Abstract
A-790742 is a potent human immunodeficiency virus type 1 (HIV-1) protease inhibitor, with 50% effective concentrations ranging from 2 to 7 nM against wild-type HIV-1. The activity of this compound is lowered by approximately sevenfold in the presence of 50% human serum. A-790742 maintained potent antiviral activity against lopinavir-resistant variants generated in vitro as well as against a panel of molecular clones containing proteases derived from HIV-1 patient isolates with multiple protease mutations. During in vitro selection, A-790742 selected two primary mutations (V82L and I84V) along with L23I, L33F, K45I, A71V/A, and V77I in the pNL4-3 background and two other mutations (A71V and V82G) accompanied by M46I and L63P in the HIV-1 RF background. HIV-1 pNL4-3 clones with a single V82L or I84V mutation were phenotypically resistant to A-790742 and ritonavir. Taking these results together, A-790742 displays a favorable anti-HIV-1 profile against both the wild type and a large number of mutants resistant to other protease inhibitors. The selection of the uncommon V82L and V82G mutations in protease by A-790742 suggests the potential for an advantageous resistance profile with this protease inhibitor.
Human immunodeficiency virus type 1 (HIV-1) protease inhibitors (PIs) are potent and effective antiretroviral agents. The current standard of care for AIDS patients involves the combination of reverse transcriptase inhibitors and/or PIs with other HIV inhibitors, which can reduce viremia to an undetectable level for an extended period of time (22, 37, 41, 42). However, 5 to 68% of patients ultimately fail therapy due to nonadherence to drug schedules, insufficient drug exposure, and resistance development (3, 8, 10, 18, 36, 38, 39). In addition, there are many cases reporting the isolation of PI-resistant HIV-1 from patients who have not received any treatment with currently available PIs, indicating that the transmission of PI-resistant HIV-1 can occur, particularly during primary infection, when the viral burden is high (1, 19, 20, 37).
Lopinavir (LPV), an HIV-1 PI, maintains reasonable potency in the presence of human serum (HS) and demonstrates favorable pharmacokinetics when coformulated with a low dose of ritonavir (RTV) (5, 34). LPV/RTV (Kaletra) has displayed efficacy in both PI-naïve and PI-experienced patients (5, 7, 14, 15, 24, 26). However, treatment with PIs, including LPV/RTV, is associated with lipid elevations in humans (23, 32). In addition, PIs such as atazanavir (ATV) and indinavir (IDV) have been shown to induce hyperbilirubinemia (17, 43). To avoid the undesirable metabolic side effects of HIV PIs, there is a need to identify new PIs with high potency, increased genetic barriers to the development of resistance, and reduced metabolic side effects. In an effort to address this need, we investigated the biological properties, antiviral activity, and resistance profile of a novel HIV-1 PI, A-790742. A-790742 had high oral bioavailability in rats and dogs, with 500-fold enhancement in the areas under the curves upon codosing with RTV (D. A. DeGoey, W. J. Flosi, D. J. Grampovnik, C. A. Flentge, L. L. Klein, C. M. Yeung, T. Dekhtyar, L. Colletti, J. F. Waring, R. Ciurlionis, K. C. Marsh, J. M. Schmidt, S. J. Swanson, V. Stoll, M. Mamo, H. Mo, W. M. Kati, A. Molla, and D. J. Kempf, presented at the 230th National Meeting of the American Chemical Society, Washington, DC, 28 August to 1 September 2005). This compound also exhibited fewer proteasome gene changes than RTV in an in vivo model for hyperlipidemia associated with PI therapy (21, 40; DeGoey et al., presented at the 230th Nat. Meet. Am. Chem. Soc.). In this study, we characterized A-790742 in a tissue culture system against wild-type (WT) and mutant HIV-1 viruses. In addition, we performed the in vitro selection and characterization of A-790742-resistant variants using two different HIV-1 strains. Finally, we describe the results of cross-resistance studies of A-790742-resistant variants with commercially available PIs.
MATERIALS AND METHODS
Generation of HIV-1 resistant to A-790742 by in vitro selection.
The generation of resistant virus by in vitro passages has been described previously (2, 4, 9, 25, 27, 35). Briefly, 2 × 106 MT-4 cells were infected with WT HIV-1 strain pNL4-3 or RF separately at a multiplicity of infection of 0.003 for 2 h, washed, and then cultured in the presence of A-790742 at an initial concentration of 1.4 nM. Viral replication was monitored by the determination of p24 antigen levels in the culture supernatants by enzyme-linked immunosorbent assay every 3 to 4 days. When p24 antigen levels exceeded 10 ng/ml, the viral supernatants were filtered and used to infect fresh MT-4 cells in the presence of an increasing concentration of A-790742, leading to the generation of virus with increased resistance to A-790742. Samples of viral supernatants from each passage were frozen at −80°C for later analyses.
Titration and 50% effective concentration (EC50) and 50% toxic dose (TD50) determinations using the MTT assay.
The titers of all viruses used for the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay were determined by infecting 2 × 105 MT-4 cells for 1 h at 37°C with serial half-log dilutions of virus. Following infection, cells were washed and plated onto a 96-well plate at a concentration of 104 cells per well in the presence of 0.5% dimethyl sulfoxide (DMSO). Five days postinfection, the level of virus-induced cytopathic effect was measured using an MTT colorimetric assay (2, 12, 16, 31), and the 50% tissue culture infectious dose of each virus was calculated using the Sperman-Karber method.
For EC50 determinations, 106 MT-4 cells were infected with viral stock at a multiplicity of infection of 0.003 (2, 16, 27, 29, 31). One hour postinfection, cells were washed and plated onto 96-well plates at concentrations of 104 cells per well in the presence of eight serial dilutions of each tested compound in triplicate. The final concentration of DMSO in all wells was 0.5%. Five days postinfection, an MTT assay was done and the EC50 for each compound was calculated using Prism software (version 4) as described previously (2, 16, 27, 29, 31).
The TD50 determination was done with MT-4 cells. Cells were plated onto 96-well plates at a concentration of 104 cells per well in the presence of eight serial dilutions of each tested compound in duplicate. The final concentration of DMSO in all wells was 0.5%. Five days later, an MTT assay was done and the TD50 for each compound was calculated using Prism software (version 4) as described before (2, 16, 27, 29, 31).
DNA sequence analyses and construction of recombinant HIV-1 molecular clones.
Culture supernatants from virus passages and plasma samples from patients who failed LPV/RTV clinical trials were analyzed by DNA sequence analysis. RNA from these samples was isolated using the QIAamp RNA extraction kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Subsequently, it was processed by reverse transcription-PCR (RT-PCR) and nested PCR to amplify the HIV-1 protease genes. The amplified samples were sequenced using the ABI Prism dye terminator cycle sequencing ready reaction kit and were analyzed on an Applied Biosystems 3100 genetic analyzer.
