Abstract
Enhancers have been functionally described for >35 years, but the molecular principles underlying the integration of regulatory inputs to alternate gene enhancers used during mammalian organogenesis remain incompletely understood. Using a combination of in vivo enhancer mapping and proteomics approaches, we have established that two distant and distinct early enhancers, each requiring different transcription complexes, are required for full activation of the gene encoding the pituitary lineage determining factor, Pit1. A transcription factor belonging to the “giant, multiple-homeodomain and zinc finger family,” Atbf1, serves as a novel pituitary regulator for one of the two required enhancers as shown by genetic and in vitro analysis.
The essence of embryonic development is to generate a regulated increase in cell number and diversity, with the identity of each cell type dictated by specific patterns of signals and expression of sets of genes Thus, a hierarchy of regulatory factors controls activation or repression of such genes with spatial/temporal precision, and such control is central to accurately unfolding genetic information to create precise patterns of developmental complexity. It involves evolutionarily conserved cis-regulatory sequences that are highly structured and organized to recruit sets of transcription activators/repressors. These transcription complexes will determine the rate and frequency of transcription initiation, generating fine control of gene expression in development, homeostasis, and disease.
The anterior pituitary gland provides an excellent model system to analyze molecular programs governing cell-specific gene regulation during embryonic development. The mature pituitary gland contains five distinct hormone-producing cell types: corticotropes, somatotropes, lactotropes, thyrotropes, and gonadotropes. All of these cell types arise from a common primordium, the Rathke's pouch, which initially develops by mouse embryonic day 9 (E9) as a single layer of epithelium; it undergoes a fast expansion attributable to intense cell proliferation and gives rise to a series of cell lineages, which eventually develop into the five cell types in response to precise spatial/temporal patterns of overlapping signaling gradients and involves several transcription factors (reviewed in refs. 1–6). Pit1, a POU-homeodomain transcription factor, is the lineage regulator responsible for the generation of somatotropes, lactotropes, and thyrotropes (7). Pit1 expression is initiated by E13.5 and is maintained in adulthood, being directly involved in the transcriptional control of the genes encoding growth hormone (GH), prolactin (Prl), and thyroid-stimulating hormone (TSHβ) (8). Our previous studies revealed that 14.8 kb of 5′-flanking sequence of the Pit1 gene was sufficient to direct the robust expression of a reporter in an identical spatial and temporal pattern as endogenous Pit1, whereas its minimal promoter (−327 bp to +13 bp) was insufficient to drive detectable reporter expression in transgenic mice (9). A distal enhancer (DE) located at −10.2 kb from Pit1 transcription start site and containing multiple functional Pit1-binding sites was shown to be involved in autoregulation (9). The 14.8 kb of 5′-flanking sequence minus the distal enhancer could still drive the activation of a reporter gene in both wt and Pit1-defective Snell (dw/dw) mutant mice, demonstrating that the elements required for Pit1 early activation were present in the same genomic region (10). Further mapping showed that the regions required for the Pit1 early activation were located between −10 kb and −3.5 kb (10). Thus, Pit1 expression appears to be under the control of multiple enhancers, at least one for its activation and one for its maintained expression. As such, Pit1 gene regulation appears as an ideal system to study fine regulation of transcription via distal cis-acting regulatory regions.
Here, we present the in vivo characterization of one of Pit1 early regulatory region/enhancers, referred to as EEα, exemplifying the complex interenhancer functional interactions required for initial developmental gene activation. A quantitative proteomics approach allowed us to identify factors capable of interacting, directly or indirectly, with this element and one of these factors, Atbf1, a transcription factor containing multiple zinc finger and homeodomain motifs, proved able to bind and activate Pit1 EEα in vitro and in vivo. Analysis of Atbf1 gene-trap mutant mice demonstrated that there was a direct genetic interaction between Pit1 and Atbf1, and that Atbf1 was required to achieve full initial activation of Pit1 gene, providing a linkage between activation of early Pit1 enhancer and subsequent Pit1-dependent autoregulatory events.
