Abstract
Cross-presentation as a fundamental pathway of activating CD8+ T cells has been well established. So far the application of this concept in vivo is limited, and the mechanisms that specialize CD8+ dendritic cells (DCs) for this task are not fully understood. Here we take advantage of the specific cytosolic export feature of cross-presenting DCs together with the property of cytosolic cytochrome c (cyt c) in initiating Apaf-1-dependent apoptosis selectively in cross-presenting DCs. A single i.v. injection of cyt c in B6 mice produced a 2- to 3-fold reduction in splenic CD8+ DCs but not in Apaf-1-deficient mice. Functional studies both in vivo and in vitro showed that cyt c profoundly abrogated OVA-specific CD8+ T cell proliferation through its apoptosis-inducing effect on cross-presenting DCs. More importantly, in vivo injection of cyt c abolished the induction of cytotoxic T lymphocytes to exogenous antigen and reduced subsequent immunity to tumor challenge. In addition, only a proportion of CD8+ DCs that express abundant IL-12 and Toll-like receptor 3 were efficient cross-presenters. Our data support the hypothesis that cross-presentation in vivo requires cytosolic diversion of endocytosed proteins, conferring cross-presentation specialization to a proportion of CD8+ DCs. We propose that DCs incapable of such transfer, even within the CD8+ DC subset, are unable to cross-present. Our model opens an avenue to specifically target cross-presenting DCs in vivo for manipulating cytotoxic T lymphocyte responses toward infections, tumors, and transplants.
Keywords: antigen presentation, apoptosome, cytotoxic T cells
Presentation of exogenous antigens (Ag) by MHC class I molecules, or cross-presentation, is an important process for generating cytotoxic T lymphocyte (CTL) responses (1). The task of cross-presentation is mainly fulfilled by dendritic cells (DCs), particularly CD8+ DCs. The reasons for this selectivity and the exact mechanisms involved in cross-presentation remain contentious although several mechanisms have been proposed (2–4). There is evidence to suggest that the specialized ability of DCs for cross-presentation may be explained in part by the presence of a unique, size-selective intracellular pathway for export of Ag from endosomal compartments into the cytosol for access to the conventional MHC class I processing pathway (2), although this has not specifically been shown for the CD8+ DC subset. Recent data also suggest that the cytosolic cross-presentation pathway is the major relevant mechanism in vivo (5) and that the machinery that confers cross-presentation specialization is located downstream from Ag uptake (6). Despite the progress in our understanding of the mechanisms of cross-presentation, in vivo application of cross-presentation to manipulate (enhance or reduce) a CD8+ T cell response remains to be exploited.
The mitochondrial intrinsic pathway is one of the most well characterized pathways of apoptosis. One initiator of this pathway in mammals is cytochrome c (cyt c), a soluble 13-kDa mitochondrial protein that also functions as a component of the electron transport chain. Upon receiving an apoptotic stimulus, cyt c is released from the intermembrane space of the mitochondrion into the cytosol where it binds to its partner protein, apoptotic protease-activating factor 1 (Apaf-1), to form a procaspase-9-activating heptameric protein complex known as the apoptosome (7). Apaf-1-mediated caspase activation is accomplished through a series of downstream effector caspases eventually resulting in cell death. Specific ablation of the apoptotic function of cyt c using a knockin mouse model has shown that the only major activator of Apaf-1 is cyt c and that the only significant protein that cyt c can activate during apoptosis is Apaf-1 (8).
Because the presence of cyt c in the cytosol is highly proapoptotic, we reasoned that if efficient cross-presenting cells have a propensity for transferring exogenous Ag to the cytosol, then exogenous cyt c would preferentially induce Apaf-1-dependent apoptosis in cross-presenting cells. Moreover, the ease of cytosolic transfer in DCs is related to Ag size. Internalized Ag with molecular masses of <40 kDa have been reported to gain access to the cytosol more readily (2), making a relatively small molecule like cyt c ideal. In this study we have investigated the in vitro and in vivo effects of horse cyt c on induction of apoptosis in DCs and consequently on the priming of CD8 T cells.
Results
Cyt c Selectively Depletes DCs in Vivo and in Vitro.
To understand the role of cross-presentation in the intact animal, we designed a mouse model that allowed functional ablation of cross-presenting cells. Our strategy used the fact that DCs are highly macropinocytic cells (9) and that efficient cross-presentation involved a specialized machinery for endosome-to-cytosol transport. Release of endogenous cyt c from the mitrochondrion of higher eukaryotic organisms into the cytosol allows engagement of Apaf-1 and initiation of apoptosis within mammalian cells (7). In contrast, cyt c from lower eukaryotic organisms—e.g., yeast—has a low affinity for Apaf-1, does not trigger activation of caspases, and fails to initiate cell death (partly because of trimethylation of lysine-72) (10). Thus, we hypothesized that introduction of exogenous horse cyt c into mice would selectively induce apoptosis in cells that possess this unique capacity for cytosolic transfer, i.e., professional cross-presenting cells.
