Abstract
Thymocyte development requires an integration of extracellular cues to enforce lineage commitment at multiple defined checkpoints in a stage-specific manner. Critical signals from the pre-TCR, Notch, and the receptor for interleukin-7 (IL-7) dictate cellular differentiation from the CD4−CD8− (double negative) stage to CD4+CD8+ (double positive) stage. The PI3K/Akt signaling pathway is required to translate these extracellular signaling events into multiple functional outcomes including cellular survival, proliferation, differentiation, and allelic exclusion at the β-selection checkpoint. However, a complete understanding of the contributions made by the PI3K/Akt pathway in thymocyte development has not been straightforward. This review highlights studies that support the model that the PI3K/Akt pathway is essential for thymocyte survival. We provide new evidence that Akt-mediated survival is not solely due to the increased expression of Bcl-xL but also is a consequence of the role played by Akt to support metabolism in proliferating thymocytes.
Keywords: Akt, PI3K, Thymocyte, TCR, Bcl-xL, metabolism
1. Early T cell Development
Acquisition of a complete peripheral T cell repertoire requires that T cell progenitors undergo a series of tightly regulated developmental events that depend on integration of signaling cascades downstream of the pre-T cell receptor (TCR) and then the mature TCR. Creating a pool of mature T cells requires that developing thymocytes interpret signals from the extracellular environment in a spatial and temporal specific manner [1]. The first step in T cell development is the emigration of early thymic progenitors (ETPs) from the bone marrow to the thymus [2]. ETPs then transit through four stages as CD4/CD8 double negative (DN) cells (DN1–4) before upregulating CD8 and CD4 to become double positive (DP) thymocytes [3]. Emergence of mature T cells requires that developing thymocytes pass through several pre- TCR/TCR-dependent selection events: the first at the DN3 stage and then two others at the DP stage. DN3 thymocytes test the newly created pre-TCRβ chain for its ability to be functionally expressed during a process known as β-selection [4, 5], while DP cells test the reactivity of the mature TCR for self peptide/MHC during positive and negative selection [6, 7]. This review focuses on the signaling events at the β-selection checkpoint that are required for thymocytes to successfully reach the DP stage.
The diverse repertoire of TCRs expressed on peripheral T cells is the result of the random reassortment of gene sections to create the polymorphic α and β chains. In addition to creating varied TCRs, this random gene rearrangement has the potential to result in proteins that cannot be expressed correctly due to insertion of stop codons or amino acids that preclude proper assembly. The suitability of the TCRβ chain is assessed at the β-selection checkpoint to ensure that only those cells that have rearranged a functional TCRβ chain survive, while thymocytes that fail to produce an appropriately rearranged receptor undergo apoptosis [8]. Survival through this checkpoint requires that cells generate a signal from the pre-TCR, which is composed of the newly rearranged TCRβ chain paired with a non-rearranged pre-Tα chain [9]. Four events occur after successful β-selection: differentiation to the DP stage, proliferation to substantially increase the number of cells with an appropriately created TCRβ chain [10, 11], prevention of apoptosis through activation of cell survival pathways [8], and allelic exclusion to ensure each cell expresses only a single TCRβ chain [12]. An active area of investigation is to determine how signals that originate from the pre-TCR dictate these four biological events [13].
2. PI3K and PDK1 are required for survival and proliferation at the β-selection checkpoint
Experimental evidence suggests that DN3 cell survival requires that signals delivered by multiple receptors, including Notch, the receptor for interleukin-7 (IL-7), and the pre-TCR, be appropriately integrated [14–17]. However, it remains unclear which signaling pathway(s) is (are) most critical for survival during the transition from the DN3 to DP stage. One pathway common to all three receptors is the phosphatidylinositol 3-kinase (PI3K) signal transduction cascade. Activation of the PI3K pathway supports survival and proliferation of multiple cell lineages [18]. PI3K activation results in the localized increase of phosphorylated lipid second messengers at the plasma membrane [19]. Key signaling intermediates are then recruited to the phosphorylated lipids via specialized lipid-binding domains, pleckstrin homology (PH) domains, and are themselves activated to initiate further signaling events. One key effector molecule that is activated in this manner is the serine/threonine kinase Akt, which, when localized to products of PI3K activation, is able to phosphorylate multiple downstream substrates that mediate cell growth, survival, and metabolism [20, 21].
PI3Ks are divided into four classes [IA, IB, II, and III] based on their subunit composition [19]. Class IA and IB PI3Ks are the best understood in the immune system and are the subject of this discussion [22]. Class IA PI3Ks are heterodimers consisting of a regulatory adaptor subunit (p85α, p55α, p50α, p85β, or p55γ) and a catalytic subunit (p110α, p110β, or p110δ[22]. Class IB PI3Ks differ in their subunit composition, as they are heterodimers of the catalytic subunit p110γ paired with a regulatory subunit p101 or p84 [23]. The catalytic and regulatory subunits exhibit complex patterns of redundant expression. Thus, it has been difficult to use genetically altered mice with single PI3K mutations to unravel the complete role for this signaling pathway in early thymocyte development or subsequent mature T cell function. Genetically modified mice, however, have provided some clues to the importance of PI3K in these events. Although mice deficient in two of the Class IA regulatory subunits, p85α or p85β, develop normal T cells [24, 25], mature p85β-deficient T cells engage in more rounds of proliferation and undergo less apoptosis when stimulated in vitro, indicating different biological functions for these subunits [25]. Interestingly, the combined loss of both p85α and p85β alter mature T cell signaling, but thymocyte development appears unaffected [26, 27], further suggesting that redundancy exists with other less well-defined PI3K regulatory subunits.
