Abstract
ATB0,+ is an amino acid transporter energized by transmembrane gradients of Na+ and Cl− and membrane potential. We cloned this transporter from mouse colon and expressed the clone functionally in mammalian (human retinal pigment epithelial, HRPE) cells and Xenopus laevis oocytes to investigate the interaction of carnitine and its acyl esters with the transporter.
When expressed in mammalian cells, the cloned ATB0,+ was able to transport carnitine, propionylcarnitine and acetylcarnitine. The transport process was Na+ and Cl− dependent and inhibitable by the amino acid substrates of the transporter. The Michaelis constant for carnitine was 0.83 ± 0.08 mm and the Hill coefficient for Na+ activation was 1.6 ± 0.1.
When expressed in Xenopus laevis oocytes, the cloned ATB0,+ was able to induce inward currents in the presence of carnitine and propionylcarnitine under voltage-clamped conditions. There was no detectable current in the presence of acetylcarnitine. Carnitine-induced currents were obligatorily dependent on the presence of Na+ and Cl−. The currents were saturable with carnitine and the Michaelis constant was 1.8 ± 0.4 mm. The analysis of Na+- and Cl−-activation kinetics revealed that 2 Na+ and 1 Cl− were involved in the transport of carnitine via the transporter.
These studies describe the identification of a novel function for the amino acid transporter ATB0,+. Since this transporter is expressed in the intestinal tract, lung and mammary gland, it is likely to play a significant role in the handling of carnitine in these tissues.
A Na+-dependent transport system for carnitine has already been described. This transporter, known as OCTN2 (novel organic cation transporter 2), is expressed in most tissues and transports carnitine with high affinity. It is energized, however, only by a Na+ gradient and membrane potential. In contrast, ATB0,+ is a low-affinity transporter for carnitine, but exhibits much higher concentrative capacity than OCTN2 because of its energization by transmembrane gradients of Na+ and Cl− as well as by membrane potential.
Carnitine (β-hydroxy-γ-trimethylaminobutyrate) is an obligate requirement for β-oxidation of long-chain fatty acids. It is synthesized endogenously in humans in the liver and kidney (Carter et al. 1995). It is also absorbed in the intestinal tract from dietary sources (Rebouche, 1992). The biological importance of this molecule is evident from the clinical consequences of carnitine deficiency encountered in a variety of genetic and acquired diseases (Kerner & Hoppel, 1998). The symptoms of carnitine deficiency include skeletal myopathy, cardiomyopathy, encephalopathy and failure to thrive (Treem et al. 1988; Kerner & Hoppel, 1998). Most tissues, including the cardiac and skeletal muscle, contain intracellular carnitine levels severalfold higher than plasma levels due to the presence of a Na+-dependent high-affinity carnitine transport system (Bremer, 1983). This transport system also exists in the brush border membrane of renal tubular epithelial cells where it plays a role in the reabsorption of carnitine (Rebouche & Mack, 1984; Huang et al. 1999). A genetic defect in this transport system results in excessive urinary loss of carnitine, causing systemic carnitine deficiency. Since the same transport system is also responsible for active accumulation of carnitine in tissues such as the heart and skeletal muscle, the genetic defect is associated with drastically reduced intracellular levels of carnitine in these tissues. The major clinical symptoms of this defect, known as primary carnitine deficiency, are skeletal and cardiac myopathy, resulting from impaired energy production from fatty acid oxidation as a consequence of reduced intracellular levels of carnitine. Recently, this transporter has been cloned (Wu et al. 1998; Tamai et al. 1998). Interestingly, this transporter also transports several organic cations and β-lactam antibiotics (Wu et al. 1998, 1999; Ohashi et al. 1999; Ganapathy et al. 2000). Furthermore, it belongs to the organic cation transporter gene family on the basis of its primary structure (Wu et al. 1998). Therefore, the transporter is named OCTN2 (novel organic cation transporter 2).
