Abstract
Exercise and contractions of isolated skeletal muscle induce phosphorylation of mitogen-activated protein kinases (MAPKs) by undefined mechanisms. The aim of the present study was to determine exercise-related triggering factors for the increased phosphorylation of MAPKs in isolated rat extensor digitorum longus (EDL) muscle.
Concentric or eccentric contractions, or mild or severe passive stretches were used to discriminate between effects of metabolic/ionic and mechanical alterations on phosphorylation of two MAPKs: extracellular signal-regulated kinase 1 and 2 (MAPKerk1/2) and stress-activated protein kinase p38 (MAPKp38).
Concentric contractions induced a 5-fold increase in MAPKerk1/2 phosphorylation. Application of the antioxidants N-acetylcysteine (20 mm) or dithiothreitol (5 mm) suppressed concentric contraction-induced increase in MAPKerk1/2 phosphorylation. Mild passive stretches of the muscle increased MAPKerk1/2 phosphorylation by 1.8-fold, whereas the combination of acidosis and passive stretches resulted in a 2.8-fold increase. Neither concentric contractions, nor mild stretches nor acidosis significantly affected phosphorylation of MAPKp38.
High force applied upon muscle by means of either eccentric contractions or severe passive stretches resulted in 5.7- and 9.5-fold increases of phosphorylated MAPKerk1/2, respectively, whereas phosphorylation of MAPKp38 increased by 7.6- and 1.9-fold (not significant), respectively.
We conclude that in isolated rat skeletal muscle an increase in phosphorylation of both MAPKerk1/2 and MAPKp38 is induced by mechanical alterations, whereas contraction-related metabolic/ionic changes (reactive oxygen species and acidosis) cause increased phosphorylation of MAPKerk1/2 only. Thus, contraction-induced phosphorylation can be explained by the combined action of increased production of reactive oxygen species, acidification and mechanical perturbations for MAPKerk1/2 and by high mechanical stress for MAPKp38.
Mitogen-activated protein kinases (MAPKs) are ubiquitous signalling proteins involved in control of cell growth, differentiation and adaptation (Fanger, 1999; Tibbles & Woodgett, 1999). Four parallel cascades of MAPKs have recently been described in mammalian cells, i.e. extracellular signal-regulated kinase 1 and 2 (MAPKerk1/2), stress-activated protein kinase p38 (MAPKp38), c-jun NH2-terminal kinase (MAPKjnk), and extracellular signal-regulated kinase 5 (MAPKerk5) (for review see Widmann et al. 1999). MAPKs are activated via dual phosphorylation on threonine and tyrosine residues by MAPK kinases (Widmann et al. 1999). Factors that have been shown to trigger MAPK activation include hormones, growth factors, reactive oxygen species (ROS), lowered pH and mechanical stress (Guyton et al. 1996; Xue & Lucocq, 1997; Williams et al. 1998; Loufrani et al. 1999). Following activation, MAPKs can either phosphorylate different cytoplasmic targets or translocate to the nucleus and directly or indirectly affect transcription. Transcription factors such as Elk1, ATF2, AP1, CREB and MEF2C have been reported to be downstream of MAPKs (Widmann et al. 1999; Zetser et al. 1999). In addition, signals conducted via MAPKerk1/2 and MAPKp38 may facilitate the initiation of translation via phosphorylation of Mnk1 kinase, which activates the eukaryotic translation initiation factor 4E (eIF4E), the limiting initiation factor in most cells (for review see Raught & Gingras, 1999).
Recent observations indicate that MAPKerk1/2 and MAPKp38 play an important role in differentiation of muscle cells (Gredinger et al. 1998; Zetser et al. 1999). Furthermore, it has been hypothesised that MAPKs contribute to the adaptive changes observed in skeletal muscle in response to exercise training. This hypothesis is supported by the fact that exercise activates MAPKerk1/2 (Aronson et al. 1997; Widegren et al. 1998) and both its upstream kinase, MEK1/2 (Widegren et al. 2000), and downstream kinases, mitogen- and stress-activated kinases (MSK) 1 and 2, and p90 ribosomal S6 kinase (p90rsk) (Krook et al. 2000) in human skeletal muscle. Furthermore, exercise in humans also activates the MAPKp38 pathway, including the upstream kinase SEK1, and one downstream kinase, mitogen-activated protein kinase-activated protein kinase 2 (MAPKAPK2) (Widegren et al. 1998; Krook et al. 2000). Thus changes in MAPKerk1/2 and MAPKp38 phosphorylation during exercise would indicate activation of these cascades. Studies where contractions have been produced in isolated skeletal muscle provide evidence that exercise-induced dual phosphorylation of MAPKerk1/2 and MAPKp38 can be of local origin and does not require any influence of systemic stimuli, such as hormones (Hayashi et al. 1999; Ryder et al. 2000; Wretman et al. 2000). Activation of MAPKerk1/2 or MAPKp38 may occur independently of each other in response to different factors (Tibbles & Woodgett, 1999; Widmann et al. 1999). It has been recently shown in the rat that severe passive stretch of isolated soleus, but not EDL, muscle induces phosphorylation of MAPKs. This effect was reported to be much larger for MAPKp38 than for MAPKerk1/2 (Boppart et al. 2001). However, other potential triggers for the activation of MAPKerk1/2 and MAPKp38 in contracting skeletal muscle remain unknown.
Muscle contraction is accompanied by simultaneous changes in many variables, including increased mechanical stress and metabolic/ionic alterations. The aim of the present study was to differentiate between the effect of mechanical stress and metabolic/ionic alterations on MAPKerk1/2 and MAPKp38 phosphorylation in isolated rat skeletal muscle. For this purpose, we exposed isolated fast-twitch extensor digitorum longus (EDL) muscle of the rat to repeated concentric or eccentric contractions or a series of passive stretches. The main result is that concentric contractions activate MAPKerk1/2 but not MAPKp38, whereas eccentric contractions increase phosphorylation of both kinases. We also show that ROS generation, mechanical stress and most likely also acidosis may induce MAPKerk1/2 phosphorylation.
METHODS
Animals
Male Wistar rats (B & K Universal AB, Sollentuna, Sweden), with a weight of 80-120 g, were housed at room temperature and fed ad libitum. Animals were killed by exposure to a rising concentration of carbon dioxide and the EDL muscles excised. In a previous study, we compared phosphorylation of MAPKs between muscles excised from rats immediately following CO2 asphyxiation and muscles excised from pentobarbital sodium anaesthetised rats (Wretman et al. 2000). These two techniques gave identical results. The study was approved by the Ethics Committee for animal research in northern Stockholm.
