraformaldehyde in 0.15 M Sørensens phosphate buffer, pH 7.4; F, female.
To analyze the effect of fixation time on the staining result, tissue array B was composed of paraffin-embedded material from specimens sampled in the postcentral gyrus of the neocortex from 29 human brains fixed by immersion, ranging from 8 days to 10 years (Table 2). The samples from Donors 1 and 8–29 were from the biobank at the Department of Pathology, OUH. Samples from Donors 2–7 were from brains donated to the Department of Anatomy and Neurobiology, The Institute of Medical Biology, University of Southern Denmark. The material from Donors 2–6 was fixed by perfusion of the head with 4% Lillies phosphate-buffered formalin solution (PBFS), pH 7.0 (Lillies PBFS: 4% w/v formaldehyde, 75 mM phosphate buffer; Sygehusapotek Fyn, OUH) through the internal carotid artery. The brain was removed from the skull and immersed in 4% paraformaldehyde (PFA) in 0.15 M Sørensens phosphate buffer, pH 7.4, and fixed for 2 weeks at 4C before routine pathology. For long-term storage, this material was kept in 0.1% PFA in 0.15 M Sørensens phosphate buffer, pH 7.4, at 4C. The brain from Donor 7 was fixed by immersion only.
Table 2.
Human brain material in tissue array B
| Donor
|
Fixation
|
|||||
|---|---|---|---|---|---|---|
| Number | Age (days) | Sex | PMI (days) | Time | Fixative | Autopsy diagnosis/death conditions |
| 1 | 63 | M | <1 | 8 days | 4% Lillies | Pneumonia, NIDDM |
| 2a | 86 | M | 2½ | 2 weeksa | 4% Lillies | NA |
| 2b | 86 | M | 2½ | 2 yearsb | 0.1% PFA | NA |
| 3a | — | M | 1 | 2 weeksa | 4% PFA | NA |
| 3b | — | M | 1 | 2½ yearsb | 0.1% PFA | NA |
| 4a | 82 | M | 1 | 2 weeksa | 4% PFA | Heart failure, coronary heart disease |
| 4b | 82 | M | 1 | 2½ yearsb | 0.1% PFA | Heart failure, coronary heart disease |
| 5a | 73 | M | <1 | 2 weeksa | 4% PFA | Respiratory and circulatory arrest after surgery |
| 5b | 73 | M | <1 | 3 yearsb | 0.1% PFA | Respiratory and circulatory arrest after surgery |
| 6a | 59 | M | 2 | 2 weeksa | 4% PFA | Heart failure after AMI |
| 6b | 59 | M | 2 | 3 yearsb | 0.1% PFA | Heart failure after AMI |
| 7a | 88 | F | 2 | 3 weeks | 4% PFA | Heart failure, ischemic heart disease |
| 7b | 88 | F | 2 | 4 yearsb | 0.1% PFA | Heart failure, ischemic heart disease |
| 8 | 71 | F | <1 | 2½ months | 4% Lillies | COLD, hypertension, hypothyroidism |
| 9 | 61 | M | <1 | 3 months | 4% Lillies | Circulatory failure, cancer, IDDM |
| 10 | 70 | M | <1 | 3 months | 4% Lillies | Heart failure, hypertension |
| 11 | 76 | M | 4 | 3 months | 4% Lillies | Acute gastrointestinal bleeding |
| 12 | 65 | M | 4 | 3 months | 4% Lillies | Pneumonia, history of alcoholism |
| 13 | 40 | F | NA | 3 months | 4% Lillies | Lung stasis and atherosclerosis, heart failure |
| 14 | 72 | M | 1 | 3½ months | 4% Lillies | Pneumonia, duodenal ulcus, history of alcoholism |
| 15 | 55 | M | 1 | 3½ months | 4% Lillies | Acute gastrointestinal bleeding |
| 16 | 75 | M | 2½ | 3½ months | 4% Lillies | Cancer |
| 16 | 31 | F | 1 | 3¾ months | 4% Lillies | Leukemia |
| 18 | 88 | F | 6 | 4 months | 4% Lillies | Circulatory failure after femoral fracture |
| 19 | 81 | M | 5 | 4 months | 4% Lillies | Multiorgan failure, history of alcoholism |
| 20 | 88 | F | 3 | 4 months | 4% Lillies | Heart failure |
| 21 | 79 | M | NA | 2 years | 4% Lillies | NA |
| 22 | 79 | M | NA | 2 years | 4% Lillies | NA |
| 23 | 86 | M | 2 | 8 years | 4% Lillies | Respiratory and circulatory failure, lung cancer |
| 24 | 76 | F | 2 | 8 years | 4% Lillies | Heart failure/AMI |
| 25 | 78 | M | 3 | 9½ years | 4% Lillies | Respiratory and circulatory failure, asthma attack |
| 26 | 84 | F | 1 | 9½ years | 4% Lillies | Heart failure, COLD |
| 27 | 81 | M | NA | 10 years | 4% Lillies | Ulcus ventriculi |
| 28 | 93 | F | NA | 10 years | 4% Lillies | Pulmonary emboli |
| 29 | 74 | M | 2 | 10 years | 4% Lillies | Respiratory and circulatory failure after surgery |
The donor was perfused with 4% Lillies PBFS through the internal carotid artery before removal of the brain from the skull, and the brain was immersed in 4% PFA solution.
The material was initially fixed by perfusion and immersion as indicated in Donor #a, but was kept in 0.1% PFA solution at 4C for long-term storage.
Specimens in tissue array B were sampled from the postcentral gyrus and embedded in paraffin. Donor numbers marked with a and b indicate that the samples are from the same brain at different stages of fixation. Donors 20 and 28 were the same as Donors 9 and 10 in tissue array A. NIDDM, non–insulin-dependent diabetes mellitus; AMI, acute myocardial infarction; COLD, chronic obstructive lung disease; IDDM, insulin-dependent diabetes mellitus; PBFS, phosphate-buffered formalin solution.
Paraffin sections were prepared as 5-μm-thick serial sections mounted on 75-μm capillary gap slides (S2024; Dako Cytomation, Dako Nordic a/s, Glostrup, Denmark). Sections were stored in sealed boxes at 4C. Sections from tissue array A were used within 6 months after sectioning, and sections from tissue array B were used within 2 weeks. Cryostate sections were prepared as 10-μm-thick serial sections, mounted on 75-μm capillary gap slides, and stored in sealed boxes containing silica gel at −20C and used within 1 year after sectioning.
Immunohistochemistry
Primary Antibodies
A total of 29 antibodies raised against antigens in neurons, astrocytes, oligodendrocytes, microglia, or proliferating cells were tested (Table 3). All antibodies are well described in the literature, with several reports describing the characterization of staining specificity and previous use in research for each antibody. For control of nonspecific binding, primary antibodies were replaced with buffer or inert isotype-specific antibodies: isotype-specific mouse-IgG1 (X0931, 100 μg/ml) and mouse-IgG2 (X0943, 100 μg/ml), inert rabbit-IgG (X0903, 20 mg/ml), and normal rabbit serum (X0902, rabbit serum format) were all from Dako Cytomation.
Table 3.
Primary antibodies
| Antigen (clone) | Type | Cat. no. | Supplier | References | Notes |
|---|---|---|---|---|---|
| β-tubulin III (Tuj1) | Ms IgG2a | 01409 | Stemcell Techn. | Geisert and Frankfurter (1989) | |
| Menezes and Luskin (1994) | |||||
| CD11b/C3bi-R (2LPM19c) | Ms IgG1 | M0741 | Dako Cytomation | Wright et al. (1988) | |
| Dominguez et al. (2001) | |||||
| CD14 (7) | Ms IgG2a | CD14-223 | Novocastra | Wright et al. (1991) | a |
| Ziegler-Heitbrock et al. (1994) | |||||
| CD34 (QBEnd/10) | Ms IgG1 | NCL-END | Novocastra | Anthony and Ramani (1991) | a |
| Krause et al. (1996) | |||||
| CD39 (A1) | Ms IgG1 | MCA1268 | AbD Serotec | Aversa et al. (1988) | |
| Wang and Guidotti (1998) | |||||
| CD45/ LCA (2B11/ PD7/26) | Ms IgG1 | M0701 | Dako Cytomation | Kurtin and Pinkus (1985) | a,b |
| Sasaki et al. (1996) | |||||
| CD68 (KP1) | Ms IgG1 | M0814 | Dako Cytomation | Pulford et al. (1989) | a,b |
| Sasaki et al. (1996) | |||||
| CD68 (PG-M1) | Ms IgG3 | M0876 | Dako Cytomation | Falini et al. (1993) | a,b |
| Rezaie et al. (2005) | |||||
| CD169 (ED3) | Ms IgG2a | MCA343R | Serotec | van den Berg et al. (1992) | |
| Ikezumi et al. (2005) | |||||
| CNPase (11-5B) | Ms IgG1 | MAB326R | Chemicon International | Kim et al. (1984) | |
| Sprinkle et al. (1987) | |||||
| CNPase (RIP) | Ms IgG | RIP | DSHB | Friedman et al. (1989) | |
| Watanabe et al. (2006) | |||||
| GFAP | Rb IgG | Z0334 | Dako Cytomation | Bignami et al. (1972) | a,b |
| Castellano et al. (1991) | |||||
| HLA-DR (LN-3) | Ms IgG2b | CM083A | Biocare Medical | Johnson et al. (1988) | |
| Swanson and Wick (1990) | |||||
| Ki67-antigen (MIB1) | Ms IgG1 | M0724 | Dako Cytomation | Gerdes et al. (1983) | a,b |
| Cattoretti et al. (1992) | |||||
| MAP-2 | Rb IgG | AB5622 | Chemicon International | Bernhardt and Matus (1984) | |
| Honig et al. (1996) | |||||
| MBP | Rb IgG | A0623 | Dako Cytomation | Johnson et al. (1988) | |
| Hardy et al. (1996) | |||||
| Nestin | Rb IgG | AB5922 | Chemicon International | Messam et al. (2000,2002) | |
| Gu et al. (2002) | |||||
| NeuN (A60) | Ms IgG1 | MAB377 | Chemicon International | Mullen et al. (1992) | |
| Lind et al. (2005) | |||||
| Neurofilament (2F11) | Ms IgG1 | M0762 | Dako Cytomation | Diepholder et al. (1991) | a,b |
| Luider et al. (1992) | |||||
| Neurofilament (DA2/FNP7/RmdO20.11) | Ms IgG1κ | 18-0171Z | Zymed | Lee et al. (1986) | a,b |
| Schmidt et al. (1990) | |||||
| NG2 | Rb IgG | AB5320 | Chemicon International | Ong and Levine (1999) | |
| Chang et al. (2000) | |||||
| Nkx-2.2 (74.6A5) | Ms IgG2b | 74.6A5 | DSHB | Qi et al. (2001) | |
| Sun et al. (2003) | |||||
| NSE (BBS / NC / VI-H14) | Ms IgG1 | M0873 | Dako Cytomation | Shimizu et al. (1983) | |
| Nogami et al. (1998) | |||||
| O4 sulfatide (81/mAB O4) | Ms IgGM | MAB345 | Chemicon International | Sommer and Schachner (1981) | |
| Bansal et al. (1989) | |||||
| PDGFα-R | Rb IgG | Sc-338 | Santa Cruz | Oumesmar et al. (1997) | |
| Zhang et al. (1999) | |||||
| p25α-antigen | Rb IgG | alfa-1 | Aarhus University | Otzen et al. (2005) | |
| Skjoerringe et al. (2006) | |||||
| S100β protein | Rb IgG | Z0311 | Dako Cytomation | Boyes et al. (1986) | a,b |
| Johnson et al. (1988) | |||||
| TOAD-64 | Rb IgG | AB5454 | Chemicon International | Minturn et al. (1995) | |
| Quinn et al. (2003) | |||||
| Vimentin (V9) | Ms IgG1 | M0725 | Dako Cytomation | Schnitzer et al. (1981) | a |
| Osborn et al. (1984) |
Antibodies are used for standardization of immunohistochemical procedures in the Nordic immunohistochemical Quality Control (NordiQC) collaboration.
Antibodies are used or standardization of immunohistochemical procedures in UK National External Quality Assessment Scheme for Immunocytochemistry (UK NEQAS).