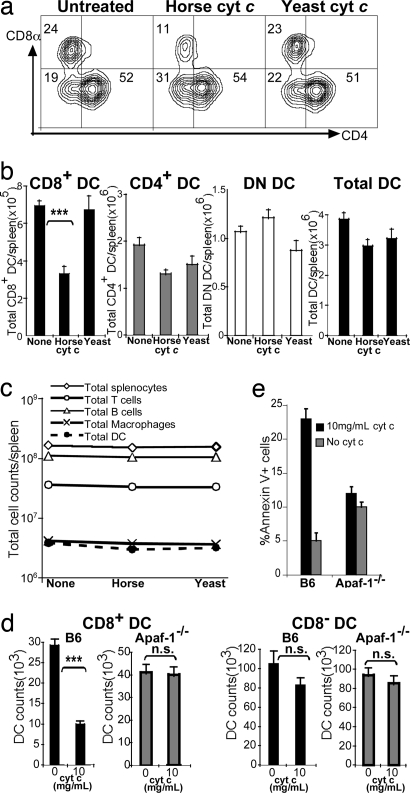
To test this hypothesis we injected C57BL/6 (B6) mice with either horse or yeast cyt c. After 16–24 h we compared the relative proportion and numbers of splenic CD11c+CD4−CD8+ (CD8+), CD11c+CD4+CD8− (CD4+), CD11c+CD4−CD8− (DN), and total CD11c+ cells by flow cytometry. As shown in Fig. 1, a 2- to 3-fold reduction in both the percentage (Fig. 1a) and number (Fig. 1b) of CD8+ DCs was consistently observed after administration of horse cyt c (P < 0.0001 compared with untreated, and P = 0.005 compared with yeast cyt c-treated). A small reduction was also seen in the CD4+ DC subset after horse cyt c treatment compared with no treatment; however, when compared with yeast cyt c treatment, no significant difference was found in absolute CD4+ DC numbers after horse cyt c treatment (P = 0.34). No reduction was observed in the CD4−CD8− subset (P = 0.2).
Fig. 1.
Horse cyt c-mediated apoptosis of cross-presenting DCs. (a) Flow cytometry of splenic DCs 24 h after treatment with 5 mg of either horse or yeast cyt c. Numbers indicate the relative percentage of CD11c+ cells. Results are typical of >10 experiments with three to five mice per group. (b) Absolute DC counts per spleen after horse or yeast cyt c treatment. Means ± SEM of three separate experiments with four to five mice per group are indicated. (c) Total cell numbers after horse or yeast cyt c treatment. (d) DC subset counts. AutoMACS-purified DCs from B6 or Apaf-1−/− mice were cultured for 24 h at the indicated doses of cyt c. DCs were gated on CD11chiCD8+ (Left) or CD11chiCD8− (Right) expression. Means of triplicate wells ± SEM are shown. ***, P ≤ 0.001; n.s., not significant. (e) Apoptosis assay. Purified CD11c+ cells were cultured in triplicate for 6 h at the indicated doses of cyt c and stained with CD8, annexin V, and propidium iodide for analysis by flow cytometry. Means ± SEM (percentage of annexin V+ propidium iodide+ cells) are shown. One representative experiment of three is depicted.
To address whether other splenic populations were affected by cyt c treatment, we repeated the above experiments and stained splenocytes in parallel with CD11b and F4/80, CD19 and B220, or TCR-β and CD3 for analysis by flow cytometry. Horse cyt c treatment resulted in a 10–30% decrease in total DC numbers, but both horse and yeast cyt c had minimal cytotoxic effects on macrophages, B cells, and T cells (Fig. 1c). Larger cyt c doses of up to 15 mg did not enhance the observed reduction in CD8+ DCs (data not shown). Hence, all subsequent experiments were performed by using an i.v. dose of 5 mg.
We next sought to establish whether cyt c treatment caused DC apoptosis. Because cyt c requires the presence of Apaf-1 to initiate apoptosis, we investigated the impact of Apaf-1 deficiency. In addition, this would dispel the possibility that our cyt c preparation was acting through a contaminant. Because most Apaf-1−/− mice die before birth, we made chimeras using mutant or WT fetal liver reconstitutions into bm1 host mice. These mice were chosen as hosts because cells from bm1 mice are incapable of directly presenting OVA257–264 to OT-I CD8+ T cells (11). Six to 12 weeks after reconstitution, the proportion of DCs/lymphocytes in Apaf-1−/−- or WT-reconstituted mice was similar to that of B6 mice (data not shown).
To determine the absolute numbers of DCs remaining in vitro after cyt c treatment, we cultured freshly isolated splenic DCs from WT or Apaf-1−/− mice in the presence of 0–10 mg/ml cyt c. After 24 h we quantified the numbers from each DC subset by calibrating against a known quantity of beads. Analysis of the absolute cell numbers revealed a 3-fold reduction in CD8+ DCs from WT B6 mice (P = 0.0001), whereas the CD8− subset (P = 0.2) and both CD8+ and CD8− DCs of Apaf-1−/− origin were not significantly affected (Fig. 1d).