Thymic development phenotypes are more apparent in mice that lack functional PI3K catalytic subunits. Although, loss of function of the Class IA catalytic subunit p110δ has no measureable effect on T cell development [28, 29], absence of p110γ, a Class IB subunit, results in decreased numbers of DP cells and a corresponding decrease in thymic cellularity [28]. This thymic phenotype correlates with the observation that p110γ−/− DP thymocytes demonstrate increased apoptosis following treatment with anti-CD3 in vivo. This TCR-induced apoptosis was not investigated further but is interesting since TCR stimulation normally drives proliferation and survival in wildtype DN3 thymocytes [30]. Mice deficient in both catalytic subunits, p110γ and p110δ, have small thymi due to a combination of increased apoptosis and decreased proliferation at the β-selection checkpoint and DP stages [31, 32]. Furthermore, mice that lack both catalytic subunits also demonstrate a paucity of large TCRβ+ thymocytes, a population representative of proliferating β-selected thymocytes that have recently received pre-TCR signals [31]. Thus, decreased accumulation of phospholipids at the plasma membrane appears to result in diminished thymocyte survival, likely at the point in which these cells should be undergoing proliferation.
Insights into the role of PI3K in lymphocyte development were further uncovered by studying loss of the lipid phosphatase PTEN [phosphatase and tensin homologue deleted on chromosome 10], the enzyme that counteracts PI3K [18]. In the absence of PTEN, PH-domain containing kinases, such as Akt, are localized basally to the membrane and become constitutively activated [19]. Activation of these critical signaling effectors initiates a cascade of events and bypasses the requirement for surface receptor engagement, such that loss of PTEN rescues development to the DP stage in mice that are deficient in components of the pre-TCR or the common γ chain cytokine receptor, a subunit required for IL-7 signaling [33]. These data suggest that the activation of the PI3K pathway is sufficient to induce differentiation to the DP stage in the absence of pre-TCR or cytokine signals.
Phosphatidylinositol accumulation by PI3K at the plasma membrane translates into survival and proliferation by triggering the activation of a cascade of serine/threonine kinases. Generally, phosphatidylinositol accumulation induces the localization of PDK1 (3-phosphoinositide-dependent protein kinase 1), a serine/threonine kinase, to the plasma membrane [19]. PDK1 then phosphorylates and activates AGC kinases, such as Akt, S6K1, RSK, and PKC isoforms [19]. The importance of PDK1 as a mediator between PI3K activation and Akt activation is illustrated by the dramatic loss in thymic cellularity and block at the DN4 stage of development in mice that have a conditional deletion in PDK1 [34]. Although the gross phenotype is similar to that of mice deficient in both PI3K catalytic subunits, p110γ and p110δ, the mechanism appears distinct, as thymocytes from these mice did not reveal increased spontaneous apoptosis but rather decreased proliferation [34]. Thus, PDK1 may be more essential for proliferation, while another PI3K-dependent but PDK1-independent pathway signals increased cell survival.
3. Akt is required to maintain thymocyte survival
Given the mounting evidence implicating the PI3K pathway in thymocyte development, several groups began to investigate the importance of Akt, a downstream effector of PI3K activation, in this process. Targeting Akt to the plasma membrane, either through the use of a myristoylation sequence or a gag sequence, bypasses the requirement for lipid accumulation at the plasma membrane and results in constitutive activation of Akt. Based on in vitro studies using the myristoylated Akt construct, it was hypothesized that activated Akt would lead to increased cell proliferation and cell survival. To extend this investigation in vivo and to determine whether constitutive activation of Akt affects T cell maturation, three groups analyzed thymocytes and mature T cells from mice that transgenically express membrane-targeted Akt. Two groups found that thymocyte survival is not directly affected by activated Akt but that mature T cells from these mice are more resistant to apoptosis [35, 36], while a third group found that activated Akt does increase thymocyte survival [37]. Differences in these results may be due to the use of the myristoylation sequence by the first two groups and the use of the gag sequence by the third, as the gag-tagged molecule may be more biologically active [37]. These gain-of-function studies are important for understanding the capacity of Akt to influence cell growth, survival, and proliferation in T cells. However, it is also of interest to determine which biological parameters are affected in the absence of Akt signaling.