CD45/LCA, leukocyte common antigen; CD169, sialoadhesin (CD169); CNPase, 2′,3′-cyclic nucleotide 3′-phosphodiesterase; GFAP, glial fibrillary acidic protein; HLA-DR, HLA-DR complex; MAP-2, microtubule associated protein-2; MBP, myelin basic protein; Ms IgG, mouse immunoglobulin G; NeuN, neuronal nuclei; NG2, NG2 chondroitin sulphate proteoglycan; NSE, neuron-specific enolase; PDGFα-R, platelet-derived growth factor-α receptor; Rb IgG, rabbit immunoglobulin G; TOAD-64, turned on after division-64/Tuc-4. Suppliers: AbD Serotec, Serotec Ltd, Oxford, UK; Biocare Medical, purchased at ProHosp Denmark a/s, Værløse, Denmark; Chemicon International, purchased at AH Diagnostics, Aarhus, Denmark; Dako Cytomation, Dako Nordic a/s, Glostrup, Denmark; DSHB, Developmental Studies Hybridoma Bank, University of Iowa, Ames, IA; Novocastra Laboratories, purchased at Trichem Aps, Frederikssund, Denmark; Santa Cruz Biotechnology, Heidelberg, Germany; Stemcell Technologies SARL, Göteborg, Sweden; Zymed, San Francisco, CA. The p25α antibody was donated by Prof. P-H Jensen at The Institute of Molecular Biology, University of Aarhus, Aarhus, Denmark.
Screening of Antibodies and Effect of Epitope Retrieval and Detection Systems
Screening of antibodies and test of the efficacy of epitope retrieval procedure and detection system were performed several times, using a standard procedure followed by adjustment of (a) antibody dilution, (b) choice of epitope retrieval technique, or (c) choice of detection system in either paraffin or cryostat sections from tissue array A. Because staining was conducted in a Dako Techmate-500 capillary gap staining instrument (Dako Cytomation), a total of 120 slides could be stained “in batch” using up to 60 different combinations of primary and secondary antibodies in each experiment. All incubations and washes were performed at room temperature unless specified.
Paraffin sections were heated for 45 min at 60C to improve adhesion of the section to the slide and deparaffinized in xylene for 4, 3, and 3 min, followed by rehydration in ethanol concentrations decreasing from 99% ethanol to 70% ethanol by several steps lasting 1 min, ending in running tap water. Sections were rinsed in two shifts of distilled water before epitope retrieval, and afterward, the sections were placed in TNT buffer (0.025 M Tris, pH 7.5, 0.03 M NaCl, and 0.05% Tween-20 in distilled water) for staining.
Cryostate sections were used to test antibodies when it was suspected that the antigens could be sensitive to organic solvents. Cryostat sections were washed in several shifts of TNT buffer, and no epitope retrieval was performed, because boiling made sections detach from the glass slides.
HIER was conducted using a microwave oven (Panasonic NN-K655; Skousen, Odense, Denmark) by heating the sections immersed in buffer in plastic Hellendahl vials (ProHosp Denmark a/s; Værløse, Denmark) for 9 min at 880 W and 15 min at 440 W, leaving the sections for 15 min in the gradually cooling buffer. Alternatively, a thermostat-regulated pressure cooker (Digital Decloaking Chamber; Biocare Medical, purchased at ProHosp) was used using the preset program: (1) initial heating and pressurizing to 125C, (2) step SP1 at 125C for 30 sec, (3) step SP2 at 90C for 10 sec, and (4) depressurizing of the pressure cooker. The lid was opened, and the sections were left in the buffer within the boiling chamber for 15 min to cool down. Four different buffers were tested: Tris-EGTA-buffer (TEG; 10 mM Tris, 0.5 mM EGTA, pH 9.0), citrate buffer (C; 10 mM citrate, pH 6.0) (both purchased from Sygehusapotek Fyn, OUH), Target Retrieval Solution diluted 1 + 9 in distilled water (TRS; S1699, Dako Cytomation), and Borg Decloak epitope retrieval buffer (Borg; Biocare Medical, purchased at ProHosp). For PrER, sections were rinsed 2 × 2 min in distilled water and 2 × 2 min in TBS at 37C. Sections were treated with pepsin solution (0.004 g/ml pepsin in 0.01 M HCl; P-7012, Sigma-Aldrich Denmark a/s, Vallensbæk Strand, Denmark) for 20 min or protease solution (0.0005 g/ml Protease type XIV in TBS; P-5147, Sigma) for 15 min at 37C, followed by rinsing in several shifts of TBS at room temperature. For some antibodies, a combination of HIER and PrER was tested.
Staining was performed in a Dako Techmate-500 capillary gap staining-instrument (Dako Cytomation) using the buffers and reagents of the Dako ChemMate system (K5001 and K5006; Dako Cytomation). Three detection systems were applied according to the vendors recommendations: Vectastain Universal Elite ABC Kit (PK-6200; Vector Laboratories, purchased from VWR, Rødovre, Denmark); Dako ChemMate LSAB system, universal mouse/rat/rabbit (K5001), and Dako Envision+ peroxidase-labeled polymer (K4061; both Dako Cytomation). The sequence of the staining program was adjusted to fit the staining procedure recommended by the manufacturer of the detection system. For experiments using Dako ChemMate LSAB system and Vectastain Universal Elite ABC, the staining sequence was (1) TNT buffer, (2) blocking of endogenous biotin (Bussolati et al. 1997) using an avidin/biotin blocking kit (SP-2001; Vector Laboratories) diluted 1 + 5 in TBS, (3) TNT buffer, (4) 30-min incubation in the primary antibodies diluted in Dako ChemMate antibody diluent (S2022; Dako Cytomation), (5) TNT buffer, (6) 30-min incubation in biotinylated secondary antibody solution, (7) TNT buffer, (8) blocking of endogenous peroxidase using the Dako ChemMate HP-Block solution (S2023; Dako Cytomation), (9) TNT buffer, (10) 25-min incubation in horseradish peroxidase (HRP)-labeled streptavidin solution, (11) TNT buffer, (12) chromogenic reaction for 3 × 5 min in Dako DAB+-chromogenic kit, and (13) TNT buffer. The program sequence for staining with Dako Envision+ peroxidase-labeled polymer (K4061; both Dako Cytomation) was modified by removing Step 2 blocking of endogenous biotin and Step 10 incubation with HRP-labeled streptavidin solution. After immunohistochemistry, sections were counterstained in Mayer acidic hemalum for 10 min, rinsed for 10 min in running tap water, and coverslipped with Aquatex (1.08562.0050; Merck, purchased at VWR International a/s, Albertslund, Denmark).
Staining for Analysis of the Effect of Fixation
For this study, three staining experiments were performed, resulting in five sets of stained sections. Paraffin sections from tissue array B were deparaffinized, rehydrated, and subjected to HIER in TEG buffer using a microwave oven for heating. The sections were stained in the Dako Techmate-500 capillary gap staining instrument using the Dako Envision+ peroxidase-labeled polymer detection system for detection of NeuN (mouse anti-neuronal nuclei, 1:400, clone A60, IgG1, MAB377; Chemicon International, purchased at AH Diagnostics, Aarhus, Denmark), GFAP (rabbit anti-GFAP, 1:2000, Z0334; Dako Cytomation), CNPase (mouse anti-CNPase, 1:300, clone 11-5B, IgG1, MAB326R; Chemicon International), and CD45/LCA (mouse anti-CD45/LCA, 1:100, clone 2B11/PD7/26, IgG1, M0701; Dako Cytomation). Finally, the sections were counterstained in Mayers acidic hemalum and coverslipped with Aquatex.
Analysis of Staining Results and Statistics
The staining results from the screening of antibodies in tissue array A were scored by two investigators (LL and IDS) on a scale from 0 to 5: 0, no staining; 1, weak specific signal; 2, weak specific signal giving the impression of lack of stained cells or structures or high level of nonspecific staining; 3, acceptable level of specific signal; 4, high level of specific staining considered optimal for cell counting; 5, very high level of staining that, however, compromised the identification of the cellular nucleus. The results of the stainings for NeuN, GFAP, CNPase, and CD45 in tissue array B were scored by two investigators (BF and HDS) blinded to the fixation time and origin of the specimens. For each staining, five sections resulting from three separate experiments were rated on a scale from 0 to 4 (Table 4). The classification was inspired by the recommendations given by NordiQC (www.nordiqc.org) for evaluation of immunohistochemical staining results. For each staining, a mean score, SD, and SEM were calculated. Statistical analysis for correlation between scores and fixation time was done using the Spearman r test in GraphPad Prism 4 for Mac OS×. The Spearman r test is non-parametric and appropriate for statistics on rank-ordered data. Because the sex of the donor, the PMI, and the method of fixation by either immersion or perfusion represent potential confounding parameters to the expression of the antigen in the specimens, the data were stratified according to these parameters before the statistical analysis. The level of statistical significance was set at p<0.05. In figures, p values are indicated as follows: *p<0.05, **p<0.01, and ***p<0.001.
Table 4.
Scale for scoring of staining results in tissue array B
| Score | Classification | Definition |
|---|---|---|
| 0 | No staining | No specific staining |
| 1 | Poor staining | Very insufficient staining: presenting a very weak staining signal, false-negative staining of cells or tissue components, or false-positive staining reaction in cells or tissue components. Incompatible with cell counting. |
| 2 | Borderline staining | Insufficient staining: presenting too weak staining, false-negative staining of cells, or false-positive staining of cells. Incompatible with cell counting |
| 3 | Good staining | Staining is acceptable visualizing the appropriate cells and tissue components. The staining still could be optimized for improving the staining intensity and signal-to-noise ratio. Suitable for cell counting. |
| 4 | Optimal staining | Perfect or close to perfect staining result visualizing the appropriate cells and tissue components with a high signal-to-noise ratio. Suitable for cell counting. |
Photodocumentation
Sections were photographed using an Olympus DP70 digital camera mounted on an Olympus BX51 microscope and connected to a PC with the Olympus DP software (Olympus Danmark a/s, Ballerup, Denmark). Adobe Photoshop CS was used to adjust contrast of and to set up figures.
Results
Identification of Immunohistochemical Markers for Visualization of Neurons and Glia
Neurons
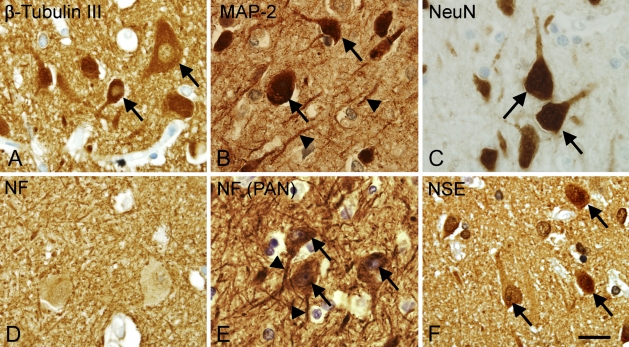
Antibodies raised against the markers β-tubulin type III, microtubule associated protein 2 (MAP-2), NeuN, neurofilament (NF), neuron-specific enolase (NSE), and Turned on after division-64 (TOAD-64) were tested in tissue array A (Table 1). NF(PAN) was only tested in tissue array B (Table 2). Optimal dilution for each antibody is given in Table 5, along with the epitope retrieval technique and the evaluation of the staining result. In adult neocortex, staining for β-tubulin III, MAP-2, NF(PAN), and NSE gave excellent results in adult brain tissue, labeling cell bodies, dendrites, and axons (Figures 1A, 1B, and 1F). Because of the strong labeling of the neuropil, these stainings were, however, considered less suitable for cellular quantification. NeuN was an excellent marker of neuronal cell bodies and nuclei (Figure 1C), and it left the neuropil unstained (Figure 1C). Staining for NF mainly located to the neuropil, whereas the neuronal cell bodies were unstained (Figure 1D). Apart from NF and NF(PAN) that were resistant to prolonged fixation, staining results were better in short-term fixed tissue. Particularly, NeuN could not be shown in tissue fixed for 4 months (Table 5). In the short-term fixed fetal tissue, β-tubulin III and NSE gave rise to ubiquitous staining of cells in the cortical plate, whereas staining for MAP-2 and NeuN detected singular neurons (data not shown). The staining for TOAD-64 resulted in a fiber-like staining of the neuropil in tissue from adult, fetal (Figure 2A), and newborn brain (Figure 2B).
Table 5.