Results and Discussion
Mapping of EEα, a cis-Regulatory Element Required for Pit1 Early Activation.
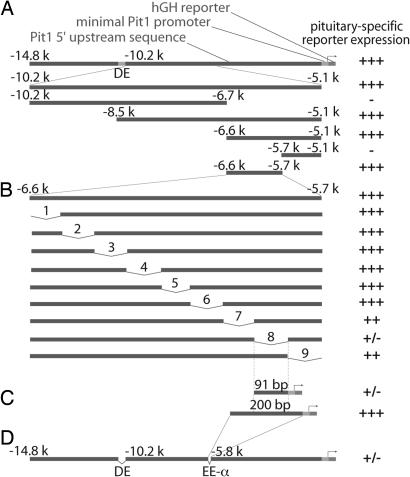
Previous studies suggested that the −10.2-kb/−5.1-kb sequence upstream Pit1 coding region could be sufficient to drive the expression of a reporter in an identical spatial and temporal pattern as Pit1 (10). A computational approach revealed a high number of highly conserved regions with lengths varying from 50 bp to 500 bp, providing few clues for the identification of specific developmental regulatory regions and making it critical to use in vivo enhancer mapping as the most reliable approach in searching for key cis-regulatory information. Analysis of a transgene containing the −10.2-kb/−5.1-kb region using human growth hormone (hGH) as reporter and the Pit1 promoter as minimal promoter showed that this 5-kb region directed pituitary-specific expression of a reporter gene (Fig. 1A). We then determined which specific subregions might be responsible for early activation. For each transgene studied, at least three integration events were generated, and the analysis was performed on the founder animals taken at E14.5–E15.5; the reporter activation was measured by in situ hybridization with a specific hGH probe. The analysis of the embryos was performed 1 or 2 days after initial Pit1 gene activation (E13.5) to preclude any technical problems, because transgene injection occasionally delays embryonic development by 1–2 days. The analysis of the first two overlapping constructs, −8.5 kb/−5.1 kb and −10 kb/−6.7 kb, showed that only the −8.5-kb/−5-kb region drove strong reporter activation in the pituitary gland of the transgenic embryos (Fig. 1A). Enhancing cis-elements appeared to be located in the −6.7-kb/−5.1-kb regions, suggesting that these 1.6 kb were sufficient for reporter activation. The 1.6-kb region was subcloned in two nonoverlapping fragments of 900 bp and 700 bp that were used for transgenic analysis. Only the transgenic embryos that had integrated the 900-bp-containing (−6.7 kb/−5.7 kb) transgene displayed reporter expression (Fig. 1A), demonstrating that enhancing activity was located between −6.7 kb and −5.7 kb.
Fig. 1.
In vivo enhancer mapping identifies a 200-bp region sufficient for initial Pit1 gene activation. (A) Scheme of successive deletions used to map a 900-bp enhancer region, located at −6.6/−5.7 kb sufficient for hGH expression. (B) Systematic deletions done in the 900-bp region: Deletion 8 abolished reporter expression, and deletions 7 and 9 mildly affect reporter expression. (C) The 91-bp core region alone was not sufficient to activate the Pit1 promoter, whereas the addition of 50 bp of 5′ and 3′ information was sufficient to drive high (hGH) expression. (D) The 14.8 kb of the Pit1 upstream sequences, carrying deletions of both DE and 191-bp ΕΕα, abolished hGH expression. The expression level of each transgene was determined by in situ hybridization by using a hGH probe at E14.5.
Further mapping was performed by generating systematic 100-bp deletions in the context of the 900-bp fragment. Only deletion 8 failed to direct reporter expression in the pituitary gland of transgenic embryos (Fig. 1B). Interestingly, the transgenes carrying the flanking deletions 7 and 9 displayed a decrease of activation, suggesting that the sequences surrounding region 8 could be required for a high level of activation. Because deletion 9 overlapped with deletion 8 by 9 bp, we deduced that the 9 bp were not required. These results permitted us to define a 91-bp core region that would be required for Pit1 early activation. Consistent with the 100-bp deletion analysis, the 91-bp element alone was not sufficient to drive detectable reporter expression in the pituitary gland (Fig. 1C). The addition of 50 bp on each side of the 91-bp core, however, was sufficient for obtaining strong reporter activation. The defined 191-bp sequence contains sufficient information to permit its early activation during pituitary development and as such will be referred to as the EEα region (Fig. 1C).
