Abstract
Background
Urinary exosomes containing apical membrane and intracellular fluid are normally secreted into the urine from all nephron segments, and may carry protein markers of renal dysfunction and structural injury. We studied effective methods for the collection, storage, and preservation of urinary exosomal proteins.
Methods
We collected first and second morning spot urines from healthy volunteers. Protease inhibitors were added, and samples were stored at 4, -20, and -80°C for one week or 7 months. Samples were thawed with and without extensive vortexing, and 3 fractions were isolated: urinary sediment, urinary supernatant, and urinary exosome fraction. Protein concentration, electrophoresis patterns, and abundance of 7 urinary exosome-associated proteins were measured.
Results
Urinary exosome-associated proteins were not detected in urinary sediment or supernatant fractions. Protease inhibitors prevented degradation of exosome-associated proteins. Freezing at -20°C caused a major loss in urinary exosomes compared to freshly collected urine. In contrast, recovery after freezing at -80°C was almost complete (86%). Extensive vortexing after thawing resulted in a markedly increased recovery of urinary exosomes in urine frozen at -20°C or -80°C, even if frozen for 7 months. The recovery from first and second morning urine was similar. The abundance of cytosolic exosome-associated proteins did not decrease during long term storage.
Conclusions
1) Protease inhibitors are essential for preservation. 2) Storage at -80°C with extensive vortexing after thawing maximizes the recovery of urinary exosomes. 3) The difference between first and second morning urine exosome-associated protein recovery was small, suggesting minimal protein degradation in the urinary tract/bladder. 4) Urinary exosomes remain intact during long term storage. These urine collection, storage, and processing conditions may be useful for future biomarker discovery efforts.
Keywords: storage, urine, exosome, biomarker, NHE3, TSG101, ALIX, AQP2, NSE, MDH, NKCC2
Introduction
Urine is an ideal non-invasive source of biomarkers to diagnose and classify kidney diseases. New urinary biomarkers will likely help speed the laboratory and clinical development of new treatments for renal diseases [1]. Exosomes containing vesicular membranes and intracellular fluid are normally secreted into the urine from all nephron segments, and contain proteins that may be altered in abundance or physical properties in association with various renal diseases. Pisitkun et. al. successfully isolated exosomal membrane proteins in fresh human urine by differential centrifugation and demonstrated the presence of several disease-related proteins [2]. A previous study found that urinary Na+/H+ exchanger isoform 3 (NHE3), a typical membrane protein, increases in patients with acute renal failure [3]. Thus, urinary exosomal proteomics may provide an avenue for the discovery of urinary biomarkers useful for early detection of kidney diseases and for monitoring of treatment [4]. However, how to store and preserve urinary exosomes remains unclear. The aim of this study is to clarify effective methods for the collection, storage, and preservation of urinary exosomal proteins.
Methods
Urine samples collection, storages and handling
Human urine samples were collected under human subject research protocols approved by Institutional Review Boards of NIDDK and Universitätsklinikum C.G. Carus, Dresden, Germany. To each 50 ml of urine, we added 4.2 ml of a protease inhibitor mixture (1.67 ml of 100 mM NaN3/ 2.5ml of 10 mM PMSF/50 :l of 1 mM Leupeptin).
Experiment 1
To confirm whether protease inhibitors are necessary during the urine collection process. Spot urines were collected with and without the above protease inhibitors from eight healthy volunteers.
Experiment 2
Three samples of first morning urine were collected from three healthy volunteers (aged 11-41, approved Research Study No. 00-DK-0107) to study effective methods for the storage and preservation of urinary exosomal proteins. Freshly obtained urine samples (300 ml each) were pooled and then subjected to 5 different protocols (100 ml per protocol in 50 ml plastic centrifuge tubes): a) store at 4 °C and processed within 1hr; store at b) -20°C or c) -80 °C for 1 week without vortexing before use; store at d) -20°C or e) -80 °C for 1 week, subject to extensive vortexing (90 seconds) after complete quick thawing. This experiment was repeated twice. In addition, we stored three individual urine samples at -80 °C for 7 months.
Experiment 3
First and second morning urine samples from three separate individuals (120 ml each) were collected to investigate the effects of urine collection time on urinary exosomes, to assess degradation of urinary exosomal proteins, and also to compare the normalization methods for umtimed/spot urine samples. Urinary creatinine (Ucr) was determined by ELISA kit (Exocell. Inc. PA). This experiment was repeated three times.
