Abstract
Six tests for the detection of West Nile virus (WNV) antibodies in the serum of experimentally infected chickens were compared. The tests included the hemagglutination-inhibition test (HIT), immunoglobulin M (IgM)-capture enzyme-linked immunosorbent assay (ELISA) with WNV-infected mouse brain antigen, immunoglobulin G (IgG) indirect ELISA with tickborne encephalitis viral antigen, the microtitre virus neutralization test, the standard plaque reduction neutralization test (PRNT), and the microtitre PRNT (micro-PRNT). Thirty adult chickens, intravenously and intramuscularly inoculated with 107 plaque-forming units (PFU) of WNV strain Egypt 101, were bled and given a booster of 107 PFU at 7, 15, and 21 d postinoculation; the final blood collection was on day 28. Although the micro-PRNT is capable of detecting the highest antibody titres during both early and late infection, because of the technical complexity and time requirements of this test a combination of IgM and IgG ELISAs is recommended for serologic screening. Serum samples that give positive results in the ELISAs can then be tested by the micro-PRNT to determine the specificity of antibodies to WNV.
West Nile virus (WNV), genus Flavivirus, family Flaviviridae, is an “old-world” arbovirus, transmitted mainly by infected mosquitoes. Wild birds are the primary amplifying hosts of the virus, but a number of species (amphibians, domestic poultry, and mammals, including humans and horses) can be infected through bites from WNV-infected mosquitoes, and disease will develop in some (1).
The virus was first detected in North America in 1999, during an encephalitis outbreak in humans and horses in New York (2). It was able to overwinter and spread the next year to various localities in the United States. In 2001, WNV was detected in 27 states and the District of Columbia; it had also spread to the Cayman Islands and Canada (southern Ontario) (3,4).
Canada conducted WNV surveillance in 2000 and 2001. One component was detection of seroconversion in sentinel chicken flocks. This approach had been successful in monitoring spread of the virus during the WNV encephalitis outbreak in Romania in 1997–1998 (5). It also has been traditionally used for surveillance of other arbovirus diseases (for example, eastern and western equine encephalitis).
All members of the genus Flavivirus share antigenic epitopes, as revealed by cross-reactivity in the hemagglutination inhibition test (HIT) (6,7). Therefore, commercially available plates, not necessarily coated with homologous antigen, can be used for antibody detection in serum by enzyme-linked immunosorbent assay (ELISA). Both HIT and ELISA should be followed by a neutralization assay to complete identification within the serologic group.
This paper describes preparation of WNV-positive control chicken serum and evaluates the following 6 tests for antibody detection: HIT, immunoglobulin M (IgM)-capture ELISA with infected mouse-brain antigen, immunoglobulin G (IgG) indirect ELISA with tickborne encephalitis (TBE) viral antigen from humans, the microtitre virus neutralization test (micro-VNT), the standard plaque reduction neutralization test (PRNT), and the microtitre PRNT (micro-PRNT).
The WNV topotype strain Egypt 101 was used for immunizing the chickens and as the test virus in the micro-VNT and the micro-PRNT. New York strain NY99 was used in the HIT and in the standard and micro-PRNT. Vero cells (ATCC) and Vero V76 (ATCC) were maintained according to standard protocols and used for virus propagation and in the neutralization assays.
Thirty specific-pathogen-free White Leghorn hens obtained from the Animal Disease Research Institute, Nepean, Ontario, were inoculated intravenously and intramuscularly with 107 plaque-forming units (PFU) of Egypt 101; boosters of 107 PFU were given intramuscularly and subcutaneously 7, 15, and 21 d after the initial inoculation. Blood (2 mL) was collected from a wing vein of each hen before the initial inoculation and on days 2, 7, 15, 21, and 28 thereafter. Serum obtained before the initial inoculation tested negative with HIT, micro-VNT, standard PRNT, and micro-PRNT; it was used to set cut-off values for the ELISAs.
The blood was allowed to clot at 37°C for 1 h, then the serum was separated by centrifugation at 2000 × g for 10 min at 4°C. The gelled serum was aspirated several times through a 16G cannula and centrifuged again at 2000 × g for 10 min at 4°C. It was then inactivated at 56°C for 30 min and stored at −20°C before testing.