The present studies describe the identification of a second energy-coupled carnitine transporter. This transporter, known as ATB0,+, is an amino acid transporter expressed in the intestine, lung and mammary gland. Functionally, ATB0,+ is a Na+- and Cl−-coupled transport system for neutral and cationic amino acids. It plays an important role in the absorption of amino acids in the intestinal tract (Ganapathy et al. 2001). The cloning of human ATB0,+ has been recently reported (Sloan & Mager, 1999). To date, the transport function of ATB0,+ has been studied only with amino acids as substrates. Its transport function is highly concentrative, energized by transmembrane gradients of Na+ and Cl− and membrane potential. ATB0,+ belongs to the gene family of Na+- and Cl−-coupled transporters for a variety of compounds such as amino acids (e.g. glycine and proline), neurotransmitters (e.g. monoamines and γ-aminobutyrate) and osmolytes (e.g. taurine and betaine). Structurally, ATB0,+ is very closely related to γ-aminobutyrate transporters and betaine transporter. Therefore, we tested whether ATB0,+ is able to recognize γ-aminobutyrate and other structurally related compounds as substrates. These studies have led to an interesting finding that ATB0,+ can transport carnitine coupled to the transmembrane gradients of Na+ and Cl−.
METHODS
Molecular cloning of ATB0,+ cDNA from mouse colon
A cDNA library was constructed using poly(A)+ RNA isolated from mouse colon. The SuperScript plasmid system (Life Technologies, Inc., Gaithersburg, MD, USA) was employed for this purpose. The cDNA probe for screening the library was prepared by RT-PCR using primers specific for the mouse ATB0,+ cDNA reported in GenBank (accession no. AF161714). The RT-PCR product (≈1.2 kbp in size) was sequenced for confirmation of its identity. Screening was done under high stringency conditions as described previously (Kekuda et al. 1996, 1998). The longest positive clone (≈3 kbp) was used for sequencing and functional analysis.
Carnitine transport via mouse ATB0,+ in a mammalian cell expression system
The cloned mouse ATB0,+ cDNA was functionally expressed in the human retinal pigment epithelial (HRPE) cell line using the vaccinia virus expression technique (Wu et al. 1998, 1999). These cells were originally provided by M. A. Del Monte (Kellog Eye Center, Ann Arbor, MI, USA) and have been used in our laboratory for functional expression of a variety of cloned transporters. Any mammalian cell can be used in the method involving the vaccinia virus-mediated heterologous expression of cloned transporter cDNAs. However, in our experience we found HRPE cells to be most suitable for the purpose because these cells withstand the infection with the vaccinia virus much better than other cell types (e.g. HeLa, COS-1, COS-7 and HEK293 cells). Initial studies of the interaction of carnitine with mouse ATB0,+ in HRPE cells were done by assessing the ability of carnitine to compete with glycine for transport via ATB0,+. Subsequent studies were carried out using [3H]carnitine to assess directly the transport of carnitine via ATB0,+. Transport measurements were made in vector-transfected cells and in mouse ATB0,+ cDNA-transfected cells in parallel using 24-well culture plates. Incubations of the cells with radiolabelled substrates were carried out at 37 °C for 15 min. The composition of the transport buffer was 25 mm Hepes-Tris (pH 7.5), containing 140 mm NaCl, 5.4 mm KCl, 1.8 mm CaCl2, 0.8 mm MgSO4 and 5 mm glucose. cDNA-specific transport was calculated by adjusting for the transport in vector-transfected cells. [3H]Glycine, [3H]carnitine, acetyl[3H]carnitine and propionyl[3H]carnitine were purchased from Moravek Biochemicals (Brea, CA, USA). Other radiolabelled amino acids were obtained from either American Radiolabeled Chemicals (St Louis, MO, USA) or DuPont-New England Nuclear (Boston, MA, USA).
Carnitine transport via mouse ATB0,+ in the Xenopus laevis oocyte expression system
Care and use of Xenopus laevis adhered to the institutional guidelines set forth by the Committee on Animal Use for Research and Education at the Medical College of Georgia. Xenopus oocytes were collected under anaesthesia from frogs that were humanely killed after the final collection. Mature oocytes from X. laevis were isolated by treatment with collagenase A (1.6 mg ml−1). Oocytes were manually defolliculated and then used for injection with mouse ATB0,+ cRNA or water (Fei et al. 2000). cRNA was synthesized using the mMESSAGE mMACHINE kit (Ambion, Austin, TX, USA). The transport of carnitine via mouse ATB0,+ in oocytes was monitored electrophysiologically using the two-microelectrode voltage-clamp technique (Fei et al. 2000). The membrane potential was held steady at -50 mV. Oocytes were perifused with carnitine and the induced current was monitored. The induced current was taken as the measure of transport rate. The composition of the perifusion buffer was 10 mm Hepes-Tris (pH 7.5), containing 100 mm NaCl, 2 mm KCl, 1 mm MgCl2 and 1 mm CaCl2.