Experiment 4
We processed 10 ml fresh first morning urine samples from three individuals to verify whether the exosome fraction could be isolated from much smaller volumes typical of clinical samples.
Isolation of urinary proteins in exosome fraction
The urinary exosome fraction was prepared using the protocol of Pisitkun et al [2]. Urine was centrifuged at 17,000 × g for 15 min at 4 °C to remove urinary sediment including whole cells, large membrane fragments, and other debris. An aliquot of the supernatant was removed and the remaining supernatant was centrifuged at 200,000 × g for 1 hr at 4 °C (L8-70M ultracentrifuge, Beckman Coulter, Inc. CA; 70.1 Ti rotor) to obtain low-density membranes. The 200,000 × g supernatants were removed and replaced with an additional 17,000 × g supernatant and ultracentrifuged again. This centrifugation step was repeated 3∼5 more times to harvest the low-density exosome pellets from 96 ml of 17,000 × g supernatant. Pellets were resuspended in 200 :l of isolation solution (10 mM triethanolamine/250 mM sucrose (pH 7.6); 0.5 mM PMSF; 1 μm Leupeptin), and pooled. After removing 10 :l for bicinchoninic acid (BCA) protein assay (Pierce), the remaining suspension was added to an equal volume of 2 × Laemmli sample buffer containing 60 mg/ml DTT and heated at 60 °C for 10 min. These samples were divided into aliquots and stored at -80 °C until use.
Some urine samples were fractionated as follows: 1) urinary sediment (pellet after 17,000 × g 15 min spin); 2) urinary supernatant (supernatant after 17,000 × g 15 min spin from 20 ml urine samples). A 10 ml aliquot of 17,000 × g supernatant was used to isolate urinary protein by acetone precipitation described by Thongboonkerd et. al. [5]. The pellets from these two fractions were resuspended in 500 :l 1 × Laemmli sample buffer. After removing 20 :l of each samples for 2-D quant protein assay (Amersham), DTT was added into each suspension (30 mg/ml) and the samples were heated at 60 °C for 10 min. Aliquots were stored at -80 °C until use (Fig. 1).
Figure 1. The isolation of three urinary fractions.
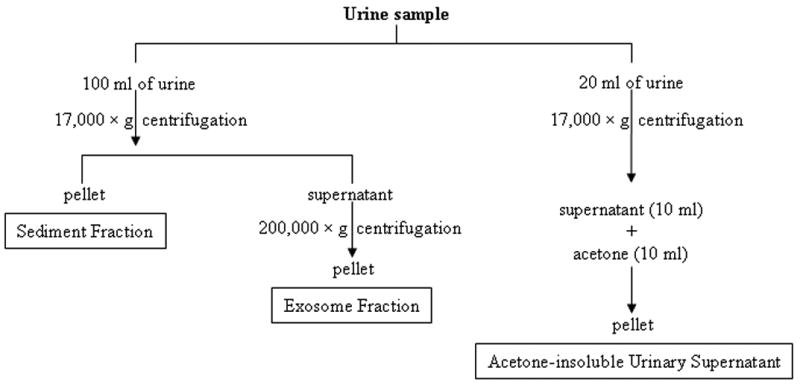
Urinary sediment (17,000 × g pellet), exosome fraction (200,000 × g pellet) and acetone insoluble supernatant (17,000 × g supernatant precipitated by acetone) were isolated as described in methods.
Gel electrophoresis and western blotting
The total amount of protein was calculated in each urinary fraction. Loading amounts for gel electrophoresis were adjusted to equalize either urine creatinine concentration (Ucr) or collection time (urine flow rate) for individual urine samples to account for differences in urine concentration or urinary flow rate. When pooling, a constant proportion of each urine sample was added as specified in Results. Protein samples were separated by 1D SDS/PAGE electrophoresis and then stained by Colloidal Blue Staining kit (Invitrogen).