Screening of candidate markers for human brains cells in tissue array A
| Fixation time
|
||||||
|---|---|---|---|---|---|---|
| Antigen (clone) | HIER | Dilution | 24 hr | 4 months | 10 years | Observed specificity |
| β-tubulin III | TEG | 1:500 | 4 | 3 | 0-1 | Neuropil and neuronal bodies |
| CD11b/C3bi-R | Cryo | 1:25 | 0 | 0 | 0 | No staining detected in paraffin or cryostat sections |
| CD14 | TEG | 1:25 | 3 | 0 | 0 | Perivascular macrophages |
| CD34 | TEG | 1:10 | 4 | 3 | 0 | Endothelium and white blood cells |
| CD39 | Cryo | 1:100 | 3 | 0 | 0 | Endothelium, astroglia, and macrophages |
| CD45/ LCA | TEG | 1:100 | 4 | 2-3 | 0 | Microglia, macrophages, and lymphocytes |
| CD68 (KP1) | C/Borg | 1:500 | 4 | 3 | 2 | Microglia and macrophages |
| CD68 (PGM1) | C | 1:400 | 4 | 3 | 0-1 | Lymphocytes, astrocytes, and microglia |
| CD169 | Cryo | 1:100 | 3 | 0 | 0 | Endothelial and perivascular macrophages in frozen tissue |
| CNPase | TEG/Borg | 1:300 | 4 | 3-4 | 0 | Myelinated fibers and round cell bodies |
| CNPase (RIP) | TEG/Borg | 1:100 | 4 | 4 | 0 | Myelinated fibers |
| GFAP | Non/TEG | 1:2000 | 4 | 3-4 | 3 | Astroglia in WM and neocortex |
| HLA-DR | TEG/TRS | 1:15 | 3-4 | 2 | 0 | Microglia/macrophages and lymphocytes in adult tissue |
| Ki67-antigen | TEG | 1:50 | 4 | 4 | 2-3 | Single or double profiles in perivascular space and SVZ |
| MAP-2 | TEG | 1:500 | 3-4 | 2-3 | 0 | Neurons and proximal part of apical dendrites |
| MBP | TEG/Borg | 1:300 | 4 | 3-4 | 3 | Myelinated fibers in adult. |
| Nestin | TEG/TRS | 1:100 | 4 | 3 | 2 | Endothelial cells/vessel wall |
| NeuN | TEG/TRS | 1:400 | 3-4 | 0 | 0 | Neuronal cell bodies |
| Neurofilament (2F11) | TEG | 1:200 | 4 | 4 | 4 | Neuronal cell processes |
| Neurofilament (DA2/FNP7/RmdO20.11) | TEG | 1:500 | 3-4 | 3 | 0-1 | Neuronal soma, apical dendrites and axons |
| NG2 | Cryo | 1:300 | 0 | 0 | 0 | No staining in neither paraffin nor cryostat sections |
| Nkx-2.2 | TEG/Borg | 1:50 | 3 | 0 | 0 | No reaction in adult tissue. Small cells in WM in newborn tissue |
| NSE | TEG | 1:1000 | 3-4 | 3-4 | 2 | Neuropil |
| O4 sulfatide | TEG/TRS | 1:80 | 1-2 | 1 | 0 | Myelinated fibers |
| PDGFα-R | TEG | 1:300 | 4-3 | 0 | 0 | No reaction in adult tissue. Small cells in WM in newborn tissue |
| p25α-antigen | TEG | 1:1000 | 4 | 4 | 0 | Neuropil and round cell bodies connected to myelin fibers |
| S100β protein | Non/TEG | 1:500 | 4 | 4 | 3 | Astroglia in WM and neocortex |
| TOAD-64 | TEG/C | 1:1000 | 1 | 0 | 0 | Neuropil |
| Vimentin | TEG | 1:200 | 4 | 2 | 1-3 | Astroglia and endothelial cells/vessel wall |
Each antibody was applied in a range of dilutions using different epitope retrieval methods in sections from tissue array A (Table 1). Staining results of repeated experiments were scored, and on this basis, the optimal staining result for each antibody was identified. For all antibodies, HIER was superior to proteolytic pretreatment, but the buffer of choice varied between antibodies, as did dilutions of antibodies. HIER, heat-induced epitope retrieval; Borg, Borg Decloak epitope retrieval buffer; C, citrate-buffer, pH 6.0; Cryo, staining in cryostat sections with no epitope retrieval; Non, no epitope retrieval; TEG, Tris-EGTA buffer, pH 9.0; TRS, target retrieval solution; SVZ, subventricular zone; WM, white matter.
Figure 1.
Visualization of neuronal cell bodies and cell processes vary with the choice of antigen/antibody combination. High-magnification micrographs showing the staining for β-tubulin III (A), microtubule-associated protein-2 (MAP-2) (B), neuronal nuclei (NeuN) (C), neurofilaments (NF) (D), NF [PAN, clone: DA2/FNP7/RmdO20.11 (PAN)] (E), and neuron-specific enolase (NSE) (F) were acquired in layer V of the parietal neocortex from an adult donor (A–D,F: Donor 6 in Table 1; E: Donor 4 in Table 2). Paraffin sections were stained using Dako ChemMate LSAB system, the heat-induced epitope retrieval (HIER) method, and antibody dilutions listed in Table 5. Arrows indicate labeled neuronal cell bodies; arrowheads indicate labeled cell processes. Bar = 20 μm.
Figure 2.
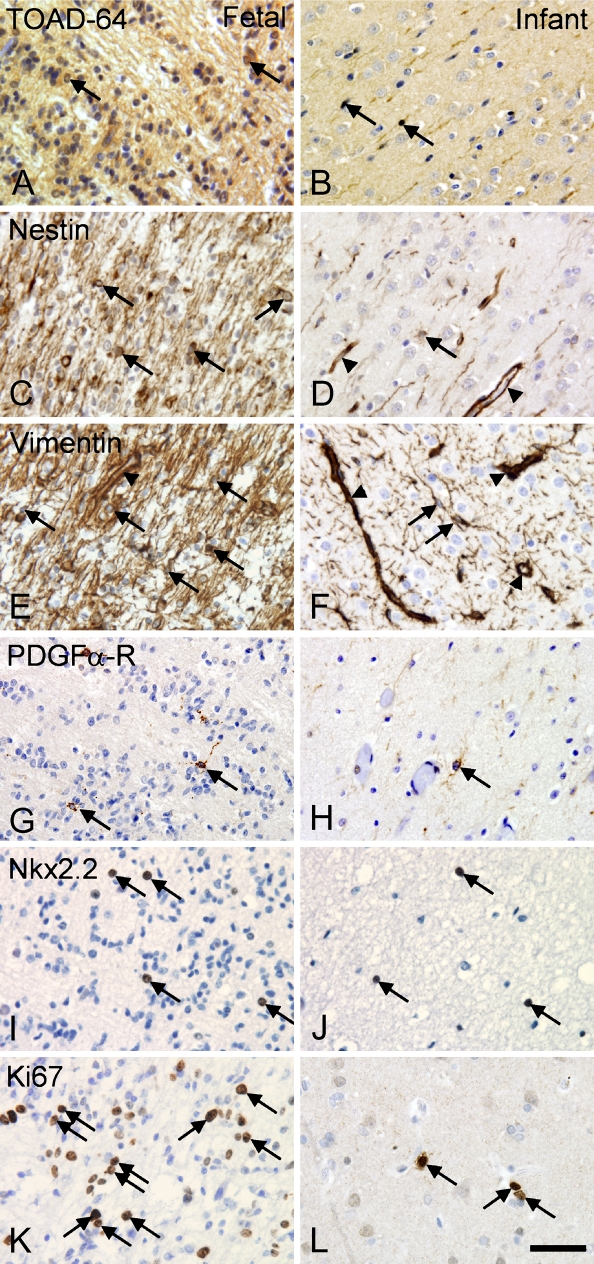
Differences in cellular expression of developmentally regulated antigens in fetal and infant tissue. (A,B) Turned on after division-64/Tuc-4 (TOAD-64), (C,D) nestin, (E,F) vimentin, (G,H) platelet-derived growth factor-α receptor (PDGFα-R), (I,J) Nkx2.2, and (K,L) Ki67. Photomicrographs were acquired in the intermediate zone in fetal brain (Donor 1 in Table 1) and in white matter in the brain from an infant (Donor 4 in Table 1). Staining was performed in paraffin sections using the Dako ChemMate LSAB system, the HIER method, and antibody dilutions listed in Table 5. Arrows indicate specifically labeled cells; arrowheads (D-F) indicate specific labeling of vascular structures. Bar = 40 μm.
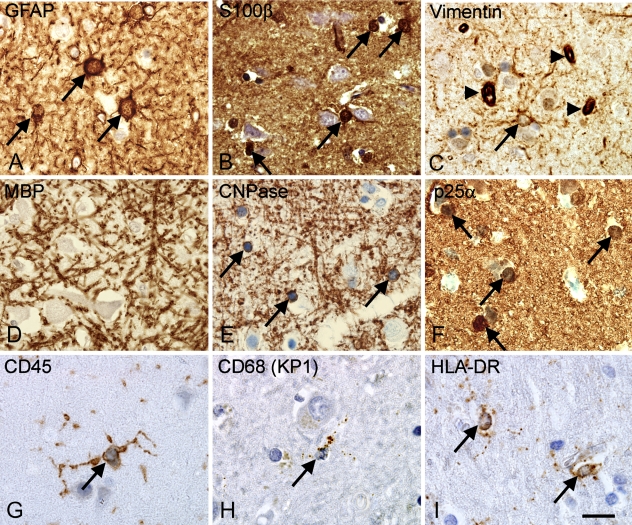
Astrocytes and Radial Glia
Antibodies against GFAP, S100β-protein, and vimentin resulted in high levels of specific staining and excellent single cell rendition in short-term fixed tissue (Figures 3A–3C). These antibodies also labeled astrocytes in tissue fixed for 10 years when applying HIER, although with a loss of staining intensity (Table 5). The antibodies to GFAP and S100β labeled process-bearing astroglia in both in neocortex and subcortical white matter. In the neocortex, the immunohistochemical signal for GFAP appeared to extend further into the distal processes than S100β, probably reflecting different intracellular location of antigens (data not shown). In tissue from fetal brains, nestin (Figure 2C) and vimentin (Figure 2E) labeled the cell bodies and processes of radial glia. Staining was reduced in tissue from newborn infants, where the endothelial cells were intensely labeled (Figures 2D and 2F).
Figure 3.
Visualization of astroglial, oligodendroglial, and microglial cell bodies in the parietal neocortex from an adult donor. (A–C) Staining for astroglial markers showing glial fibrillary acidic protein (GFAP)+ astroglia (A), S100β+ glia (B), and vimentin+ astrocytes and cells of the capillary walls (arrowheads) (C). (D–F) Markers of oligodendroglia and myelin showing myelin basic protein (MBP)+ myelin fibers but no labeled cell bodies (D), 2′3′-cyclic nucleotide 3′-phosphodiesterase (CNPase)+ myelin fibers and round cell bodies (arrows) (E), and round cell bodies labeled by P25 α-antigen/tubulin polymerization promoting protein (p25α) (F). (G–I) Staining for microglia showing CD45+ ramified microglia (G), CD68+ (KP1) microglia showing a more punctuate labeling of their cell processes (H), and HLA-DR labeling the cellular processes (I). Paraffin sections from tissue array A were stained using the Dako ChemMate LSAB system, HIER method, and antibody dilutions listed in Table 5. Micrographs were acquired in neocortical layer VIa in samples from Donor 6 (Table 1). Labeled cell bodies are indicated by arrows. Bar = 20 μm.
Oligodendrocytes and Oligodendrocyte Precursor Cells
Eight different antibodies labeling oligodendrocytes, myelin-antigens, and oligodendrocyte precursor cells were evaluated (Table 5). In the adult neocortex only, the antibody against CNPase and the anti-oligodendroglia antibody (RIP), also recognizing an epitope in CNPase (Watanabe et al. 2006), labeled the cell bodies and the myelinated fibers (Figure 3E, shown for CNPase only). The staining for MBP labeled the myelinated fibers, and in contrast to the staining for CNPase, MBP+ myelinated fibers were still detected in tissue fixed in formalin for 10 years (Table 5). In agreement with recent studies locating the P25α-antigen/tubulin polymerization promoting protein (p25α) to oligodendrocytes (Skjoerringe et al. 2006), the anti-p25α antibody labeled round cell bodies with the nuclear morphology and distribution of oligodendrocytes but not myelinated fibers (Figure 3F). Staining for O4-sulfatide resulted in an inconsistent, weak staining of myelinated fibers and pyramidal neurons in adult tissues when applied to cryostat and paraffin sections (data not shown). Antibodies against Nkx-2.2 and platelet-derived growth factor-α receptor (PDGFα-R) expressed by oligodendrocyte precursor cells, labeled small cells with round nuclei in the intermediate zone in fetal tissue (Figures 2G and 2I) and subcortical white matter from infant brain (Figures 2H and 2J); only very few cells were shown in adult brain (data not shown). Despite the use of different protocols of staining and antigen retrieval, and the additional use of cryostat sections, we were unable to show staining for NG2 (Table 5).