HIV-1 molecular clones containing mutations in the protease gene were constructed for study in single-cycle assays. For LPV-resistant HIV-1 patient samples, the protease coding region was first RT-PCR amplified with primers that incorporated ApaI and XmaI restriction sites and then was cloned into a pNL4-3 replication-defective viral vector with a luciferase gene (pNL4-3-Luc), which contains an ApaI site upstream and an XmaI site downstream of the protease coding region (27, 28). In addition, HIV-1 molecular clones with mutations selected by A-790742 in the protease gene were constructed by generating PCR fragments from the protease region of different mutant viruses derived from passage in the presence of A-790742, cloning the fragments into the pNL4-3-Luc vector as ApaI-XmaI fragments, and selecting individual clones containing the mutation(s) of interest. The RF-Luc constructs contained the WT or mutant RF protease gene inserted into the pNL4-3-Luc vector as an ApaI-XmaI fragment.
Antiviral activity of PIs against HIV-1 molecular clones with mutations in the protease gene.
Antiviral activities of HIV-1 PIs against HIV-1 molecular clones with and without mutations in the protease gene were measured using a single-cycle assay. Human embryonic kidney 293 (HEK-293) cells were cotransfected with HIV-1 molecular clones containing a luciferase reporter gene plus a vesicular stomatitis virus G envelope protein expression vector by using Lipofectamine 2000 and Plus reagent (Invitrogen). The transfection conditions were optimized to achieve >90% transfection efficiency according to the manufacturer's instructions. Four hours after transfection, cells were trypsinized and 2 × 104 cells per well were plated onto 96-well plates containing serial dilutions of each tested PI in triplicate. Forty-eight hours posttransfection, pseudotyped viral stocks generated in the presence of inhibitors were used to infect fresh HEK-293 cells plated on 96-well plates at a density of 104 cells per well. Replication in each well was monitored by measuring luciferase expression in infected target cells 48 h after infection. The percent inhibition of luciferase signal in cells treated with inhibitors relative to that in the untreated cells was calculated. EC50s were determined using Prism software (11, 30, 33). Replication capacities (RCs) of mutant constructs were calculated by comparing the luciferase activity generated by a mutant to that generated by the WT. The RCs were expressed as percentages of the replication of the WT construct.
(This work was presented in part at the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy, 2005 [28a].)
RESULTS
Antiviral activity of A-790742 against WT HIV-1 and LPV-resistant HIV-1 mutant viruses.
The anti-HIV activity of A-790742 (Fig. 1) against WT HIV-1 and HIV-1 mutant viruses with LPV-resistant mutations was compared to those of LPV, ATV, darunavir (DRV), and tipranavir (TPV). A-790742 had an EC50 of 3 nM against WT pNL4-3 and 5 nM against WT RF in the absence of HS (Table 1). Thus, its activity was similar to or slightly better than that of ATV against pNL4-3 and RF. It was six times more active than LPV against both viruses and four times better than DRV against pNL4-3. A-790742 was 61 and 41 times more active than TPV against pNL4-3 and RF, respectively. In the presence of 50% HS, A-790742 was approximately seven times less potent than without HS, with an EC50 of 20 nM. It was 7.5 and 392 times more potent than LPV and TPV, respectively, under the same conditions (Table 1).
FIG. 1.
Structure of A-790742.
TABLE 1.
Activity of A-790742, ATV, LPV, DRV, and TPV against WT HIV-1a
| Inhibitor | EC50 ± SD (nM) against:
|
TD50 ± SD (μM) | ||
|---|---|---|---|---|
| pNL4-3
|
RF (0% HS) | |||
| 0% HS | 50% HS | |||
| A-790742 | 3 ± 1 | 20 ± 7 | 5 ± 2 | 22 ± 4 |
| ATV | 4 ± 2 | 11 ± 3 | 7 ± 5 | 67 ± 14 |
| LPV | 18 ± 4 | 151 ± 12 | 32 ± 13 | 30 ± 7 |
| DRV | 12 ± 4 | 40 ± 15 | ND | >100 |
| TPV | 184 ± 42 | 7,839 ± 888 | 204 ± 34 | 66 ± 11 |
Values shown are representatives of at least 10 experiments for the EC50s of A-790742, ATV, and LPV against pNL4-3, three experiments for EC50s of DRV and TPV against pNL4-3, and three experiments for all tested PIs against RF. ND, not determined.
The activities of A-790742, ATV, LPV, DRV, and TPV were evaluated against a panel of passaged viruses derived from in vitro selection with LPV. These viruses had key mutations in protease at the following positions: 32, 46, 47, and 84 for A17; 46, 50, 54, and 82 for B26; and 32, 46, 47, and 84 for P25 (Table 2). The EC50s of A-790742 against A17-, B26-, and P25-passaged viruses were 3- to 15-fold higher than that of the WT pNL4-3. A-790742 was the least active against the P25 virus, with a 15-fold change in its EC50. ATV, on the other hand, was 13 to 89 times less active against the same panel of viruses than against WT pNL4-3. It was more potent against the B26 virus and less potent against the A17 and P25 viruses. DRV was 28- to 71-fold less potent against this panel of viruses than against WT pNL4-3. It was more active against the A17 virus than the B26 and P25 viruses. TPV was tested against A17 only and was two times less potent than it was against the WT. As reported before, all of these mutant viruses were highly resistant to LPV, with 59- to more than 500-fold reductions in susceptibility relative to that of the WT virus (2, 27). Taking these results together, A-790742 displayed anti-HIV activity superior to those of LPV, ATV, DRV, and TPV for WT pNL4-3 and RF as well as for LPV-resistant HIV-1 mutants.
TABLE 2.
Activity of A-790742, ATV, LPV, DRV, and TPV against LPV-resistant HIV-1a
| Inhibitor | EC50 ± SD (nM) against:
|
||
|---|---|---|---|
| A17b | B26c | P25d | |
| A-790742 | 10 ± 2 | 22 ± 3 | 45 ± 9 |
| ATV | 296 ± 61 | 51 ± 17 | 355 ± 67 |
| LPV | 1,057 ± 85 | 3,790 ± 187 | >10,000 |
| DRV | 338 ± 53 | 587 ± 207 | 857 ± 170 |
| TPV | 360 ± 15 | ND | ND |
Values shown are representatives of at least three experiments. A17, B26, and P25 viruses were derived from in vitro passages with LPV. ND, not determined.
A17 contains the mutations L10F, V32I, M46I, I47V, Q58E, and I84V.
B26 contains the mutations L33F, K45I, M46I, I50V, I54V, A71V, and V82F.