Muscle preparation and solutions
In experiments with mechanical recordings, small stainless steel loops were tied, using thin nylon thread, to the tendons of the isolated EDL muscle. The muscle was then mounted between a force transducer and a lab-built stretch/shortening device. The length of the muscle was adjusted to allow maximum tetanic force to be generated. This optimal length (lo) of the muscle was 19-22 mm. During the experiment the muscles were bathed in continuously stirred Tyrode solution of the following composition (mm): 121 NaCl, 5 KCl, 0.5 MgCl2, 1.8 CaCl2, 0.4 NaH2PO4, 0.1 NaEDTA, 24 NaHCO3, 5.5 glucose. Fetal calf serum (0.2 %) was added to the solution. The solution was bubbled with 95 % O2-5 % CO2 (pH 7.4) and experiments were performed at room temperature (24-26 °C). At the end of each experiment, muscles were frozen in liquid nitrogen and stored at -80 °C.
In some experiments muscles were exposed to drugs. In these experiments muscles were allowed to equilibrate for 1 h in the standard Tyrode solution. This period was included in order to reduce basal phosphorylation levels, which could have been increased by the dissection procedure. Then one of the muscles was moved to a separate Tyrode solution supplemented with 20 mmN-acetylcysteine (NAC), 5 mm dithiothreitol (DTT), 1 mm 2,3-butanedione monoxime (BDM), or 10 μm dantrolene and incubated for 75 min (in NAC or DTT), 45 min (in BDM), or 5 min (in dantrolene). All these chemicals were from Sigma (St Louis, MO, USA). The effect of acidosis was studied in some experiments. Muscles were then exposed to a Tyrode solution gassed with 30 % CO2-70 % O2 (pH 6.6) for 15 min. During experiments with altered Tyrode solution, the contralateral muscle always remained in the regular Tyrode solution for the same period of time.
When skeletal muscles are incubated in vitro, there is the inherent risk of a hypoxic central core developing inside the muscle. In the present study, this risk was minimised by using small animals throughout the study and, in additon, by choosing the EDL rather than the soleus muscle, because the former is less dependent on oxidative metabolism. Although there is no direct evidence against the presence of a hypoxic central core in our model, the following observations argue against that: (1) the EDL muscle exhibited robust force generation capacity for more than 12 h in a pilot study, with only 3 % isometric force decline over this time period (A. Lionikas, unpublished observation), (2) EDL has been shown to have the same contraction-induced phosphorylation and similar fatiguability as the epithrochlearis muscle from animals of the same weight (Wretman et al. 2000). In the latter study, the epitrochlearis muscle was chosen because of its thinness, < 1 mm at its thickest site, thereby making it unlikely that a hypoxic central core would develop. The reason for not using the epitrochlearis muscle in the present study was its lack of a distinct distal tendon, which we considered increased the risk of damage when the muscle was being mounted for stimulation.
Stimulation and mechanical recording
Tetanic contractions were produced by trains of supramaximal current pulses (0.5 ms duration), which were applied every third second for 15 min via platinum plate electrodes placed on each side of the muscle. The stimulation frequency was 100 Hz and the train duration 170 ms. Stimulation was started following at least 1 h of rest. In concentric contraction experiments, the stimulated muscle was allowed to shorten by approximately 30 % of lo at a speed of approximately 7 lo s−1, thereby minimising force generation (Fig. 1). In eccentric contraction experiments, the stimulated muscle was initially stretched by approximately 10 % of lo at a speed of approximately 1 lo s−1; as force production declined during fatiguing stimulation, the amplitude and speed of the stretch increased to about 15 % and 1.5 lo s−1, respectively (Fig. 6). In experiments with mild passive stretch (mild stretch), a resting muscle was stretched every third second for 15 min by approximately 30 % of lo at a speed of approximately 3 lo s−1 (Fig. 5). In order to induce high passive force (severe stretch), a resting muscle was prestretched until resting force was equivalent to 50 % of the isometric tetanic force at lo, which occurred with a stretch of about 30 % of lo. The muscle was then stretched every third second for 15 min by approximately 20 % of lo at a speed of approximately 2.5 lo s−1 (Fig. 8).
Figure 1. Original records of muscle force (top) and length (bottom) in a concentric contraction experiment.
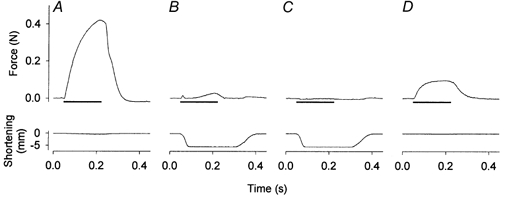
An isometric contraction was induced before (A) and at the end (D) of a series of concentric contractions (B and C). In concentric contractions, a rapid ramp release allowed the muscle to shorten at near maximal speed, thereby minimising force generation in the beginning of the series of contractions (B). By the end of the series, concentric force was undetectable (C). Horizontal bar represents 170 ms trains of stimuli at 100 Hz.
Figure 6. Original records of muscle force (top) and length (bottom) from an experiment with eccentric contractions.
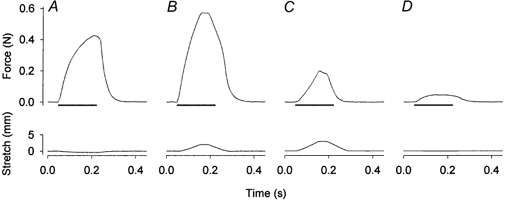
An isometric contraction was induced before (A) and at the end (D) of a series of eccentric contractions (B and C, the first and the last eccentric contraction, respectively). The muscle was initially stretched by 10 % of its optimal length at a speed 1 lo s−1. Horizontal bars represent 170 ms trains of stimuli at 100 Hz.
Figure 5. Original records of muscle force (top) and length (bottom) from an experiment with mild stretches.