Microglia
The antibodies against CD45/LCA, CD68 (KP1), and the HLA-DR complex stained process-bearing microglial cells and cells with a perivascular location (Figures 3G–3I). The CD68(PGM1) antibody labeled cells with astroglial morphology in addition to perivascular cells and microglial cells (Table 5), giving a result different to the CD68(KP1) antibody. The intensity of staining for CD45, CD68(KP1), and HLA-DR decreased with increasing fixation time (Table 5). In the intermediate zone of short-term fixed fetal tissue, CD45 and CD68(KP1) visualized cells with macrophage morphology, whereas HLA-DR expression was not observed at this age (data not shown). Similar to the markers described above, CD14 was detected in cells with a perivascular location, whereas CD34, CD39, and CD169 appeared to be expressed by endothelial cells (Table 5).
Proliferating Cells
The antibody against Ki67 visualized cell nuclei in the subventricular and intermediate zone in fetal tissue (Figure 2K), in the white matter and neocortex of newborn brain (Figure 2L), and in the perivascular space in adult brains (Table 5). There was a visible decline in intensity of staining with increasing fixation time (Table 5).
Effect of Epitope Retrieval and Choice of Detection System
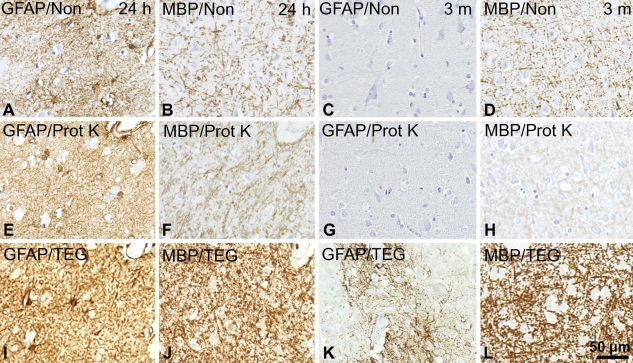
In the initial screening, checkerboard titration was performed for each of the antibodies. The results were evaluated qualitatively selecting the combination of epitope retrieval and antibody concentration, resulting in a high level of specific signal and low level of background staining. In our hands, HIER yielded better results than PrER, even in antibodies in which the manufacturer suggested the use of proteolytic pretreatment such as MBP, GFAP, CD68(KP1), CD68(PGM1), and Ki67 (shown for GFAP and MBP in Figure 4). This observation was in agreement with results in recent test reports on www.ukneqasicc.ucl.ac.uk. No difference was observed using the microwave oven or the pressure cooker as a heating source (data not shown), whereas the choice of epitope retrieval buffer (TEG, TRS, Borg, or citrate buffer) greatly influenced the resultant staining depending on the specific antibody (Table 5). The antibodies against CD39 and CD169 gave rise to specific signal in cryostat sections only.
Figure 4.
Influence of epitope retrieval technique on staining quality. The effect of HIER and proteolytic epitope retrieval (PrER) were tested on stainings for GFAP and MBP in paraffin sections of adult neocortical tissue fixed for 24 hr and 3 months. (A–D) No epitope retrieval (Non), (E–H) pretreatment by PrER with proteinase K (Prot K), and (I–L) HIER by heating in microwave oven in Tris-EGTA buffer, pH 9.0 (TEG buffer). Binding of the primary rabbit-anti-GFAP-antibody (1:1000) or rabbit-anti-MBP-antibody (1:200) was visualized using the Dako LSAB-ChemMate detection system. Photomicrographs were acquired in neocortical layer VI in specimens from Donor 6 (A,B,E,F,I,J) and Donor 8 (C,D,G,H,K,L) from tissue array A (Table 1). Results were superior using epitope retrieval with HIER.
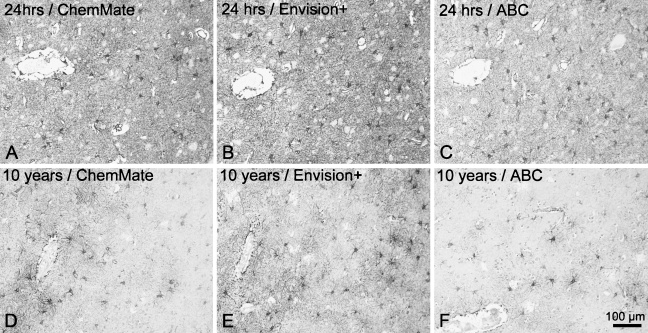
The performance of three detection systems for staining of short- and long-term fixed material was evaluated qualitatively. The Dako ChemMate LSAB-kit gave rise to a high signal level, with considerable background staining in tissue fixed for 24 hr (Figure 5A). The background staining did not compromise results in tissue stored in 4% Lillies PBFS, but the signal level was lower than that of both the Envision+ peroxidase-labeled polymer detection system and the Vectastain Universal Elite ABC kit (Figures 5D–5F). The Envision+ peroxidase-labeled polymer detection system resulted in very high signal levels, visualizing even fine astroglial processes both in tissue fixed in 4% Lillies PBFS for 24 hr (Figure 5B) and 10 years (Figure 5E). The Vectastain Universal Elite ABC kit produced slightly weaker specific signal, yet very low background staining both in short- and long-term fixed specimens (Figures 5C and 5F). Finally, to unveil nonspecific staining caused by the secondary antibodies or detection reagents, sections incubated without the primary antibody or with an inert isotype-specific antibody were included in each staining experiment. With few exceptions, these sections presented no signal, but when applying HIER and the Dako ChemMate LSAB system for staining of samples from adults fixed for 24 hr, a weak staining of white matter, neuropil, and pyramidal neurons was observed (Donors 6 and 7 in tissue array A; data not shown).
Figure 5.
Influence of detection system on staining quality. The visualization of cell bodies and cell processes, illustrated by the visualization of neocortical GFAP+ astrocytes in paraffin sections of adult human brain material fixed for 24 hr (A–C) and 10 years (D–F), depended on the choice of detection system. (A,D) Detection by Dako LSAB-ChemMate detection system. (B,E) Detection by Dako Envision+ peroxidase-labeled polymer. (C,F) Detection by the Vectastain Universal Elite ABC kit. Horseradish peroxidase activity was detected using Dako DAB+-chromogenic kit for 3 × 5 min. Staining was carried out on paraffin sections that were subjected to HIER by heating in a microwave oven in TEG buffer before incubation in rabbit-anti-GFAP-antibody (1:2000). Photomicrographs were acquired in neocortical layers V–VI in specimens from Donor 6 and Donor 10 in tissue array A (Table 1). Results were superior using the Dako Envision+ peroxidase-labeled polymer.
Effect of Fixation Method and Tissue Storage for Visualization of Specific Antigens
During the initial screening, we identified GFAP, NeuN, CNPase, and CD45/LCA as candidate markers for visualization of the cell bodies of the four major classes of brain cells, noting that the staining for GFAP and CD45/LCA appeared to be relatively robust, whereas NeuN and CNPase were sensitive to formalin fixation. We applied these antibodies in a protocol involving HIER in TEG buffer and detection with the Envision+ peroxidase-labeled polymer detection system to paraffin sections from tissue array B (Table 2). The staining quality of individual samples was scored on a scale from 0 to 4 (Table 4; Figure 6) from five different slides and by two independent observers blinded to the arrangement of the tissue array. For each sample, the mean score and SEM was calculated, whereafter the data were stratified for fixation method (Figures 7 and 8), sex (♂: n=16; ♀: n=9), and PMI (PMI ≤ 24 hr, n=7; PMI of 25–48 hr, n=6; PMI ≥ 49 hr, n=12; Figure 7). Results were analyzed by the non-parametric Spearman r test.
Figure 6.
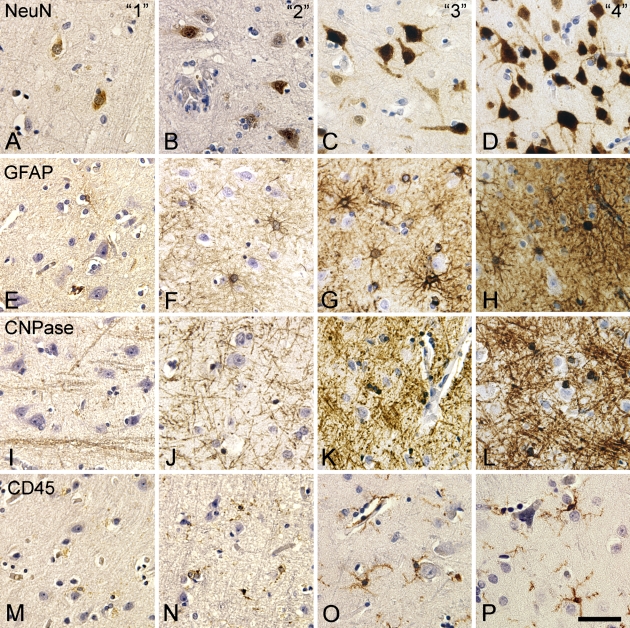
Scoring of immunohistochemical stainings for NeuN, GFAP, CNPase, and CD45. Photomicrographs were acquired in neocortical layer VI in specimens from tissue array B (Table 2) scored as 1 (A,E,I,M), 2 (B,F,J,N), 3 (C,G,K,O), and 4 (D,H,L,P) according to the scale in Table 4. Staining was performed using the HIER method and antibody dilutions in Table 5. Bar = 50 μm.
Figure 7.
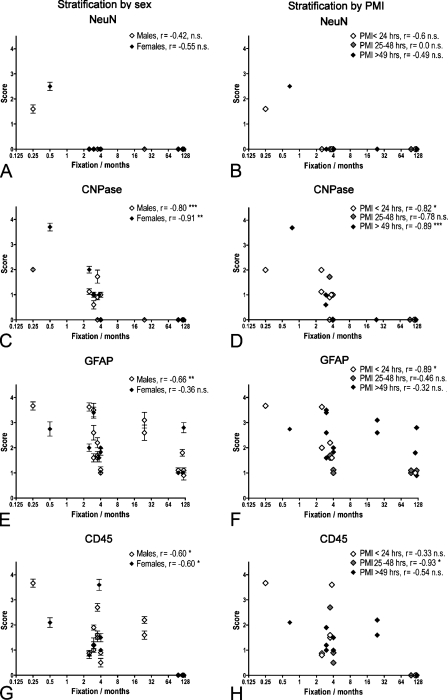
Correlation between immunohistochemical staining result and storage time in 4% Lillies phosphate-buffered formalin solution (PBFS) at room temperature. (A) NeuN, (B) CNPase, (C) GFAP, and (D) CD45. Specimens from tissue array B were stained using HIER and detected by the Dako Envision+ peroxidase-labeled polymer. Stainings were scored using the scale given in Table 4 and shown in Figure 6, and the mean score (points) and SEM (error bars) were calculated. The data were stratified for sex (A,C,E,G) and PMI (B,D,F,H) and analyzed for correlation using the Spearman r test. The r value is given for each group along with the p value. NS, not significant. *p<0.05; **p<0.01; ***p<0.001.
Figure 8.