To independently assess the role of EEα in early Pit1 activation, we constructed a transgene containing the original 14.8 kb with deletions of both the DE and EEα. No expression of the reporter could be detected in six out of seven transgenic embryos, and only a modest expression was observed in the final founder mouse (Fig. 1D). The fact that only a single embryo displayed a modest reporter activation suggested that, in specific insertional contexts, other regulatory regions might exert additional effects. Although EEα is required for efficient early Pit1 activation, other regulatory elements would be predicted to contribute to the initial activation of Pit1 promoter. This is in agreement with our findings that another well conserved region, EEβ, located at −8.3 kb, is also initially involved in Pit1 early activation (11). Prop1, an early pituitary-specific transcription factor binds to the EEβ region, and its interaction with the β-catenin coactivator is required to initiate Pit1 gene transcription. Genetic analysis demonstrated that Prop1 and β-catenin were required for Pit1 gene activation and the subsequent differentiation of Pit1-dependent cell lineages (11). Interestingly, a −10.2-kb/−6.7-kb transgene, which encompasses EEβ alone, failed to display significant reporter activity when analyzed in three integration events, which correlates the data obtained with the 14.8-kb/ΔDΕ/ΔEEα transgene. These data argue that, although it is required, EEβ alone is not sufficient for robust initial activation of the Pit1 gene, and both EEβ and EEα are combinatorially required to achieve effective, full early activation of the Pit1 transcription unit.
Multiple Sites Within EEα Contribute to Its Function.
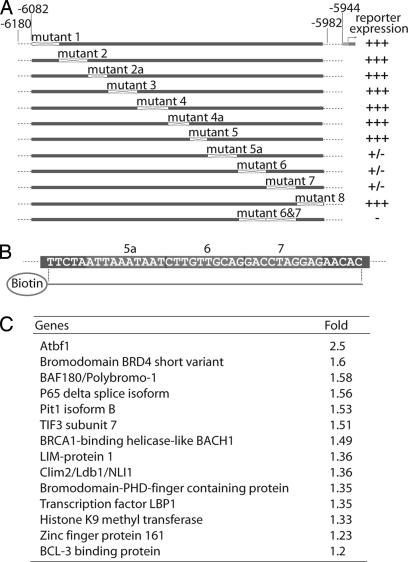
Sequence alignment of 5′-flanking genomic regions of the Pit1 gene between multiple species showed that EEα was located in a highly conserved region [supporting information (SI) Fig. 6]. To define required binding sites, we inserted segmented mutations across the entire length of the 91-bp core region in the context of the 191-bp minimal promoter hGH reporter, replacing 5 to 10 bp at a time with a nonrelevant sequence devoid of any biological activity (8) (Fig. 2A). Transgene analysis showed that only the mutations of sites 5a, 6, and 7 caused dramatic decrease of reporter activity (Fig. 2A). Interestingly, none of the mutations completely abolished promoter activation, and the three sites appeared to be critical in facilitating a high level of pituitary-gland-specific reporter expression. These data were confirmed by the fact that a double mutation of sites 6 and 7 was sufficient to completely abolish reporter expression (Fig. 2A). That the three sites were adjacent suggested that they could act cooperatively in instructing EEα function.
Fig. 2.
Characterization of functional sites within EEα and purification of EEα binding factors by ICAT analysis. (A) Mutations replacing 5- to 10-bp segments of the 91-bp core region in the context of 191-bp EEα: Mutations of sites 5a, 6, or 7 severely decreased reporter expression. Double mutation of sites 6 and 7 completely abolished hGH expression. (B) DNA sequence used for DNA affinity protein purification encompasses sites 5a, 6, and 7. (C) List of the transcription-related factors isolated by quantitative proteomics approach with the 5a/6/7 regions.