Data analysis
Experiments were repeated at least three times. Results are given as means ±s.e.m. The kinetic parameters, Michaelis constant (Kt) and maximal velocity (Vmax), were calculated by fitting the ATB0,+-specific transport data to the Michaelis-Menten equation describing a single saturable transport system. Na+- and Cl−-activation kinetics were analysed by fitting the ATB0,+-specific transport data to the Hill equation for the determination of K0.5 values for Na+ and Cl− (concentration of Na+ or Cl− necessary for half-maximal activation) and the Hill coefficient (nH; the number of Na+ or Cl− ions involved in the activation process). The kinetic parameters were first determined by non-linear regression methods and subsequently confirmed by linear regression methods using the commercially available computer program SigmaPlot, version 6.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Structural features of mouse ATB0,+
The mouse ATB0,+ cDNA isolated from the colon cDNA library is 3007 bp long (GenBank accession no. AF320226). The open reading frame is flanked by a 192 bp long 5′-untranslated region and an 898 bp long 3′-untranslated region. The coding region is exactly the same as the sequence of mouse ATB0,+ previously reported in GenBank (accession no. AF161714) except for one amino acid substitution at position 192 (Ser instead of Asn). However, the two clones differ in their 3′-untranslated region. The mouse ATB0,+ consists of 638 amino acids and the sequence is highly homologous to the human ATB0,+ (88 % identity) (Fig. 1). The protein sequence contains 12 putative transmembrane domains. Based on the amino acid sequence, mouse ATB0,+ belongs to the gene family of Na+- and Cl−-coupled neurotransmitter transporters.
Figure 1. Comparison of the amino acid sequence between mouse ATB0,+ and human ATB0,+.
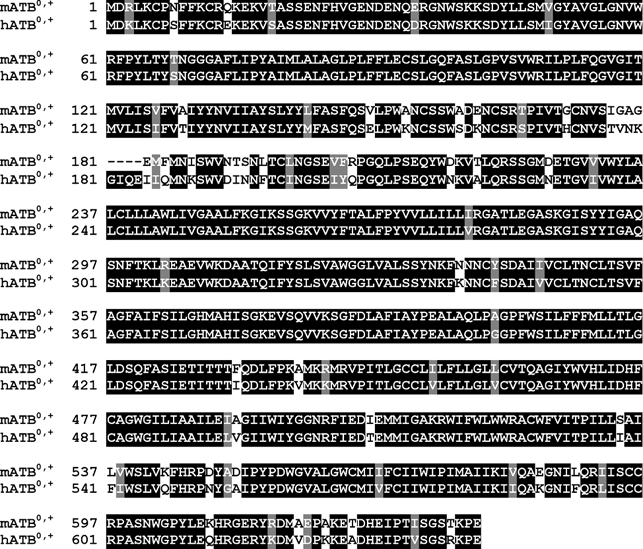
Regions of sequence identity between the two species homologues are shaded.
Analysis of carnitine transport via mouse ATB0,+ in the mammalian cell expression system
The recently cloned human ATB0,+ is a Na+- and Cl−-coupled transporter for zwitterionic and cationic amino acids. Therefore, we first assessed the transport function of the ATB0,+ cDNA isolated from mouse colon by comparing the transport of 12 different zwitterionic and cationic amino acids in cDNA-transfected cells and in vector (pSPORT1)-transfected cells. The cDNA-induced increase in transport was demonstrable for all amino acids examined (Gly, Ala, Ser, Thr, His, Gln, Asn, Leu, Ile, Phe, Trp and Arg). The increase was, however, the highest for glycine, the transport in cDNA-transfected cells being ≈30-fold higher than in vector-transfected cells.