P25 contains the mutations L10F, G16E, V32I, M46I, I47A, H69Y, I84V, and T91S.
Antiviral activity of A-790742 against HIV-1 molecular clones with protease genes derived from patient isolates.
To determine if A-790742 could effectively inhibit different PI-resistant viruses, it was tested along with LPV and ATV in single-cycle assays against HIV molecular clones containing protease genes derived from HIV-1 isolates from patients who failed different PI treatments (Table 3). The first three constructs in Table 3 were derived from samples from patients who failed LPV treatment. The rest of the constructs were from a panel of molecular clones displaying reduced susceptibility to more than one PI. Each of these tested isolates contained a mixture of primary and secondary mutations as designated by the International AIDS Society-USA resistance panel (11, 13). In general, all clones were less resistant to A-790742 than to LPV and ATV. A-790742 was active against clones with V82A/F/T mutations. However, combined mutations at positions 71 and 84 appeared to confer some degree of resistance to A-790742. Of note, mutations at positions 71 and 84 were selected during the passage of pNL4-3 in the presence of A-790742 as described below. Other secondary mutations also contributed to the generation of resistance to all tested PIs.
TABLE 3.
Activity of A-790742, ATV, and LPV against HIV-1 molecular clones containing proteases derived from PI-resistant patient isolates
| Virus | Mutations | Fold change in EC50 fora:
|
||
|---|---|---|---|---|
| A-790742 | LPV | ATV | ||
| HIV-Luc | WT | 1 | 1 | 1 |
| 1 | L10V, I15V, G16E, K20R, E35D, M36I, R41K, M46I, I54V, R57K, Q61N, I64L, A71V, I72R, V82A, L89I, L90M, Q92K | 3 | 247 | 9 |
| 2 | L10I, I15V, G16E, K20R, E35D, M36I, R41K, I54A, R57K, Q61D, A71V, I72R, V82A, L89I, L90M, Q92K | 12 | 222 | 172 |
| 3 | L10I, E35D, N37D, M461, I54V, L63P, A71V, T74P, I84V, L90M, I93L | 33 | 64 | 89 |
| 4 | N37T, M46I, Q58E, I64V, T74K, V77I, V82F, L90M | 1 | 6 | 5 |
| 5 | L10F, I15V, M46I, L63P, A71V, I72L, V82T, I84V, I85V, L90M | 2 | 16 | 18 |
| 6 | L10I, L24I, M46I, I54V, K55R, Q58E, L63P, I64V, A71V, V82A, T91A | 1 | 40 | 15 |
| 7 | L10I, M46M/L, G48V, I54V, L63P, A71V, I72M, V77I, V82A, L90M, I93L | 2 | 40 | 25 |
| 8 | L10I, I13V, L33F, E34Q, R41K, M46I, I54V, K55R, R57K, Q58E, I62V, L63P, A71V, I72I/T, G73S, V82A, I84V, L90M | 4 | 91 | 32 |
| 9 | L10I, I15I/V, K20R, L33F, E35D, M36I, N37D, R41K, K43I, M46I, I54V, I62V, L63P, A71T, I72T, V82A, I84V, L90M, I93L | 6 | 50 | 22 |
| 10 | L10I, I13V, K20R, L33F, E35D, M36I, N37D, I54L, Q58E, I62V, L63P, A71V, V82A, I84V, L90M | 10 | 58 | 56 |
| 11 | L10I, I15V, K20R, E21Q, E35D, M36I, N37D, R41K, M46L, I54V, I62V, L63P, A71V, T74D, P79A, V82T, I84V, I85V, L90M, I93L | 11 | 58 | 43 |
| 12 | L10I, I13V, K20I, E35D, M36I, M46I, I50V, I54V, R57K, I62V, L63P, A71V, V82A, L89V, L90M, Q92K | 1 | 98 | 3 |
| 13 | L10I, K14R, L33F, E34Q, R41K, K45R, M46I, I50V, K55R, L63P, A71V, G73T, V77I, V82A, L90M, I93L | 2 | 81 | 5 |
Shown are the changes in resistance relative to the EC50 of the WT molecular clone pNL4-3-Luc.
Selection of A-790742-resistant virus by in vitro passages.
In order to understand the importance of the genetic background in the selection of viruses resistant to A-790742, two different strains of HIV-1 (pNL4-3 and RF) were passaged in the presence of increasing concentrations of A-790742 (Tables 4 and 5) . Viral replication was monitored every 3 to 4 days by the detection of viral antigen (p24) before increasing the concentration of the inhibitor. pNL4-3 and RF initially were grown in the presence of 1.4 nM A-790742 (i.e., one-half of the EC50 for pNL4-3 virus and one-quarter of the EC50 for RF virus). There also was an attempt to grow RF virus at an initial concentration of 3 nM A-790742 (one-half of the EC50), but viral replication was very low (data not shown). During the selection process, the A-790742 concentration was increased gradually to a final concentration of 2 μM (pNL4-3) (Table 4) or 0.5 μM (RF) (Table 5). It took approximately 3.5 months to complete the selection. The selection with pNL4-3 progressed faster than that with RF virus. A total of 21 passages were performed for pNL4-3, but only 15 passages were performed for RF. This difference might be due to the different genetic backgrounds of the two viruses and suggests that resistance in the RF background developed less rapidly than in the pNL4-3 background.
TABLE 4.
In vitro selection and genotype of mutant variants during passage of HIV-1 pNL4-3 in the presence of A-790742
| Passage no. | A-790742 concna (nM) (fold change in EC50) | Mutation(s)b |
|---|---|---|
| 5 | 12 (4) | I84V |
| 6 | 16 (5.3) | I84V |
| 7 | 32 (10.7) | I84V |
| 8 | 50 (16.7) | V82V/L, I84V |
| 9 | 80 (26.7) | V82V/L, I84V |
| 10 | 140 (46.7) | V82V/L, I84V |
| 13 | 400 (133.3) | V82L, I84V |
| 15 | 560 (186.7) | L33L/F, K45I, V82L, I84V |
| 18 | 900 (300) | L33F, K45I, A71A/V, V77V/I, V82L, I84V |
| 21 | 2,000 (666.7) | L23I, L33F, K45I, A71A/V, V77I, V82L, I84V |
The concentration of A-790742 was increased in each passage on the basis of the p24 value of the previous passage.
Mutations were detected by population sequencing.
TABLE 5.