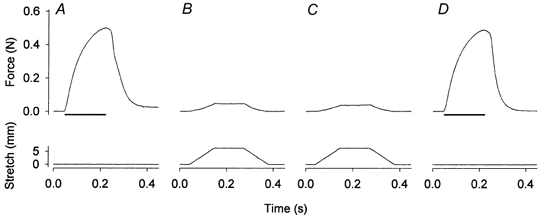
An isometric contraction was induced before (A) and at the end (D) of a series of intermittent passive stretches (B and C, the first and the last stretch, respectively). The muscle was stretched by 30 % of its optimal length at a speed 3 lo s−1. Horizontal bars represent 170 ms trains of stimuli at 100 Hz.
Figure 8. Original records of muscle force (top) and length (bottom) from an experiment with severe stretches.
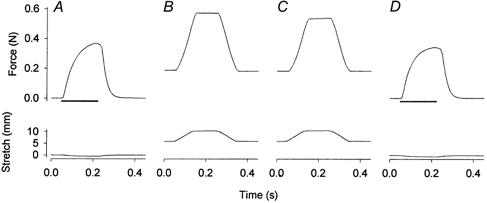
An isometric contraction was induced before (A) and at the end (D) of a series of severe stretches (B and C, the first and the last stretch, respectively). In order to induce high passive force, a resting muscle was prestretched to a passive tension close to half of isometric force. Then the muscle was intermittently stretched by 23 % of its optimal length at a speed ≈2.6 lo s−1. Horizontal bars represent 170 ms trains of stimuli at 100 Hz.
In a series of experiments where the effects of decreased force and/or myoplasmic free Ca2+ concentration ([Ca2+]i) were tested, both muscles were subjected to repeated tetanic stimulation under isometric conditions. One muscle was stimulated in standard solution and the other after addition of 1 mm BDM, which inhibits cross-bridge force production (Horiuti et al. 1988), or 10 μm dantrolene, which inhibits sarcoplasmic reticulum Ca2+ release (Song et al. 1993).
Western blot
The muscles were weighed and homogenised in cold lysis buffer (2 mm Hepes (pH 7.4), 1 mm EDTA, 5 mm EGTA, 10 mm MgCl2, 50 mmβ-glycerophosphate, 1 mm Na3VO4, 2 mm DTT, 1 % Triton X-100, 20 μg ml−1 leupeptin, 50 μg ml−1 aprotinin, 40 μg ml−1 phenylmethylsulfonyl fluoride). Homogenates were rotated for 60 min at 4°C and clarified by centrifugation at 15 000 g for 10 min at 6°C. The supernatants were collected and protein content was determined by the Coomassie protein assay reagent with bovine serum albumin as a standard (Bradford, 1976). Samples were solubilised in Laemmli buffer (62.5 mm Tris-HCl (pH 6.8), 5 % β-mercaptoethanol, 2 % SDS, 10 % glycerol, 0.1 % bromphenol blue), boiled for 4 min, and 10 μg protein per lane of each sample was applied to 10 % SDS-PAGE gels. Proteins were transferred electrophoretically to a nitrocellulose membrane and reversibly stained with Ponceau S to verify equal transfer and loading. The membrane was blocked in a Tris-buffered saline solution containing 5 % non-fat dry milk, and 0.02 % Tween-20 and incubated with antibodies against either dual phosphorylated MAPKerk1/2 (1:500) and MAPKp38 (1:250), or total MAPKerk1/2 (1:500). Membranes were washed in a buffer (Tris-buffered saline, 0.02 % Tween-20) and incubated with horseradish peroxidase-labelled antibodies (1:2000). All antibodies were from New England Biolabs (Beverly, MA, USA). Antibodies were visualised by LumiGLO chemiluminescent substrate. The optical density of the protein bands was measured between approximately 50 and 25 kDa using Scion Image for Windows (Scion Corp., Frederick, MD, USA). Background was subsequently subtracted. The optical density of each muscle was normalised to the mean density of control muscles in each group. This way was preferred to relating values to the contralateral muscle of the same rat, in order to avoid large relative effects of small absolute differences in basal phosphorylation between individual control muscles.
Statistical analysis
Results are presented as means ± s.e.m. To test for significant differences in phosphorylation between the different treatments, a two-way ANOVA (repeated measures design) was applied, following logarithmic data transformation. The latter was performed in order to make the distribution of the data more symmetrical. When the ANOVA resulted in a significant F ratio (P < 0.05), differences were assessed via Student's paired or unpaired t test where appropriate. Bonferroni's method was applied for multiple comparisons. The data from the BDM and dantrolene experiment were tested for significance using the paired t test.
RESULTS
Effect of concentric contractions on MAPKerk1/2 and MAPKp38 phosphorylation
Concentric contractions were used in order to minimise mechanical stress and thereby to investigate the effect of metabolic changes on MAPKerk1/2 and MAPKp38 in contracting skeletal muscle. Figure 1 shows isometric and concentric force records obtained at the start and end of the 15 min period of repeated contractions. The maximum force of the first concentric contraction ranged between 0 and 10 % of isometric force. Although the force produced in concentric contractions was low because of high shortening velocity, by the end of the stimulation period muscles were severely fatigued and isometric force was reduced to about 20 % of the original, whereas concentric force was undetectable.
Concentric contractions increased phosphorylation of MAPKerk1/2 5-fold but did not have a significant effect on phosphorylation of MAPKp38 (Fig. 2). The increase in MAPKerk1/2 phosphorylation obtained with repeated concentric contractions in the present study (5-fold) appears clearly larger than that obtained previously in EDL muscles with repeated isometric contractions, 1.5-fold (Wretman et al. 2000). It could be speculated that the larger phosphorylation in the present study might be related to the lower force production during concentric contractions as compared with isometric contractions. The assumption that high force would negatively influence MAPKerk1/2 phosphorylation was tested in control experiments with repeated isometric contractions in the presence of 1 mm BDM, which inhibits cross-bridge force production (Horiuti et al. 1988), or 10 μm dantrolene, which inhibits force production by reducing [Ca2+]i (Song et al. 1993). Application of BDM or dantrolene reduced isometric force production to 76 ± 2 % (n = 5) and 80 ± 2 % (n = 5) of the control, respectively. However, the phosphorylation of MAPKerk1/2 induced by a series of repeated isometric contractions in the presence of either of these drugs did not show an increase but rather tended to decrease compared with the phosphorylation without drugs: 78 ± 12 % of the control with BDM (P = 0.12) and 79 ± 11 % with dantrolene (P = 0.5). Furthermore, these drugs had no effect on MAPKerk1/2 phosphorylation in resting muscles (data not shown). Thus, the larger increase in MAPKerk1/2 phosphorylation with concentric compared with isometric contractions is more likely to be due to the increased metabolic stress than the difference in force production. In subsequent experiments we therefore focused on two exercise-related metabolic factors that might induce the increase in MAPKerk1/2 phosphorylation: generation of ROS during cellular respiration and acidification.