The micro-PRNT was carried out in the following manner. First, 100 μL of serial dilutions of serum (starting with 1:10) was incubated with 100 μL of 200 PFU of Egypt 101 for 1 h at 37°C in 5% CO2 in cell-free 96-well microtitre plates (Costar; Corning Inc., Corning, New York, USA). Then 100 μL/well of the mixture was transferred onto cells grown in 96-well plates that had been seeded 24 h earlier with Vero V76 cells, 105 cells/cm2; the medium was removed just before the transfer. The plates were incubated for 1 h at 37°C in 5% CO2. An overlay of 100 μL/well of 3% carboxymethylcellulose (CMC sodium salt, medium viscosity; Sigma Chemical Company, St. Louis, Missouri, USA) in phosphate-buffered saline (PBS) was added to the plates, which were then incubated for 3 d at 37°C in 5% CO2. Next the cells were fixed with 4% formaldehyde and stained with 0.5% crystal violet in 80% methanol/20% PBS. The number of plaques per well was verified by virus back-titration. Serum dilutions causing at least 70% plaque reduction were considered positive for anti-WNV antibodies.
For the IgG indirect ELISA, we used commercially obtained plates coated with antigen of the Western subtype of TBE isolated from humans in Eastern Europe (TBE IgG and IgM, quantitative; Serion, Wurtzburg, Germany), not to be confused with the Eastern subtype, which causes Russian spring–summer encephalitis. The plates were blocked with 100 μL of 3% skim milk/PBS for 1 h at 37°C. Then 100 μL of 2-fold serial dilutions of serum in 3% skim milk/PBS were incubated on the plates for 1 h at 37°C, and the bound antibodies were detected by incubation for 1 h at 37°C with 100 μL of horseradish-peroxidase-labelled goat anti-chicken IgG antibodies (Kirkegaard and Perry, Gaithersburg, Maryland, USA) diluted 1:5000 in 3% skim milk/PBS. Next, 100 μL of 1 mM 2,2'-azino-di-3-ethyl-benzthiazoline-6-sulfonic acid (ABTS) in citrate buffer was added to the wells, and the (optical density) OD415 of the substrate in the plates was read after 3 min of incubation at room temperature. The plates were washed 3 times between the steps with PBS/0.05% Tween 20.
The IgM-capture ELISA was carried out as previously described (8). Briefly, anti-chicken IgM-capture antibody was coated on 96-well plates and incubated overnight at 4°C. Serum diluted 1:250 was then added to the wells, and inactivated suckling mouse WNV antigen and horseradish-peroxidase-conjugated WNV monoclonal antibody (Centers for Disease Control, Fort Collins, Colorado, USA) were added sequentially. The colorimetric reaction produced by the addition of tetramethyl benzidine was recorded. Specimens were considered IgM positive if the P/N ratio was at least 2.0 (P = OD of test specimen reacted with viral antigen; N = OD of negative control serum reacted with viral antigen). For the P/N ratio to be valid, P had to be at least twice the OD of the test specimen reacted with normal antigen derived from noninfected mice.
The micro-VNT was performed as follows. First, 50 μL of 103 TCID (tissue-culture infective dose)50 of virus was mixed with 50 μL of 2-fold serial dilutions of serum in 96-well cell-culture microtitre plates (Costar) and incubated at 37°C in 5% CO2 for 1 h. Then 50 μL of 2 × 105/mL Vero cells was added to each well. The plates were incubated for 4 d at 37°C in 5% CO2. Serum dilutions causing at least 90% reduction in cytopathic effect were considered positive for anti-WNV antibodies.
The standard PRNT was performed as previously described (9). Mixtures of virus and dilutions of chicken serum were incubated at 37°C for 1 h, then added to 6-well plates containing Vero cell monolayers. After the plates had been incubated at 37°C for 1 h, a nutrient-agar overlay was added, and the plates were placed in a CO2 incubator for approximately 3 d, after which another overlay, containing neutral red as a vital stain, was added. The plates were checked periodically over the next few days for plaque formation. The highest serum dilution with a plaque reduction of at least 90% was defined as the titration end point.