In vitro selection and genotype of mutant variants during passage of HIV-1 RF in A-790742
| Passage no. | A-790742 concna (nM) (fold change in EC50) | Mutation(s)b |
|---|---|---|
| 6 | 16 (3.2) | WT |
| 8 | 52 (10.4) | V75I |
| 10 | 160 (32) | A71V, V82G |
| 13 | 340 (68) | M46I, L63P, A71V, V82G |
| 15 | 500 (100) | M46I, L63P, A71V, V82G |
The concentration of A-790742 was increased in each passage on the basis of the p24 value of the previous passage.
Mutations were detected by population sequencing.
Sequence analyses of the protease-coding region from HIV-1 passaged in A-790742.
To monitor the protease mutations selected by A-790742, RNA was isolated from the culture supernatant of passages 5 to 10 and 13, 15, 18, and 21 for pNL4-3 selection (Table 4) and from those of passages 6, 8, 10, 13, and 15 for RF selection (Table 5). The RNA was amplified by RT-PCR and nested PCR, and then the population sequences of the PCR products were determined. In the selection with pNL4-3, two primary mutations initially were observed: I84V (passages 5 to 10 and 13) and V82L (passages 8, 9, 10, and 13). These two mutations also were detected in passages 15, 18, and 21, suggesting that they were important for the generation of viral resistance. In passages 15, 18, and 21, the additional mutations L23I, L33F, K45I, A71V, and V77I also were detected. The A-790742 resistance selection using RF virus produced a different pattern of mutations. The mutation V75I was found in passage 8, but this mutation became undetectable in passage 10. Instead, mutations A71V and V82G were found in passage 10. In passages 13 and 15, the additional mutations M46I and L63P were observed. The cleavage sites of viruses derived from passage 21 of pNL4-3 selection and passage 15 of RF selection were sequenced, and no mutations were detected.
Susceptibility of HIV-1 selected by A-790742 to different PIs.
To determine the susceptibility of HIV-1 mutants selected by A-790742 to different PIs, the titers of several pNL4-3 passage viruses (passages 9, 15, and 21) and one RF passage virus (passage 13) were determined, and their levels of resistance to A-790742 and a number of commercially available PIs, such as ATV, LPV, saquinavir (SQV), RTV, amprenavir (APV), IDV, and nelfinavir (NFV), were determined using an MTT assay (Table 6). Virus from pNL4-3 passage 9 was 12.7-fold less susceptible to A-790742 than the WT pNL4-3 virus. This passage was the most resistant to RTV, with a 26.7-fold increase in its EC50 compared to that of the WT pNL4-3 virus. ATV, LPV, SQV, APV, IDV, and NFV demonstrated three- to ninefold increases in their EC50s at passage 9 compared to that of the WT. Virus selected in pNL4-3 passage 15 had similar levels of resistance to A-790742 and RTV, with a 28-fold change in the EC50. In addition, ATV and APV had 13.6- and 10.5-fold changes in their respective EC50s for virus from passage 15. LPV, SQV, IDV, and NFV had about five- to sixfold changes in their EC50s with this passaged virus. pNL4-3 passage 21 virus had the highest degree of resistance to all tested PIs. It was highly resistant to A-790742 (>2,500-fold resistance), RTV (>141-fold resistance), ATV (145-fold resistance), and NFV (>93-fold resistance). However, it had only a low to moderate level of resistance to the other tested PIs. In particular, it retained most of its sensitivity to SQV, LPV, and IDV.
TABLE 6.
Susceptibility of A-790742-passaged HIV-1 to different PIs
| Virus | Fold change in EC50 fora:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| A-790742 | ATV | LPV | SQV | RTV | APV | IDV | NFV | |
| pNL4-3 (WT) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| P9b | 13 ± 4 | 6 ± 2 | 4 ± 1 | 3 ± 1 | 26.7 ± 3 | 9 ± 2 | 3 ± 1 | 5 ± 2 |
| P15c | 29 ± 3 | 14 ± 3 | 6 ± 2 | 4 ± 1 | 29 ± 3 | 11 ± 3 | 5 ± 2 | 6 ± 2 |
| P21d | >2,500 ± 250 | 145 ± 10 | 14 ± 4 | 5 ± 2 | >141 ± 22 | 34 ± 6 | 14 ± 4 | >93 ± 15 |
| RF (WT) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| P13e | 72 ± 6 | 31 ± 3 | >301 ± 31 | 21 ± 3 | 79 ± 6 | 41 ± 4 | 31 ± 3 | 67 ± 4 |
Values are the change in resistance relative to that of WT pNL4-3 or RF EC50 and represent average values from three experiments. P9, P15, and P21 viruses were derived from in vitro passages of pNL4-3 with A-790742. P13 was derived from the in vitro passage of RF with A-790742.
P9 contains the mutations V82V/L and I84V.
P15 contains the mutations L33L/F, K45I, V82L, and I84V.
P21 contains the mutations L23I, L33F, K45I, A71A/V, V77I, V82L, and I84V.
P13 contains the mutations M46I, L63P, A71V, and V82G.
Virus from passage 13 selected in the RF background had similar levels of resistance to A-790742, RTV, and NFV, with 72-, 79-, and 67-fold changes in EC50s, respectively, compared to that of the WT RF virus. APV, ATV, and IDV had about 31- to 41-fold changes in their EC50s with this passaged virus. In addition, the passage 13 virus displayed the greatest resistance to LPV, with a >301-fold increase in its EC50 compared to that of the WT RF virus, and it was the least resistant to SQV, with a 21-fold increase in its EC50 compared to that of the WT RF virus.
Phenotypic analyses of HIV-1 molecular clones resistant to A-790742.
To determine the effect of genotypic changes found in the passaged variants on the susceptibility of HIV-1 to A-790742 and commercially available PIs, HIV-1 molecular clones with protease mutations selected by A-790742 in pNL4-3 and RF were engineered in the pNL4-3-Luc vector (Table 7). These constructs permitted the rapid and sensitive quantification of drug susceptibility during a single round of viral replication in the presence of increasing concentrations of drugs (30, 33).
TABLE 7.