Figure 2. Concentric contractions of isolated muscle induce MAPKerk1/2 but not MAPKp38 phosphorylation.
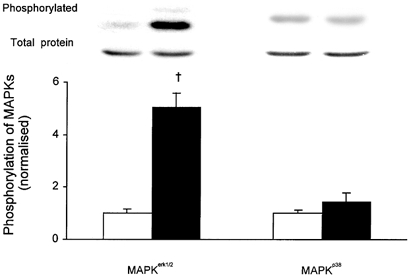
EDL muscles were subjected to concentric contractions (▪) and contralateral muscles served as resting controls (□). Representative immunoblots of phosphorylated and total proteins are presented above corresponding bars. Data are normalised to the corresponding average of resting controls. Results are presented as means ± s.e.m. for 6 muscles per bar. † P < 0.01 versus resting control.
Effects of ROS and acidification on MAPKerk1/2 phosphorylation
Intensive contractile activity of skeletal muscle results in an augmented generation of ROS (Reid et al. 1992; Kolbeck et al. 1997). To investigate if an increase in ROS production during contractions might play a role in phosphorylation of MAPKerk1/2, we conducted the concentric contraction protocol on muscles that had been incubated for 1 h in Tyrode solution supplemented with either of two different antioxidants: NAC (20 mm) or DTT (5 mm). Incubation in NAC did not alter contractility of the muscles, whereas DTT caused a slight force depression (by ≈10 %) that was reversed after 15 min of washout (cf. Khawli & Reid, 1994). Incubation in NAC or DTT markedly attenuated the concentric contraction-induced increase in MAPKerk1/2 phosphorylation from 5-fold to 1.7- and 1.3-fold, respectively (Fig. 3). Neither NAC nor DTT increased fatigue resistance of the muscles: isometric force at the end of the stimulation was depressed to 13 ± 5 % and 14 ± 2 % of the initial force, respectively, as compared with about 20 % without antioxidants (see above). Neither of the two antioxidants affected MAPKerk1/2 phosphorylation at rest (Fig. 3).
Figure 3. Effect of antioxidants on concentric contraction-induced phosphorylation of MAPKerk1/2.
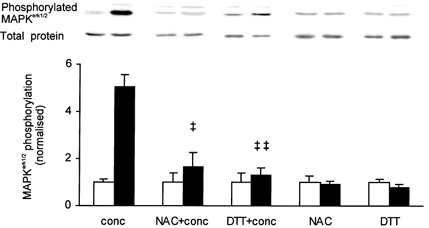
EDL muscles were incubated in 20 mm of N-acetylcysteine (NAC) or 5 mm of dithiothreitol (DTT) and subjected to concentric contractions (+conc) or an equally long rest period. Muscles treated with NAC or DTT (▪) are compared with contralateral muscles serving as resting controls (□). Representative immunoblots of phosphorylated and total proteins are presented above corresponding bars. Concentric contractions only (conc) from Fig. 2 are added for comparison. Data are normalised to the corresponding average of resting controls. Results are presented as means ± s.e.m. for 6 muscles per bar in all groups except DTT (5 muscles per bar). ‡ P < 0.05, ‡‡ P < 0.01 versus concentric contractions only.
During intense exercise the intracellular milieu becomes acidic due to lactic acid accumulation (Fitts, 1994). In order to investigate whether acidosis has an effect on phosphorylation of MAPKerk1/2, the Tyrode solution was gassed with 30 % CO2 (instead of the normal 5 % CO2) for 15 min. This will result in a decrease in intracellular pH of about 0.5 pH units (Westerblad & Allen, 1992), which is similar to the acidification observed in severe muscle fatigue (Fitts, 1994). Exposure to 30 % CO2 tended to increase the phosphorylation of MAPKerk1/2 but the increase was not significant (Fig. 4).
Figure 4. Effect of acidosis and mild passive stretches on phosphorylation of MAPKerk1/2.
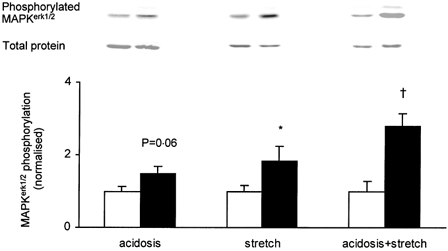
EDL muscles were acidified by exposure to 30 % CO2 (acidosis) and/or subjected to intermittent passive stretches (stretch) (▪); contralateral muscles served as resting controls (□). Representative immunoblots of phosphorylated and total proteins are presented above corresponding bars. Data are normalised to the corresponding average of resting controls. Results are presented as means ± s.e.m. for 6 muscles per bar. † P < 0.01, *P < 0.05 versus resting control.
Effects of mild stretch on MAPKerk1/2 phosphorylation
Despite the negligible force production during the concentric contractions, some mechanical stress would be imposed on the fibres due to the changes of the muscle length during contraction. To mimic this, we subjected muscles to intermittent passive stretches for 15 min. The passive tension developed during stretches was equal to 10-20 % of the isometric tetanic force (Fig. 5). The series of mild stretches did not result in any reduction of force generating capacity: in two experiments isometric force after the stretches was 97 % and 101 % of the original. Mild stretches, however, induced a 1.8-fold increase in phosphorylation of MAPKerk1/2 (Fig. 4).
To address the question of whether metabolic alterations and mechanical stress have additive effects on MAPKerk1/2 phosphorylation, mild stretches were imposed on muscles acidified by incubation in 30 % CO2. This combined treatment increased phosphorylation of MAPKerk1/2 by 2.8-fold (Fig. 4).