Identification of ATBF1 as the Main EEα Binding Factor by Quantitative Proteomics.
To identify factors that bind EEα and potentially contribute to regulating early Pit1 activation, we developed a biochemical approach using GHFT1 cells. These cells are derived from pituitary tumors that were induced by a transgene expressing the SV40 viral transforming protein large T under control of the 14.8-kb Pit1 regulatory region (12). They express low levels of Pit1 but none of the hormone genes (e.g., GH and PRL), suggesting that GHFT1 cells may reflect an early stage in Pit1 lineage.
We tested by transient transfection whether EEα would respond in GHFT1 cells as it did in vivo. Wt EEα could drive a consistent 3- to 5-fold activation of a Luciferase reporter when compared with Pit1 minimal promoter alone, whereas mutations within EEα of sites 5a, 6, and 7 markedly reduced reporter activity (SI Fig. 7). Mutations in other sites did not decrease reporter activity (SI Fig. 7). Interestingly, mutation of site 4 increased the activation by 2-fold, which would not be detected in the transgene studies because in situ hybridization is only semiquantitative (SI Fig. 7). Only mutation of site 5 behaved differently than it did in vivo by showing a decreased reporter activity (data not shown). Despite these few quantitative distinctions, EEα behaved similarly enough in GHFT1 cells in comparison with the in vivo data to permit us to use these cells for the biochemical purification of factors binding to EEα that might be potentially involved in early Pit1 gene activation.
To purify the EEα-binding proteins, we performed a single-step procedure of DNA affinity purification of interacting protein complexes from GHFT1 nuclear extracts followed by a quantitative proteomic approach. Protein complexes were first bound to biotinylated DNA probes containing specific or nonspecific sequence. The specific probe was a trimer of the sequence encompassing the 5a, 6, and 7 sites plus 5 bp on each end (Fig. 2B). The nonspecific probe was of the same length and contained tandem arrays of the nonrelevant sequence used to mutate sites in EEα. The protein–DNA complexes were isolated on streptavidin beads, and the bound factors were eluted and labeled with cleavable cysteine-reactive ICAT (isotope-coded affinity tag) reagents. Mass spectrometry allowed us to compare the relative abundance of tryptic peptides derived from the two protein samples, permitting the identification of specific factors against a high background of copurifying molecules (13). The ICAT analysis identified a large number of peptides with a significant ratio between specific and nonspecific probes. A high number of peptides purified appeared to be transcription-related as part of basic transcriptional machinery, chromatin remodeling, or transcription factors (Fig. 2C). A smaller number were involved in signal transduction and cell structure, and a number of unknown molecules were present (data not shown). By RT-PCR, we showed that several of the molecules characterized in this biochemical screen displayed pituitary expression, confirming the potential relevance of proteins identified by this approach (data not shown).
Of the numerous candidate proteins identified, only three were present in >2-fold higher abundance in the specific-versus-nonspecific sample, similar to the data obtained in another similar screen (14). Atbf1 exhibited a 2.5-fold increase (Fig. 2C) and was selected for further investigation, because it is a transcription factor that might exert regulatory roles in development.
Atbf1 Is Expressed in the Developing Pituitary Gland.