The HIT was performed as described by Clarke and Casals (10), with a microtitre-plate format. In brief, nonspecific inhibitors and natural hemagglutinins were removed by acetone extraction and adsorption with goose erythrocytes, respectively. Dilutions of acetone-treated chicken serum were mixed with 8 hemagglutination (HA) units of suckling mouse WNV antigen and incubated at 4°C overnight. Goose erythrocytes were then added to the mixture, and the solution was incubated for an additional hour at room temperature. The HI titre was determined as the highest dilution of chicken serum that caused complete inhibition of erythrocyte agglutination by 8 HA units of viral antigen.
Means and standard errors were determined for the serum titres at each time point in the individual tests. The graphs were plotted with the SigmaPlot5 program. Differences between high and low antibody responders were confirmed with Student's t-test for comparison of 2 population means.
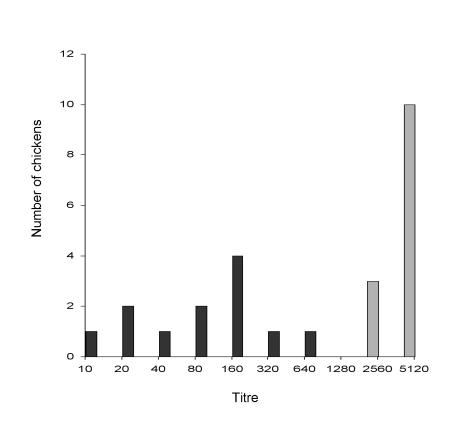
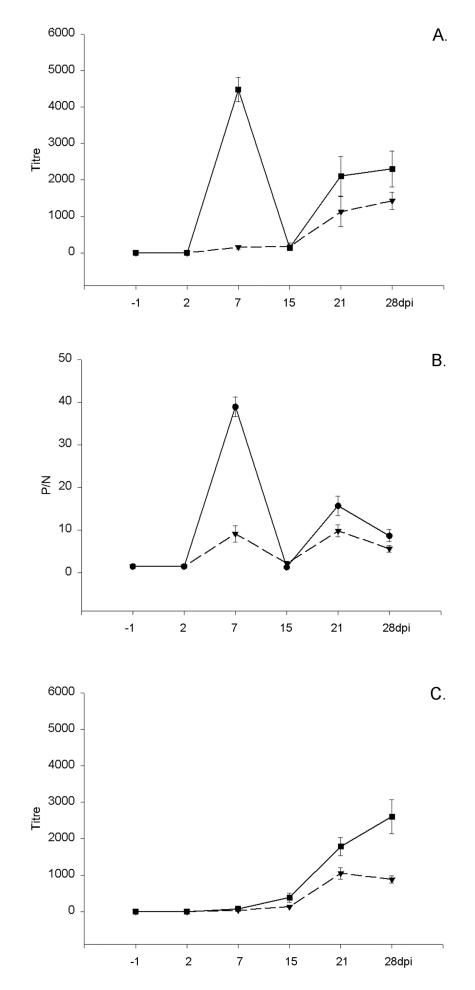
A full set of data was obtained for 25 of the 30 chickens; some samples from 5 of the birds were considered unfit for testing owing to cell toxicity or serum gelling. The initial experiments were with Egypt 101, but since there was no observable difference in the results of the micro-PRNT between Egypt 101 and NY99 the latter strain was used in the remaining assays as the more relevant in vivo strain for North America. Overall, comparing the earliest time of antibody detection, the number of positive samples at different times after inoculation, and the mean serum antibody titres, our data indicated that, among the 6 assays, the micro-PRNT detected the highest titres and the highest number of antibody-positive serum samples at different times after inoculation. This observation probably reflects the ability of the micro-PRNT to detect both IgM and IgG antibodies. Interestingly, with respect to the results of the IgM ELISA, the chickens formed 2 almost equal groups: 1 with very high titres on day 7 (13 chickens), and the other with initially lower titres (12 chickens). The observation was even more obvious with the micro-PRNT: on day 7 the group with high IgM titres also had high micro-PRNT titres, and the group with low IgM titres also had low micro-PRNT titres (Figure 1). The chickens were still within their respective groups on day 28 when tested by micro-PRNT and by IgG ELISA, as confirmed by the Student's t-test (P < 0.1). Three serum samples did not titrate to the end point even at a dilution of 1:5120; they were assigned a titre of 5120 to facilitate representation of the data (Figure 2).