Apoptosis of DCs from naïve B6 or Apaf-1−/−-reconstituted mice was also measured by annexin V assays of purified DCs cultured with 0–10 mg/ml cyt c for 6 h. At the highest concentration used, cyt c induced apoptosis in almost 25% of splenic CD8+ DC from B6 mice, as detected by annexin V binding (Fig. 1e). In contrast, cyt c failed to induce early apoptosis in DCs of Apaf-1−/− origin (Fig. 1e). The observed effect was dose-dependent (data not shown).
It should be noted that the preferential cyt c-induced suicide of CD8+ DC was not due to preferential initial uptake. Radio-iodinated cyt c was taken up equally by CD8+ DCs and CD8− DCs (1,976 ± 676 vs. 1,720 ± 814 cpm) within 2 h; we used yeast cyt c to avoid any apoptogenic effects. Moreover, FITC-labeled horse cyt c bound equivalently to both DC subsets after 30 min at 37°C.
Cyt c Treatment Impairs Cross-Presentation in Vivo and in Vitro.
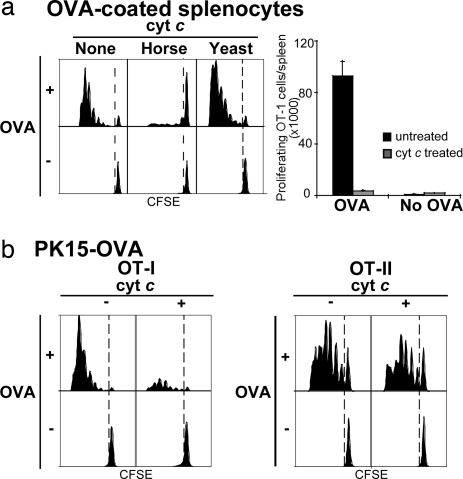
To verify whether the cyt c-induced suicide of CD8+ DCs was functionally significant, we determined whether cross-presentation of cellular Ag was preferentially impaired in vivo. We adoptively transferred 1 × 106 CFSE-labeled OT-I cells into B6 mice on day −2, then injected mice with three daily doses of cyt c starting the day before i.v. injection of two forms of cellular Ag: either OVA-coated bm1 splenocytes (OCS) (Fig. 2a) or OVA expressed in a pig xenogeneic cell line, PK15-OVA (Fig. 2b). Three injections of cyt c were used because of its very short circulating half-life of 4 min (12) and because DCs have a rapid turnover rate. We then assessed cross-presentation 60 h after mice had received Ag by measuring OT-I proliferative responses in the spleen. Cross-presentation was profoundly inhibited in cyt c-treated mice; the overall number of expanded OT-I cells was up to 27-fold lower in cyt c-treated mice than that of untreated control B6 mice (Fig. 2a Right).
Fig. 2.
Cyt c treatment preferentially inhibits cross-presentation in vivo. B6 mice were left untreated or given three daily doses of i.v. cyt c starting the day before injection of OCS (a) or PK15-OVA (b). CFSE-labeled OT-I cells were adoptively transferred into all mice (a and b Left). Some mice were coinjected with equal numbers of CFSE-labeled OT-II (b Right). The FACS profiles of OT-II proliferation were similar for both types of cellular Ag, and for the sake of clarity only the profiles for PK15-OVA are shown. Results are representative of multiple experiments with four to five mice per group.
Next we investigated the effect on CD4+ proliferative responses. Adoptive transfer of equal numbers of CFSE-labeled OT-I and OT-II cells were injected into the same animal. When we compared MHC class II presentation to MHC class I (cross)-presentation, we observed that cyt c treatment preferentially abrogated OT-I proliferation but resulted in a modest reduction in OT-II proliferation (Fig. 2b). This effect was consistently seen when OVA transfectants (Fig. 2b) or OVA-coated cells (data not shown) were used as immunogens.
To test whether the suppression of CD8+ T cell proliferation in vivo was nonspecific, we performed similar experiments in CD11c-mOVA mice, which express membrane-bound OVA in DCs under the control of the CD11c promoter. Thus, OVA is constitutively synthesized and presented via the classical MHC class I pathway. When CFSE-labeled OT-I cells were adoptively transferred into these mice, equivalent proliferative responses were observed in mice treated with cyt c compared with mice that were not (data not shown).
To extend our in vivo observations, splenic CD8+, CD4+, and CD4−CD8− DC subsets were cultured continuously in vitro in the presence of 2 mg/ml cyt c and OVA. Cyt c selectively abrogated the ability of CD8+ DC to cross-present OVA to OT-I cells [supporting information (SI) Fig. 8a] in a dose-dependent manner (data not shown). The inability of CD8+ DCs to stimulate OT-I proliferation in the presence of cyt c was not related to direct toxicity of cyt c on T cells in vitro, because all subsets of DCs were able to stimulate robust OT-I proliferative responses when incubated with OVA257–264 peptide in vitro (SI Fig. 8b). Thus, our in vitro studies confirmed our in vivo cross-presentation results.
Cyt c-Mediated Inhibition of Cross-Presentation Is Apaf-1-Dependent.