Duplicated gels were transferred to PVDF membranes for western blotting. After blocking with 5% milk blot (1hr), membranes were probed overnight at 4 °C with antibodies: polyclonal antibodies to NHE3 (1: 1000), aquaporin 2 (AQP2) (1:2000), Na-K-Cl cotransporter isoform 2 (NKCC2) (1:1000) [2], neuron specific enolase (NSE) (1:1000) (Biogenesis, NH); monoclonal antibodies to tumor susceptibility gene (TSG101) (1:1000) (Abcam, Cambridge, MA) or apoptosis-linked gene-2 interacting protein X (ALIX) (1:250) (AIP1, BD Bioscience); sheep anti-malate dehydrogenase (MDH) (1:4000) (Rockland, PA). Peroxidase-conjugated, affinity-purified donkey anti-rabbit, anti-mouse, or anti-sheep IgG (1:100,000) (Jackson ImmunoReseach) were used for 90 min at room temperature. The antibody-antigen reactions were visualized by using ECL plus western blotting detection system (Amersham Biosciences) and light-sensitive film (Kodak BioMax XAR).
Results
Effects of protease inhibitors
Samples obtained from healthy individuals were prepared with and without protease inhibitors. Western blot analysis of NKCC2 showed that the samples without protease inhibitors had no signal or decreased signal compared to the samples with protease inhibitors (Fig. 2A and 2B).
Figure 2. The effect of protease inhibitors.
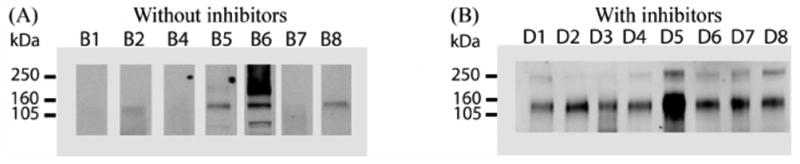
Eight fresh urine samples were collected without (A) and with (B) protease inhibitors, and exosome fractions were prepared, and evaluated by western blotting for NKCC2. Sample loading was normalized by urine creatinine. B1-B8 and D1-D8 represent 2 sets of different volunteers.
Effects of storage and vortexing
Samples were pooled from 3 individuals, and then processed 5 different ways (see methods). Freezing at -20 °C caused a major loss of exosome-associated protein measured by BCA protein assay (27.4 % recovery compared to urine stored at 4°C). In contrast, the recovery after freezing at -80°C was 86%. However, extensive vortexing after thawing resulted in 87.4% and 100% recovery in urine frozen at -20 °C and -80 °C, respectively (Fig. 3A). These changes in exosome-associated protein were verified using gel electrophoresis and Commassie-Blue staining (Fig. 3B). Western blot analysis showed that normal urine had easily detectable amounts of NHE3, TSG101, ALIX and AQP2. In contrast, NHE3, TSG101 and ALIX could not be detected, and less AQP2 was present in urine samples frozen at -20°C without vortexing after thawing. Freezing at -80°C preserved almost all of the specific urinary exosome-associated proteins. Extensive vortexing resulted in the complete recovery of the above four specific urinary exosome-associated proteins in pooled urine samples after freezing at either -20°C or -80°C (Fig. 3C).
Figure 3. The effect of storage and vortexing.
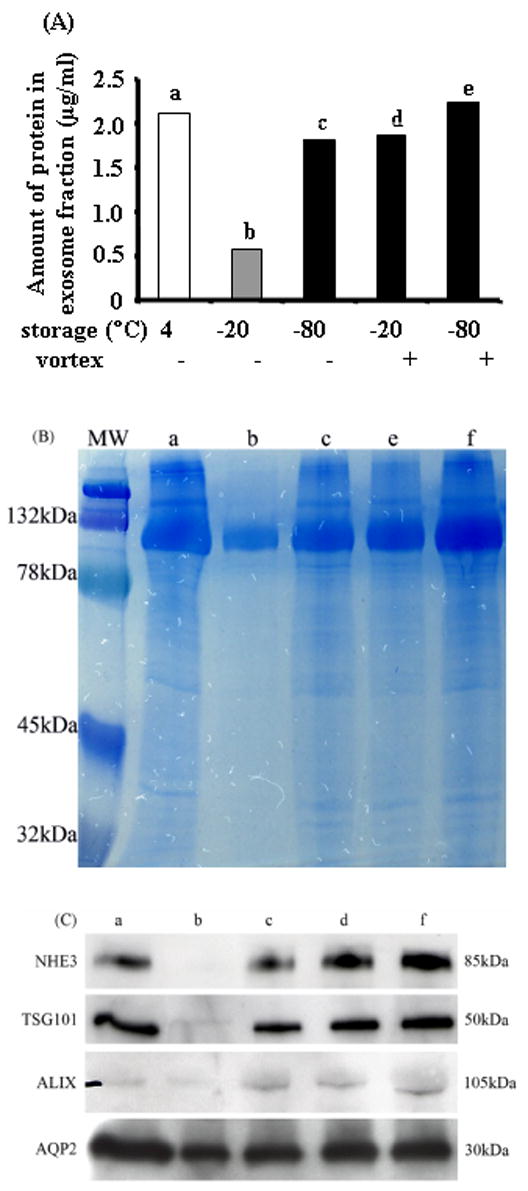
Samples were pooled from 3 individuals. Amount of urinary exosome-associated protein (200,000 × g pellet) (A), Coomassie blue-stained gel of equal fraction volume of protein (B), Western blot (10 :l aliquot) for NHE3, TSG101, ALIX and AQP2 (C).