There is a significant structural similarity between carnitine and γ-aminobutyrate as well as between carnitine and betaine. Carnitine is a derivative of γ-aminobutyrate with an addition of a hydroxyl group at the β carbon and the replacement of the amino group with the trimethylamino group. Because of the presence of the trimethylamino group at the terminal carbon atom, carnitine is also structurally similar to betaine. Since the primary structure of ATB0,+ is closely related to that of γ-aminobutyrate transporters and betaine transporter in the Na+- and Cl−-coupled neurotransmitter transporter gene family, we investigated whether ATB0,+ is capable of interacting with γ-aminobutyrate and betaine. In the same experiment, we also tested the ability of ATB0,+ to interact with carnitine and its acetyl and propionyl esters. In these studies, the transport function of ATB0,+ was monitored by measuring the transport of glycine in HRPE cells expressing the cloned mouse ATB0,+. The expression of ATB0,+ in these cells increased the transport of glycine by 30-fold (Fig. 2A). The interaction of the transporter with the test compounds was investigated by assessing their ability to inhibit ATB0,+-mediated glycine transport (Fig. 2B). These studies produced interesting, but quite unexpected, results. γ-Aminobutyrate and betaine showed little or no effect on ATB0,+-mediated glycine transport. In contrast, carnitine and propionylcarnitine inhibited ATB0,+-mediated glycine transport markedly. The IC50 values (concentration of the compound at which the inhibition was 50 %) for carnitine and propionylcarnitine were 0.6 ± 0.1 and 0.9 ± 0.1 mm, respectively. Acetylcarnitine was also able to inhibit ATB0,+-mediated glycine transport, but surprisingly the inhibitory potency of this ester was much less than that of carnitine and its propionyl ester (IC50 for acetylcarnitine was 15 ± 3 mm).
Figure 2. Inhibition of mouse ATB0,+-mediated glycine transport by carnitine and its acyl esters (A and B) and transport of carnitine and its acyl esters by mouse ATB0,+ and the ion dependence of the process (C and D) in HRPE cells.
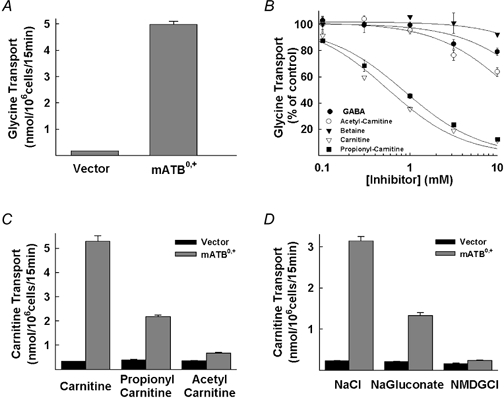
A, transport of glycine (10 μm) in vector-transfected cells and in cells transfected with mouse ATB0,+ cDNA (mATB0,+). B, inhibition of ATB0,+-mediated glycine (10 μm) transport by γ-aminobutyrate (GABA), betaine, carnitine, acetylcarnitine and propionylcarnitine. Transport in the absence of inhibitors was taken as 100 %. C, transport of carnitine (25 μm), propionylcarnitine (25 μm) and acetylcarnitine (25 μm) in vector-transfected cells and in cells transfected with mouse ATB0,+ cDNA. D, transport of carnitine (15 μm) in vector-transfected cells and in cells transfected with mouse ATB0,+ cDNA in the presence of NaCl, in the presence of Na+ but in the absence of Cl− (sodium gluconate), and in the presence of Cl− but in the absence of Na+ (NMDG-Cl).
To determine whether or not carnitine and its esters are transportable substrates for ATB0,+, we compared the transport of [3H]carnitine and its esters between vector-transfected cells and ATB0,+ cDNA-transfected cells (Fig. 2C). Expression of ATB0,+ in HRPE cells induced the transport of carnitine (16-fold) and propionylcarnitine (6-fold) compared to transport in vector-transfected cells. The transport of acetylcarnitine was also increased by ATB0,+ expression but to a much smaller extent (2-fold). These data show that ATB0,+ recognizes carnitine, propionylcarnitine and acetylcarnitine as transportable substrates. Since ATB0,+ is a member of the Na+- and Cl−-coupled transporter gene family, we investigated the influence of these two ions on the transport of carnitine mediated by ATB0,+ (Fig. 2D). The transport was completely abolished when Na+ in the uptake buffer was replaced with N-methyl-d-glucamine. Removal of Cl− from the uptake buffer by replacement with gluconate reduced the transport by ≈60 %. These results show that ATB0,+-mediated carnitine transport is coupled to both Na+ and Cl−. The removal of Cl− did not abolish the transport completely because of the possible release of Cl− from the cells during transport measurements. The transporter interacts with Cl− with high affinity and therefore the transporter is significantly active even at low concentrations of Cl− (see below).
Since ATB0,+ transports several zwitterionic and cationic amino acids, we tested whether the ATB0,+-mediated carnitine transport is inhibitable by the amino acid substrates of the transporter (Fig. 3A). All zwitterionic and cationic amino acids examined inhibited carnitine transport mediated by ATB0,+. The anionic amino acid aspartate and the N-methylated amino acid α-(methylamino)isobutyric acid (MeAIB), which are not substrates for ATB0,+, did not inhibit ATB0,+-mediated carnitine transport.