Effects of ROS, acidification and mild stretch on MAPKp38 phosphorylation
The series of concentric contractions did not induce a significant increase in phosphorylation of MAPKp38 (see Fig. 2). Thus, MAPKp38 does not appear to be activated by contraction-induced metabolic changes or mild mechanical stress, and hence we did not expect any of the above interventions to have an effect on MAPKp38 phosphorylation. In accordance, MAPKp38 phosphorylation was not affected by the presence of NAC or DTT or by acidification during the series of concentric contractions (data not shown). Furthermore, the presence of BDM or dantrolene did not significantly affect MAPKp38 phosphorylation during a series of isometric contractions and a series of mild passive stretches did not induce MAPKp38 phosphorylation (data not shown). Unexpectedly, application of NAC and DTT increased the MAPKp38 phosphorylation in rested muscles by ≈2- to 3-fold. The mechanism(s) underlying this increase was not further investigated.
Effects of eccentric contractions and severe stretches on MAPKerk1/2 and MAPKp38 phosphorylation
In order to study the effect of high forces on MAPK phosphorylation, experiments were performed with repeated eccentric contractions or severe passive stretches. Original force records from an experiment with repeated eccentric contractions are shown in Fig. 6. The peak force in the initial eccentric contraction was 139 ± 5 % of the isometric force (n = 6). During the series of eccentric contractions peak force decreased to 41 ± 3 % of the original isometric force. The isometric force at the end of the series of eccentric contractions was also markedly depressed (8 ± 1 % of the original). Eccentric contractions caused 5.7- and 7.6-fold increases in phosphorylation of MAPKerk1/2 and MAPKp38, respectively (Fig. 7).
Figure 7. Effect of eccentric contractions and severe stretch on phosphorylation of MAPKerk1/2 and MAPKp38.
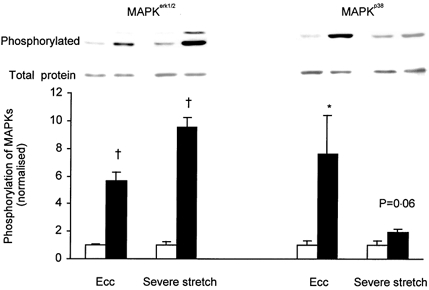
EDL muscles were subjected (▪) to eccentric contractions (Ecc) or severe stretch. Contralateral muscles served as resting controls (□). Representative immunoblots of phosphorylated and total proteins are presented above corresponding bars. Data are normalised to the corresponding average of resting controls. Results are presented as means ± s.e.m. for 6 muscles per bar. * P < 0.05, † P < 0.01 versus resting control.
Muscles were also subjected to severe stretches, i.e. prestretch followed by a series of repeated stretches (Fig. 8). The passive force developed during the severe stretch protocol was ≈150 % of the tetanic force at lo but despite this, the series of stretches did not have any major effect on the tetanic force (see Fig. 8). The series of severe stretches increased MAPKerk1/2 phosphorylation 9.5-fold (Fig. 7) and tended to increase in MAPKp38 phosphorylation (1.9-fold, P = 0.06).
DISCUSSION
There are three major novel results of the present study. First, a series of concentric contractions has divergent effects on MAPKs; it induces a marked increase in MAPKerk1/2 phosphorylation, whereas MAPKp38 is not significantly affected. Second, the increase in phosphorylation of MAPKerk1/2, but not of MAPKp38, can be induced by metabolic changes that occur during repeated contractions (i.e. increased ROS production and most likely also acidification) and by mild mechanical perturbations. Third, eccentric contractions markedly increase phosphorylation of both MAPKerk1/2 and MAPKp38.
Mechanisms underlying MAPK phosphorylation with concentric contractions
Although supramaximal stimulation was used, the repeated concentric contractions were characterised by low force production, secondary to the large and rapid shortening step (see Fig. 1). In spite of the low force production, metabolic demand during these contractions may be large (Woledge et al. 1985; Ryschon et al. 1997). ROS generation in skeletal muscle increases during periods of repeated contractions (Reid et al. 1992; Kolbeck et al. 1997) and ROS have been shown to induce MAPKerk1/2 phosphorylation in a variety of cell types (Guyton et al. 1996; Rao, 2000; Nishida et al. 2000). We used two distinctly different antioxidants, NAC and DTT, in order to address the question whether ROS have effects on MAPKerk1/2 phosphorylation during the series of concentric contractions. NAC is a non-specific antioxidant, which has been shown to reduce hydroxyl radicals, hydrogen peroxide and hypochlorous acid (Moldeus et al. 1986; Aruoma et al. 1989). DTT, on the other hand, is a thiol reducing agent capable of preventing oxidation of SH groups (Cheng & Kang, 1998; Hu et al. 2000). Neither of the two antioxidants affected MAPKerk1/2 phosphorylation at rest. However, both of these antioxidants markedly suppressed concentric contraction-induced increase in phosphorylation of MAPKerk1/2, from 5-fold to 1.3- to 1.7-fold (Fig. 3). This indicates that ROS, generated by the contracting muscle, is one important factor for increased MAPKerk1/2 phosphorylation during exercise.
Although the concentric contraction-induced MAPKerk1/2 phosphorylation was markedly reduced in the presence of antioxidants, it still tended to be higher than the control value (Fig. 3). This suggests that either buffering of ROS was incomplete or that other trigger(s) contributed to the increased phosphorylation. When energy turnover in contracting muscle is high, the intracellular milieu becomes acidic due to lactic acid accumulation. There are few and somewhat contradictory data on the effect of intracellular acidification on MAPKerk1/2 signalling. In PC12 cells, Thomas et al. (1996) reported a role of reduced intracellular pH in the mitogenic effect of growth factors, which involves the MAPKerk1/2 pathway. On the other hand, activation of MAPKerk1/2 was unrelated to intracellular pH changes in human small lung cancer cells (Merryman et al. 1997). The results of Shrode et al. (1997) suggest a permissive role for cytosolic acidification in cell growth rather than a role as a trigger in the mitogenic signal transduction cascade. Our in vitro results indicate that acidosis, of the magnitude similar to acidosis in severe fatigue (Fitts, 1994), can induce MAPKerk1/2 phosphorylation in skeletal muscle cells, although an effect of CO2 as a messenger molecule cannot be ruled out (Merryman et al. 1997).