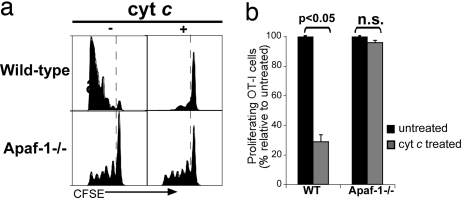
To confirm the mechanistic specificity of the observed reduction in cross-presentation, we compared how exogenous cyt c affected the ability to stimulate naïve OT-I proliferation in Apaf-1−/−- or WT-reconstituted mice. As expected, cross-presentation was inhibited in WT-reconstituted mice that received cyt c (Fig. 3a Upper). In contrast, cyt c treatment did not reduce the frequency (Fig. 3a Lower) and overall numbers (Fig. 3b) of expanded OT-I cells in Apaf-1−/− mice. The level of cross-presentation in untreated Apaf-1−/−-reconstituted mice was slightly lower than that observed in WT-reconstituted mice, but this was not attributable to lower hematopoietic reconstitution because the reconstitution efficiency of the blood and DC compartments was comparable in Apaf-1−/− and WT mice. Regardless, the key issue is that there was no difference in the internal comparison between the presence or absence of cyt c within the Apaf-1−/−-reconstituted mice. Thus, these results cogently indicate that exogenous cyt c resulted in apoptosis of cross-presenting cells through an Apaf-1-dependent pathway.
Fig. 3.
Effects of cyt c are mediated through an Apaf-1-dependent mechanism. CFSE-labeled OT-I cells were adoptively transferred into WT- or Apaf-1−/−-reconstituted bm1 mice on day 2. Mice were untreated or given three daily doses of cyt c starting the day before receiving OCS. Proliferation was assessed 60 h later with representative flow cytometry plots (a) and quantitation of proliferating cells (b) shown. Bars represent the average number of proliferating cells relative to values obtained in untreated mice ± SEM. Results are representative of more than six experiments with four to five mice per group. n.s., not significant.
Cyt c Treatment Preferentially Reduces CTL Induction and Ag-Specific Proliferative Response.
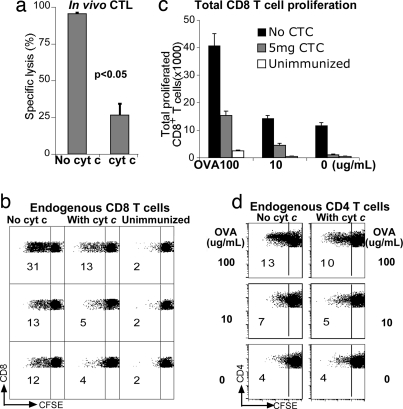
Although we have shown that cyt c treatment inhibited cross-presentation of cell-associated Ag to OT-I cells in vivo, it was unclear whether this phenomenon was merely idiosyncratic of transgenic T cells. Thus, we designed a model whereby both T cells and DCs were of endogenous origin. We pretreated B6 mice with cyt c on three consecutive days, commencing a day before injection of OCS, and assessed in vivo CTL activity 7 d after T cell priming. There was almost 4-fold less OVA-specific CTL activity in cyt c-treated mice compared with untreated mice (Fig. 4a).
Fig. 4.
Cyt c inhibits endogenous anti-OVA CD8+ responses. Cyt c-treated or untreated B6 mice were primed in vivo with OCS. (a) Endogenous in vivo CTL responses were determined after 7 d. Means ± SEM are shown. (b) Endogenous CD8+ T cell proliferation. Spleens were harvested 7 d after in vivo priming, CFSE-labeled, and cultured in duplicate for 4 d with 100, 10, or 0 μg/ml OVA. Dot plots are gated on live CD8+ cells. Numbers indicate the percentage of proliferated CD8+ cells. (c) Total numbers of CFSEdimCD8+ cells from cyt c-treated immunized, untreated immunized, or unimmunized mice (mean ± SEM). (d) Endogenous CD4+ T cell proliferation. Dot plots are gated on live CD4+ cells. Numbers indicate the percentage of proliferated CD4+ cells. Data are representative of three independent experiments with three to four mice per group.
To assess endogenous anti-OVA proliferative responses (compared with OT-I responses), mice were pretreated with cyt c as described above. CFSE-labeled splenocytes were restimulated in vitro 7 d later with OVA. After 4 d we assessed proliferation of endogenous CD4+ and CD8+ T cells by CFSE dye dilution. A 2- to 3-fold impairment of CD8+ T cell expansion was observed in mice treated with cyt c with minimal nonspecific proliferation in unimmunized mice (Fig. 4 b and c). In contrast, there was minimal impairment of endogenous CD4+ T cell responses (Fig. 4d). These results strongly indicate that cyt c inhibited endogenous CD8+ T cell responses (both proliferative and killer responses) to cross-presentation in vivo.
Cyt c Impairs Immune Rejection of B Cell Lymphoma.