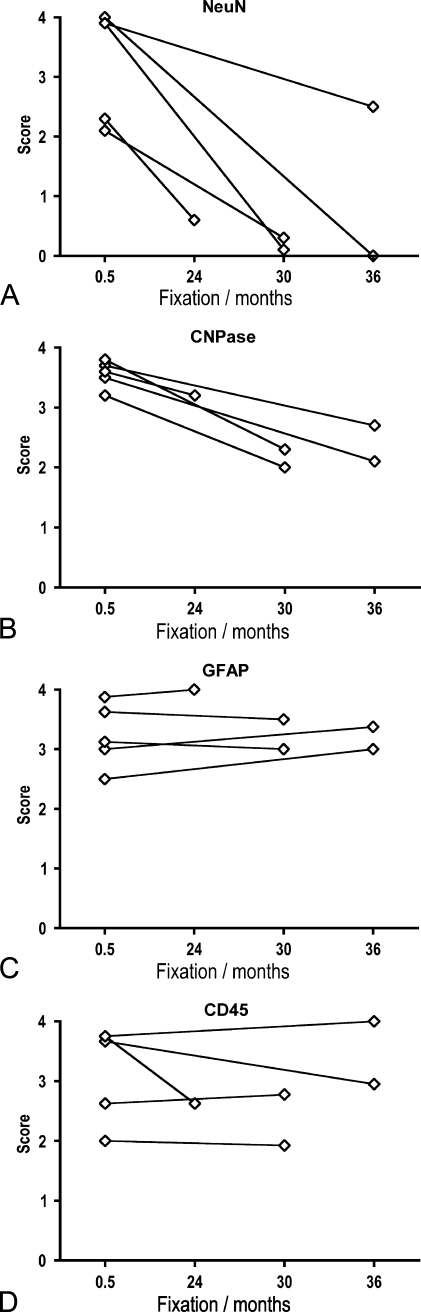
Immunohistochemical staining result in perfusion-fixed brain material. Graphic illustration of changes in immunohistochemical staining results for NeuN (A), CNPase (B), GFAP (C), and CD45 (D) resulting from long-term storage of perfusion fixed brain material in 0.1% paraformaldehyde in 0.15 M Sørensens phosphate buffer, pH 7.4 (PFA) solution at 4C. The stainings were performed on specimens in tissue array B (Donors 2–6 in Table 2), prepared as paraffin sections, stained for NeuN, CNPase, GFAP, and CD45 using HIER, and detected by Dako Envision+. Stainings were scored using the scale given in Table 4 and shown in Figure 6. Data are presented as mean scores of individual specimens (I) at 2 weeks and 3–4 years of fixation. Mean scores of specimens deriving from the same donor are connected by a straight line.
NeuN staining was observed in only two of the specimens fixed by simple immersion in 4% Lillies PBFS (Table 2, Donors 1 and 7a), having mean scores of 1.7 and 2.6, respectively. All specimens fixed by immersion for >2.5 months were scored 0, making stratification for sex and PMI redundant (Figures 7A and 7B). Specimens that had been fixed by perfusion before immersion in 4% PFA for 2 weeks obtained mean scores >2 (two of five specimens: 2–2.4; three of five specimens: 3.8–4; Figure 8A). However, although one specimen maintained a score of 3, most specimens had dropped to a mean score of <1 (four of five specimens: 0–0.6) after 36 months of storage in 0.1% PFA at 4C (Figure 8A).
The mean scores of the CNPase stainings correlated negatively to the storage time in 4% Lillies PBFS at room temperature (Figures 7C and 7D) in both sexes (♂: r = −0.80, p<0.001; ♀: r = −0.91, p<0.01; Figure 7C) and for specimens with a PMI ≤ 24 hr and a PMI ≥ 49 hr (r = −0.89, p<0.001; Figure 7D). The analysis of specimens from the perfusion- and immersion-fixed brains showed that the staining for CNPase deteriorated with storage time (Figure 8B), yet not as pronounced as observed for specimens from non-perfused brain stored in 4% Lillies PBFS at room temperature (Figures 7C and 7D).
In case of GFAP, the mean scores for the GFAP stainings correlated negatively with storage time in 4% Lillies PBFS at room temperature in specimens from male donors (r = −0.66, p<0.01) but not female donors (r = −0.36; Figure 7E) and in specimens with a PMI < 24 hr (r = −0.89, p<0.05; Figure 7F). Unlike CNPase, the analysis of the specimens from the perfusion- and immersion-fixed brains showed that the GFAP staining was well preserved, even with long-term storage of the specimens in 0.1% PFA at 4C (Figure 8C).
The mean score of the staining result for CD45/LCA was negatively correlated to the time stored in 4% Lillies PBFS at room temperature (Figures 7E and 7F). As shown for CNPase, this was independent of the sex of the donor (♂: r = −0.60, p<0.05; ♀: r = −0.60, p<0.05; Figure 7G). Regarding PMI, the mean scores were only negatively correlated to time of fixation for specimens with a PMI from 25 to 48 hr (r = −0.93, p<0.05). As observed for the GFAP staining, the mean scores for the CD45 staining of specimens from the perfusion- and immersion-fixed brains showed that CD45 was well preserved in this material, even with long-term storage of the specimens in 0.1% PFA at 4C (Figure 8D).
In the case of GFAP and CD45, the staining sensitivity seemed to be influenced by the cellular activation state. In some samples, astrocytes and microglia showed an activated phenotype with hypertrophic cell bodies and hypertrophic or blunted processes throughout sections, making these cells very easy to identify. In other specimens, microglia expressed very low levels of CD45 and showed a resting phenotype, with thin angulated processes (Figures 5G and 5H). In such samples, there were occasionally small areas corresponding to the territory of one or two microglial cells with no staining, suggesting that CD45 might not be expressed by all resting-like microglia in human brain.
Discussion
As a first step in successfully applying stereology and immunohistochemistry to the human brain, the intent of the study was to identify candidate immunohistochemical markers for visualization of the cell bodies of neurons, astrocytes, oligodendrocytes, and microglia, which yield reproducible staining results when applied in human brain tissue obtained from autopsies and stored in formalin fixative solutions.
Even well-designed studies in human postmortem brain tissue based on matched groups will face variations deriving from differences in the quality of the tissue because of differences in biology and conservation of the tissue (Lewis 2002). Several variables influence the conformation of an antigen in a tissue section. Autolysis occurring during the PMI is known to degrade or change tissue antigens, making this an important factor to control for (Lewis 2002; Maleszewski et al. 2007; Schmitt et al. 2007). In this context, the choice of fixation method and fixative solution is of concern, with several reports pointing at perfusion with fixative to yield a more rapid and even fixation, beneficial for immunohistochemistry (Adickes et al. 1997; Lyck et al. 2006; Sharma and Grieve 2006). Finally, the storage time in formaldehyde fixative solutions (Beckstead 1994; Nadji et al. 2005) and the method of embedding or tissue stabilization before sectioning is known to influence the availability of tissue antigens (Werner et al. 1996). The specificity and sensitivity of an immunohistochemical staining method is influenced by the choice of epitope retrieval technique (Pileri et al. 1997; Shi et al. 1997; Frost et al. 2000; Boenisch 2005), by the use of detergents (Weruaga et al. 1998; Heffer-Lauc et al. 2007), or by blocking of disturbing endogenous molecules (Bussolati et al. 1997; Shi et al. 1997). Furthermore, the specificity and avidity of the primary or secondary antibodies can be altered by the conditions of the incubation (Boenisch 1999,2001,2002) and by the choice of detection system (Ellis and Halliday 1992; Werner et al. 1996; Pileri et al. 1997).
By checkerboard titration, we systematically tested 29 antibodies in combination with HIER or PrER and three different detection systems in tissue arrays, prepared as either paraffin or cryostat sections. The staining results were then evaluated qualitatively. This strategy has previously been used for establishment and standardization of immunohistochemical protocols for other purposes and in other tissue types (Pileri et al. 1997; Boenisch 2001,2005; Maleszewski et al. 2007) and in international standardization collaborations (www.nordiqc.com; www.ukneqasicc.ucl.ac.uk). We took advantage of an automated staining procedure. This allowed simultaneous staining of large batches of sections, but required the sections to be maximally 10 μm thick, which is not appropriate for cell counting by either the optical or physical dissector, but suitable for a qualitative evaluation of staining results. Furthermore, the staining protocols, involving 30-min incubation with primary antibodies and secondary reagents at room temperature, were selected on the basis of protocols used in international programs for standardization of immunohistochemical methods (www.nordiqc.com; www.ukneqasicc.ucl.ac.uk).
We tested two PrER procedures and HIER in four different buffers, observing that HIER gave better results than PrER (Figure 4; Table 5). Because we observed no difference in the staining results when conducting HIER using a microwave oven or a thermostat-regulated pressure cooker, we performed HIER in the microwave oven, which was easy to handle with large batches of sections. The result of individual antibodies improved by HIER in different buffers, yet for most of the tested antibodies, HIER with TEG was most effective. Although HIER was efficient in increasing the sensitivity of staining in long-term fixed material, the use of HIER in tissue fixed for 24 hr induced nonspecific staining. Notably, when using the Dako ChemMate LSAB detection system in short-term fixed specimens, we observed weak nonspecific staining of myelinated fibers, neuropil, and pyramidal neurons, similar to nonspecific staining patterns reported earlier due to cross-binding of the secondary antibodies in sections treated with HIER (Ellis and Halliday 1992; Shi et al. 1997; Horobin 1998; Weruaga et al. 1998). This background staining was not present in specimens fixed for 2 weeks or longer, and it was absent in staining performed with the Dako Envision+ peroxidase-labeled polymer detection system or Vectastain Universal Elite ABC kit. Because the Dako Envision+ peroxidase-labeled polymer circumvents the problem with false-positive detection of endogenous biotin after HIER treatment (Bussolati et al. 1997) and gives the highest signal-to-noise ratio, we used this detection system to test the effect of tissue fixation on staining intensity.
Prolongation of the incubation period with the active reagents can to some extent improve the sensitivity of an immunohistochemical staining (Boenisch 2002). This strategy is mainly used to allow for the use of highly dilute antibodies (Heinsen et al. 2000), to improve the signal-to-noise ratio when using polyclonal antibodies and high stringency rinsing (Larsson 1989), or to improve the penetration of staining in thick, free-floating sections (Evers and Uylings 1994,1997). We believe that the incubation period of 30 min was sufficient to allow binding of primary antibody for the following reasons: (1) titration of the antibody concentration was performed for all antibodies resulting in reproducible results between experiments, (2) tissue array B was designed so that specimens with short and long storage time in fixative were placed next to each other to control for an effect of the arrangement on the array, (3) the scoring was performed by investigators blinded to the arrangement of the specimens in the tissue array, and (4) the use of 30-min incubation during automated staining of sections originally had previously been shown to result in optimal staining through systematic testing in the Department of Pathology, OUH.
Immunohistochemistry in Tissue Stored in Fixative Solution
Most of the literature describing staining with the antibodies tested in this study are based on staining of material fixed in formaldehyde fixatives for hours or a few days, including studies of the expression of NeuN (Sarnat et al. 1998b), vimentin (Sarnat 1998a), MBP (Zecevic et al. 1998), O4, and NG-2 (Back et al. 2001). Even studies testing the effect of HIER on immunostaining focused on specimens fixed up to 1 week (Pileri et al. 1997; Nadji et al. 2005). Only a few studies have addressed the use of immunohistochemistry on tissue kept in formalin for >1 year (Evers and Uylings 1994,1997). Evers and Uylings (1994,1997) reported good results for detection of neuronal antigens MAP-2, phosphorylated and non-phosphorylated neurofilaments, calbindin, parvalbumin, calretinin, and neuropeptide Y, but also reported that it was impossible to obtain satisfactory staining results in tissue kept in fixative for ≥8 years. In the initial screening, staining results for most antigens were excellent when applied to specimens from brains stored in fixative for 24 hr (Table 5), yielding results comparable to literature reports. Exceptions to this were the antibodies against CD11b, NG-2, and O4 yielding no staining, neither in paraffin nor cryostat sections. The absence of signal could be caused by degradation of the antigens because both NG-2 and O4 have been detected in unfixed, frozen sections of human brain material sampled with only very short postmortem delay (Chang et al. 2000; Back et al. 2001). In this study, most tissue was collected with a longer postmortem delay, raising the possibility that these antigens might be particularly sensitive to postmortem autolysis. With longer time of fixation, staining results varied, raising a demand for a more rigorous test of the effect of fixation time on the staining result than could be achieved with the suboptimally matched tissue samples in tissue array A (Table 1).