Mice that lack PI3K signals have defective thymocyte survival [28, 31, 32], while constitutive activation of Akt increases thymocyte survival [37]. Thus, we [38] and others [39, 40] studied mice deficient in Akt to determine if Akt is essential for thymocyte development. Three isoforms of Akt are expressed in mice: Akt1, Akt2, and Akt3. We decided to study both the single Akt1 and Akt2 knockout animals in addition to the doubly deficient mice, since Akt1 and Akt2 are the isoforms that have the highest expression in thymocytes [38]. We found that adult mice that lack Akt1 have subtle decreases in thymic cellularity but grossly normal thymic subsets, while Akt2-deficient thymocytes reveal no detectable differences compared to wildtype animals. However, a different pattern emerged when we examined thymi from fetuses. In this case, we found that Akt1-null thymi were nearly 2-fold less cellular than Akt wildtype or Akt2-null fetuses. Additionally, Akt1−/−Akt2−/− fetal thymi were 8-fold less cellular than heterozygous controls, and thymic subsets were skewed to a higher percentage of cells at the DN3 stage and a lower percentage of cells at the DP stage, suggesting defective β-selection in the absence of these two Akt isoforms. Defective thymic development in the absence of both Akt1 and Akt2 is exacerbated in a radiation bone marrow chimera system. To extend the analysis of Akt1−/−Akt2−/− thymocytes, fetal liver cells were injected into irradiated hosts and given sufficient time to repopulate the hematopoietic compartment. Bone marrow chimeras reconstituted with hematopoietic cells that lack both Akt1 and Akt2 have a 70-fold decrease in DP cells but only a 2-fold decrease in DN3 cells, suggesting a loss of cells during β-selection [38].
Since thymic cellularity is maintained by both an increase in proliferation and increased survival during the DN3 to DP transition, we next sought to determine if the loss of Akt affected survival or proliferation. Thymocyte proliferation was only subtly decreased in the DN3 and DN4 subsets of Akt1−/−Akt2−/− bone marrow chimeras as measured by BrdU (5-bromo-2-deoxyuridine) incorporation. However, we found that the majority of CD8+, intermediate single positive cells, a transient stage between the DN4 and DP stage, undergo spontaneous apoptosis in the absence of Akt1 and Akt2. Additionally, Akt1−/−Akt2−/− DN3 cells cultured in vitro undergo apoptosis following pre-TCR stimulation, while wildtype DN3 cells respond with increased survival [38]. The results of these last experiments are similar to those found in vivo when thymocytes deficient in p110γ undergo apoptosis following anti-CD3 stimulation [28]. Thus, in the absence of PI3K/Akt signals, thymocytes transition through β-selection and respond to the signal to proliferate, but during this proliferation, they are susceptible to apoptosis.
Activation of Akt may increase cell survival in multiple ways, but one mechanism that has been suggested is through increasing the expression of anti-apoptotic Bcl-2 family members, such as Bcl-xL or A1 [41, 42]. A recent report showed that the mechanism for gag-Akt-induced survival in DP thymocytes was due to increased protein levels of Bcl-xL, likely through increased activation of the NF-κB pathway [37]. NF-κB transcriptional activation sites are located in the promoters of these pro-survival molecules [43], and likewise, thymocytes have diminished survival in the absence of NF-κB signals [44]. However, other studies have implicated an Akt-independent regulation of the anti-apoptotic Bcl-2 family member A1 at the DN3 stage. One group found that pre-TCR signals lead directly to the activation of A1 and, while this signal may be activated by PKC and NF-κB-mediated pathways, it appears independent of Akt [41]. Mature T cells from mice deficient in the p110δ catalytic subunit of PI3K lack Akt activation but retain NF-κB signals, providing evidence that NF-κB signaling is not dependent on PI3K or Akt activation [45]. Thus, although there is likely crosstalk between the NF-κB and PI3K/Akt pathways, the biological relevance in thymocyte development is unclear.
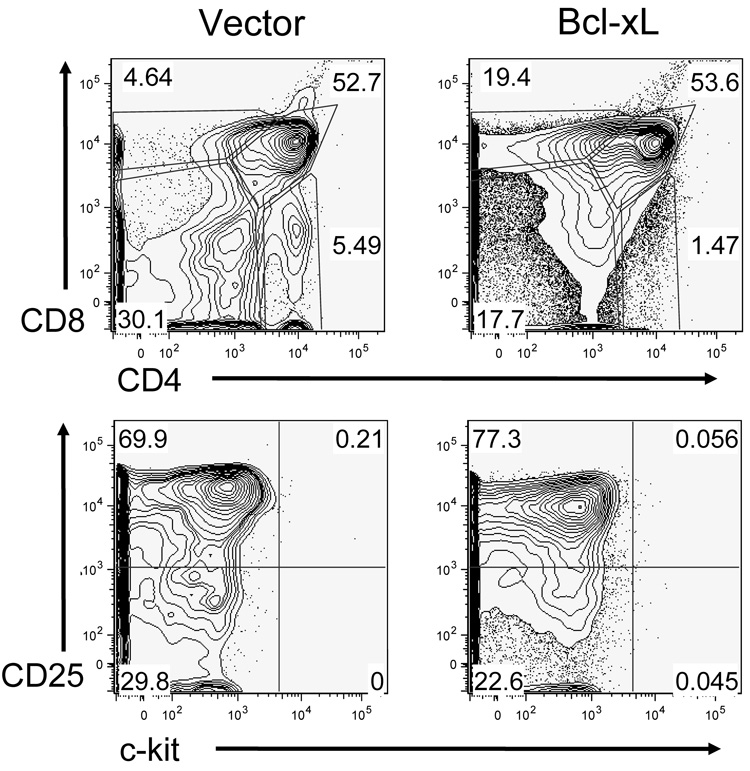
The mechanism for decreased survival due to loss of PI3K/Akt activation in thymocytes may be due to decreased expression of anti-apoptotic Bcl-2 family members. To test this model, we hypothesized that if the survival defect found in Akt1−/−Akt2−/− cells was due to failed upregulation of Bcl-xL, ectopic expression of Bcl-xL would rescue development to the DP stage in Akt1−/−Akt2−/− thymocytes. We transduced Akt1−/−Akt2−/− fetal liver progenitor cells with a retrovirus that encoded for Bcl-xL or the vector alone. We found that ectopic expression of Bcl-xL in Akt1−/−Akt2−/− fetal liver bone marrow chimeras did not rescue development of Akt1−/−Akt2−/− thymocytes [Fig. 1]. Bcl-xL did not increase the percentage of DP cells or overall thymic cellularity in the absence of Akt1 and Akt2. Additionally, if Bcl-xL protein levels are dependent on Akt activation, then Akt1−/−Akt2−/− thymocytes should have reduced levels of Bcl-xL. However, there was no measureable difference in endogenous Bcl-xL protein levels in Akt1−/−Akt2−/− thymocytes derived either from bone marrow chimeras or from an in vitro culture system (Jeffrey C. Rathmell, Duke University, personal communication). The increased apoptosis in Akt1−/−Akt2−/− thymocytes is thus not likely due to a loss of expression of Bcl-xL.