Figure 3. Characteristics of mouse ATB0,+-mediated carnitine transport in HRPE cells.
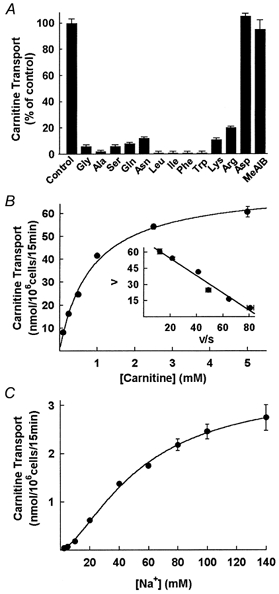
A, inhibition of ATB0,+-mediated carnitine (10 μm) transport by zwitterionic and cationic amino acids (2.5 mm). Transport in the absence of inhibitors was taken as 100 %. B, transport of carnitine via mouse ATB0,+ over a carnitine concentration range of 0.1-5 mm (inset, Eadie-Hofstee plot; V, carnitine transport in nmol (106 cells)−1 (15 min)−1; S, carnitine concentration in mm). C, transport of carnitine (15 μm) via mouse ATB0,+ over a Na+ concentration range of 2.5-140 mm. Concentration of Cl− was kept constant (140 mm) and concentration of Na+ was varied by appropriately replacing NaCl with N-methyl-d-glucamine chloride.
The transport of carnitine mediated by ATB0,+ was saturable (Fig. 3B). The values for the kinetic parameters Kt and Vmax were 0.83 ± 0.08 mm and 72 ± 2 nmol (106 cells)−1 (15 min)−1. The relationship between ATB0,+-mediated carnitine transport and Na+ concentration was sigmoidal (Fig. 3C). The K0.5 for Na+ was 54 ± 4 mm and the Hill coefficient (nH) was 1.6 ± 0.1. The Cl−-activation kinetics were not investigated in this expression system because of the efflux of significant amounts of Cl− from the cells during the experiment.
To determine whether the ability to transport carnitine is also a characteristic of human ATB0,+, we isolated a functional ATB0,+ cDNA from a MCF-7 (a human mammary tumour cell line) cDNA library and examined its ability to transport carnitine in HRPE cells following heterologous expression. The amino acid sequence of ATB0,+ cloned from the MCF-7 cell line was exactly the same as the recently published sequence of human ATB0,+ (Sloan & Mager, 1999). The transport of carnitine was increased 4.5-fold in cells transfected with human ATB0,+ cDNA compared to transport in cells transfected with vector alone. These data show that human ATB0,+ is also capable of carnitine transport.
Analysis of carnitine transport via mouse ATB0,+ in the X. laevis oocyte expression system
Transport of amino acid substrates via mouse ATB0,+ is electrogenic (Sloan & Mager, 1999). To investigate the electrogenic nature of ATB0,+-mediated carnitine transport, we employed the X. laevis oocyte expression system. When oocytes expressing the mouse ATB0,+ were perifused with carnitine, marked inward currents were detectable by the two-microelectrode voltage-clamp technique (≈300 nA at 10 mm carnitine). Under similar conditions, propionylcarnitine induced ≈200 nA currents. In contrast, acetylcarnitine was unable to induce any detectable currents. With carnitine and propionylcarnitine, the induced currents were absolutely dependent on the presence of Na+ as well as Cl−. Removal of either of the two ions abolished the currents completely.
We analysed the kinetic parameters of ATB0,+-mediated carnitine transport using the carnitine-induced inward currents as a measure of the transporter function. The currents were saturable with increasing concentrations of carnitine (Fig. 4A). The K0.5 for carnitine was 1.8 ± 0.4 mm. The Na+-activation kinetics of carnitine-induced currents showed a sigmoidal relationship (Fig. 4B). The K0.5 for Na+ was 25 ± 4 mm and the Hill coefficient (nH) was 1.9 ± 0.5. Since the removal of Cl− abolished completely the carnitine-induced currents, there was apparently no efflux of Cl− from the oocytes under the experimental conditions. Therefore, we used this expression system to analyse the Cl−-activation kinetics. The relationship between carnitine-induced currents and Cl− concentration was hyperbolic (Fig. 4C). The K0.5 for Cl− was 15 ± 5 mm and the Hill coefficient (nH) was 0.9 ± 0.2. These data show that the Na+:Cl−:carnitine stoichiometry for the ATB0,+-mediated transport process was 2:1:1.