Based on the initial screenings, we tested the effect of fixation time on NeuN, CNPase, GFAP, and CD45, applying the HIER/TEG protocol for epitope retrieval and the Dako Envision+ peroxidase-labeled polymer detection system to tissue array B, which was composed of 29 different specimens sampled in the postcentral gyrus from adult donor brains fixed for 8 days to 10 years. Although perfect matching of the donors was not possible, all donors were selected on the basis of their medical history and diagnoses, avoiding inclusion of donors with conditions that could severely impact the brain. Most of the donor material was fixed by simple immersion of the brain in 4% Lillies PBFS at room temperature, as is the routine at the Department of Pathology, OUH, and at the Brain Bank at Bispebjerg Hospital (Pakkenberg and Gundersen 1997; Samuelsen et al. 2003). In addition, we included five sets of specimens sampled from donor brains that had been fixed by perfusion before immersion-fixation for 2 weeks in 4% PFA and storage in 0.1% PFA solution at 4C for 2–3 years. For both types of fixation, the staining results were excellent when brains had been fixed for 2–3 weeks, yet the results pointed at better preservation of sensitive antigens like NeuN and CNPase in perfusion-fixed specimens. NeuN was very sensitive to fixation, with complete disappearance of immunohistochemical signal after 2–3 months of storage in 4% Lillies PBFS, and the staining signal also deteriorated with storage time in 0.1% PFA at 4C. For GFAP and CD45, excellent staining was obtained in several specimens from immersion-fixed brains stored in 4% Lillies PBFS for up to 4 months, yet preservation of these antigens was improved in perfusion-fixed material stored in 0.1% PFA at 4C, which was also pronounced for CNPase. This difference could be caused by several things. First, the perfusion with fixative resulted in a more even and quick fixation of the brain tissue, preventing degradation of tissue antigens. This observation is in line with recent literature suggesting the use of perfusion fixation for human brains to improve the neuropathological examination caused by better preservation of morphology and tissue antigens (Adickes et al. 1997; Sharma and Grieve 2006; Schmitt et al. 2007). Second, it might be better to store the already fixed tissue at 4C in a solution containing less formaldehyde than the usual conduct of storage. The recent consensus report from The Consortium of Brainnet Europe II set a focus on the importance the preparation, fixation, and storage of brain tissue collected by brain banks to better preserve the tissue molecules (Schmitt et al. 2007), and our data add to the discussion on how to fix and store brain tissue.
Selection of Candidate Markers for Future Cell Counting Studies
The histological material used in this study did not conform to the demands set up for unbiased stereology. First, the 5-μm paraffin sections were not thin enough to qualify for counting of neurons or glial cells using the physical disector (Sterio 1984). For this purpose, sets of serial sections cut at 1–2 μm should be used. Second, the 10-μm-thick cryostat sections did not qualify as thick sections for cell counting by the optical disector (Gundersen 1986, West et al. 1991). To use this tool for counting of large cells like neurons, the final section thickness should be at least 25 μm (Andersen and Gundersen 1999; Dorph-Petersen et al. 2001), and penetration of the staining through the section thickness should be validated by analysis of the z-axis distribution of labeled cells (Dorph-Petersen et al. 2001; Gardella et al. 2003). Recent studies in mice have combined the use of immunohistochemistry and stereology for estimation of total numbers of NeuN+ neurons (Lyck et al. 2007), CD11b+ microglia (Wirenfeldt et al. 2003,2007), and GABAergic neurons (Muller et al. 2001; Lifshitz et al. 2007) in specific brain regions by using the optical fractionator.
Based on our results, we suggest NeuN for identification of neurons in the human neocortex, because β-tubulin III–, MAP-2–, NF(PAN)-, and NSE-labeled epitopes are also present in neuronal cell processes. GFAP and S100β can be used to label astroglia, although both markers might be suboptimal. GFAP labeling is low in the protoplasmic astrocytes in the neocortex (Korzhevskii et al. 2005) and high in activated astrocytes (Schmidt-Kastner and Szymas 1990, Jessen 2006). It was recently shown that the binding of the polyclonal rabbit anti-GFAP antibody from Dako Cytomation is sensitive to phosphorylation (Tramontina et al. 2007). S100β has been reported to also label subpopulations of oligodendroglial cells (Rickmann and Wolff 1995; Tiu et al. 2000a,b). Only CNPase and p25α identified the cell bodies of oligodendroglia in adult brain. Nkx2.2+ and PDGFα-R+ oligodendroglial precursor cells were observed in only fetal and newborn brain. Because the anti-p25α-antibody was characterized as a marker for oligodendrocytes just recently (Otzen et al. 2005; Skjoerringe et al. 2006), more knowledge on its specificity is needed before applying this marker in quantitative studies, rendering CNPase the candidate for identification of oligodendrocytes. In case of microglia, staining for CD45/LCA provided better visualization of the cell body than staining for CD68(KP1) and labeled much more microglia than staining for HLA-DR. Microglial expression of hematogenous cell markers is known to depend on the state of activation (Mittelbronn et al. 2001; Perry 2003; Raivich and Banati 2004; Ladeby et al. 2005). We observed some variation in intensity of staining and morphology of microglia, with appearance of coarse, intensely stained profiles in samples from some brains. This raises the question if perimortal activation of microglia may occur, influencing the detection of these cells. Finally, monocytes and perivascular cells also express these markers (Sasaki et al. 1996; Mittelbronn et al. 2001; Ladeby et al. 2005), and the investigator should be able to distinguish between these cells and microglia during quantitative studies. A recent paper suggested the use of an antibody against IBA-1 for labeling of microglia in human brain (Ahmed et al. 2007). This was not tested in this study but could be another potential marker for cell counting purposes.
Although the optical disector has already proven its value for counting of immunohistochemically labeled cells, it may be advantageous to use the physical disector because of transparency of sections with intense specific labeling of the neuropil. The presented staining methodology could easily be implemented in sets of parallel 1- to 2-μm paraffin sections for counting using the physical disector, because the staining method is already optimized for staining of thin sections in large batches. For example, it may be possible to use the protocols for labeling of neurons expressing β-tubulin III, MAP-2, NF(PAN), or NSE in a physical disector design, just as counting of GFAP+ astrocytes should be done using the physical disector because of limited penetration of staining into thick sections (Lyck et al. 2006). Furthermore, in a recent stereological study of microglial responses to axonal lesion, it was noted that reactive microglia were present in the tissue as “clusters,” making it difficult to distinguish individual cells when using the optical disector (Wirenfeldt et al. 2007). Thus, it is necessary to test the immunohistochemical staining in conjunction to actual application to clarify whether the staining is reproducible between subjects and whether it allows identification of the individual cells in the specimen in a way that conforms with the rules for stereological counting.
In conclusion, based on a screening of a range of frequently used antibodies along with epitope retrieval techniques and detection systems, we identified a panel of candidate markers for future stereological studies. We also observed considerable loss of immunohistochemical staining signal in tissue specimens stored for long periods of time in 4% Lillies PBFS at room temperature, which has been widely used in tissue banks. For some markers, this loss could be delayed and possibly reduced in the long term by perfusion fixation before storage in 0.1% PFA at 4C. We believe that the application of immunohistochemistry in future stereological studies of the human brain will be possible with careful selection and validation of staining methods and by the use of well-preserved material.
Acknowledgments
The Augustinus Foundation, The Beckett-Foundation, The Carlsberg Foundation, The Danish Multiple Sclerosis Society, Fonden til Lægevidenskabens Fremme, The Gangsted Family Foundation, the Lundbeck Foundation, The Danish MRC, and The Velux Foundation of 1981 supported this work by grants to L.L. and B.F.
We thank Ole Nielsen, Lisbeth Mortensen, Inger Nissen, and Lene Jørgensen for technical assistance and Dr. Pakkenberg, Research Laboratory for Stereology and Neuroscience, Bispebjerg Hospital, and Dr. Kock, Department of Pathology, Odense University Hospital, for donation of tissue samples. Dr. Fenger, University of Southern Denmark, donated anti-Nkx-2.2 antibody, and the anti-Rip antibody was a gift from Dr. Owens, Montreal Neurological Institute, McGill University, Montreal, Canada.
References
- Abitz M, Nielsen RD, Jones EG, Laursen H, Graem N, Pakkenberg B (2007) Excess of neurons in the human newborn mediodorsal thalamus compared with that of the adult. Cereb Cortex 17:2573–2578 [DOI] [PubMed] [Google Scholar]
- Adickes ED, Folkerth RD, Sims KL (1997) Use of perfusion fixation for improved neuropathologic examination. Arch Pathol Lab Med 121:1199–1206 [PubMed] [Google Scholar]
- Ahmed Z, Shaw G, Sharma VP, Yang C, McGowan E, Dickson DW (2007) Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J Histochem Cytochem 55:687–700 [DOI] [PubMed] [Google Scholar]
- Andersen BB, Gundersen HJ (1999) Pronounced loss of cell nuclei and anisotropic deformation of thick sections. J Microsc 196:69–73 [PubMed] [Google Scholar]
- Anthony PP, Ramani P (1991) Endothelial markers in malignant vascular tumours of the liver: superiority of QB-END/10 over von Willebrand factor and Ulex europaeus agglutinin 1. J Clin Pathol 44:29–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aversa GG, Suranyi MG, Waugh JA, Bishop AG, Hall BM (1988) Detection of a late lymphocyte activation marker by A1, a new monoclonal antibody. Transplant Proc 20:49–52 [PubMed] [Google Scholar]
- Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC (2001) Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci 21:1302–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R, Warrington AE, Gard AL, Ranscht B, Pfeiffer SE (1989) Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. J Neurosci Res 24:548–557 [DOI] [PubMed] [Google Scholar]
- Beckstead JH (1994) A simple technique for preservation of fixation-sensitive antigens in paraffin-embedded tissues. J Histochem Cytochem 42:1127–1134 [DOI] [PubMed] [Google Scholar]
- Bernhardt R, Matus A (1984) Light and electron microscopic studies of the distribution of microtubule-associated protein 2 in rat brain: a difference between dendritic and axonal cytoskeletons. J Comp Neurol 226:203–221 [DOI] [PubMed] [Google Scholar]
- Bignami A, Eng LF, Dahl D, Uyeda CT (1972) Localisation of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res 2:429–435 [DOI] [PubMed] [Google Scholar]
- Boenisch T (1999) Diluent buffer ions and pH: their influence on the performance of monoclonal antibodies in immunohistochemistry. Appl Immunohistochem Mol Morphol 7:300 [Google Scholar]
- Boenisch T (2001) Formalin-fixed and heat-retrieved tissue antigens: a comparison of their immunoreactivity in experimental antibody diluents. Appl Immunohistochem Mol Morphol 9:176–179 [DOI] [PubMed] [Google Scholar]
- Boenisch T (2002) Heat-induced antigen retrieval restores electrostatic forces: prolonging the antibody incubation as an alternative. Appl Immunohistochem Mol Morphol 10:363–367 [DOI] [PubMed] [Google Scholar]
- Boenisch T (2005) Effect of heat-induced antigen retrieval following inconsistent formalin fixation. Appl Immunohistochem Mol Morphol 13:283–286 [DOI] [PubMed] [Google Scholar]
- Boyes BE, Kim SU, Lee V, Sung SC (1986) Immunohistochemical co-localisation of S100b and the glial fibrillary acidic protein in rat brain. Neuroscience 17:857–865 [DOI] [PubMed] [Google Scholar]