To test if the exosme fraction could be isolated from clinically relevant samples, we examined abundance of these four specific urinary exosome-associated proteins in a much smaller volume (10 ml) of fresh first morning urine from three individual volunteers. Samples were normalized by Ucr. We found that 10 ml of urine was sufficient to detect these four specific urinary exosome-associated proteins by western blot analysis (Fig. 4A lane 1-3).
Figure 4. Effect of long-term storage.
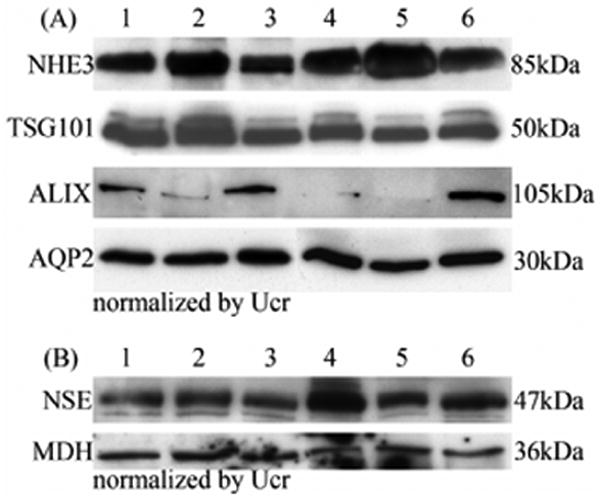
(A) Western blot of NHE3, TSG101, ALIX and AQP2 abundance in exosome fraction normalized by urine creatinine from 10 ml freshly-collected urine samples (lane 1-3) or after long-term storage (-80°C for 7 months; lane 4-6) from 3 different individuals. Lane 1 and 4, lane 2 and 5, lane 3 and 6 are from the same volunteer. (B) Western blot of cytosolic exosome-associated proteins (NSE and MDH) normalized by urine creatinine in fresh (lane 1-3) and long-term stored (-80°C for 7 months; lane 4-6) urine samples from 3 different individuals. Lanes as in A.
To determine the effect of long-term storage on the recovery of the urinary exosome fraction, we examined the abundance of the above four specific urinary exosome-associated proteins (normalized by Ucr) in the individual urine samples stored at -80°C for 7 months. The results showed that extensive vortexing also could result in almost complete recovery of the above four specific urinary exosomes compared to fresh urine samples (Fig. 4A lane 4-6). However, recovery of ALIX showed some variablity in individual urine samples (Fig. 4A).
To investigate whether storage at -80°C can cause urinary exosomes to rupture or remain intact, we examined the abundance of two cytosolic proteins (NES and MDH) isolated from the exosome fraction in fresh urine and frozen urine (-80°C for 7 months) from three individual volunteers. We hypothesized that cytosolic (internal) exosome-associated proteins would not be detected if urinary exosomes were damaged by prolonged freezing. We found no difference in the abundance of NES and MDH in urine stored at (-80°C for 7 months) compared to the fresh urine samples (Fig. 4B).
The effect of urine collection protocol
The exosome fraction accounted for only 3.0 ± 0.6% of total protein excreted in the urine; whereas, the soluble protein (supernatant fraction) contained 49.3 ± 2.4% and the sediment fraction contained 47.7 ± 2.5% of total protein excreted in the urine (Fig. 5). To determine if urine exosome-associated proteins are stable during a prolonged overnight collection, we compared overnight (first urine) and fresh (second urine) collection methods. We used urinary creatinine or urine flow rate to normalize the gel loading of protein. The protein staining patterns of 17,000 × g urinary sediment pellets (Fig. 6C and D) or 17,000 × g urinary supernatant (Fig. 6E and F) are almost the same by using either normalization method. However, some minor differences were seen in the 200,000 × g pellet (Fig. 6A and B). Both the 200,000 × g pellet and the 17,000 × g urinary sediment were dominated by a single species, presumably Tamm-Horsfall protein (THP). In contrast, the 17,000 × g urinary supernatant had fewer bands with much less THP in this fraction. The major band was presumably albumin.