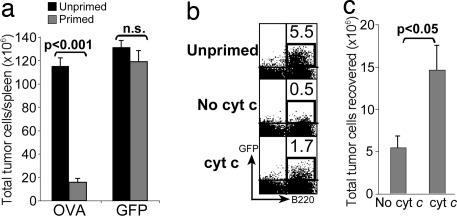
We also analyzed the effect of cyt c on the elimination of OVA-expressing Eμ-myc B cell lymphomas. Mice were either left untreated or given three doses of cyt c at the time of priming with OCS. After 7 d, 1 × 106 Eμ-myc-OVA or Eμ-myc-GFP cells were inoculated i.v. into primed or naïve mice. Mice were left for 7 d to allow spontaneous lymphoma development. A robust Ag-specific tumor rejection response was detected in the spleens of mice that had been primed with Eμ-myc-OVA but not Eμ-myc-GFP (Fig. 5a). Cyt c-treated mice had 3-fold more tumor cells than untreated controls (Fig. 5 b and c), indicating that in vivo disruption of cross-presentation by cyt c impaired immunity to tumor Ag.
Fig. 5.
Cyt c impairs subsequent immunity to B cell lymphoma. B6 mice were untreated or given three daily injections of cyt c starting the day before in vivo priming with OCS. After 7 d recipient mice were inoculated with Eμ-myc-GFP or Eμ-myc-OVA cells. After another 7 d spleens were analyzed for GFP+ B220+ tumor cells. Total tumor numbers per spleen (a and c) and representative FACS plots (b) are shown (representative of three experiments with three to five mice per group). n.s., not significant. Numbers indicate the percentage of tumor cells of all live cells.
Cyt c-Resistant DCs Are Incapable of Cross-Presentation but Retain a Capacity for MHC Class II Presentation.
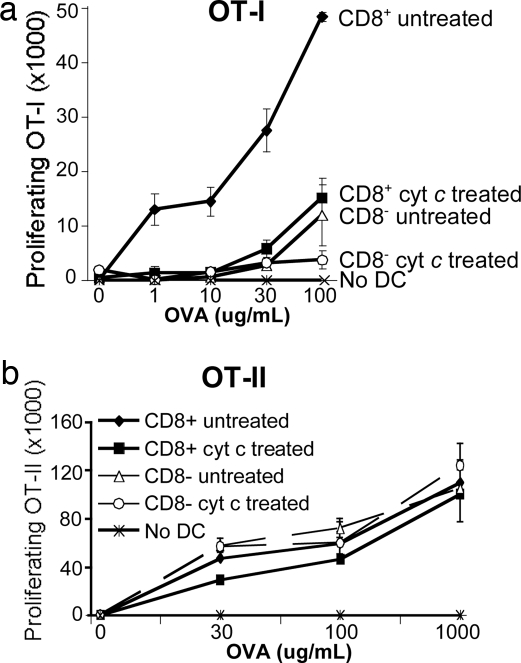
Despite a considerable remaining population of CD8+ DCs after cyt c treatment (Fig. 1a), cross-presentation was still markedly impaired. Therefore, it was important to determine the Ag presentation capacity of residual DCs. To this end, we treated B6 mice with cyt c for 24 h and identified a 50% decrease in the proportion of CD8+ DCs (see Fig. 1a). We cultured equal numbers of sorted CD8+ or CD8− DCs from untreated or cyt c-treated mice with various concentrations of OVA protein and CFSE-labeled OT-I cells. CD8+ DCs from cyt c-treated mice were far less capable of stimulating OT-I proliferation in vitro than were CD8+ DCs from untreated mice (Fig. 6a). Indeed, the level of proliferation seen in cyt c-treated mice approximated that of CD8− DCs from untreated mice, which are known for their poor cross-presentation capacity. Using CFSE-labeled OT-II cells, we showed that the remaining (cyt c-resistant) DCs were defective only in MHC class I but not MHC class II presentation ability because these DCs could present newly encountered OVA protein to OT-II cells equally well compared with DCs from untreated mice (Fig. 6b).
Fig. 6.
Cyt c-resistant DCs are incapable of cross-presentation but retain the capacity for MHC class II presentation. Residual splenic CD11c+ DCs from pooled cyt c-treated (after 24 h) or untreated mice were sorted into CD8+ or CD8− subsets. Each subset was cultured continuously in duplicate for 60 h with the indicated doses of OVA together with CFSE-labeled OT-I (a) or OT-II (b) cells. Numbers of proliferating cells are shown (mean ± SEM). Data are representative of three individual experiments.
Cyt c Treatment Preferentially Reduces IL-12 Production and Toll-Like Receptor 3 (TLR3) Expression by CD8+ DCs.
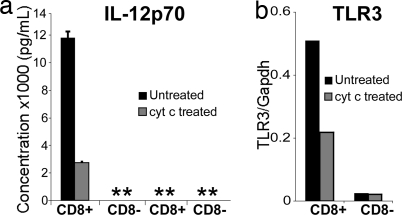
Apart from their efficiency at cross-presentation, another functional specialization of CD8+ DCs is their propensity for IL-12 production in response to TLR engagement. Given that CD8+ DCs are the main targets of cyt c-mediated depletion, we compared the IL-12-producing capacity of residual cyt c-treated CD8+ DCs to their untreated counterparts. Twenty four hours after treatment with cyt c, remaining CD8+ or CD8− DC populations from untreated or cyt c-treated B6 mice were stimulated with CpG and IFN-γ. The presence of IL-12p40 and IL-12p70 was assayed from cultured supernatants after 24 h. On a per-cell basis, CD8+ DCs from cyt c-treated mice produced nearly 2-fold less IL-12p40 (data not shown) and ≈4-fold less IL-12p70 than did CD8+ DCs from untreated mice (Fig. 7a). IL-12p70 was undetectable from CD8− DCs stimulated with CpG and IFN-γ or from all unstimulated DCs (Fig. 7a).