Activity of A-790742 and other PIs against HIV-1 molecular clones containing protease mutations selected by A-790742
| Clone no. | Fold change in EC50 fora:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A-790742 | ATV | LPV | SQV | RTV | APV | IDV | NFV | DRV | TPV | |
| pNL4-3-Luc (WT) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 1b | 2 ± 0.1 | 1 ± 0.4 | 1 ± 0.4 | 1 ± 0.2 | 3 ± 1 | 1 ± 0.2 | 1 ± 0.1 | 1 ± 0.1 | 1 ± 0.2 | ND |
| 2c | 22 ± 2 | 1 ± 0.2 | 1 ± 0.1 | 1 ± 0.2 | 7 ± 2 | 1 ± 0.1 | 1 ± 0.2 | 1 ± 0.7 | 1 ± 0.1 | ND |
| 3d | 23 ± 3 | 34 ± 4 | 10 ± 3 | 0.4 ± 0.1 | 79 ± 5 | 12 ± 2 | 5 ± 2 | 3 ± 0.8 | 12 ± 3 | ND |
| 4e | 158 ± 11 | 16 ± 3 | 4 ± 1 | 1 ± 0.3 | 14 ± 2 | 2 ± 0.3 | 2 ± 0.2 | 2 ± 0.3 | 7 ± 1.4 | 6 ± 1.4 |
| RF-Luc (WT) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ND |
| 5f | 72 ± 8 | 39 ± 3 | >1788 ± 161 | 28 ± 4 | 1386 ± 65 | 41 ± 6 | 76 ± 18 | 203 ± 33 | 59 ± 2 | ND |
Values are the changes for clones 1 to 4 relative to those of the WT HIV pNL4-3-Luc EC50 and represent average values from three experiments. The levels of change for clone 5 are relative to those of the WT HIV RF-Luc EC50 and represent average values from three experiments. ND, not determined.
Clone 1 contains the mutation I84V.
Clone 2 contains the mutation V82L.
Clone 3 contains the mutations L33F, K45I, V82L, and I84V.
Clone 4 contains the mutations L33F, A71V, G73S, V77I, V82L, and I84V.
Clone 5 contains the mutations L63P, A71V, and V82G.
Molecular clones containing the single mutation V82L or I84V in the pNL4-3 background displayed resistance only to A-790742 and RTV (Table 7). In particular, clone 1, which contained an I84V mutation, was two- and threefold less susceptible to A-790742 and RTV, respectively, than WT pNL4-3-Luc. In contrast, clone 2, which harbored a V82L mutation, displayed 22-fold more resistance to A-790742 but only 7-fold more resistance to RTV. Mutant clone 3, which had secondary mutations L33F and K45I in addition to primary mutations V82L and I84V, was 23-fold less susceptible to A-790742. Clone 3 was more resistant than clone 2 to all other tested PIs except SQV (Table 7). Clone 4 contained secondary mutations L33F, A71V, G73S, and V77I in addition to primary mutations V82L and I84V. Mutations L33F, A71V, and V77I were secondary mutations detected by population sequencing in the passaged variants (Table 4). However, G73S was not detected by population sequencing; it was identified by sequencing individual TA clones derived from pNL4-3 passaged in the presence of A-790742. Clone 4 was more resistant to A-790742 (158-fold change in the EC50) but less resistant to all tested PIs than clone 3 (Table 7). The difference in the resistance profiles of clones 3 and 4 suggests that K45I is important for the resistance to all PIs except A-790742 and SQV. Furthermore, individual or combined A71V, G73S, and V77I mutations contributed to the increase in resistance to A-790742. Among these three mutations, A71V was detected in both pNL4-3 and RF after selection using A-790742 and probably contributed most of the additional resistance to A-790742. It is of note that molecular clone 3, which contained most of the mutations of passaged HIV-1 P21 (Table 6), showed the same trend of resistance as P21. Both clone 3 and P21 were more resistant to A-790742, ATV, and RTV than the other tested PIs.
Clone 5, which contained A-790742-selected PI mutations in the RF background, also was evaluated in the single-cycle assay (Table 7). This clone contained the primary mutation V82G and the other mutations L63P and A71V. It was 72-fold less susceptible to A-790742 than the WT construct and was highly resistant to LPV and RTV, with more than 1,300-fold resistance to these two PIs. However, it had only moderate levels of resistance to the other tested PIs. Importantly, it was only 28 times more resistant to SQV than the WT construct. Interestingly, overall this construct conferred a higher level of resistance to all tested PIs than all of the molecular clones in the pNL4-3 background (clones 1, 2, 3, and 4) (Table 7).
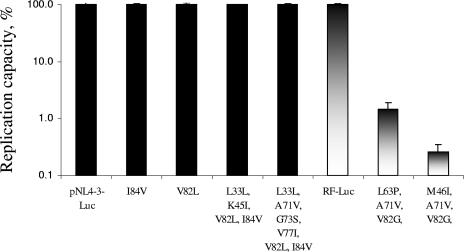
During phenotypic testing, significant differences in replication efficiencies between molecular clones with WT and mutant protease genes in the RF background were observed (Fig. 2). Constructs with the combined mutations L63P, A71V, and V82G or M46I, A71V, and V82G replicated 99% less efficiently than the WT HIV-RF-luc construct based on the difference in luciferase signal. The luciferase signal of the RF molecular clone harboring the mutations M46I, A71V, and V82G was so low that its phenotype could not be determined in the single-cycle assay. One of the possible reasons for the lower replication capacity of these constructs with the V82G mutation is the negative impact of this mutation on the fitness of the constructs. The presence of this mutation also might explain the slow growth of virus during the selection with A-790742 in the RF background. All other clones with mutations in the protease gene replicated at the same level as the virus with WT protease (pNL4-3-Luc).
FIG. 2.
RC of pNL4-3-Luc and RF-Luc molecular clones containing different protease mutations. The RCs of mutant molecular clones were calculated by comparing the median luciferase signal generated by mutants to that generated by WT pNL4-3-Luc and RF-Luc, respectively, in at least three experiments. The RCs were expressed as percentages of the value for the WT and reflected the levels of replication for mutant clones compared to that of the WT control. The RC of the WT pNL4-3-Luc molecular clone was approximately two times higher than that of the WT RF-Luc molecular clone. Black boxes represent WT pNL4-3 and pNL4-3-derived mutants, and gradient boxes represent WT RF and RF-derived mutants. Error bars represent the standard deviations.
DISCUSSION
The current standard of care for AIDS patients involves the combination of drugs with different mechanisms of action against HIV-1. Due to the metabolic side effects induced by some PIs, there is a significant need for novel HIV-1 PIs with superior potency against both WT and mutant viruses as well as fewer metabolic side effects. A-790742 is a novel PI that exhibited fewer proteasome gene changes that could lead to lipid elevation than RTV (DeGoey et al., presented at the 230th Nat. Meet. Am. Chem. Soc.) and showed only a modest elevation of bilirubin levels compared to those shown by ATV and IDV (43). In this study, we characterized the antiviral activity, resistance profile, and development of resistance to A-790742. A-790742 is a potent PI, with activity ranging from 2 to 7 nM against WT HIV-1. With few exceptions, this compound retained its activity against LPV-resistant viruses as well as molecular clones with protease genes derived from LPV-resistant viruses and a panel of HIV-1 patient isolates with multiple mutations in the protease gene.