Figure 1.
Thymocyte profiles of Akt1−/−Akt2−/− chimeras retrovirally transduced with Bcl-xL. Akt1−/−Akt2−/− fetal liver cells were retrovirally transduced with Bcl-xL or vector alone and injected into lethally irradiated congenic recipient mice. Thymuses were harvested 8 weeks post-transplant and analyzed for surface expression of CD4, CD8, CD25, c-kit, and lineage markers. Upper panels are gated on lineage negative thymocytes, lower panels are gated on CD4−,CD8−, lineage− thymocytes. Lineage markers included NK1.1, CD11b, GR-1, CD11c, B220, and Ter119.
The critical targets of Akt at the DN3 stage have yet to be elucidated, but restoration of Bcl-xL is not sufficient to rescue the absence of Akt signals. Another Bcl-2 family member, A1, is a more physiologic anti-apoptotic protein at the β-selection checkpoint [41], and its expression in Akt1−/−Akt2−/− cells may yield different results. Additionally, Akt and Bcl-xL mediate survival in different ways [46]. Bcl-xL maintains mitochondrial membrane stability and does not directly affect glycolytic processes, whereas Akt maintains mitochondrial membrane potential and is a critical mediator of cellular metabolism both by increasing glucose transport into the cell and by regulating key glycolytic enzymes [47]. We found that Akt1−/−Akt2−/− DN3 cells have decreased glucose transport and decreased cell size, both indicators of affected cellular metabolism [38]. Transgenic expression of myristoylated Akt increases basal glucose transport and cell size in mature T cells [36]. Supporting the model that increased cell size and metabolism translates to a greater ability of cells to proliferate, T cells expressing myristoylated-Akt engage in more cell divisions after TCR and CD28 stimulation in vitro compared to their non-transgenic counterparts [36]. Thus, Akt1−/−Akt2−/− thymocytes have decreased cell survival and decreased metabolic capacity resulting in a phenotype that is not rescued by ectopic expression of Bcl-xL, but it is not clear whether maintaining metabolic capacity in the absence of Akt would be sufficient to increase thymocyte survival.
A biological role for Akt in thymocyte metabolism was recently established by studying Notch signaling in DN3 thymocytes. Notch signals are required at the DN3 to DP transition and are likely required to enhance cell survival and proliferation [48, 49]. Using an in vitro system, Notch was found to be required for cell survival at the DN3 stage, specifically by maintaining Akt activation and cell metabolism [17]. A current model suggests that two extracellular signals, Notch and the pre-TCR, converge on the PI3K/Akt signaling pathway during the DN3 to DP transition to initiate several rounds of cell division. This proliferative burst at the β-selection checkpoint places a large metabolic demand on DN3 cells to rapidly accumulate enough cellular substrates for repeated mitosis, and activation of the Akt signaling pathway is crucial to maintain cellular metabolism for this continued division [20].
4. Do PI3K and Akt contribute to allelic exclusion?
In-frame rearrangements at the TCRβ loci and the subsequent signal from the pre-TCR dictate the fate of DN3 cells. An estimated four out of nine DN3 cells fail to produce a TCRβ chain and eventually die due to neglect [50]. The current model for allelic exclusion is that a signal from the pre-TCR negatively regulates V-DJ rearrangements at the TCRβ loci. In support of this model, PKC theta and Lck promote proliferation and differentiation but also enforce allelic exclusion at the TCRβ locus [51, 52]. However, constitutive activation of the MAPK pathway through Ras or Raf does not affect V-DJ rearrangement [52–54]. The role of the PI3K pathway in allelic exclusion at the TCRβ loci has not been addressed and will be an interesting area to pursue.
Studies in mice with conditional deletion of PTEN in thymocytes indicate that PI3K may affect V-DJ feedback inhibition during β-selection. TCRβ− cells aberrantly survive in mice that lack both PTEN and the CD3γ chain [33]. Close to half of all DP cells from PTEN−/− CD3γ−/− thymocytes lack intracellular TCRβ, indicating a failure of the β-selection checkpoint to eliminate cells without productive TCRβ chain rearrangements. These results suggest that the expansion of intracellular TCRβ− cells is likely due to a rescue of cells that should have otherwise been deleted [33]. Alternatively, the abundance of intracellular TCRβ− cells may also reflect a premature discontinuation of rearrangements or early feedback inhibition at the TCRβ locus.