Fig. 7.
Cyt c treatment preferentially reduces IL-12 production and TLR3 expression by CD8+ DCs. DC subsets were prepared as for Fig. 6 and cultured in duplicate for 24 h with a mixture of CpG, IFN-γ, and GM-CSF or media alone. (a) IL-12p70 production as measured by ELISA of culture supernatants (mean ± SEM). Data are representative of three individual experiments. **, undetectable. (b) TLR3 expression as measured by quantitative real-time PCR. Values are expressed relative to GAPDH levels.
CD8+ DCs have also been reported to express high levels of TLR3, and this has been linked to their cross-priming ability (13). When residual CD8+ DCs from cyt c-treated mice were quantitatively analyzed for their TLR3 expression by real-time PCR, we found a 1.5- to 2-fold reduction in the expression of this receptor, whereas very low levels of TLR3 were expressed on CD8− DCs (Fig. 7b).
Thus, we concluded that cyt c reduced the proportion of CD8+ DCs that abundantly expressed IL-12 and TLR3.
Discussion
Surface CD4 and CD8 expression has been useful in classifying DC subsets (14, 15). However, these markers are merely phenotypic markers and are not causally associated with function. The CD8+ subset, at least ex vivo, is the major DC population mediating cross-presentation (16–18). Our knowledge of the function of the various DC subsets is largely based on in vitro or ex vivo studies. This is because currently available murine models usually lack more than one DC subset (19) and commonly have profound defects in cytokine expression and/or functional maturation (20–22). Our strategy of differential induction of apoptosis in cross-presenting cells has two advantages. First, this strategy correlates with cross-presenting function; i.e., apoptosis is triggered only when exogenous cyt c is delivered to the cytosol. Second, it is completely performed in vivo; i.e., the processing/presentation of Ag does not contain any ex vivo steps and hence avoids any potential artifacts during isolation and culture of DCs. This system not only provides a tool for further study into the in vivo contribution of cross-presentation for induction of a CD8 T cell responses but also opens an avenue for the first time to specifically target cross-presenting DCs for manipulation of CTL responses.
The CD8+ DC was the major DC subset reduced by cyt c treatment. This is in line with established evidence that this subset is the main DC mediating cross-presentation (16). Although only half of the CD8+ DCs were killed by cyt c administration, this corresponded to a dramatic reduction in Ag-specific CD8 T cell proliferation for both transgenic OT-I cells (Fig. 2) and endogenous CD8 T cells (Fig. 4). We therefore argue that the cyt c-sensitive population is the cohort of efficient cross-presenters. This was evidenced by the results in Fig. 5a where the residual CD8+ DCs (cyt c-resistant) were inefficient at cross-presentation and indeed were only as effective as CD8− DCs. Additionally, experiments at different time points when incubating DCs with cyt c in vitro revealed that only a proportion of CD8+ DCs were found to be apoptotic at any given point in time (Fig. 1 d and e). A major conclusion from our work is that only a subset of CD8+ DCs possesses a highly efficient mechanism for endosome-to-cytosol transport [where access to the TAP/proteasome pathway is gained (5)] that makes it particularly specialized at cross-presentation of exogenous Ag.
IL-12 production and TLR3 expression are hallmarks of CD8+ DCs (13, 23). TLR3 has been shown to augment cross-priming during viral infection (13), and signaling through this receptor has been reported to cause up-regulation of costimulatory molecules and production of antiviral cytokines (24, 25). The fact that both IL-12 and TLR3 expression were attenuated by cyt c treatment indicates that the cyt c-sensitive DCs were the major producers of IL-12 and TLR3 and hence consistent with the tenet that they are the efficient cross-presenters. Systematic comparison of CD8+ DCs from untreated or cyt c-treated animals may reveal further molecular differences between these two cohorts of CD8+ DCs.
We argue that apoptosis is the mechanism by which exogenously administered cyt c works. This is first supported by the reduction in CD8+ DC numbers in vivo and in vitro (Fig. 1). Second, there is an increase in annexin V staining after cyt c treatment (Fig. 1e). As expected, this was not as dramatic as the findings measuring absolute cell numbers, presumably because annexin V staining detects early events of apoptosis and is a snapshot of cell death whereas cell number quantification is cumulative. Moreover, DCs can phagocytose dying cells that would not be accounted for during annexin V staining. Third, the abrogation of cyt c's effect in the absence of Apaf-1 strongly argues for its mechanistic specificity via the apoptosome, i.e., by exclusively inducing apoptosis in DCs that have transferred cyt c into the cytosol. Furthermore, yeast cyt c, a form that fails to initiate apoptosis, did not result in a significant in vivo reduction of DC numbers and cross-presentation.