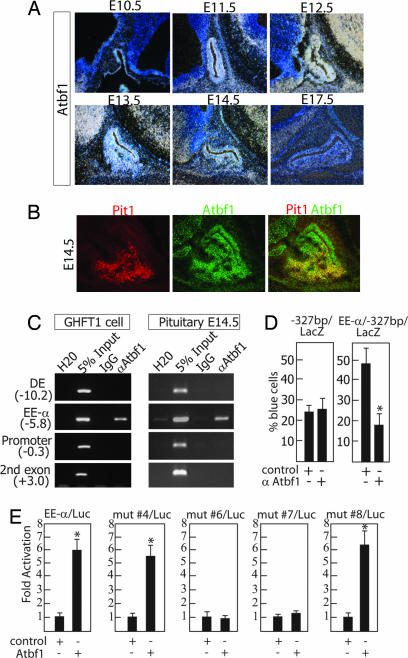
Atbf1 was originally identified as a factor that binds the AT-rich element in the human α-fetoprotein gene (AFP) enhancer (15). The Atbf1 gene encodes two proteins of 306 and 404 kDa, respectively. The peptide characterized in the proteomic analysis corresponded to the 404-kDa isoform that contains four homeodomains and 23 zinc fingers (16). Atbf1 mRNA is highly expressed in the developing brain (17). In the mouse, its expression peaks around E13–E15 and progressively decreases to an undetectable level by postnatal day 28 (18). In the developing pituitary gland, Atbf1 transcripts appeared around E11.5, peaked by E12.5–E13.5, and diminished after E14.5 (Fig. 3A), which coincided with the timing of Pit1 initial activation. Immunostaining experiments confirmed that Atbf1 protein was specifically expressed in pituitary cells, although at a lower level than in the brain (Fig. 3B). Costaining of Pit1 and Atbf1 at E13.5 revealed that virtually all Pit1-expressing cells were Atbf1-positive. However, many Atbf1-positive cells in dorsal-anterior and intermediate pituitary were Pit1-negative, suggesting that Atbf1 was also present in non-Pit1 lineages.
Fig. 3.
Atbf1 is expressed in the developing pituitary gland and binds EEα in vivo. (A) Atbf1 expression peaks at E12–E13 as shown by in situ hybridization. (B) Double immunostaining indicates that Pit1 (red) colocalizes with Atbf1 (green). Note the high level of Atbf1 expression in the adjacent brain tissue. (C) ChIP assays showed that Atbf1 specifically binds to EEα in both GHFT1 cells and E14.5 microdissected pituitaries. (D) Microinjection experiments in GHFT1 cells show that the EEα//Pit1 minimal promoter drives the activation of a LacZ reporter. Coinjection with anti-Atbf1 suppresses this activation. In contrast, coinjection of anti-Atbf1 did not affect the basal level of activation driven by only the Pit1 minimal promoter. (E) Cotransfection of Luciferase reporters containing EEα (wt or mutants) in front of the Pit1 minimal promoter with an ATBF1 expression vector in 293 cells showed that Atbf1 can activate EEα reporters, except for those harboring mutations 5a, 6, and 7. Error bars indicate the SEM. *, P < 0.001, the statistically significant difference between the control and anti-Atbf1 or Atbf1 expression plasmid (Student's t test).
Atbf1 Binds and Activates EEα.
To confirm the in vivo binding of Atbf1 to EEα, we carried out chromatin immunoprecipitation (ChIP) analysis first in GHFT1 cells. ChIP assays showed that Atbf1 was present specifically on EEα, but not on Pit1 DE or minimal promoter, demonstrating that ATBF1 binds EEα in vivo (Fig. 3C). We then examined Atbf1 presence on Pit1 EEα during pituitary development by performing ChIP analysis on microdissected embryonic E14.5 pituitary glands. The ChIP assays revealed that Atbf1 was present exclusively on EEα, as observed in GHFT1 cells (Fig. 3C). These results confirmed that Atbf1, despite being purified by an in vitro biochemical approach, was actually present in vivo on the Pit1 EEα during pituitary development. A direct functional interaction between the Pit1 EEα and Atbf1 was demonstrated by single-cell nuclear microinjection assays in GHFT1 cells. An EEα–LacZ reporter construct was consistently activated when