Further evidence that Akt is involved in allelic exclusion was found by studying the effects of myristoylated Akt on Rag activation. Constitutively active Akt can modulate Rag expression during T cell development, possibly through NF-AT (nuclear factor of activated T cells) [55]. Thymocytes that express a constitutively active form of calcineurin, thus maintaining NF-AT nuclear localization, are blocked at the DN3 stage of development. Constitutive NF-AT activation also causes decreased Rag1 protein expression, and thymocytes subsequently develop into DP cells without detectable intracellular TCRβ chain [55]. Transgenic expression of myristoylated Akt rescues the calcineurin induced block and restores expression of Rag proteins and the TCRβ chain in DP thymocytes. It remains to be determined whether Akt directly phosphorylates NF-AT or if there is an alternative pathway that influences the transcriptional activation of Rag. Furthermore, the PI3K pathway may be involved in feedback inhibition of antigen receptor rearrangements, as PI3K activation is required for Rag repression in immature B cells [56, 57]. Thus, the PI3K pathway is likely involved in allelic exclusion, but further studies are required to determine if the mechanism is through early differentiation to the DP stage or through direct inactivation of the recombination machinery.
5. Conclusion
At the DN3-DP transition, the formation of the pre-TCR at the β-selection checkpoint triggers a proliferative burst, estimated to be about 10 cell divisions [58]. One model posits that highly proliferative cells become more susceptible to apoptosis to limit tissue expansion [59, 60]. In this model, parallel signals induce proliferation and apoptosis, ensuring that tissue size is homeostatically preserved. However, when there is a blockade of apoptosis, the tissue undergoes expansion, and in the thymus, this expansion occurs during the DN3 to DP transition. Thymocytes that fail to proliferate or survive result in dramatically decreased organ size, and thymocytes that fail to die or are hyperproliferative result in larger thymi [61, 62].
Although Akt appears to be essential in this process, the critical mediators in thymocytes downstream of this kinase have yet to be elucidated. Akt can directly phosphorylate GSK3 [63], a target of the Wnt pathway [63]. The main nuclear effector of the Wnt pathway, TCF-1, is especially important for survival at the DN3 to DP stage of development. TCF-1−/− mice have a severe defect in development due to death at the DN3 stage, most pronounced in the intracellular TCRβ+ DN3 population [64]. Additionally, there is limited information on isoform-specific targets; however Akt1, not Akt2, has been shown to directly phosphorylate CREB [65]. CREB−/− mice also have a deficiency in the TCRβ high population and have decreased fetal thymic cellularity [66]. Ongoing studies are attempting to identify the exact targets of Akt at the DN3 to DP transition, as these same pathways likely are utilized by proto-oncogenic cells that must also increase metabolism, proliferation, survival, and differentiation to undergo successful transformation to malignancy.
Acknowledgement
This work was supported by NIH grants P01 CA93615 (G.A.K. and M.M.J.) and F31 A1056671 (M.M.J.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aifantis I, Mandal M, Sawai K, Ferrando A, Vilimas T. Regulation of T-cell progenitor survival and cell-cycle entry by the pre-T-cell receptor. Immunol Rev. 2006;209:159–169. doi: 10.1111/j.0105-2896.2006.00343.x. [DOI] [PubMed] [Google Scholar]
- 2.Bhandoola A, von Boehmer H, Petrie HT, Zuniga-Pflucker JC. Commitment and developmental potential of extrathymic and intrathymic T cell precursors: plenty to choose from. Immunity. 2007;26:678–689. doi: 10.1016/j.immuni.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3−CD4−CD8− triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- 4.Levelt CN, Ehrfeld A, Eichmann K. Regulation of thymocyte development through CD3. I. Timepoint of ligation of CD3 ε determines clonal deletion or induction of developmental program. J Exp Med. 1993;177:707–716. doi: 10.1084/jem.177.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levelt CN, Carsetti R, Eichmann K. Regulation of thymocyte development through CD3. II. Expression of T cell receptor β CD3 ε and maturation to the CD4+8+ stage are highly correlated in individual thymocytes. J Exp Med. 1993;6:1867–1875. doi: 10.1084/jem.178.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starr TK, Jameson SC, Hogquist KA. Positive and Negative Regulation of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 7.Werlen G, Hausmann B, Naeher D, Palmer E. Signaling Life and Death in the Thymus: Timing Is Everything. Science. 2003;299:1859–1863. doi: 10.1126/science.1067833. [DOI] [PubMed] [Google Scholar]