At least two routes of cross-presentation have been proposed. One pathway involves the transport of Ag from endosomes into the cytosol, whereas, in the alternative pathway, Ag fails to escape from endosomes and is processed and degraded into antigenic peptides within these vesicles (26, 27). The cytosolic route appears to be the predominant pathway used by DCs (2), although an alternative noncytosolic pathway has been described (3). The molecular mechanisms endowing CD8+ DCs with superior cross-presentation capacity are unknown. It has been argued that antigenic capture confers this specialization to CD8+ DCs (28), but our group and others have refuted this notion (6, 16). Recent studies have correlated the efficiency of class II (29) and class I (30, 31) presentation by DCs with enhanced Ag retention within endocytic compartments. However, this has not been demonstrated specifically within DC subsets. Additionally, the unique capacity for rapid endosome-to-cytosol protein translocation within 1–2 h of internalization (32, 33) may endow the superiority of CD8+ DCs for cross-presentation. Our finding that cyt c can induce suicide in cross-presenting DCs supports the hypothesis that cytosolic diversion is required for cross-presentation in vivo and extends it to CD8+ DCs because this is the only way cyt c induces apoptosis. We argue that this is the mechanism that confers specialization to CD8+ DCs because cells incapable of such transfer, even within the CD8+ DC subset, are unable to cross-present and are not depleted.
We also conclude that only a proportion of CD8+ DCs are efficient at cross-presentation, at least by the cytosolic route. Our model allows assessment of cross-presentation that is totally in vivo, and this functional model of depleting cross-presenting DCs can be used for studying and ultimately manipulating cross-priming responses in the pathophysiology of transplantation, viral, or tumor immunity in vivo.
Materials and Methods
Mice and Reagents.
C57BL/6 (B6), bm1 (11), OT-I (34), OT-II (35), and CD11c-mOVA (36) (from R. Steptoe, Centre for Immunology and Cancer Research, Brisbane, Australia) mice have been described. Reconstitution of lethally irradiated bm1 hosts with embryonic day 14.5 Apaf-1−/− fetal liver cells was performed as described (37). OVA protein, cyt c from horse heart, and Saccharomyces cerevisiae were obtained from Sigma. Mice received 5 mg of horse or yeast cyt c i.v. dissolved in PBS.
Splenic DC Isolation and Analysis.
Splenic DCs isolated as previously described (15) were used for (i) high-speed sorting (MoFlo; Cytomation), (ii) immunomagnetic bead purification (AutoMACS; Miltenyi Biotec), or (iii) immunofluorescent labeling before analysis.
Immunofluorescent Staining.
Anti-CD11c, CD4, CD8, CD3, CD11b, CD19, Vα2, B220, F4/80, TCRβ, annexin V, and streptavidin were purchased from Pharmingenor Caltag. Propidium iodide was added at 1 μg/ml just before analysis.
Cell Lines.
The pig kidney cell line PK15 (American Type Culture Collection) was stably lipofected with OVA DNA (designated PK15-OVA) and maintained in the presence of 1.1 mg/ml geneticin (G418; GIBCO/BRL) and 4 μg/ml puromycin (Sigma). Stable Eμ-myc cell lines (38) bearing GFP (Eμ-myc-GFP) or GFP and OVA (Eμ-myc-OVA) were generated by retroviral transduction (39).
Proliferation and CTL Assays.
Usually OCS (36) were used as the immunogen. In vivo OT-I and OT-II proliferation assays, in vitro proliferation, and in vivo CTL assays were performed as described (36).
Endogenous Proliferative Responses.
A modified CFSE dilution method was used (40). B6 mice received three daily doses of cyt c starting the day before i.v. immunization with 2 × 107 OCS. A week later, equivalent numbers of splenocytes were labeled with 5 μM CFSE (Molecular Probes) for 10 min at 37°C. CFSE-labeled cells were plated at 5 × 106 per milliliter with OVA at 37°C for 4 d. Cells were enriched for viable lymphocytes by using density centrifugation. FACS analysis was performed (FACSAria; BD Biosciences).
RNA Extraction and Quantitative Real-Time PCR.
RNA extraction and quantitative real-time RT-PCR analysis were performed as described (41).
IL-12 ELISA.
ELISAs for murine IL-12 by Pharmingen were used on culture supernatants of DCs stimulated with 0.5 μM CpG1688 (Geneworks), 20 ng/ml IFN-γ (Pharmingen), and 100 ng/ml GM-CSF (PeproTech).
Statistical Analysis.
Student's t test was used to compare two sets of data.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Drs. R. Kluck and T. Kaufmann for advice. This work was supported by the National Health and Medical Research Council of Australia, the Juvenile Diabetes Research Foundation, the Howard Hughes Medical Institute, and the Leukemia and Lymphoma Society.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712394105/DC1.