Figure 5. The amount of total protein in three different urinary fractions of human first and second morning urine.
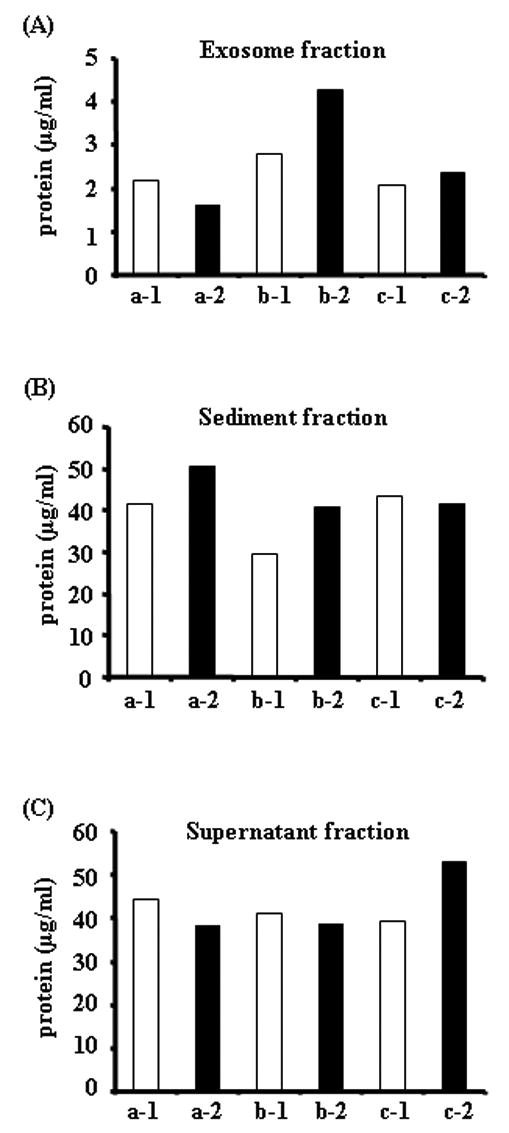
The amount of total protein in first (open bar) and second (closed bar) morning urine samples in different urinary fractions from three volunteers (a, b and c), 1: morning first urine, 2: morning second urine. Protein content in the exosome fraction (after 200,000 × g 1hr spin, A), urinary sediment proteins (after 17,000 × g 15 min spin, B), and supernatant proteins (after 17,000 × g 15 min spin, C).
Figure 6. Protein electrophoretic patterns of three different urinary fractions of human first and second morning urine.
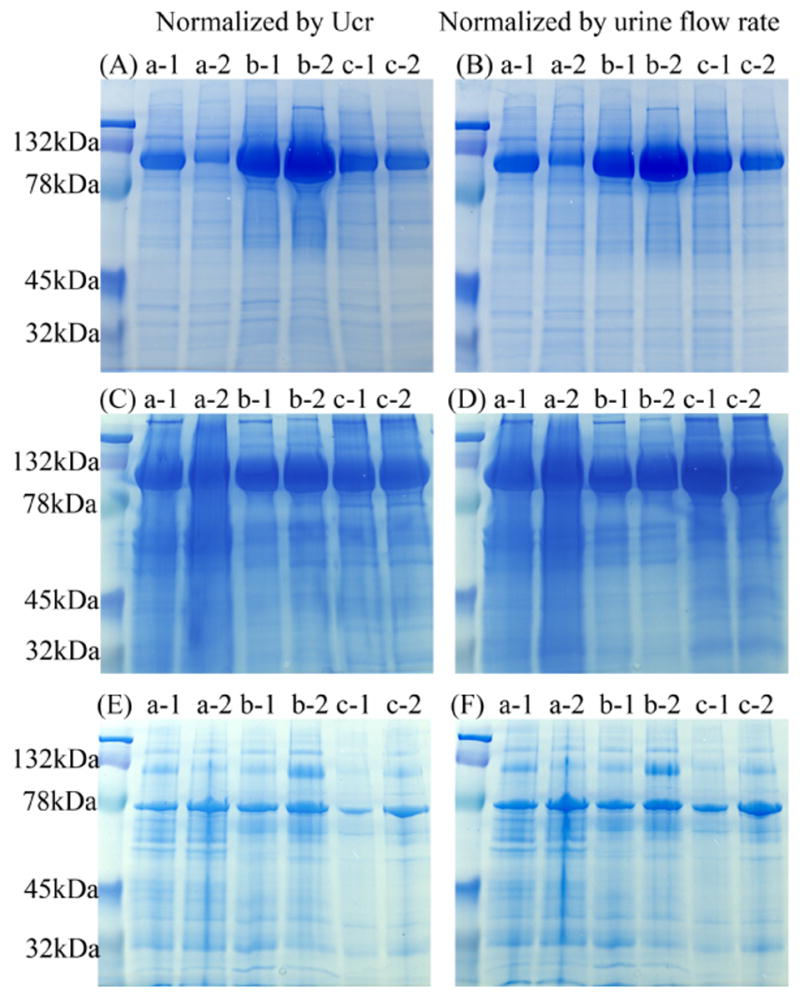
Coomassie blue-stained gels of urinary proteins in exosome fraction after 200,000 × g 1hr spin (A and B), urinary sediment proteins after 17,000 × g 15 min spin (C and D) and supernatant proteins after 17,000 × g 15 min spin (E and F) normalized by urinary creatinine (A, C, E) or urine flow rate (B, D, F). Legend: a, b and c represent different volunteers, 1: first morning urine, 2: second morning urine.
Specific urinary exosome-associated proteins were not detected in all urinary fractions. Urinary exosome-associated proteins (NHE3, TSG101, ALIX and AQP2) were easily detected only in the 200,000 × g pellet (Fig. 7), but not in 17,000 × g urinary sediment or 17,000 × g urinary supernatant by western blot analysis (data not shown).
Figure 7. Specific exosome-associated proteins in first and second morning urine.