The force output was low in the present concentric contractions, but still some intramuscular mechanical perturbation would occur in each contraction. Effects of mechanical stretch on MAPKerk1/2 phosphorylation have been observed in cardiac fibroblasts (MacKenna et al. 1998), mesangial cells (Ingram et al. 1999), blood vessels (Loufrani et al. 1999), cardiac myocytes (Yamazaki et al. 1995) and most recently in isolated skeletal muscle (Boppart et al. 2001). In the present study, we used two protocols of intermittent passive stretches to address the question of whether mechanical perturbations may induce phosphorylation of MAPKs in skeletal muscle. The mild stretch protocol was designed to simulate the minor mechanical changes that would occur in concentric contractions. The amplitude (0.3 lo) and speed (3 lo s−1) of mild stretch are clearly within the physiological range. The mild stretches did not cause any gross structural damage since muscle force generating capacity was fully preserved after the stretches (Fig. 5). We demonstrate that intermittent, mild passive stretches are sufficient to induce phosphorylation of MAPKerk1/2 in skeletal muscle, especially when the muscle is acidified. This phosphorylation of MAPKerk1/2 might be triggered by integrins, transmembrane heterodimeric glycoproteins bound to the extracellular matrix, which mediate signal transduction in a number of signalling pathways (Clark & Brugge, 1995; Carson & Wei, 2000).
Mechanisms underlying MAPK phosphorylation with eccentric contractions
In contrast to the previous observations where isometric contractions increased MAPKp38 phosphorylation in isolated rat fast-twitch muscle (Ryder et al. 2000; Wretman et al. 2000), concentric contractions did not induce a significant increase in MAPKp38 phosphorylation in the present study. This implies that MAPKp38 phosphorylation is little affected by metabolic alterations and the present results show no effect of acidification or presence of antioxidants during the series of contractions. Furthermore, we show that MAPKp38 phosphorylation is not induced by mild mechanical stress. Thus, by exclusion it appears that the higher mechanical stress imposed upon muscle in isometric contractions is required to induce the increase in MAPKp38 phosphorylation. In line with this, eccentric contractions markedly induced phosphorylation of MAPKp38, an effect that also tended to occur with severe stretches. Both protocols induced phosphorylation of MAPKerk1/2, confirming the importance of mechanical stress for increase in phosphorylation. The extent of the effect, however, differed. Severe stretches induced a larger effect on MAPKerk1/2 phosphorylation than eccentric contractions (9.5- vs. 5.7-fold, respectively), whereas the reverse was true for MAPKp38 (1.9- vs. 7.6-fold, respectively). Muscles were subjected to similar peak forces during the eccentric and severe stretch protocols (about 140 and 150 % of isometric force, respectively). However, the origin and transmission of force in the muscles would differ between the two protocols. In eccentric contractions, the contractile elements (i.e. cross-bridges) and the elastic elements in series (e.g. Z-line, tendon) are involved in force generation and transmission, whereas in severe stretch, stiffness of elastic components in parallel (e.g. sarcolemma, endomysium, perimysium, epimysium) and in series generates force (Woledge et al. 1985). Based on the present results it may be suggested that generation of high force by the cross-bridges and transduction of this force by elastic elements in series is critical for MAPKp38 phosphorylation. Stretch of parallel elastic components, on the other hand, appears to be more important for phosphorylation of MAPKerk1/2. However, a similar increase in MAPKerk1/2 phosphorylation induced by isometric contractions and strenuous static stretch (1.5- to 1.7-fold and ≈2-fold, respectively) has recently been reported in rat soleus muscle (Boppart et al. 2001). Compared with the results of the severe stretch applied in the present study (9.5-fold), this suggests that the pattern of passive stretch (static vs. intermittent) and/or muscle type (slow twitch vs. fast twitch) might also be of importance.
The role of [Ca2+]i in MAPK phosphorylation
Increased cytoplasmic [Ca2+] has been shown to induce phosphorylation of MAPKerk1/2 in smooth muscle (Katoch et al. 1999) and MAPKp38 in human peripheral mononuclear cells (Kreideweiss et al. 1999). Taken together, the present and previous results from isolated muscle do not support a central role of [Ca2+]i in the contraction-induced activation of MAPKerk1/2 or MAPKp38 in skeletal muscle. [Ca2+]i transients during contraction are similar during isometric and concentric conditions (Vandenboom et al. 1998) and if increased [Ca2+]i were a key trigger, phosphorylation of the two MAPKs would be expected to be similar in these two situations. However, while both MAPKs are phosphorylated by isometric contractions (Ryder et al. 2000; Wretman et al. 2000), concentric contractions induced a large MAPKerk1/2 phosphorylation whereas MAPKp38 phosphorylation was not significantly increased. The lack of an important role of [Ca2+]i in MAPKs phosphorylation is further implied by the experiment with inhibition of Ca2+ release. Incubation in 1 μm dantrolene reduces tetanic Ca2+ (Westerblad et al. 1998) but barely had an effect on phosphorylation of MAPKerk1/2. Nevertheless, adaptive changes in muscle can still be related to Ca2+ but will then be mediated by other signalling pathways, such as the Ca2+-calmodulin activated phosphatase calcineurin (Olson & Williams, 2000) or protein kinase C (Richter et al. 1987).
Concluding remarks
Based on the present results in isolated rat skeletal muscle, we conclude that contraction-induced MAPKerk1/2 phosphorylation in skeletal muscle can be explained by the combined action of increased ROS production, acidification and mechanical perturbations. Phosphorylation of MAPKp38 is not significantly affected by metabolic/ionic alterations but is induced by high mechanical stress.
The present results imply different roles of MAPKerk1/2 and MAPKp38 in skeletal muscle long-term adaptations to exercise. It could be speculated that MAPKerk1/2, which is activated by metabolic/ionic changes, is likely to be involved in the adaptive changes that occur as a consequence of endurance training, i.e. increased capacity and efficiency for oxygen utilisation. Conversely, activation of MAPKp38, which seems to require more extensive mechanical stress, might have a role in the adaptive changes occurring in response to strength training, i.e. increased number of myofibrils and muscle hypertrophy.
Acknowledgments
This work was supported by grants from the Swedish Medical Research Council (Projects 14X-07917 and 14X-10842), the Swedish National Centre for Research in Sports, the Karolinska Institutet Foundation and the Research Foundations of Clas Groschinsky, Edla och Eric Smedberg and Magn Bergvall. Arimantas Lionikas was supported by a research scholarship from the Swedish Institute.