- 8.Falk I, Nerz G, Haidl I, Krotkova A, Eichmann K. Immature thymocytes that fail to express TCRβ and/or TCRγδ proteins die by apoptotic cell death in the CD44−CD25− (DN4) subset. Eur J Immunol. 2001;31:3308–3317. doi: 10.1002/1521-4141(200111)31:11<3308::aid-immu3308>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Aifantis I, Buer J, von Boehmer H, Azogui O. Essential Role of the Pre-T Cell Receptor in Allelic Exclusion of the T Cell Receptor β Locus. Immunity. 1997;7:601–607. doi: 10.1016/s1074-7613(00)80381-7. [DOI] [PubMed] [Google Scholar]
- 10.Dudley EC, Petrie HT, Shah LM, Owen MJ, Hayday AC. T cell receptor β chain gene rearrangement and selection during thymocyte development in adult mice. Immunity. 1994;1:83–93. doi: 10.1016/1074-7613(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman ES, Passoni L, Crompton T, Leu TM, Schatz DG, Koff A, et al. Productive T-cell receptor beta-chain gene rearrangement: coincident regulation of cell cycle and clonality during development in vivo. Genes Dev. 1996;10:948–962. doi: 10.1101/gad.10.8.948. [DOI] [PubMed] [Google Scholar]
- 12.Michie AM, Zuniga-Pflucker JC. Regulation of thymocyte differentiation: pre-TCR signals and β -selection. Semin Immunol. 2002;14:311–323. doi: 10.1016/s1044-5323(02)00064-7. [DOI] [PubMed] [Google Scholar]
- 13.Kruisbeek AM, Haks MC, Carleton M, Wiest DL, Michie AM, Zuniga-Pflucker JC. Branching out to gain control: how the pre-TCR is linked to multiple functions. Immunol Today. 2000;21:637–644. doi: 10.1016/s0167-5699(00)01744-8. [DOI] [PubMed] [Google Scholar]
- 14.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman IL. Bcl-2 Rescues T Lymphopoiesis in Interleukin-7 Receptor-Deficient Mice. Cell. 1997;89:1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 15.Wurch A, Biro J, Falk I, Mossmann H, Eichmann K. Reduced Generation but Efficient TCR β-Chain Selection of CD4+8+ Double-Positive Thymocytes in Mice with Compromised CD3 Complex Signaling. J Immunol. 1999;162:2741–2747. [PubMed] [Google Scholar]
- 16.Trigueros C, Hozumi K, Silva-Santos B, Bruno L, Hayday AC, Owen MJ, et al. Pre-TCR signaling regulates IL-7 receptor α expression promoting thymocyte survival at the transition from the double-negative to double-positive stage. Eur J Immunol. 2003;33:1968–1977. doi: 10.1002/eji.200323831. [DOI] [PubMed] [Google Scholar]
- 17.Ciofani M, Zuniga-Pflucker JC. Notch promotes survival of pre-T cells at the β-selection checkpoint by regulating cellular metabolism. Nat Immunol. 2005;6:881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- 18.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 19.Cantrell DA. Phosphoinositide 3-kinase signalling pathways. J Cell Sci. 2001;114:1439–1445. doi: 10.1242/jcs.114.8.1439. [DOI] [PubMed] [Google Scholar]
- 20.Plas DR, Thompson CB. Akt-dependent transformation: there is more to growth than just surviving. Oncogene. 2005;24:7435–7442. doi: 10.1038/sj.onc.1209097. [DOI] [PubMed] [Google Scholar]
- 21.Manning BD, Cantley LC. AKT/PKB Signaling: Navigating Downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fruman DA. The role of class I phosphoinositide 3-kinase in T-cell function and autoimmunity. Biochem Soc Trans. 2007;035:177–180. doi: 10.1042/BST0350177. [DOI] [PubMed] [Google Scholar]
- 23.Andrews S, Stevens LR, Hawkins PT. PI3K class IB pathway. Sci STKE. 2007 doi: 10.1126/stke.4072007cm2. 2007:cm2. [DOI] [PubMed] [Google Scholar]
- 24.Fruman DA, Snapper SB, Yballe CM, Davidson L, Yu JY, Alt FW, et al. Impaired B Cell Development and Proliferation in Absence of Phosphoinositide 3-Kinase p85. Science. 1999;283:393–397. doi: 10.1126/science.283.5400.393. [DOI] [PubMed] [Google Scholar]
- 25.Deane JA, Trifilo MJ, Yballe CM, Choi S, Lane TE, Fruman DA. Enhanced T Cell Proliferation in Mice Lacking the p85β Subunit of Phosphoinositide 3-Kinase. J Immunol. 2004;172:6615–6625. doi: 10.4049/jimmunol.172.11.6615. [DOI] [PubMed] [Google Scholar]