Figure 4. Characteristics of carnitine transport mediated by mouse ATB0,+ in X. laevis oocytes.
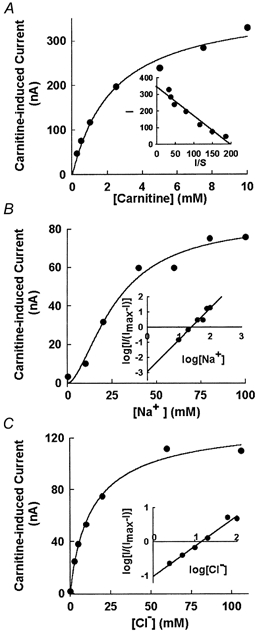
A, saturation kinetics for carnitine-induced current in oocytes expressing mouse ATB0,+ (inset, Eadie-Hofstee plot; I, carnitine-induced current in nA; S, carnitine concentration in mm). B, Na+ dependence of carnitine (1 mm)-induced current in oocytes expressing mouse ATB0,+ (inset, Hill plot; I, carnitine-induced current; Imax, maximal current induced by 1 mm carnitine). Concentration of Cl− was kept constant (100 mm) and concentration of Na+ was varied by appropriately replacing NaCl with N-methyl-d-glucamine chloride. C, Cl− dependence of carnitine (1 mm)-induced current in oocytes expressing mouse ATB0,+ (inset, Hill plot; I, carnitine-induced current; Imax, maximal current induced by 1 mm carnitine). Concentration of Na+ was kept constant (100 mm) and concentration of Cl− was varied by appropriately replacing NaCl with sodium gluconate.
DISCUSSION
We have demonstrated in the present study that the amino acid transporter ATB0,+ is able to mediate the transport of carnitine in a Na+- and Cl−-coupled manner. This property is demonstrable with mouse ATB0,+ cloned from colon and human ATB0,+ cloned from a mammary tumour cell line. The characteristics of carnitine transport via mouse ATB0,+ were investigated using two different expression systems: a mammalian cell expression system and the X. laevis oocyte expression system. In the mammalian cell expression system, carnitine transport via ATB0,+ was obligatorily dependent on Na+. The transport process was also Cl− dependent even though removal of Cl− from the uptake buffer did not abolish carnitine transport completely. The persistence of significant carnitine transport activity in a Cl−-free uptake medium is most likely to be due to Cl− ions that might be released from the cells into the extracellular medium during the 15 min period used in uptake measurements. This is supported by the findings that in the X. laevis oocyte expression system in which the transport-associated currents were measured over a very short time period, removal of Cl− from the perifusion buffer abolished the carnitine-induced currents almost completely. There are additional differences in the kinetic parameters of ATB0,+-mediated carnitine transport between the two expression systems. Possible reasons for these differences might include the different time periods employed in the two expression systems to measure carnitine transport and also the fact that the transport was measured in HRPE cells without clamping the membrane potential whereas transport in X. laevis oocytes was measured under voltage-clamped conditions.
To date, OCTN2 is the only transporter that has been shown to transport carnitine in an ion gradient-coupled manner (Wu et al. 1998, 1999; Tamai et al. 1998). The transport function of OCTN2 is dependent on the presence of Na+. Cl− does not have any role in the function of this transporter. The transporter is, however, likely to be electrogenic due to the zwitterionic nature of carnitine and the coupling of the transport process with Na+ cotransport. OCTN2-mediated transport of carnitine is therefore energized by the transmembrane Na+ gradient and membrane potential. The present studies describe the identification of a second ion gradient-coupled transporter for carnitine. ATB0,+ transports carnitine in a Na+- and Cl−-coupled manner. The transport process is electrogenic. Thus, the transport of carnitine via ATB0,+ is energized by transmembrane gradients of Na+ and Cl− as well as membrane potential. The concentrative capacity of ATB0,+ for carnitine is much greater than that of OCTN2. However, ATB0,+ is a low-affinity transporter for carnitine (Kt= 1-2 mm). In contrast, OCTN2 is a high-affinity transporter for carnitine (Kt= 5-15 μm) (Tamai et al. 1998; Wu et al. 1999). The concentrations of carnitine in blood are in the range of 30-50 μm and therefore OCTN2 is more important that ATB0,+ for cellular uptake of carnitine in most tissues under physiological conditions. Interestingly, the tissue distribution of the two transporters is quite different. OCTN2 is expressed in most tissues whereas ATB0,+ is expressed primarily in the mammary gland, lung and intestinal tract (Sloan & Mager, 1999). ATB0,+ is likely to play a significant role in tissues in which it is expressed. Recent studies with JVS mice, which have a genetic defect in OCTN2 transport function, have shown that the intestinal absorption of carnitine is reduced by only about 50 % due to the defect (Yokogawa et al. 1999). The finding that defects in OCTN2 function do not eliminate intestinal absorption completely suggests that some additional, hitherto unidentified, transporters participate in the intestinal absorption of carnitine. Since functional studies have shown that system B0,+ is expressed in the brush border membrane of the absorptive cells of the intestinal tract, it is possible that ATB0,+, along with OCTN2, participates in the intestinal absorption of carnitine. Furthermore, ATB0,+ is expressed not only in the small intestine but also in the colon. The present studies were done with ATB0,+ cloned from mouse colon. Microbial flora utilize carnitine as a carbon source (Rebouche & Seim, 1998). Therefore, we speculate that ATB0,+ in the colon may play a role in the absorption of carnitine and thus compete with colonic bacteria for carnitine in the lumen. In addition, the intestinal transport of carnitine via ATB0,+ is likely to be very relevant to carnitine homeostasis in patients with genetic defects in OCTN2.