Based on the activity of A-790742 against HIV-1 molecular clones containing protease derived from PI-resistant patient isolates (Table 3), we speculate that A-790742 is likely to retain most of its antiviral activity against patient isolates with multiple mutations in the protease gene. Multiple combinations of mutations could confer resistance to A-790742, but their degree of resistance to A-790742 appeared to be less than that to LPV and ATV. In particular, patient isolates with mutation A71V combined with mutation I84V displayed resistance to A-790742, whereas mutations V82A/F/T did not seem to confer much resistance to A-790742 (Table 3). Consistently with these results, mutations A71V and I84V also were detected in the selection of resistant viruses with pNL4-3 in the presence of A-790742 in vitro (Table 4).
To better understand the resistance profile of A-790742, we performed the selection of HIV-1 mutant viruses in two different genetic backgrounds with this PI. Passage in a pNL4-3 background initially selected the I84V mutation (Table 4). Further passages selected an additional primary mutation, V82L (passage 8), and a number of other mutations, e.g., A71V, in later passages. In contrast, the V75I mutation was first detected in the selection in the RF background (Table 5). However, the combination of A71V and V82G mutations was later observed in the RF selection at passage 10, accompanied by the appearance of other mutations in passage 13. In two studies conducted previously, the passage of viruses in the presence of LPV in a pNL4-3 background resulted in the selection of mutant viruses with different mutation patterns, including primary mutations I84V and I50V/M46I (2, 27).
The selection of different major mutations with A-790742 in different strains of HIV-1 points to the importance of the genetic background for the selection of resistant mutants. In addition, the selection with pNL4-3 progressed faster than that with RF virus in the presence of A-790742, suggesting that resistance developed more readily in the pNL4-3 background than the RF background. Slower selection with the RF strain than with the pNL4-3 strain is consistent with the observation that molecular clones with the RF protease gene containing mutations A71V plus V82G had greatly reduced replication capacities compared to those of the molecular clone containing the WT RF protease (Fig. 2). Our results are consistent with previous reports demonstrating that the development of resistance was affected by the viral strain used in the selection (4, 9).
Primary mutations V82L and V82G are not commonly selected with existing PIs. The single mutation V82L (clone 2) (Table 7), and probably the V82G mutation, did not confer a significant degree of resistance to A-790742. In contrast, mutants harboring V82L or V82G, in combination with other mutations, e.g., A71V, were highly resistant to A-790742. These mutants were mainly cross-resistant to RTV and LPV as well as ATV (Tables 6 and 7). However, they were only moderately resistant to most other tested PIs, and they remained quite susceptible to SQV, APV, IDV, DRV, and TPV.
In summary, A-790742 displays favorable anti-HIV-1 potency against both the WT and most PI-resistant mutants. In vitro passage of HIV-1 with A-790742 led to the selection of the primary mutation I84V, as well as the uncommon mutations V82L and V82G in protease. Molecular clones harboring these primary mutations in combination with other mutations were highly resistant to A-790742 as well as to LPV and RTV. These clones, however, were only moderately resistant to most other PIs and retained their susceptibility to SQV. In addition, mutation V82G in the RF background appeared to have a significant negative impact on the replication capacity of the virus. A-790742 thus has promising antiviral activity and a potentially advantageous resistance profile.
Acknowledgments
We thank Tami Pilot-Matias for the critical reading of the manuscript and Monogram Biosciences for testing compounds against some of the molecular clones listed in Table 3.
Footnotes
Published ahead of print on 22 January 2008.
REFERENCES
- 1.Boden, D., A. Hurley, L. Zhang, Y. Cao, Y. Guo, E. Jones, J. Tsay, J. Ip, C. Farthing, K. Limoli, N. Parkin, and M. Markowitz. 1999. HIV-1 drug resistance in newly infected individuals. JAMA 282:1135-1141. [DOI] [PubMed] [Google Scholar]
- 2.Carrillo, A., K. D. Stewart, H. L. Sham, D. W. Norbeck, W. E. Kohlbrenner, J. M. Leonard, D. J. Kempf, and A. Molla. 1998. In vitro selection and characterization of human immunodeficiency virus type 1 variants with increased resistance to ABT-378, a novel protease inhibitor. J. Virol. 72:7532-7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cingolani, A., A. Antinori, M. G. Rizzo, R. Murri, A. Ammassari, F. Baldini, S. Di Giambenedetto, R. Cauda, and A. De Luca. 2002. Usefulness of monitoring HIV drug resistance and adherence in individuals failing highly active antiretroviral therapy: a randomized study. AIDS 16:369-379. [DOI] [PubMed] [Google Scholar]
- 4.Colonno, R., R. Rose, C. McLaren, A. Thiry, N. Parkin, and J. Friborg. 2004. Identification of I50L as the signature atazanavir (ATV)-resistance mutation in treatment-naive HIV-1-infected patients receiving ATV-containing regimens. J. Infect. Dis. 189:1802-1810. [DOI] [PubMed] [Google Scholar]
- 5.Cvetkovic, R. S., and K. L. Goa. 2003. Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs 63:769-802. [DOI] [PubMed] [Google Scholar]
- 6.Reference deleted.