References
- Aronson D, Violan M A, Dufresne S D, Zangen D, Fielding R A, Goodyear L J. Exercise stimulates the mitogen-activated protein kinase pathway in human skeletal muscle. Journal of Clinical Investigation. 1997;99:1251–1257. doi: 10.1172/JCI119282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruoma O I, Halliwell B, Hoey B M, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radical Biology and Medicine. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- Boppart M D, Hirshman M F, Sakamoto K, Fielding R A, Goodyear L J. Static stretch increases c-Jun NH2-terminal kinase activity and p38 phosphorylation in rat skeletal muscle. American Journal of Physiology - Cell Physiology. 2001;280:C352–358. doi: 10.1152/ajpcell.2001.280.2.C352. [DOI] [PubMed] [Google Scholar]
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carson J A, Wei L. Integrin signaling's potential for mediating gene expression in hypertrophying skeletal muscle. Journal of Applied Physiology. 2000;88:337–343. doi: 10.1152/jappl.2000.88.1.337. [DOI] [PubMed] [Google Scholar]
- Cheng Y W, Kang J J. Emodin-induced muscle contraction of mouse diaphragm and the involvement of Ca2+ influx and Ca2+ release from sarcoplasmic reticulum. British Journal of Pharmacology. 1998;123:815–820. doi: 10.1038/sj.bjp.0701677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E A, Brugge J S. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Fanger G R. Regulation of the MAPK family members: role of subcellular localization and architectural organization. Histology and Histopathology. 1999;14:887–894. doi: 10.14670/HH-14.887. [DOI] [PubMed] [Google Scholar]
- Fitts R H. Cellular mechanisms of muscle fatigue. Physiological Reviews. 1994;74:49–93. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Gredinger E, Gerber A N, Tamir Y, Tapscott S J, Bengal E. Mitogen-activated protein kinase pathway is involved in the differentiation of muscle cells. Journal of Biological Chemistry. 1998;273:10436–10444. doi: 10.1074/jbc.273.17.10436. [DOI] [PubMed] [Google Scholar]
- Guyton K Z, Liu Y, Gorospe M, Xu Q, Holbrook N J. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. Journal of Biological Chemistry. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hirshman M F, Dufresne S D, Goodyear L J. Skeletal muscle contractile activity in vitro stimulates mitogen-activated protein kinase signaling. American Journal of Physiology. 1999;277:C701–707. doi: 10.1152/ajpcell.1999.277.4.C701. [DOI] [PubMed] [Google Scholar]
- Horiuti K, Higuchi H, Umazume Y, Konishi M, Okazaki O, Kurihara S. Mechanism of action of 2,3-butanedione 2-monoxime on contraction of frog skeletal muscle fibres. Journal of Muscle Research and Cell Motility. 1988;9:156–164. doi: 10.1007/BF01773737. [DOI] [PubMed] [Google Scholar]
- Hu C M, Cheng H W, Cheng Y W, Kang J J. Induction of skeletal muscle contracture and calcium release from isolated sarcoplasmic reticulum vesicles by sanguinarine. British Journal of Pharmacology. 2000;130:299–306. doi: 10.1038/sj.bjp.0703279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram A J, Ly H, Thai K, Kang M J, Scholey J W. Mesangial cell signaling cascades in response to mechanical strain and glucose. Kidney International. 1999;56:1721–1728. doi: 10.1046/j.1523-1755.1999.00743.x. [DOI] [PubMed] [Google Scholar]
- Katoch S S, Su X, Moreland R S. Ca2+- and protein kinase C-dependent stimulation of mitogen-activated protein kinase in detergent-skinned vascular smooth muscle. Journal of Cellular Physiology. 1999;179:208–217. doi: 10.1002/(SICI)1097-4652(199905)179:2<208::AID-JCP11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Khawli F A, Reid M B. N-acetylcysteine depresses contractile function and inhibits fatigue of diaphragm in vitro. Journal of Applied Physiology. 1994;77:317–324. doi: 10.1152/jappl.1994.77.1.317. [DOI] [PubMed] [Google Scholar]
- Kolbeck R C, She Z W, Callahan L A, Nosek T M. Increased superoxide production during fatigue in the perfused rat diaphragm. American Journal of Respiratory and Critical Care Medicine. 1997;156:140–145. doi: 10.1164/ajrccm.156.1.9610041. [DOI] [PubMed] [Google Scholar]
- Kreideweiss S, Ahlers C, Nordheim A, Ruhlmann A. Ca2+-induced p38/SAPK signalling inhibited by the immunosuppressant cyclosporin A in human peripheral blood mononuclear cells. European Journal of Biochemistry. 1999;265:1075–1084. doi: 10.1046/j.1432-1327.1999.00830.x. [DOI] [PubMed] [Google Scholar]
- Krook A, Widegren U, Jiang X J, Henriksson J, Wallberg-Henriksson H, Alessi D, Zierath J R. Effects of exercise on mitogen- and stress-activated kinase signal transduction in human skeletal muscle. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2000;279:R1716–1721. doi: 10.1152/ajpregu.2000.279.5.R1716. [DOI] [PubMed] [Google Scholar]
- Loufrani L, Lehoux S, Tedgui A, Levy B I, Henrion D. Stretch induces mitogen-activated protein kinase activation and myogenic tone through 2 distinct pathways. Arteriosclerosis, Thrombosis and Vascular Biology. 1999;19:2878–2883. doi: 10.1161/01.atv.19.12.2878. [DOI] [PubMed] [Google Scholar]
- MacKenna D A, Dolfi F, Vuori K, Ruoslahti E. Extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activation by mechanical stretch is integrin-dependent and matrix-specific in rat cardiac fibroblasts. Journal of Clinical Investigation. 1998;101:301–310. doi: 10.1172/JCI1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merryman J I, Park P G, Schuller H M. Carbon dioxide, an important messenger molecule for small cell lung cancer. Chest. 1997;112:779–784. doi: 10.1378/chest.112.3.779. [DOI] [PubMed] [Google Scholar]
- Moldeus P, Cotgreave I A, Berggren M. Lung protection by a thiol-containing antioxidant: N-acetylcysteine. Respiration. 1986;50:31–42. doi: 10.1159/000195086. [DOI] [PubMed] [Google Scholar]
- Nishida M, Maruyama Y, Tanaka R, Kontani K, Nagao T, Kurose H. Gαi and Gαo are target proteins of reactive oxygen species. Nature. 2000;408:492–495. doi: 10.1038/35044120. [DOI] [PubMed] [Google Scholar]