Figure 1. Distribution of serum titres of antibodies to West Nile virus (WNV) in the microtitre plaque reduction neutralization test (micro-PRNT) in blood collected 7 d after inoculation. Chickens considered as low responders are represented by black columns, high responders by grey columns.
Figure 2. Mean antibody titres and standard errors, determined by the micro-PRNT (A) and the immunoglobulin G enzyme-linked immunosorbent assay (IgG ELISA) (C). Graph B shows IgM ELISA P/N values (see text for explanation) for serum specimens obtained on various days postinoculation (dpi): the group of 13 chickens with high IgM response at 7 d is represented by the solid line; the group of 12 chickens with a lower IgM response at 7 d is represented by the dashed line.
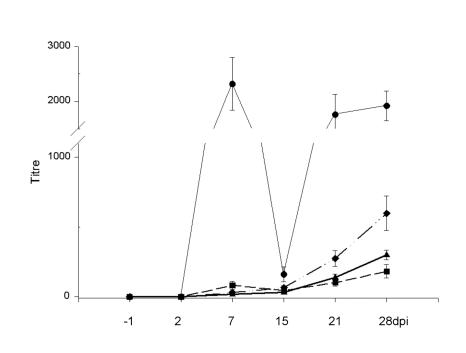
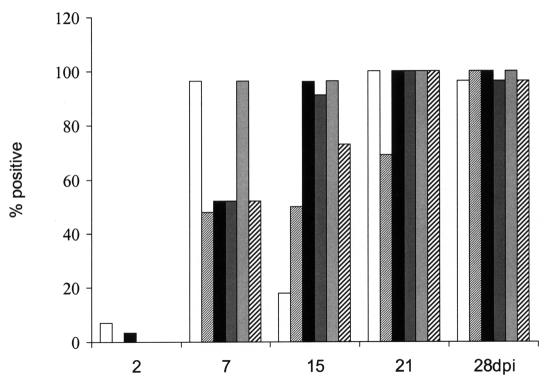
The micro-VNT and the standard PRNT showed comparable antibody titres (Figure 3), with dynamics similar to the development of IgG antibody, as measured by the IgG ELISA. The HIT also has the potential to detect both IgM and IgG antibodies; however, despite showing higher titres when compared with the standard PRNT (Figure 2), the HIT detected lower titres and lower numbers of antibody-positive samples than the micro-PRNT. Figure 4 summarizes the numbers of positive samples detected by the individual methods at each time after inoculation. The micro-VNT, standard PRNT, and HIT detected antibodies in all samples only on days 21 and 28, the peak titre reaching about 640.
Figure 3. Means and standard errors of serum antibody titres for 25 chickens, determined by the micro-PRNT (circles), the hemagglutination-inhibition test (HIT) (diamonds), the standard PRNT (triangles), and the microtitre virus neutralization test (micro-VNT) (squares).
Figure 4. Rates of detection of WNV antibodies in the serum from all chickens at different times after inoculation with the following tests: IgM ELISA (white bars), micro-VNT (finely cross-hatched bars), IgG ELISA (black bars), HIT (dark grey bars), micro-PRNT (light grey bars), and standard PRNT (thickly cross-hatched bars).
The apparent ability of the micro-PRNT to detect IgM antibodies on day 7 was in contrast to the ability of the standard PRNT and the micro-VNT to detect only very low neutralizing activity in about 50% of the serum samples at this time point. The mechanism of IgM antibody interaction with flaviviruses is not known; however, the early detection of IgM antibody by the micro-PRNT may be explained with the model of the interaction of avian influenza virus and IgM antibodies (11,12). Once the IgM antibodies reach certain levels, attachment of the virus to cells is prevented by steric hindrance. The ability of a neutralization assay to detect this would be a function of the test's design and the mechanism by which virus and IgM bind to form a complex. In the micro-PRNT the serum–virus mixture is not removed from the cells and is only diluted in half with the addition of the overlay; thus, the IgM antibodies have a prolonged opportunity to react with the virus. This reaction may prevent the virus from attaching to cells not only during the first cycle of replication but also subsequently, thereby limiting replication and thus increasing the perceived neutralizing titre of serum in the micro-PRNT. The prolonged interaction of the virus with not only IgM but also IgG antibodies may contribute to the higher titres detected by the micro-PRNT as compared with the standard PRNT and the micro-VNT.