- 7.de Mendoza, C., L. Martin-Carbonero, P. Barreiro, B. Diaz, E. Valencia, I. Jimenez-Nacher, O. Gallego, M. Nunez, J. Gonzalez-Lahoz, and V. Soriano. 2002. Salvage treatment with lopinavir/ritonavir (Kaletra) in HIV-infected patients failing all current antiretroviral drug families. HIV Clin. Trials 3:304-309. [DOI] [PubMed] [Google Scholar]
- 8.Demeter, L. M., M. D. Hughes, R. W. Coombs, J. B. Jackson, J. M. Grimes, R. J. Bosch, S. A. Fiscus, S. A. Spector, K. E. Squires, M. A. Fischl, S. M. Hammer, et al. 2001. Predictors of virologic and clinical outcomes in HIV-1-infected patients receiving concurrent treatment with indinavir, zidovudine, and lamivudine. Ann. Intern. Med. 135:954-964. [DOI] [PubMed] [Google Scholar]
- 9.Gong, Y. F., B. S. Robinson, R. E. Rose, C. Deminie, T. P. Spicer, D. Stock, R. J. Colonno, and P. F. Lin. 2000. In vitro resistance profile of the human immunodeficiency virus type 1 protease inhibitor BMS-232632. Antimicrob. Agents Chemother. 44:2319-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grabar, S., C. Pradier, E. Le Corfec, R. Lancar, C. Allavena, M. Bentata, P. Berlureau, C. Dupont, P. Fabbro-Peray, I. Poizot-Martin, and D. Costagliola. 2000. Factors associated with clinical and virological failure in patients receiving a triple therapy including a protease inhibitor. AIDS 14:141-149. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch, M. S., F. Brun-Vezinet, R. T. D'Aquila, S. M. Hammer, V. A. Johnson, D. R. Kuritzkes, C. Loveday, J. W. Mellors, B. Clotet, B. Conway, L. M. Demeter, S. Vella, D. M. Jacobsen, and D. D. Richman. 2000. Antiretroviral drug resistance testing in adult HIV-1 infection: recommendations of an International AIDS Society-USA panel. JAMA 283:2417-2426. [DOI] [PubMed] [Google Scholar]
- 12.Ho, D. D., T. Toyoshima, H. Mo, D. J. Kempf, D. Norbeck, C. M. Chen, N. E. Wideburg, S. K. Burt, J. W. Erickson, and M. K. Singh. 1994. Characterization of human immunodeficiency virus type 1 variants with increased resistance to a C2-symmetric protease inhibitor. J. Virol. 68:2016-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kantor, R., W. J. Fessel, A. R. Zolopa, D. Israelski, N. Shulman, J. G. Montoya, M. Harbour, J. M. Schapiro, and R. W. Shafer. 2002. Evolution of primary protease inhibitor resistance mutations during protease inhibitor salvage therapy. Antimicrob. Agents Chemother. 46:1086-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kempf, D. J., J. D. Isaacson, M. S. King, S. C. Brun, J. Sylte, B. Richards, B. Bernstein, R. Rode, and E. Sun. 2002. Analysis of the virological response with respect to baseline viral phenotype and genotype in protease inhibitor-experienced HIV-1-infected patients receiving lopinavir/ritonavir therapy. Antivir. Ther. 7:165-174. [PubMed] [Google Scholar]
- 15.Kempf, D. J., J. D. Isaacson, M. S. King, S. C. Brun, Y. Xu, K. Real, B. M. Bernstein, A. J. Japour, E. Sun, and R. A. Rode. 2001. Identification of genotypic changes in human immunodeficiency virus protease that correlate with reduced susceptibility to the protease inhibitor lopinavir among viral isolates from protease inhibitor-experienced patients. J. Virol. 75:7462-7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kempf, D. J., K. C. Marsh, J. F. Denissen, E. McDonald, S. Vasavanonda, C. A. Flentge, B. E. Green, L. Fino, C. H. Park, X. P. Kong, et al. 1995. ABT-538 is a potent inhibitor of human immunodeficiency virus protease and has high oral bioavailability in humans. Proc. Natl. Acad. Sci. USA 92:2484-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kempf, D. J., J. F. Waring, D. C. Morfitt, P. Werner, B. Ebert, M. Mitten, B. Nguyen, J. T. Randolph, D. A. DeGoey, L. L. Klein, and K. Marsh. 2006. Practical preclinical model for assessing the potential for unconjugated hyperbilirubinemia produced by human immunodeficiency virus protease inhibitors. Antimicrob. Agents Chemother. 50:762-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ledergerber, B., M. Egger, M. Opravil, A. Telenti, B. Hirschel, M. Battegay, P. Vernazza, P. Sudre, M. Flepp, H. Furrer, P. Francioli, R. Weber, et al. 1999. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Lancet 353:863-868. [DOI] [PubMed] [Google Scholar]
- 19.Little, S. J. 2000. Transmission and prevalence of HIV resistance among treatment-naive subjects. Antivir. Ther. 5:33-40. [DOI] [PubMed] [Google Scholar]
- 20.Little, S. J., E. S. Daar, R. T. D'Aquila, P. H. Keiser, E. Connick, J. M. Whitcomb, N. S. Hellmann, C. J. Petropoulos, L. Sutton, J. A. Pitt, E. S. Rosenberg, R. A. Koup, B. D. Walker, and D. D. Richman. 1999. Reduced antiretroviral drug susceptibility among patients with primary HIV infection. JAMA 282:1142-1149. [DOI] [PubMed] [Google Scholar]
- 21.Lum, P. Y., Y. D. He, J. G. Slatter, J. F. Waring, N. Zelinsky, G. Cavet, X. Dai, O. Fong, R. Gum, L. Jin, G. E. Adamson, C. J. Roberts, D. B. Olsen, D. J. Hazuda, and R. G. Ulrich. 2007. Gene expression profiling of rat liver reveals a mechanistic basis for ritonavir-induced hyperlipidemia. Genomics 90:464-473. [DOI] [PubMed] [Google Scholar]
- 22.MacArthur, R. D., R. M. Novak, G. Peng, L. Chen, Y. Xiang, K. H. Hullsiek, M. J. Kozal, M. van den Berg-Wolf, C. Henely, B. Schmetter, and M. Dehlinger. 2006. A comparison of three highly active antiretroviral treatment strategies consisting of non-nucleoside reverse transcriptase inhibitors, protease inhibitors, or both in the presence of nucleoside reverse transcriptase inhibitors as initial therapy (CPCRA 058 FIRST study): a long-term randomised trial. Lancet 368:2125-2135. [DOI] [PubMed] [Google Scholar]
- 23.Manfredi, R., and F. Chiodo. 2001. Disorders of lipid metabolism in patients with HIV disease treated with antiretroviral agents: frequency, relationship with administered drugs, and role of hypolipidaemic therapy with bezafibrate. J. Infect. 42:181-188. [DOI] [PubMed] [Google Scholar]
- 24.Marcelin, A. G., I. Cohen-Codar, M. S. King, P. Colson, E. Guillevic, D. Descamps, C. Lamotte, V. Schneider, J. Ritter, M. Segondy, H. Peigue-Lafeuille, L. Morand-Joubert, A. Schmuck, A. Ruffault, P. Palmer, M. L. Chaix, V. Mackiewicz, V. Brodard, J. Izopet, J. Cottalorda, E. Kohli, J. P. Chauvin, D. J. Kempf, G. Peytavin, and V. Calvez. 2005. Virological and pharmacological parameters predicting the response to lopinavir-ritonavir in heavily protease inhibitor-experienced patients. Antimicrob. Agents Chemother. 49:1720-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Markowitz, M., H. Mo, D. J. Kempf, D. W. Norbeck, T. N. Bhat, J. W. Erickson, and D. D. Ho. 1995. Selection and analysis of human immunodeficiency virus type 1 variants with increased resistance to ABT-538, a novel protease inhibitor. J. Virol. 69:701-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masquelier, B., D. Breilh, D. Neau, S. Lawson-Ayayi, V. Lavignolle, J. M. Ragnaud, M. Dupon, P. Morlat, F. Dabis, and H. Fleury. 2002. Human immunodeficiency virus type 1 genotypic and pharmacokinetic determinants of the virological response to lopinavir-ritonavir-containing therapy in protease inhibitor-experienced patients. Antimicrob. Agents Chemother. 46:2926-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mo, H., L. Lu, T. Dekhtyar, K. D. Stewart, E. Sun, D. J. Kempf, and A. Molla. 2003. Characterization of resistant HIV variants generated by in vitro passage with lopinavir/ritonavir. Antivir. Res. 59:173-180. [DOI] [PubMed] [Google Scholar]
- 28.Mo, H., L. Lu, R. Pithawalla, D. J. Kempf, and A. Molla. 2004. Complementation in cells cotransfected with a mixture of wild-type and mutant human immunodeficiency virus (HIV) influences the replication capacities and phenotypes of mutant variants in a single-cycle HIV resistance assay. J. Clin. Microbiol. 42:4169-4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Mo, H., T. Dekhtyar, L. Lu, S. Masse, T. I. Ng, D. A. DeGoey, L. L. Klein, D. J. Kempf, and A. Molla. 2005. In vitro selection and characterization of HIV-1 with reduced susceptibility to A-790742, a novel HIV-1 protease inhibitor potent against multi-PI resistant mutants, abstr. H-1092. Abstr. 45th Int. Conf. Antimicrob. Agents. Chemother. American Society for Microbiology, Washington, DC.