- Olson E N, Williams R S. Remodeling muscles with calcineurin. Bioessays. 2000;22:510–519. [Google Scholar]
- Rao G N. Oxidant stress stimulates phosphorylation of eIF4E without an effect on global protein synthesis in smooth muscle cells. Lack of evidence for a role of H2O2 in angiotensin II-induced hypertrophy. Journal of Biological Chemistry. 2000;275:16993–16999. doi: 10.1074/jbc.275.22.16993. [DOI] [PubMed] [Google Scholar]
- Raught B, Gingras A C. eIF4E activity is regulated at multiple levels. International Journal of Biochemistry and Cell Biology. 1999;31:43–57. doi: 10.1016/s1357-2725(98)00131-9. [DOI] [PubMed] [Google Scholar]
- Reid M B, Shoji T, Moody M R, Entman M L. Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. Journal of Applied Physiology. 1992;73:1805–1809. doi: 10.1152/jappl.1992.73.5.1805. [DOI] [PubMed] [Google Scholar]
- Richter E A, Cleland P J F, Rattigan S, Clark M G. Contraction-associated translocation of protein kinase C in rat skeletal muscle. FEBS Letters. 1987;217:232–236. doi: 10.1016/0014-5793(87)80669-5. [DOI] [PubMed] [Google Scholar]
- Ryder J W, Fahlman R, Wallberg-Henriksson H, Alessi D R, Krook A, Zierath J R. Effect of contraction on mitogen-activated protein kinase signal transduction in skeletal muscle. Involvement of the mitogen- and stress-activated protein kinase 1. Journal of Biological Chemistry. 2000;275:1457–1462. doi: 10.1074/jbc.275.2.1457. [DOI] [PubMed] [Google Scholar]
- Ryschon T W, Fowler M D, Wysong R E, Anthony A, Balaban R S. Efficiency of human skeletal muscle in vivo: comparison of isometric, concentric, and eccentric muscle action. Journal of Applied Physiology. 1997;83:867–874. doi: 10.1152/jappl.1997.83.3.867. [DOI] [PubMed] [Google Scholar]
- Shrode L D, Tapper H, Grinstein S. Role of intracellular pH in proliferation, transformation, and apoptosis. Journal of Bioenergetics and Biomembranes. 1997;29:393–399. doi: 10.1023/a:1022407116339. [DOI] [PubMed] [Google Scholar]
- Song S K, Karl I E, Ackerman J J, Hotchkiss R S. Increased intracellular Ca2+: a critical link in the pathophysiology of sepsis? Proceedings of the National Academy of Sciences of the USA. 1993;90:3933–3937. doi: 10.1073/pnas.90.9.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Ritz M F, Malviya A N, Gaillard S. Intracellular acidification mediates the proliferative response of PC12 cells induced by potassium ferricyanide and involves MAP kinase activation. International Journal of Cancer. 1996;68:547–552. doi: 10.1002/(SICI)1097-0215(19961115)68:4<547::AID-IJC22>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Tibbles L A, Woodgett J R. The stress-activated protein kinase pathways. Cellular and Molecular Life Sciences. 1999;55:1230–1254. doi: 10.1007/s000180050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenboom R, Claflin D R, Julian F J. Effects of rapid shortening on rate of force regeneration and myoplasmic [Ca2+] in intact frog skeletal muscle fibres. Journal of Physiology. 1998;511:171–180. doi: 10.1111/j.1469-7793.1998.171bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen D G. Changes of intracellular pH due to repetitive stimulation of single fibres from mouse skeletal muscle. Journal of Physiology. 1992;449:49–71. doi: 10.1113/jphysiol.1992.sp019074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Dahlstedt A J, Lannergren J. Mechanisms underlying reduced maximum shortening velocity during fatigue of intact, single fibres of mouse muscle. Journal of Physiology. 1998;510:269–277. doi: 10.1111/j.1469-7793.1998.269bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widegren U, Wretman C, Lionikas A, Hedin G, Henriksson J. Influence of exercise intensity on ERK/MAP kinase signalling in human skeletal muscle. Pflügers Archiv. 2000;441:317–322. doi: 10.1007/s004240000417. [DOI] [PubMed] [Google Scholar]
- Widegren U, Jiang X J, Krook A, Chibalin A V, Björnholm M, Tally M, Roth R A, Henriksson J, Wallberg-Henriksson H, Zierath J R. Divergent effects of exercise on metabolic and mitogenic signaling pathways in human skeletal muscle. FASEB Journal. 1998;12:1379–1389. doi: 10.1096/fasebj.12.13.1379. [DOI] [PubMed] [Google Scholar]
- Widmann C, Gibson S, Jarpe M B, Johnson G L. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiological Reviews. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- Williams N G, Zhong H, Minneman K P. Differential coupling of α1-, α2-, and β-adrenergic receptors to mitogen-activated protein kinase pathways and differentiation in transfected PC12 cells. Journal of Biological Chemistry. 1998;273:24624–24632. doi: 10.1074/jbc.273.38.24624. [DOI] [PubMed] [Google Scholar]
- Woledge R C, Curtin N A, Homsher E. Energetic aspects of muscle contraction. Monographs of The Physiological Society. 1985;41:201–209. [PubMed] [Google Scholar]
- Wretman C, Widegren U, Lionikas A, Westerblad H, Henriksson J. Differential activation of mitogen-activated protein kinase signalling pathways by isometric contractions in isolated slow- and fast-twitch rat skeletal muscle. Acta Physiologica Scandinavica. 2000;170:45–49. doi: 10.1046/j.1365-201x.2000.00752.x. [DOI] [PubMed] [Google Scholar]
- Xue L, Lucocq J M. Low extracellular pH induces activation of ERK 2, JNK, and p38 in A431 and swiss 3T3 cells. Biochemical and Biophysical Research Communications. 1997;241:236–242. doi: 10.1006/bbrc.1997.7759. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Komuro I, Yazaki Y. Molecular mechanism of cardiac cellular hypertrophy by mechanical stress. Journal of Molecular and Cellular Cardiology. 1995;27:133–140. doi: 10.1016/s0022-2828(08)80013-2. [DOI] [PubMed] [Google Scholar]
- Zetser A, Gredinger E, Bengal E. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. Journal of Biological Chemistry. 1999;274:5193–5200. doi: 10.1074/jbc.274.8.5193. [DOI] [PubMed] [Google Scholar]