- Bussolati G, Gugliotta P, Volante M, Pace M, Papotti M (1997) Retrieved endogenous biotin: a novel marker and a potential pitfall in diagnostic immunohistochemistry. Histopathology 31:400–407 [DOI] [PubMed] [Google Scholar]
- Cameron RS, Rakic P (1991) Glial cell lineage in the cerebral cortex: a review and synthesis. Glia 4:124–137 [DOI] [PubMed] [Google Scholar]
- Castellano B, Gonzalez B, Jensen MB, Pedersen EB, Finsen BR, Zimmer J (1991) A double staining technique for simultaneous demonstration of astrocytes and microglia in brain sections and astroglial cell cultures. J Histochem Cytochem 39:561–568 [DOI] [PubMed] [Google Scholar]
- Cattoretti G, Becker MH, Key G, Duchrow M, Schluter C, Galle J, Gerdes J (1992) Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol 168:357–363 [DOI] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD (2000) NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci 20:6404–6412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delalle I, Evers P, Kostovic I, Uylings HB (1997) Laminar distribution of neuropeptide Y-immunoreactive neurons in human prefrontal cortex during development. J Comp Neurol 379:515–522 [DOI] [PubMed] [Google Scholar]
- Diepholder HM, Schwechheimer K, Mohadjer M, Knoth R, Volk B (1991) A clinicopathologic and immunomorphologic study of 13 cases of ganglioglioma. Cancer 68:2192–2201 [DOI] [PubMed] [Google Scholar]
- Dominguez J, Alvarez B, Alonso F, Thacker E, Haverson K, McCullough K, Summerfield A, et al. (2001) Workshop studies on monoclonal antibodies in the myeloid panel with CD11 specificity. Vet Immunol Immunopathol 80:111–119 [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Nyengaard JR, Gundersen HJ (2001) Tissue shrinkage and unbiased stereological estimation of particle number and size. J Microsc 204:232–246 [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Pierri JN, Wu Q, Sampson AR, Lewis DA (2007) Primary visual cortex volume and total neuron number are reduced in schizophrenia. J Comp Neurol 501:290–301 [DOI] [PubMed] [Google Scholar]
- Ellis J, Halliday G (1992) A comparative study of avidin-biotin-peroxidase complexes for the immunohistochemical detection of antigens in neural tissue. Biotech Histochem 67:367–371 [DOI] [PubMed] [Google Scholar]
- Evers P, Uylings HB (1994) Effects of microwave pretreatment on immunocytochemical staining of vibratome sections and tissue blocks of human cerebral cortex stored in formaldehyde fixative for long periods. J Neurosci Methods 55:163–172 [DOI] [PubMed] [Google Scholar]
- Evers P, Uylings HB (1997) An optimal antigen retrieval method suitable for different antibodies on human brain tissue stored for several years in formaldehyde fixative. J Neurosci Methods 72:197–207 [DOI] [PubMed] [Google Scholar]
- Evers P, Uylings HB (2000) Microwave-stimulated antigen retrieval in neuroscience. In Shi S-R, Gu J, Taylor CR, eds. Antigen Retrieval Techniques. Natick, MA, Eaton Publishing, 139–150
- Falini B, Flenghi L, Pileri S, Gambacorta M, Bigerna B, Durkop H, Eitelbach F, et al. (1993) PG-M1: a new monoclonal antibody directed against a fixative-resistant epitope on the macrophage-restricted form of the CD68 molecule. Am J Pathol 142:1359–1372 [PMC free article] [PubMed] [Google Scholar]
- Friedman B, Hockfield S, Black JA, Woodruff KA, Waxman SG (1989) In situ demonstration of mature oligodendrocytes and their processes: an immunocytochemical study with a new monoclonal antibody, Rip. Glia 2:380–390 [DOI] [PubMed] [Google Scholar]
- Frost AR, Sparks D, Grizzle WE (2000) Methods of antigen recovery vary in their usefulness in unmasking specific antigens in immunohistochemistry. Appl Immunohistochem Mol Morphol 8:236–243 [DOI] [PubMed] [Google Scholar]
- Gardella D, Hatton WJ, Rind HB, Rosen GD, von Bartheld CS (2003) Differential tissue shrinkage and compression in the z-axis: implications for optical disector counting in vibratome-, plastic- and cryosections. J Neurosci Methods 124:45–59 [DOI] [PubMed] [Google Scholar]
- Geisert EE Jr, Frankfurter A (1989) The neuronal response to injury as visualized by immunostaining of class III β-tubulin in the rat. Neurosci Lett 102:137–141 [DOI] [PubMed] [Google Scholar]
- Gerdes J, Schwab U, Lemke H, Stein H (1983) Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 31:13–20 [DOI] [PubMed] [Google Scholar]
- Gu H, Wang S, Messam CA, Yao Z (2002) Distribution of nestin immunoreactivity in the normal adult human forebrain. Brain Res 943:174–180 [DOI] [PubMed] [Google Scholar]
- Gundersen HJ (1986) Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc 143:3–45 [PubMed] [Google Scholar]
- Hardy RJ, Lazzarini RA, Colman DR, Friedrich VL (1996) Cytoplasmic and nuclear localization of myelin basic proteins reveals heterogeneity among oligodendrocytes. J Neurosci Res 46:246–257 [DOI] [PubMed] [Google Scholar]
- Heffer-Lauc M, Viljetić B, Vajn K, Schnaar RL, Lauc G (2007) Effects of detergents on redistribution of gangliosides and GPI-anchored proteins in brain tissue sections. J Histochem Cytochem 55:805–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsen H, Arzberger T, Schmitz C (2000) Celloidin mouting (embedding without infiltration): a new, simple and reliable method for producing serial sections of of high thickness through complete human brains and its application to stereological and immunohistochemical investigations. J Chem Neuroanat 20:49–59 [DOI] [PubMed] [Google Scholar]
- Honig LS, Herrmann K, Shatz CJ (1996) Developmental changes revealed by immunohistochemical markers in human cerebral cortex. Cereb Cortex 6:794–806 [DOI] [PubMed] [Google Scholar]
- Horobin RW (1998) Problems and artifacts of microwave accelerated procedures in neurohistotechnology and resolutions. Methods 15:101–106 [DOI] [PubMed] [Google Scholar]
- Ikezumi Y, Suzuki T, Hayafuji S, Okubo S, Nikolic-Paterson DJ, Kawachi H, Shimizu F, et al. (2005) The sialoadhesin (CD169) expressing a macrophage subset in human proliferative glomerulonephritis. Nephrol Dial Transplant 20:2704–2713 [DOI] [PubMed] [Google Scholar]
- Jessen KR (2006) A brief look at glial cells. Novartis Found Symp 276:5–14 [PubMed] [Google Scholar]
- Johnson MD, Glick AD, Davis BW (1988) Immunohistochemical evaluation of Leu-7, myelin basic-protein, S100-protein, glial-fibrillary acidic-protein, and LN3 immunoreactivity in nerve sheath tumors and sarcomas. Arch Pathol Lab Med 112:155–160 [PubMed] [Google Scholar]
- Kahveci Z, Minbay FZ, Noyan S, Cavusoglu I (2003) A comparison of microwave heating and proteolytic pretreatment antigen retrieval techniques in formalin fixed, paraffin embedded tissues. Biotech Histochem 78:119–128 [DOI] [PubMed] [Google Scholar]
- Kim SU, McMorris FA, Sprinkle TJ (1984) Immunofluorescence demonstration of 2′:3′-cyclic-nucleotide 3′-phosphodiesterase in cultured oligodendrocytes of mouse, rat, calf and human. Brain Res 300:195–199 [DOI] [PubMed] [Google Scholar]
- Korzhevskii DE, Otellin VA, Grigor'ev IP (2005) Glial fibrillary acidic protein in astrocytes in the human neocortex. Neurosci Behav Physiol 35:789–792 [DOI] [PubMed] [Google Scholar]
- Kostovic I, Judas M (2002) The role of the subplate zone in the structural plasticity of the developing human cerebral cortex. Neuroembryology 1:145–153 [Google Scholar]
- Kostovic I, Judas M, Kostovic-Knezevic L, Simic G, Delalle I, Chudy D, Sajin B, et al. (1991) Zagreb research collection of human brains for developmental neurobiologists and clinical neuroscientists. Int J Dev Biol 35:215–230 [PubMed] [Google Scholar]
- Krause DS, Fackler MJ, Civin CI, May WS (1996) CD34: structure, biology, and clinical utility. Blood 87:1–13 [PubMed] [Google Scholar]
- Kreczmanski P, Heinsen H, Mantua V, Woltersdorf F, Masson T, Ulfig N, Schmidt-Kastner R, et al. (2007) Volume, neuron density and total neuron number in five subcortical regions in schizophrenia. Brain 130:678–692 [DOI] [PubMed] [Google Scholar]
- Kurtin PJ, Pinkus GS (1985) Leukocyte common antigen: a diagnostic discriminant between hematopoietic and nonhematopoietic neoplasms in paraffin sections using monoclonal antibodies: correlation with immunologic studies and ultrastructural localization. Hum Pathol 16:353–365 [DOI] [PubMed] [Google Scholar]
- Ladeby R, Wirenfeldt M, Garcia-Ovejero D, Fenger C, Dissing-Olesen L, Dalmau I, Finsen B (2005) Microglial cell population dynamics in the injured adult central nervous system. Brain Res Brain Res Rev 48:196–206 [DOI] [PubMed] [Google Scholar]
- Larsson L-I (1989) Immunocytochemistry: Theory and Practice. 2nd ed. Boca Raton, CRC Press
- Lee VMY, Carden MJ, Schlaepfer WW (1986) Structural similarities and differences between neurofilament proteins from five different species as revealed using monoclonal antibodies. J Neurosci 6:2179–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA (2002) The human brain revisited: opportunities and challenges in post mortem studies of psychiatric disorders. Neuropsychopharmacology 26:143–154 [DOI] [PubMed] [Google Scholar]
- Lifshitz J, Witgen BM, Grady MS (2007) Acute cognitive impairment after lateral fluid percussion brain injury recovers by 1 month: evaluation by conditioned fear response. Behav Brain Res 177:347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind D, Franken S, Kappler J, Jankowski J, Schilling K (2005) Characterization of the neuronal marker NeuN as a multiply phosphorylated antigen with discrete subcellular localization. J Neurosci Res 79:295–302 [DOI] [PubMed] [Google Scholar]
- Luider TM, van Dommelen MW, Tibboel D, Meijers JH, Ten Kate FJ, Trojanowski JQ, Molenaar JC (1992) Differences in phosphorylation state of neurofilament proteins in ganglionic and aganglionic bowel segments of children with Hirschsprung's disease. J Pediatr Surg 27:815–819 [DOI] [PubMed] [Google Scholar]
- Lyck L, Jelsing J, Jensen PS, Lambertsen KL, Pakkenberg B, Finsen B (2006) Immunohistochemical visualization of neurons and specific glial cells for stereological application in the porcine neocortex. J Neurosci Methods 152:229–242 [DOI] [PubMed] [Google Scholar]
- Lyck L, Kroigaard T, Finsen B (2007) Unbiased cell quantification reveals a continued increase in the number of neocortical neurons during early postnatal development in mice. Eur J Neurosci 26:1749–1764 [DOI] [PubMed] [Google Scholar]
- Maleszewski J, Lu J, Fox-Talbot K, Malushka MK (2007) Robust immunohistochemical staining of several classes of proteins in tissue subjected to autolysis. J Histochem Cytochem 55:597–606 [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B (2003) Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol 462:144–152 [DOI] [PubMed] [Google Scholar]
- Mason JT, O'Leary TJ (1991) Effects of formaldehyde fixation on protein secondary structure: a calorimetric and infrared spectroscopic investigation. J Histochem Cytochem 39:225–229 [DOI] [PubMed] [Google Scholar]
- Menezes JR, Luskin MB (1994) Expression of neuron-specific tubulin defines a novel population in the proliferative layers of the developing telencephalon. J Neurosci 14:5399–5416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messam CA, Hou J, Berman JW, Major EO (2002) Analysis of the temporal expression of nestin in human fetal brain derived neuronal and glial progenitor cells. Brain Res Dev Brain Res 134:87–92 [DOI] [PubMed] [Google Scholar]
- Messam CA, Hou J, Major EO (2000) Coexpression of nestin in neural and glial cells in the developing human CNS defined by a human-specific anti-nestin antibody. Exp Neurol 161:585–596 [DOI] [PubMed] [Google Scholar]
- Minturn JE, Geschwind DH, Fryer HJ, Hockfield S (1995) Early postmitotic neurons transiently express TOAD-64, a neural specific protein. J Comp Neurol 355:369–379 [DOI] [PubMed] [Google Scholar]
- Mittelbronn M, Dietz K, Schluesener HJ, Meyermann R (2001) Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol (Berl) 101:249–255 [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM (1992) NeuN, a neuronal specific nuclear protein in vertebrates. Development 116:201–211 [DOI] [PubMed] [Google Scholar]
- Muller GJ, Moller A, Johansen FF (2001) Stereological cell counts of GABAergic neurons in rat dentate hilus following transient cerebral ischemia. Exp Brain Res 141:380–388 [DOI] [PubMed] [Google Scholar]