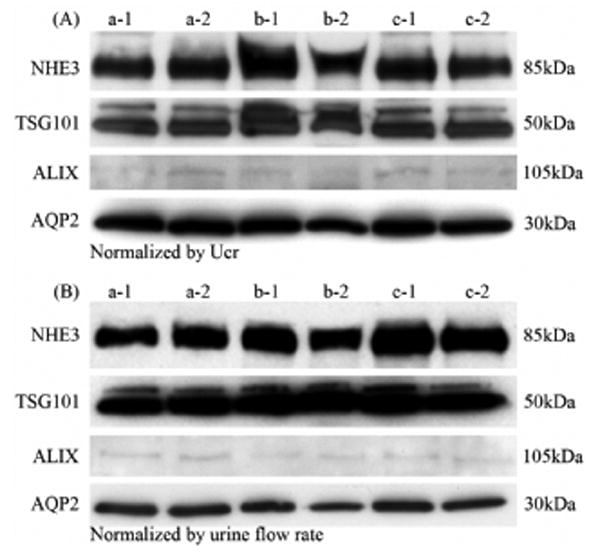
Abundance of NHE3, TSG101, ALIX and AQP2 by western blotting in the urinary exosome fraction normalized by urinary creatinine (A) or urine flow rate (B). Legend: a, b and c represent different volunteers, 1: first morning urine, 2: second morning urine.
First and second morning urine had similar amounts of total protein in the three urinary fractions (Fig. 5). The protein patterns matching the change of total protein amounts were verified by Coomassie-Blue-stained SDS/PAGE (Fig. 6A-F) after normalization. To investigate the effect of protein degradation in bladder/urinary tract on urinary exosomes, we compared the abundance of four specific urinary exosmes by western blot analysis. We found that the abundance of four specific exosome-associated proteins (TSG101, NHE3, ALIX and AQP2) are similar between first and second morning urine after normalizing by Ucr (Fig. 7A) or urine flow rate (Fig. 7B).
Discussion
The results of the present study demonstrate that 1) protease inhibitors are necessary for preservation of exosome-associated proteins during urine collection process; 2) freezing urine samples at -80°C preserved urinary exosome-associated proteins better than -20 °C; 3) extensive vortexing after thawing improved recovery of the urinary exosome fraction; 4) human urine exosome-associated proteins were detectable in 10 ml urine samples; 5) first and second morning urine can be used for exosome isolation.