Although it was not our aim to investigate the immune response of the chickens to WNV, it was interesting to observe the grouping of the chickens with respect to their early IgM response. Somewhat similar observations have been made in other studies (13,14). The double IgM peak may be linked to the hyperimmunization schedule used in this study.
In summary, a combination of IgM and IgG ELISAs is recommended for the screening of serum from chickens or other species for which commercial conjugated secondary antibodies are available, such as rabbits, to cover both early and late stages of infection. The ELISAs are more sensitive than the HIT and are simple and rapid when compared with the PRNTs. However, the utility of IgM ELISA in chickens could be limited in surveillance conditions, depending on the bleeding schedule, because during the natural infection one would expect the IgM antibodies to be detected for a limited time. The specificity of positive serum should be confirmed by micro-PRNT. The micro-PRNT could also be the test of choice for serum from species for which conjugated secondary antibodies are not available.
Footnotes
Acknowledgments
The authors thank Drs. J. Copps, J. Gren, and S. Halayko for providing animal work support.
Address all correspondence and reprint requests to Dr. Hana M. Weingartl; telephone: (204) 789-2027; fax: (204) 789-2038; e-mail: hweingartl@inspection.gc.ca
Received March 18, 2002. Accepted August 22, 2002.
References
- 1.Hubalek Z, Halouzka J. West Nile fever — a reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis 1999;5:643–650. [DOI] [PMC free article] [PubMed]
- 2.Outbreak of West Nile-like viral encephalitis — New York, 1999. Morbid Mortal Wkly Rep 1999;48:845–849. [PubMed]
- 3.Nasci RS, Savage HM, White DJ, et al. West Nile virus in overwintering Culex mosquitoes, New York City, 2000. Emerg Infect Dis 2001;7:742–744. [DOI] [PMC free article] [PubMed]
- 4.Weekly Update: West Nile virus activity — United States, November 14–20, 2001. Morbid Mortal Wkly Rep 2001;50:1061–1062. [PubMed]
- 5.Cernescu C, Nedelcu NI, Tardei G, Ruta S, Tsai TF. Continued transmission of West Nile virus to humans in Southeastern Romania, 1997–1998. J Infect Dis 2000;181:710–712. [DOI] [PubMed]
- 6.Casals J, Brown LV. Hemagglutination with arthropod-borne viruses. J Exp Med 1954;99:429–449. [DOI] [PMC free article] [PubMed]
- 7.Calisher CH, Karabatsos N, Dalrymple JM, et al. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J Gen Virol 1989;70:37–43. [DOI] [PubMed]
- 8.Martin DA, Muth DA, Brown T, Johnson AJ, Karabatsos N, Roehrig JT. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J Clin Microbiol 2000;38:1823–1826. [DOI] [PMC free article] [PubMed]
- 9.Beaty BJ, Calisher CH, Shope RS. Arboviruses. In: Schmidt NJ, Emmons RW, eds. Diagnostic Procedures for Viral, Rickettsial and Chlamydial infections. 6th ed. Washington, DC: American Public Health Association, 1989:797–856.
- 10.Clarke DH, Casals J. Techniques for hemagglutination and hemagglutination inhibition with arthropod-borne virus. Am J Trop Med Hyg 1958;7:561–573. [DOI] [PubMed]
- 11.Outlaw MC, Dimmock NJ. Mechanisms of neutralization of influenza virus on mouse trachea epithelial cells by mouse monoclonal polymeric IgA and polyclonal IgM directed against the virus haemagglutinin. J Gen Virol 1990;71:69–76. [DOI] [PubMed]
- 12.Armstrong SJ, Outlaw MC, Dimmock NJ. Morphological studies of the neutralization of influenza virus by IgM. J Gen Virol 1990;71:2313–2319. [DOI] [PubMed]
- 13.Pevzner IY, Stone HA, Nordskog AW. Immune response and disease resistance in chickens. I. Selection for high and low titer to Salmonella pullorum antigen. Poult Sci 1981;60:920–926. [DOI] [PubMed]
- 14.Heller ED, Zrhava U. Serological evidence for major histocompatibility complex (B complex) antigens in broilers selected for humoral immune response. Poult Sci 1991;70:726–732. [DOI] [PubMed]