- 29.Molla, A., S. Vasavanonda, G. Kumar, H. L. Sham, M. Johnson, B. Grabowski, J. F. Denissen, W. Kohlbrenner, J. J. Plattner, J. M. Leonard, D. W. Norbeck, and D. J. Kempf. 1998. Human serum attenuates the activity of protease inhibitors toward wild-type and mutant human immunodeficiency virus. Virology 250:255-262. [DOI] [PubMed] [Google Scholar]
- 30.Parkin, N., C. Chappey, L. Maroldo, M. Bates, N. S. Hellmann, and C. J. Petropoulos. 2002. Phenotypic and genotypic HIV-1 drug resistance assays provide complementary information. J. Acquir. Immun. Defic. Syndr. 31:128-136. [DOI] [PubMed] [Google Scholar]
- 31.Pauwels, R., J. Balzarini, M. Baba, R. Snoeck, D. Schols, P. Herdewijn, J. Desmyter, and E. De Clercq. 1988. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 20:309-321. [DOI] [PubMed] [Google Scholar]
- 32.Périard, D., A. Telenti, P. Sudre, J. J. Cheseaux, P. Halfon, M. J. Reymond, S. M. Marcovina, M. P. Glauser, P. Nicod, R. Darioli, V. Mooser, et al. 1999. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. Circulation 100:700-705. [DOI] [PubMed] [Google Scholar]
- 33.Petropoulos, C. J., N. T. Parkin, K. L. Limoli, Y. S. Lie, T. Wrin, W. Huang, H. Tian, D. Smith, G. A. Winslow, D. J. Capon, and J. M. Whitcomb. 2000. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 44:920-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sham, H. L., D. J. Kempf, A. Molla, K. C. Marsh, G. N. Kumar, C. M. Chen, W. Kati, K. Stewart, R. Lal, A. Hsu, D. Betebenner, M. Korneyeva, S. Vasavanonda, E. McDonald, A. Saldivar, N. Wideburg, X. Chen, P. Niu, C. Park, V. Jayanti, B. Grabowski, G. R. Granneman, E. Sun, A. J. Japour, J. M. Leonard, J. J. Plattner, and D. W. Norbeck. 1998. ABT-378, a highly potent inhibitor of the human immunodeficiency virus protease. Antimicrob. Agents Chemother. 42:3218-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tisdale, M., R. E. Myers, B. Maschera, N. R. Parry, N. M. Oliver, and E. D. Blair. 1995. Cross-resistance analysis of human immunodeficiency virus type 1 variants individually selected for resistance to five different protease inhibitors. Antimicrob. Agents Chemother. 39:1704-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Leeuwen, R., C. Katlama, R. L. Murphy, K. Squires, J. Gatell, A. Horban, B. Clotet, S. Staszewski, A. van Eeden, N. Clumeck, M. Moroni, A. T. Pavia, R. E. Schmidt, J. Gonzalez-Lahoz, J. Montaner, F. Antunes, R. Gulick, D. Banhegyi, M. van der Valk, P. Reiss, L. van Weert, F. van Leth, V. A. Johnson, J. P. Sommadossi, and J. M. Lange. 2003. A randomized trial to study first-line combination therapy with or without a protease inhibitor in HIV-1-infected patients. AIDS 17:987-999. [DOI] [PubMed] [Google Scholar]
- 37.Van Vaerenbergh, K. 2001. Study of the impact of HIV genotypic drug resistance testing on therapy efficacy. Verh. K. Acad. Geneeskd. Belg. 63:447-473. [PubMed] [Google Scholar]
- 38.Volberding, P. A. 1999. Advances in the medical management of patients with HIV-1 infection: an overview. AIDS 13(Suppl. 1):S1-S9. [PubMed] [Google Scholar]
- 39.Walmsley, S., B. Bernstein, M. King, J. Arribas, G. Beall, P. Ruane, M. Johnson, D. Johnson, R. Lalonde, A. Japour, S. Brun, and E. Sun. 2002. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N. Engl. J. Med. 346:2039-2046. [DOI] [PubMed] [Google Scholar]
- 40.Waring, J., R. Ciurlionis, K. Marsh, L. L. Klein, D. A. DeGoey, J. T. Randolph, B. Spear, and D. J. Kempf. 2005. Identification of proteasome gene regulation in a rat model for HIV-induced hyperlipidemia using microarray analysis, abstr. 839. 12th Conf. Retrovir. Opportun. Infect., Boston, MA.
- 41.Yeni, P., D. A. Cooper, J. P. Aboulker, A. G. Babiker, D. Carey, J. H. Darbyshire, M. Floridia, P. M. Girard, R. L. Goodall, M. H. Hooker, A. Mijch, V. Meiffredy, and B. Salzberger. 2006. Virological and immunological outcomes at 3 years after starting antiretroviral therapy with regimens containing non-nucleoside reverse transcriptase inhibitor, protease inhibitor, or both in INITIO: open-label randomised trial. Lancet 368:287-298. [DOI] [PubMed] [Google Scholar]
- 42.Yeni, P. G., S. M. Hammer, M. S. Hirsch, M. S. Saag, M. Schechter, C. C. Carpenter, M. A. Fischl, J. M. Gatell, B. G. Gazzard, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, R. T. Schooley, M. A. Thompson, S. Vella, and P. A. Volberding. 2004. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA panel. JAMA 292:251-265. [DOI] [PubMed] [Google Scholar]
- 43.Zell, S. C. 2003. Clinical vignette in antiretroviral therapy: jaundice. J. Int. Assoc. Physicians AIDS Care 2:133-139. [DOI] [PubMed] [Google Scholar]