- Nadji M, Nassiri M, Vincek V, Kanhoush R, Morales AR (2005) Immunohistochemistry of tissue prepared by a molecular-friendly fixation and processing system. Appl Immunohistochem Mol Morphol 13:277–282 [DOI] [PubMed] [Google Scholar]
- Nogami M, Takatsu A, Ishiyama I (1998) Immunohistochemical study of neuron-specific enolase in human brains from forensic autopsies. Forensic Sci Int 94:97–109 [DOI] [PubMed] [Google Scholar]
- Ong WY, Levine JM (1999) A light and electron microscopic study of NG2 chondroitin sulfate proteoglycan-positive oligodendrocyte precursor cells in the normal and kainate-lesioned rat hippocampus. Neuroscience 92:83–95 [DOI] [PubMed] [Google Scholar]
- Osborn M, Debus E, Weber K (1984) Monoclonal antibodies specific for vimentin. Eur J Cell Biol 34:137–143 [PubMed] [Google Scholar]
- Otzen DE, Lundvig DM, Wimmer R, Nielsen LH, Pedersen JR, Jensen PH (2005) p25α is flexible but natively folded and binds tubulin with oligomeric stoichiometry. Protein Sci 14:1396–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oumesmar BN, Vignais L, Baron-Van Evercooren A (1997) Developmental expression of platelet-derived growth factor α-receptor in neurons and glial cells of the mouse CNS. J Neurosci 17:125–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJG (1997) Neocortical neuron number in humans: effect of sex and age. J Comp Neurol 384:312–320 [PubMed] [Google Scholar]
- Pelvig DP, Pakkenberg H, Regeur L, Oster S, Pakkenberg B (2003) Neocortical glial cell numbers in Alzheimers disease. Dement Geriatr Cogn Disord 16:212–219 [DOI] [PubMed] [Google Scholar]
- Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B (In Press) Neocortical glial cell numbers in human brains. Neurobiol Aging. Published online May 31, 2007 (DOI: 10.1016/:j.neurobiolaging. 2997.04.013) [DOI] [PubMed]
- Perl DP, Good PF, Bussiere T, Morrison JH, Erwin JM, Hof PR (2000) Practical approaches to stereology in the setting of aging- and disease-related brain banks. J Chem Neuroanat 20:7–19 [DOI] [PubMed] [Google Scholar]
- Perry VH (2003) Microglia in the developing and mature central nervous system. In Jessen KR, Richardson WD, eds. Glial Cell Development: Basic Principles and Clinical Relevance. Oxford, Oxford University Press, 75–90
- Pileri SA, Roncador G, Ceccarelli C, Piccioli M, Briskomatis A, Sabattini E, Ascani S, et al. (1997) Antigen retrieval techniques in immunohistochemistry: comparison of different methods. J Pathol 183:116–123 [DOI] [PubMed] [Google Scholar]
- Puchtler H, Meloan SN (1985) On the chemistry of formaldehyde fixation and its effects on immunohistochemical reactions. Histochemistry 82:201–204 [DOI] [PubMed] [Google Scholar]
- Pulford KA, Rigney EM, Micklem KJ, Jones M, Stross WP, Gatter KC, Mason DY (1989) KP1: a new monoclonal antibody that detects a monocyte/macrophage associated antigen in routinely processed tissue sections. J Clin Pathol 42:414–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Cai J, Wu Y, Wu R, Lee J, Fu H, Rao M, et al. (2001) Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development 128:2723–2733 [DOI] [PubMed] [Google Scholar]
- Quinn CC, Chen E, Kinjo TG, Kelly G, Bell AW, Elliott RC, McPherson PS, et al. (2003) TUC-4b, a novel TUC family variant, regulates neurite outgrowth and associates with vesicles in the growth cone. J Neurosci 23:2815–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivich G, Banati R (2004) Brain microglia and blood-derived macrophages: molecular profiles and functional roles in multiple sclerosis and animal models of autoimmune demyelinating disease. Brain Res Brain Res Rev 46:261–281 [DOI] [PubMed] [Google Scholar]
- Rakic S, Zecevic N (2003) Early oligodendrocyte progenitor cells in the human fetal telencephalon. Glia 41:117–127 [DOI] [PubMed] [Google Scholar]
- Rezaie P, Dean A, Male D, Ulfig N (2005) Microglia in the cerebral wall of the human telencephalon at second trimester. Cereb Cortex 15:938–949 [DOI] [PubMed] [Google Scholar]
- Rickmann M, Wolff JR (1995) S100 immunoreactivity in a subpopulation of oligodendrocytes and Ranvier's nodes of adult rat brain. Neurosci Lett 186:13–16 [DOI] [PubMed] [Google Scholar]
- Samuelsen GB, Larsen KB, Bogdanovic N, Laursen H, Graem N, Larsen JF, Pakkenberg B (2003) The changing number of cells in the human fetal forebrain and its subdivisions: a stereological analysis. Cereb Cortex 13:115–122 [DOI] [PubMed] [Google Scholar]
- Sarnat HB (1998a) Vimentin immunohistochemistry in human fetal brain: methods of standard incubation versus thermal intensification achieve different objectives. Pediatr Dev Pathol 1:222–229 [DOI] [PubMed] [Google Scholar]
- Sarnat HB, Nochlin D, Born DE (1998b) Neuronal nuclear antigen (NeuN): a marker of neuronal maturation in early human fetal nervous system. Brain Dev 20:88–94 [DOI] [PubMed] [Google Scholar]
- Sasaki A, Nakazato Y, Ogawa A, Sugihara S (1996) The immunophenotype of perivascular cells in the human brain. Pathol Int 46:15–22 [DOI] [PubMed] [Google Scholar]
- Schmidt ML, Lee VMY, Trojanowski JQ (1990) Relative abundance of Tau and neurofilament epitopes in hippocampal neurofibrillary tangles. Am J Pathol 136:1069–1075 [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Szymas J (1990) Immunohistochemistry of glial fibrillary acidic protein, vimentin and S-100 protein for study of astrocytes in hippocampus of rat. J Chem Neuroanat 3:179–192 [PubMed] [Google Scholar]
- Schmitt A, Bauer M, Heinsen H, Feiden W, The Consortium of Brainnet Europe II, Falkai P (2007) How a neuropsychiatric brain bank should be run: a consensus paper of Brainnet Europe II. J Neural Transm 114:527–537 [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR (2005) Design-based stereology in neuroscience. Neuroscience 130:813–831 [DOI] [PubMed] [Google Scholar]
- Schnitzer J, Franke WW, Schachner M (1981) Immunocytochemical demonstration of vimentin in astrocytes and ependymal cells of developing and adult mouse nervous system. J Cell Biol 90:435–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Grieve JH (2006) Rapid fixation of brains: a viable alternative? J Clin Pathol 59:393–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SR, Cote RJ, Taylor CR (1997) Antigen retrieval immunohistochemistry: past, present, and future. J Histochem Cytochem 45:327–343 [DOI] [PubMed] [Google Scholar]
- Shi SR, Key ME, Kalra KL (1991) Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 39:741–748 [DOI] [PubMed] [Google Scholar]
- Shimizu A, Suzuki F, Kato K (1983) Characterization of alpha alpha, beta beta, gamma gamma and alpha gamma human enolase isozymes, and preparation of hybrid enolases (alpha gamma, beta gamma and alpha beta) from homodimeric forms. Biochim Biophys Acta 748:278–284 [DOI] [PubMed] [Google Scholar]
- Skjoerringe T, Lundvig DM, Jensen PH, Moos T (2006) P25α/Tubulin polymerization promoting protein expression by myelinating oligodendrocytes of the developing rat brain. J Neurochem 99:333–342 [DOI] [PubMed] [Google Scholar]
- Sommer I, Schachner M (1981) Monoclonal antibodies (O1 to O4) to oligodendrocyte cell surfaces: an immunocytological study in the central nervous system. Dev Biol 83:311–327 [DOI] [PubMed] [Google Scholar]
- Sprinkle TJ, Agee JF, Tippins RB, Chamberlain CR, Faguet GB, De Vries GH (1987) Monoclonal antibody production to human and bovine 2′:3′-cyclic nucleotide 3′-phosphodiesterase (CNPase): high-specificity recognition in whole brain acetone powders and conservation of sequence between CNP1 and CNP2. Brain Res 426:349–357 [DOI] [PubMed] [Google Scholar]
- Stark AK, Uylings HB, Sanz-Arigita E, Pakkenberg B (2004) Glial cell loss in the anterior cingulate cortex, a subregion of the prefrontal cortex, in subjects with schizophrenia. Am J Psychiatry 161:882–888 [DOI] [PubMed] [Google Scholar]
- Sterio DC (1984) The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc 134:127–136 [DOI] [PubMed] [Google Scholar]
- Sun T, Dong H, Wu L, Kane M, Rowitch DH, Stiles CD (2003) Cross-repressive interaction of the Olig2 and Nkx2.2 transcription factors in developing neural tube associated with formation of a specific physical complex. J Neurosci 23:9547–9556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson PE, Wick MR (1990) HLA-DR (Ia-like) reactivity in tumors of bone and soft tissue: an immunohistochemical comparison of monoclonal antibodies LN3 and LK8D3 in routinely processed specimens. Mod Pathol 3:113–119 [PubMed] [Google Scholar]
- Tiu SC, Chan WY, Heizmann CW, Schafer BW, Shu SY, Yew DT (2000a) Differential expression of S100B and S100A6(1) in the human fetal and aged cerebral cortex. Brain Res Dev Brain Res 119:159–168 [DOI] [PubMed] [Google Scholar]
- Tiu SC, Chan WY, Heizmann CW, Schafer BW, Shu SY, Yew DT (2000b) Erratum to: differential expression of S100B(1) and S100A6(1) in the human fetal and aged cerebral cortex. Brain Res Dev Brain Res 124:153. [DOI] [PubMed] [Google Scholar]
- Tiu SC, Yew DT, Chan WY (2003) Development of the human cerebral cortex: a histochemical study. Prog Histochem Cytochem 38:3–149 [DOI] [PubMed] [Google Scholar]
- Tosic M, Rakic S, Matthieu JM, Zecevic N (2002) Identification of Golli and myelin basic proteins in human brain during early development. Glia 37:219–228 [DOI] [PubMed] [Google Scholar]
- Tramontina F, Leite MC, Cereser K, Fraga de Souza D, Tramontina AC, Nardin P, Andreazza AC, et al. (2007) Immunoassay for glial fibrillary acidic protein: antigen recognition is affected by its phosphorylation state. J Neurosci Methods 162:282–286 [DOI] [PubMed] [Google Scholar]
- van den Berg TK, Breve JJ, Damoiseaux JG, Dopp EA, Kelm S, Crocker PR, Dijkstra CD, et al. (1992) Sialoadhesin on macrophages: its identification as a lymphocyte adhesion molecule. J Exp Med 176:647–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TF, Guidotti G (1998) Widespread expression of ecto-apyrase (CD39) in the central nervous system. Brain Res 790:318–322 [DOI] [PubMed] [Google Scholar]
- Watanabe M, Sakurai Y, Ichinose T, Aikawa Y, Kotani M, Itoh K (2006) Monoclonal antibody Rip specifically recognizes 2′,3′-cyclic nucleotide 3′-phosphodiesterase in oligodendrocytes. J Neurosci Res 84:525–533 [DOI] [PubMed] [Google Scholar]
- Werner M, Von WR, Komminoth P (1996) Antigen retrieval, signal amplification and intensification in immunohistochemistry. Histochem Cell Biol 105:253–260 [DOI] [PubMed] [Google Scholar]
- Weruaga E, Alonso JR, Porteros A, Crespo C, Arevalo R, Brinon JG, Velasco A, et al. (1998) Nonspecific labelling of myelin with secondary antisera and high concentrations of triton X-100. J Histochem Cytochem 46:109–117 [DOI] [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC (1994) Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet 344:769–772 [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ (1991) Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec 231:482–497 [DOI] [PubMed] [Google Scholar]
- Wirenfeldt M, Dalmau I, Finsen B (2003) Estimation of absolute microglial cell numbers in mouse fascia dentata using unbiased and efficient stereological cell counting principles. Glia 44:129–139 [DOI] [PubMed] [Google Scholar]
- Wirenfeldt M, Dissing-Olesen L, Babcock AA, Nielsen M, Meldgaard M, Zimmer J, Azcoitia I, et al. (2007) Population control of resident and immigrant microglia by mitosis and apoptosis. Am J Pathol 171:617–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SD, Ramos RA, Hermanowski-Vosatka A, Rockwell P, Detmers PA (1991) Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med 173:1281–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SD, Weitz JI, Huang AJ, Levin SM, Silverstein SC, Loike JD (1988) Complement receptor type three (CD11b/CD18) of human polymorphonuclear leukocytes recognizes fibrinogen. Proc Natl Acad Sci USA 85:7734–7738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Andjelkovic A, Matthieu J, Tosic M (1998) Myelin basic protein immunoreactivity in the human embryonic CNS. Brain Res Dev Brain Res 105:97–108 [PubMed] [Google Scholar]
- Zhang SC, Ge B, Duncan ID (1999) Adult brain retains the potential to generate oligodendroglial progenitors with extensive myelination capacity. Proc Natl Acad Sci USA 96:4089–4094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock HW, Pechumer H, Petersmann I, Durieux JJ, Vita N, Labeta MO, Strobel M (1994) CD14 is expressed and functional in human B cells. Eur J Immunol 24:1937–1940 [DOI] [PubMed] [Google Scholar]