- 26.Deane JA, Kharas MG, Oak JS, Stiles LN, Luo J, Moore TI, et al. T-cell function is partially maintained in the absence of class IA phosphoinositide 3-kinase signaling. Blood. 2007;109:2894–2902. doi: 10.1182/blood-2006-07-038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oak JS, Deane JA, Kharas MG, Luo J, Lane TE, Cantley LC, et al. Sjogren's syndrome-like disease in mice with T cells lacking class 1A phosphoinositide-3-kinase. Proc Nat Acad Sci USA. 2006;103:16882–16887. doi: 10.1073/pnas.0607984103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, et al. Function of PI3K in Thymocyte Development, T Cell Activation, and Neutrophil Migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 29.Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, et al. Impaired B and T Cell Antigen Receptor Signaling in p110delta PI 3-Kinase Mutant Mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 30.Levelt CN, Mombaerts P, Iglesias A, Tonegawa S, Eichmann K. Restoration of early thymocyte differentiation in T-cell receptor β-chain-deficient mutant mice by transmembrane signaling through CD3ε. Proc Nat Acad Sci USA. 1993;90:11401–11405. doi: 10.1073/pnas.90.23.11401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webb LMC, Vigorito E, Wymann MP, Hirsch E, Turner M. Cutting Edge: T Cell Development Requires the Combined Activities of the p110γ and p110δ Catalytic Isoforms of Phosphatidylinositol 3-Kinase. J Immunol. 2005;175:2783–2787. doi: 10.4049/jimmunol.175.5.2783. [DOI] [PubMed] [Google Scholar]
- 32.Swat W, Montgrain V, Doggett TA, Douangpanya J, Puri K, Vermi W, et al. Essential role of PI3Kδ and PI3Kγ in thymocyte survival. Blood. 2006;107:2415–2422. doi: 10.1182/blood-2005-08-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagenbeek TJ, Naspetti M, Malergue F, Garcon F, Nunes JA, Cleutjens KBJM, et al. The Loss of PTEN Allows TCR αβ Lineage Thymocytes to Bypass IL-7 and Pre-TCR-mediated Signaling. J Exp Med. 2004;200:883–894. doi: 10.1084/jem.20040495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinton HJ, Alessi DR, Cantrell DA. The serine kinase phosphoinositide-dependent kinase 1 (PDK1) regulates T cell development. Nat Immunol. 2004;5:539–545. doi: 10.1038/ni1062. [DOI] [PubMed] [Google Scholar]
- 35.Na S-Y, Patra A, Scheuring Y, Marx A, Tolaini M, Kioussis D, et al. Constitutively Active Protein Kinase B Enhances Lck and Erk Activities and Influences Thymocyte Selection and Activation. J Immunol. 2003;171:1285–1296. doi: 10.4049/jimmunol.171.3.1285. [DOI] [PubMed] [Google Scholar]
- 36.Rathmell JC, Elstrom RL, Cinalli RM, Thompson CB. Activated Akt promotes increased resting T cell size, CD28-independent T cell growth, and development of autoimmunity and lymphoma. Eur J Immunol. 2003;33:2223–2232. doi: 10.1002/eji.200324048. [DOI] [PubMed] [Google Scholar]
- 37.Jones RG, Parsons M, Bonnard M, Chan VSF, Yeh W-C, Woodgett JR, et al. Protein Kinase B Regulates T Lymphocyte Survival, Nuclear Factor κ Activation, and Bcl-XL Levels In Vivo. J Exp Med. 2000;191:1721–1734. doi: 10.1084/jem.191.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juntilla MM, Wofford JA, Birnbaum MJ, Rathmell JC, Koretzky GA. Akt1 and Akt2 are required for αβ thymocyte survival and differentiation. Proc Nat Acad Sci USA. 2007;104:12105–12110. doi: 10.1073/pnas.0705285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fayard E, Gill J, Paolino M, Hynx D, Holländer GA, Hemmings BA. Deletion of PKBalpha/Akt1 Affects Thymic Development. PLoS ONE. 2007;2:e992. doi: 10.1371/journal.pone.0000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mao C, Tili EG, Dose M, Haks MC, Bear SE, Maroulakou I, et al. Unequal Contribution of Akt Isoforms in the Double-Negative to Double-Positive Thymocyte Transition. J Immunol. 2007;178:5443–5453. doi: 10.4049/jimmunol.178.9.5443. [DOI] [PubMed] [Google Scholar]
- 41.Mandal M, Borowski C, Palomero T, Ferrando AA, Oberdoerffer P, Meng F, et al. The BCL2A1 gene as a pre-T cell receptor-induced regulator of thymocyte survival. J Exp Med. 2005;201:603–614. doi: 10.1084/jem.20041924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veis DJ, Sentman CL, Bach EA, Korsmeyer SJ. Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. J Immunol. 1993;151:2546–2554. [PubMed] [Google Scholar]
- 43.Zong W-X, Edelstein LC, Chen C, Bash J, Gelinas C. The prosurvival Bcl-2 homolog Bfl- 1/A1 is a direct transcriptional target of NF-κB that blocks TNFα-induced apoptosis. Genes Dev. 1999;13:382–387. doi: 10.1101/gad.13.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voll RE, Jimi E, Phillips RJ, Barber DF, Rincon M, Hayday AC, et al. NF-κB Activation by the Pre-T Cell Receptor Serves as a Selective Survival Signal in T Lymphocyte Development. Immunity. 2000;13:677–689. doi: 10.1016/s1074-7613(00)00067-4. [DOI] [PubMed] [Google Scholar]
- 45.Okkenhaug K, Patton DT, Bilancio A, Garcon F, Rowan WC, Vanhaesebroeck B. The p110δ Isoform of Phosphoinositide 3-Kinase Controls Clonal Expansion and Differentiation of Th Cells. J Immunol. 2006;177:5122–5128. doi: 10.4049/jimmunol.177.8.5122. [DOI] [PubMed] [Google Scholar]