Exosomes are secreted bioactive vesicles of 50-100nm in diameter, formed in late endocytic compartments [multivesicular bodies (MVB)]. Currently, many features remain unclear about exosomes, but research has begun to highlight their potential physiological roles and clinical uses [8]. Exosomes have immunoregulatory roles and potent antitumor effects in B lymphocytes and dendritic cells [6, 7]. Urinary exosomes are the internal vesicles of MVB that are delivered to the urinary space by fusion of the outer membrane of MVB with the apical plasma membrane of renal tubule epithelial cells [4]. Pisitkun et. al. [2] first verified the existence of exosomes in human urine from healthy subjects by electron microscopy and identified 295 proteins in urinary exosomes by proteomic analysis. Du Cheyron reported that urinary NHE3, a typical apical membrane protein, increases in patients with acute renal failure (despite storage of urine samples at -20°C, see below) [3]. These results suggest that the urinary exosome-associated proteins may serve as biomarkers for early diagnosis and treatment of kidney diseases. However, before proceeding with large scale discovery efforts, there are a number of important barriers that need to be overcome. The first barrier is to determine how to effectively collect, preserve and recover urinary exosome-associated proteins [4].
Numerous studies have reported the effect of storage temperature on the individual major urinary proteins. Uto et. al. reported that storage at -70 °C did not alter the concentration of Tamm-Horsfall protein (THP) compared to fresh urine, while storage -30°C resulted in a 3-fold increase of THP in human urine [9]. Innanen found that one or two freeze-thaw cycles decreased the concentration of microalbumin; however, mixing after thawing restored microalbumin concentration to normal [10]. Klasen et. al. reported that storage at − 70 °C is more stable than -20 °C for preserving urinary albumin, transferrin, and globulin [11]. The previous studies did not address the collection, storage and preservation of human urinary exosome-associated proteins. In the present study, we found that protease inhibitors are necessary to prevent the degradation of exosome-associated proteins during collection and processing steps. We found that freezing at -20°C caused a major loss of urinary exosome-associated proteins, whereas, -80°C storage only caused a mild loss (14%) of urinary exosome-associated proteins. However, of greater importance, we found that extensive vortexing could restore to normal the recovery of urinary exosome-associated proteins in the urine frozen at -80°C, and greatly improve the recovery of urinary exosome-associated proteins even in urine stored at -20°C. In addition, we found that urinary exosome-associated proteins were preserved almost completely after long-term storage at -80 °C although some proteins, such as ALIX maybe more labile. We hypothesize that urinary exosomes may be trapped in a proteinous meshwork and then co-precipitate with urinary constituents such as THP, or attach to the wall of plastic tubes after freezing. Eventually, extensive vortexing releases exosome-associated proteins after thawing. Thus, long term storage is a viable option, despite initial losses, because urinary exosomes can be recovered with extensive vortexing.
To evaluate collection procedures, we compared first and second morning urine. We found that human first and second morning urine are very similar with respect to total protein in different urinary fractions (sediment, supernatant, and exosomes) or exosome-associated proteins, suggesting that there is only minimal degradation of urinary exosomes in the bladder/urinary tract. Untimed or spot urine collections are more easily obtained than timed urine collection in both in-patient and out-patient settings. In theory, the best biomarker quantification scheme would express biomarker excretion in units of mass excretion rate. Since the excretion rate cannot be determined from a spot urine sample, instead, a typical control for urinary concentration is estimated by the urinary creatinine concentration which assumes creatinine excretion rate is constant. Assadi et al found that urinary microalbumin to creatinine ratios in second morning urine correlated well with 24hr urine protein excretion [12]. Ginsberg et al reported that the determination of ratios of urinary protein to creatinine concentration in a random single voided urine sample during daytime activities can replace the measurement of protein excretion in 24hrs urine collection [13]. To our knowledge, this is the first report to compare urinary exosome secretion between first and second morning urine samples. Also small clinically relevant urine volumes (10 ml) are sufficient to detect urinary exosomal markers.
Our results may appear to differ from those recently reported by du Cheyron et. al. [3]. They only found NHE3 in the urinary exosome fraction from patients with acute renal failure, but did not, in general, detect NHE3 in normal subjects. In contrast, we could easily detect NHE3 in normal subjects. However, du Cheyron et. al. stored their urine samples at -20°C, and did not mention a vortexing step. Thus, both studies agree that NHE3 is difficult to detect in urine samples stored at -20°C and processed without vortexing. This highlights the critical importance of optimizing urine collection, storage, and processing conditions.