References
- 1.Heath WR, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez A, et al. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat Cell Biol. 1999;1:362–368. doi: 10.1038/14058. [DOI] [PubMed] [Google Scholar]
- 3.Shen L, Sigal LJ, Boes M, Rock KL. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity. 2004;21:155–165. doi: 10.1016/j.immuni.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Ackerman AL, Giodini A, Cresswell P. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity. 2006;25:607–617. doi: 10.1016/j.immuni.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Palmowski MJ, et al. Role of immunoproteasomes in cross-presentation. J Immunol. 2006;177:983–990. doi: 10.4049/jimmunol.177.2.983. [DOI] [PubMed] [Google Scholar]
- 6.Schnorrer P, et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci USA. 2006;103:10729–10734. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schafer ZT, Kornbluth S. The apoptosome: physiological, developmental, and pathological modes of regulation. Dev Cell. 2006;10:549–561. doi: 10.1016/j.devcel.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Hao Z, et al. Specific ablation of the apoptotic functions of cytochrome c reveals a differential requirement for cytochrome c and Apaf-1 in apoptosis. Cell. 2005;121:579–591. doi: 10.1016/j.cell.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Norbury CC, et al. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur J Immunol. 1997;27:280–288. doi: 10.1002/eji.1830270141. [DOI] [PubMed] [Google Scholar]
- 10.Kluck RM, et al. Determinants of cytochrome c pro-apoptotic activity: The role of lysine 72 trimethylation. J Biol Chem. 2000;275:16127–16133. doi: 10.1074/jbc.275.21.16127. [DOI] [PubMed] [Google Scholar]
- 11.Nikolic-Zugic J, Carbone FR. The effect of mutations in the MHC class I peptide binding groove on the cytotoxic T lymphocyte recognition of the Kb-restricted ovalbumin determinant. Eur J Immunol. 1990;20:2431–2437. doi: 10.1002/eji.1830201111. [DOI] [PubMed] [Google Scholar]
- 12.Kunitomo R, Miyauchi Y, Inoue M. Synthesis of a cytochrome c derivative with prolonged in vivo half-life and determination of ascorbyl radicals in the circulation of the rat. J Biol Chem. 1992;267:8732–8738. [PubMed] [Google Scholar]
- 13.Schulz O, et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 14.Pulendran B, et al. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vremec D, et al. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978–2986. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- 16.den Haan JMM, Lehar SM, Bevan MJ. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iyoda T, et al. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belz GT, et al. Cutting edge: Conventional CD8 alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol. 2004;172:1996–2000. doi: 10.4049/jimmunol.172.4.1996. [DOI] [PubMed] [Google Scholar]
- 19.Hacker C, et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol. 2003;4:380–386. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki S, et al. Critical roles of interferon regulatory factor 4 in CD11bhighCD8alpha- dendritic cell development. Proc Natl Acad Sci USA. 2004;101:8981–8986. doi: 10.1073/pnas.0402139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zenke M, Hieronymus T. Towards an understanding of the transcription factor network of dendritic cell development. Trends Immunol. 2006;27:140–145. doi: 10.1016/j.it.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Schiavoni G, et al. ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8{alpha}+ dendritic cells. J Exp Med. 2002;196:1415–1425. doi: 10.1084/jem.20021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hochrein H, et al. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J Immunol. 2001;166:5448–5455. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 24.Reis e Sousa C. Toll-like receptors and dendritic cells: For whom the bug tolls. Semin Immunol. 2004;16:27–34. doi: 10.1016/j.smim.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Sen GC, Sarkar SN. Transcriptional signaling by double-stranded RNA: Role of TLR3. Cytokine Growth Factor Rev. 2005;16:1–14. doi: 10.1016/j.cytogfr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Shen L, Rock KL. Priming of T cells by exogenous antigen cross-presented on MHC class I molecules. Curr Opin Immunol. 2006;18:85–91. doi: 10.1016/j.coi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Guermonprez P, Amigorena S. Pathways for antigen cross presentation. Springer Semin Immunopathol. 2005;26:257–271. doi: 10.1007/s00281-004-0176-0. [DOI] [PubMed] [Google Scholar]
- 28.Schulz O, Reis e Sousa C. Cross-presentation of cell-associated antigens by CD8alpha+ dendritic cells is attributable to their ability to internalize dead cells. Immunology. 2002;107:183–189. doi: 10.1046/j.1365-2567.2002.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delamarre L, et al. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–1634. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 30.Accapezzato D, et al. Chloroquine enhances human CD8+ T cell responses against soluble antigens in vivo. J Exp Med. 2005;202:817–828. doi: 10.1084/jem.20051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savina A, et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 32.Guermonprez P, et al. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- 33.Houde M, et al. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–406. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- 34.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 35.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 36.Wilson NS, et al. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. 2006;7:165–172. doi: 10.1038/ni1300. [DOI] [PubMed] [Google Scholar]
- 37.Marsden VS, et al. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature. 2002;419:634–637. doi: 10.1038/nature01101. [DOI] [PubMed] [Google Scholar]
- 38.Adams JM, et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- 39.Pear W, Nolan G, Scott M, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mannering SI, et al. A sensitive method for detecting proliferation of rare autoantigen-specific human T cells. J Immunol Methods. 2003;283:173–183. doi: 10.1016/j.jim.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Lahoud MH, et al. Signal regulatory protein molecules are differentially expressed by CD8− dendritic cells. J Immunol. 2006;177:372–382. doi: 10.4049/jimmunol.177.1.372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.