OCTN2 and ATB0,+ differ not only in their affinity and driving forces but also in their substrate specificity. OCTN2 transports carnitine, acetylcarnitine and propionylcarnitine with comparable affinity (Wu et al. 1999). In contrast, ATB0,+ transports only carnitine and propionylcarnitine. The transporter shows very low affinity for acetylcarnitine. Acetylcarnitine is the predominant acylcarnitine ester inside the cell as well as in the circulation. It is a key intermediate in anabolic and catabolic pathways of metabolism. The differential affinity of OCTN2 and ATB0,+ for acetylcarnitine may have physiological implications. OCTN2 belongs to the organic cation transporter gene family, the members of which mediate the transport of structurally diverse xenobiotics (Burckhardt & Wolff, 2000). In contrast, ATB0,+ belongs to the neurotransmitter transporter gene family, the members of which mediate Na+- and Cl−-coupled transport of several cationic and zwitterionic organic solutes including monoamines, amino acids and osmolytes. The structural differences between OCTN2 and ATB0,+ must underlie the differential substrate specificities of these two transporters.
Acknowledgments
This work was supported by the National Institutes of Health grant HL64196.
References
- Bremer J. Carnitine - metabolism and function. Physiological Reviews. 1983;63:1420–1480. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- Burckhardt G, Wolff NA. Structure of renal organic anion and cation transporters. American Journal of Physiology. 2000;278:F853–866. doi: 10.1152/ajprenal.2000.278.6.F853. [DOI] [PubMed] [Google Scholar]
- Carter AL, Abney TO, Lapp DF. Biosynthesis and metabolism of carnitine. Journal of Child Neurology. 1995;10(suppl. 2):S3–S7. [PubMed] [Google Scholar]
- Fei YJ, Sugawara M, Nakanishi T, Huang W, Wang H, Prasad PD, Leibach FH, Ganapathy V. Primary structure, genomic organization, and functional and electrogenic characteristics of human system N (SN1), a Na+- and H+-coupled glutamine transporter. Journal of Biological Chemistry. 2000;275:23707–23717. doi: 10.1074/jbc.M002282200. [DOI] [PubMed] [Google Scholar]
- Ganapathy ME, Huang W, Rajan DP, Carter AL, Sugawara M, Iseki K, Leibach FH, Ganapathy V. β-Lactam antibiotics as substrates for OCTN2, an organic cation/carnitine transporter. Journal of Biological Chemistry. 2000;275:1699–1707. doi: 10.1074/jbc.275.3.1699. [DOI] [PubMed] [Google Scholar]
- Ganapathy V, Ganapathy ME, Leibach FH. Intestinal transport of peptides and amino acids. In: Barrett KE, Donowitz M, editors. Current Topics in Membranes. Vol. 50. Academic Press; 2001. pp. 379–412. [Google Scholar]
- Huang W, Shaikh SN, Ganapathy ME, Hopfer U, Leibach FH, Carter AL, Ganapathy V. Carnitine transport and its inhibition by sulfonylureas in human kidney proximal tubular epithelial cells. Biochemical Pharmacology. 1999;58:1361–1370. doi: 10.1016/s0006-2952(99)00219-1. [DOI] [PubMed] [Google Scholar]
- Kekuda R, Prasad PD, Fei YJ, Torres-Zamorano V, Sinha S, Yang-Feng TL, Leibach FH, Ganapathy V. Cloning of the sodium-dependent broad-scope neutral amino acid transporter B0 from a human placental choriocarcinoma cell line. Journal of Biological Chemistry. 1996;271:18657–18661. doi: 10.1074/jbc.271.31.18657. [DOI] [PubMed] [Google Scholar]