- 46.Parcellier A, Tintignac LA, Zhuravleva E, Hemmings BA. PKB and the mitochondria: AKTing on apoptosis. Cell Signal. 2008;20:21–30. doi: 10.1016/j.cellsig.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Plas DR, Talapatra S, Edinger AL, Rathmell JC, Thompson CB. Akt and Bcl-xL Promote Growth Factor-independent Survival through Distinct Effects on Mitochondrial Physiology. J Biol Chem. 2001;276:12041–12048. doi: 10.1074/jbc.M010551200. [DOI] [PubMed] [Google Scholar]
- 48.Maillard I, Tu L, Sambandam A, Yashiro-Ohtani Y, Millholland J, Keeshan K, et al. The requirement for Notch signaling at the β-selection checkpoint in vivo is absolute and independent of the pre-T cell receptor. J Exp Med. 2006;203:2239–2245. doi: 10.1084/jem.20061020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ciofani M, Schmitt TM, Ciofani A, Michie AM, Cuburu N, Aublin A, et al. Obligatory Role for Cooperative Signaling by Pre-TCR and Notch during Thymocyte Differentiation. J Immunol. 2004;172:5230–5239. doi: 10.4049/jimmunol.172.9.5230. [DOI] [PubMed] [Google Scholar]
- 50.Mallick CA, Dudley EC, Viney JL, Owen MJ, Hayday AC. Rearrangement and diversity of T cell receptor β chain genes in thymocytes: A critical role for the β chain in development. Cell. 1993;73:513–519. doi: 10.1016/0092-8674(93)90138-g. [DOI] [PubMed] [Google Scholar]
- 51.Michie AM, Soh J-W, Hawley RG, Weinstein IB, Zuniga-Pflucker JC. Allelic exclusion and differentiation by protein kinase C-mediated signals in immature thymocytes. Proc Nat Acad Sci USA. 2001;98:609–614. doi: 10.1073/pnas.021288598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gartner F, Alt FW, Monroe R, Chu M, Sleckman BP, Davidson L, et al. Immature Thymocytes Employ Distinct Signaling Pathways for Allelic Exclusion versus Differentiation and Expansion. Immunity. 1999;10:537–546. doi: 10.1016/s1074-7613(00)80053-9. [DOI] [PubMed] [Google Scholar]
- 53.Jackson AM, Krangel MS. A Role for MAPK in Feedback Inhibition of TCRβ Recombination. J Immunol. 2006;176:6824–6830. doi: 10.4049/jimmunol.176.11.6824. [DOI] [PubMed] [Google Scholar]
- 54.Iritani BM, Alberola-Ila J, Forbush KA, Perimutter RM. Distinct Signals Mediate Maturation and Allelic Exclusion in Lymphocyte Progenitors. Immunity. 1999;10:713–722. doi: 10.1016/s1074-7613(00)80070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patra AK, Drewes T, Engelmann S, Chuvpilo S, Kishi H, Hunig T, et al. PKB Rescues Calcineurin/NFAT-Induced Arrest of Rag Expression and Pre-T Cell Differentiation. J Immunol. 2006;177:4567–4576. doi: 10.4049/jimmunol.177.7.4567. [DOI] [PubMed] [Google Scholar]
- 56.Verkoczy L, Duong B, Skog P, Ait-Azzouzene D, Puri K, Vela JL, et al. Basal B Cell Receptor-Directed Phosphatidylinositol 3-Kinase Signaling Turns Off RAGs and Promotes B Cell-Positive Selection. J Immunol. 2007;178:6332–6341. doi: 10.4049/jimmunol.178.10.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Llorian M, Stamataki Z, Hill S, Turner M, Martensson I-L. Cutting Edge: The PI3K p110δ Is Required for Down-Regulation of RAG Expression in Immature B Cells. J Immunol. 2007;178:1981–1985. doi: 10.4049/jimmunol.178.4.1981. [DOI] [PubMed] [Google Scholar]
- 58.Egerton M, Scollay R, Shortman K. Kinetics of Mature T-Cell Development in the Thymus. Proc Nat Acad Sci USA. 1990;87:2579–2582. doi: 10.1073/pnas.87.7.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1:19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 60.Ogasawara J, Suda T, Nagata S. Selective apoptosis of CD4+CD8+ thymocytes by the anti-Fas antibody. J Exp Med. 1995;181:485–491. doi: 10.1084/jem.181.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malstrom S, Tili E, Kappes D, Ceci JD, Tsichlis PN. Tumor induction by an Lck-MyrAkt transgene is delayed by mechanisms controlling the size of the thymus. Proc Nat Acad Sci USA. 2001;98:14967–14972. doi: 10.1073/pnas.231467698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chao DT, Korsmeyer SJ. BCL-XL-regulated apoptosis in T cell development. Int Immunol. 1997;9:1375–1384. doi: 10.1093/intimm/9.9.1375. [DOI] [PubMed] [Google Scholar]
- 63.Cross DAE, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 64.Goux D, Coudert JD, Maurice D, Scarpellino L, Jeannet G, Piccolo S, et al. Cooperating pre-T-cell receptor and TCF-1-dependent signals ensure thymocyte survival. Blood. 2005;106:1726–1733. doi: 10.1182/blood-2005-01-0337. [DOI] [PubMed] [Google Scholar]
- 65.Kato S, Ding J, Du K. Differential activation of CREB by Akt1 and Akt2. Biochem Biophys Res Com. 2007;354:1061–1066. doi: 10.1016/j.bbrc.2007.01.094. [DOI] [PubMed] [Google Scholar]
- 66.Rudolph D, Tafuri A, Gass P, Hammerling GJ, Arnold B, Schutz G. Impaired fetal T cell development and perinatal lethality in mice lacking the cAMP response element binding protein. Proc Nat Acad Sci USA. 1998;95:4481–4486. doi: 10.1073/pnas.95.8.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]