In additional to renal disease or injury, several variables such as collection time, volume status, age, gender, nutritional status, physical activity, etc., might modulate exosome excretion and hence confound the comparison between different groups of subjects. A uniform normalization method is required to compare subjects with widely different hydration states or urinary flow rates. Normalization by urine flow rate is theoretically the best method but it is rarely practical; therefore, normalization by Ucr is currently the best option for clinical study. With the isolation of urinary exosomal biomarkers from spot urine collection, it may be possible to collect urine samples to screen for kidney diseases detection prognoses or guide treatment of known kidney diseases. Also, wide range studies can be conducted in either inpatients or outpatients to understand the pathophysiological mechanisms of various kidney diseases by the biological information included in urinary exosomes.
Conclusion
Recovery of urinary exosome-associated proteins is enhanced by addition of protease inhibitors soon after collection, storage at -80°C and extensive vortexing during thawing. Spot urine can be normalized by urine creatinine or urine flow rate. Urinary exosome-associated proteins can be detected in clinically relevant urine samples (10 ml). The difference between first and second morning urine protein recovery was small, suggesting minimal protein degradation in the urinary tract and bladder. These collection, storage, and processing conditions of urinary samples may be useful for future biomarker discovery efforts.
Acknowledgments
This research was supported by the intramural research program of NIH, NIDDK and NHLBI.
References
- 1.Hewitt SM, Dear J, Star RA. Discovery of protein biomarkers for renal diseases. J Am Soc Nephrol. 2004;15:1677–89. doi: 10.1097/01.asn.0000129114.92265.32. [DOI] [PubMed] [Google Scholar]
- 2.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101:13368–73. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.du Cheyron D, Daubin C, Poggioli J, et al. Urinary measurement of Na+/H+ exchanger isoform 3 (NHE3) protein as new marker of tubule injury in critically ill patients with ARF. Am J Kidney Dis. 2003;42:497–506. doi: 10.1016/s0272-6386(03)00744-3. [DOI] [PubMed] [Google Scholar]
- 4.Hoorn EJ, Pisitkun T, Zietse R, et al. Prospects for urinary proteomics: Exosomes as a source of urinary biomarkers. Nephrology (Carlton) 2005;10:283–90. doi: 10.1111/j.1440-1797.2005.00387.x. [DOI] [PubMed] [Google Scholar]
- 5.Thongboonkerd V, Klein E, Klein JB. Sample preparation for 2-D proteomic analysis. Contrib Nephrol. 2004;141:11–24. doi: 10.1159/000074587. [DOI] [PubMed] [Google Scholar]
- 6.Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–72. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zitvogel L, Regnault A, Lozier A, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 8.Chaput N, Taieb J, Andre F, Zitvogel L. The potential of exosomes in immunotherapy. Expert Opin Biol Ther. 2005;5:737–47. doi: 10.1517/14712598.5.6.737. [DOI] [PubMed] [Google Scholar]
- 9.Uto I, Ishimatsu T, Hirayama H, et al. Determination of urinary Tamm-Horsfall protein by ELISA using a maleimide method for enzyme-antibody conjugation. J Immunol Methods. 1991;138:87–94. doi: 10.1016/0022-1759(91)90067-p. [DOI] [PubMed] [Google Scholar]
- 10.Innanen VT, Groom BM, de Campos FM. Microalbumin and freezing. Clin Chem. 1997;43:1093–4. [PubMed] [Google Scholar]
- 11.Klasen IS, Reichert LJ, de Kat Angelino CM, Wetzels JF. Quantitative determination of low and high molecular weight proteins in human urine: influence of temperature and storage time. Clin Chem. 1999;45:430–2. [PubMed] [Google Scholar]
- 12.Assadi FK. Quantitation of microalbuminuria using random urine samples. Pediatr Nephrol. 2002;17:107–10. doi: 10.1007/s00467-001-0762-5. [DOI] [PubMed] [Google Scholar]
- 13.Ginsberg JM, Chang BS, Matarese RA, Garella S. Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med. 1983;309:1543–6. doi: 10.1056/NEJM198312223092503. [DOI] [PubMed] [Google Scholar]