- Kekuda R, Prasad PD, Wu X, Wang H, Fei YJ, Leibach FH, Ganapathy V. Cloning and functional characterization of a potential-sensitive, polyspecific organic cation transporter (OCT3) most abundantly expressed in placenta. Journal of Biological Chemistry. 1998;273:15971–15979. doi: 10.1074/jbc.273.26.15971. [DOI] [PubMed] [Google Scholar]
- Kerner J, Hoppel C. Genetic disorders of carnitine metabolism and their nutritional management. Annual Review of Nutrition. 1998;18:179–206. doi: 10.1146/annurev.nutr.18.1.179. [DOI] [PubMed] [Google Scholar]
- Ohashi R, Tamai I, Yabuuchi H, Nezu J, Oku A, Sai Y, Shimane M, Tsuji A. Na+-dependent carnitine transport by organic cation transporter (OCTN2): Its pharmacological and toxicological relevance. Journal of Pharmacology and Experimental Therapeutics. 1999;291:778–784. [PubMed] [Google Scholar]
- Rebouche CJ. Carnitine function and requirements during the life cycle. FASEB Journal. 1992;6:3379–3386. [PubMed] [Google Scholar]
- Rebouche CJ, Mack D. Sodium gradient-stimulated transport of L-carnitine into renal brush border membrane veiscles: kinetics, specificity, and regulation by dietary carnitine. Archives of Biochemistry and Biophysics. 1984;235:393–402. doi: 10.1016/0003-9861(84)90212-1. [DOI] [PubMed] [Google Scholar]
- Rebouche CJ, Seim H. Carnitine metabolism and its regulation in microorganisms and mammals. Annual Review of Nutrition. 1998;18:39–61. doi: 10.1146/annurev.nutr.18.1.39. [DOI] [PubMed] [Google Scholar]
- Sloan JL, Mager S. Cloning and functional expression of a human Na+- and Cl−-dependent neutral and cationic amino acid transporter. Journal of Biological Chemistry. 1999;274:23740–23745. doi: 10.1074/jbc.274.34.23740. [DOI] [PubMed] [Google Scholar]
- Tamai I, Ohashi R, Nezu J, Yabuuchi H, Oku A, Shimane M, Sai Y, Tsuji A. Molecular and functional identification of sodium-dependent, high affinity human carnitine transporter OCTN2. Journal of Biological Chemistry. 1998;273:20378–20382. doi: 10.1074/jbc.273.32.20378. [DOI] [PubMed] [Google Scholar]
- Treem WR, Stanley CA, Finegold DN, Hale DE, Coates PM. Primary carnitine deficiency due to a failure of carnitine transport in kidney, muscle and fibroblasts. New England Journal of Medicine. 1998;319:1331–1336. doi: 10.1056/NEJM198811173192006. [DOI] [PubMed] [Google Scholar]
- Wu X, Huang W, Prasad PD, Seth P, Rajan DP, Leibach FH, Chen J, Conway SJ, Ganapathy V. Functional characteristics and tissue distribution pattern of organic cation transporter 2 (OCTN2), an organic cation/carnitine transporter. Journal of Pharmacology and Experimental Therapeutics. 1999;290:1482–1492. [PubMed] [Google Scholar]
- Wu X, Prasad PD, Leibach FH, Ganapathy V. cDNA sequence, transport function, and genomic organization of human OCTN2, a new member of the organic cation transporter family. Biochemical and Biophysical Research Communications. 1998;246:589–595. doi: 10.1006/bbrc.1998.8669. [DOI] [PubMed] [Google Scholar]
- Yokogawa K, Higashi Y, Tamai I, Nomura M, Hashimoto N, Nikaido H, Hayakawa JI, Miyamoto KI, Tsuji A. Decreased tissue distribution of L-carnitine in juvenile visceral steatosis mice. Journal of Pharmacology and Experimental Therapeutics. 1999;289:224–230. [PubMed] [Google Scholar]