microinjected into GHFT1 cells as compared with Pit1 minimal promoter construct (Fig. 3D). This activation was dramatically reduced by coinjection of anti-Atbf1 IgG. As a control for functional specificity, anti-Atbf1 IgG did not affect the basal activation of Pit1 minimal promoter (Fig. 3D). Therefore, Atbf1 appeared to be a main component of Pit1 EEα activation. Moreover Atbf1 could directly activate an EEα–Luciferase reporter construct by 6-fold as shown in cotransfection experiments in 293 cells, a heterologous cell line (Fig. 3E). In parallel transfection experiments, Atbf1 failed to activate EEα reporter constructs carrying the mutations 5a, 6, or 7, whereas mutations on other sites of EEα did not appear to significantly alter the activation (Fig. 3E and data not shown). We concluded that Atbf1 was directly involved in EEα activation and that all three sites, 5a, 6, and 7, were required for Atbf1 to be fully functional in regulating EEα. Because Atbf1 was originally identified as a factor that binds to the AT motif of human AFP (15), we assumed that Atbf1 would bind the AT-rich site 5a. However, given the complexity the protein, with 4 homeodomains and 23 zinc fingers, Atbf1 might well interact with multiple factors on the EEα site, which would explain the requirement for such large binding site. Moreover, Atbf1 is expressed in a significant number of tissues during development (19–21), it is likely that it synergizes with other transcription factors to regulate a variety of developmental processes. Atbf1 also displays DNA/RNA-dependent ATPase activity (15). We are tempted to speculate that the simultaneous binding of these three sites by Atbf1 might lead to its enzymatic activation, perhaps with a switch of protein complexes associated with EEα, leading to Pit1 activation.
Generation of Atbf1 Mutant Mice by Using Gene-Trap ES Cells.
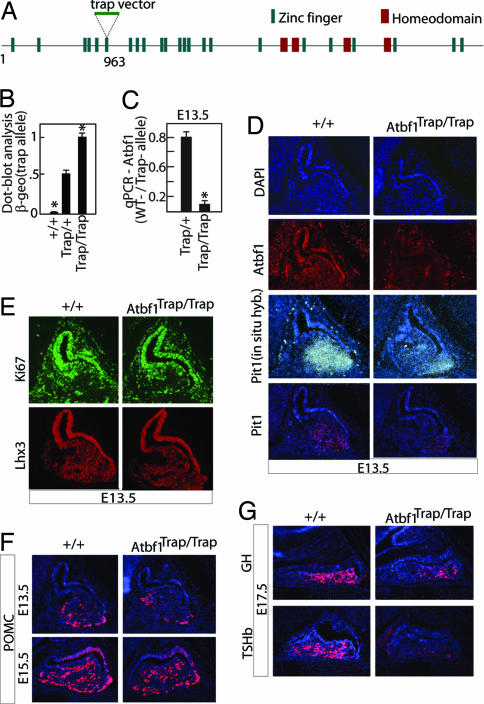
To assess the genetic interaction between Atbf1 and Pit1, we generated an Atbf1 mutant mouse line by using the Atbf1 gene-trap ES cells (RRJ548, BayGenomics). Analysis of the 5′ RACE sequence (BayGenomics) showed that the insertion occurred in 60 kb of intronic region between the 3rd and 4th exons of the Atbf1 gene (Fig. 4A). This insertion should result in the expression of a protein truncated at amino acid 963 that would contain only 5 zinc fingers instead of 23 and no homeodomains and that might be expected to be functionally impaired. To genotype the Atbf1 trap allele, dot blots of genomic DNA isolated from embryos were hybridized with β-geo (present in the trap vector) and wt Atbf1 probes. The signal intensity of β-geo was then measured and normalized by that of Atbf1 (Fig. 4B). Atbf1Trap/Trap embryos bearing two β-geo copies were easily differentiated from Atbf1Trap/+ embryos bearing only one β-geo copy. The levels of mutant and wt transcripts in trap mutants were determined by quantitative PCR (q-PCR). Surprisingly, ≈10% of total Atbf1 transcripts were wt in Atbf1Trap/Trap embryos at E13.5, 13% at E15.5, and 20% at E17.5 (Fig. 4C and data not shown). These ratios of wt/mutant transcripts were consistently observed among many litters of embryos at the same embryonic stages. Also, the truncated Atbf1 protein did not appear to function as a dominant-negative as heterozygotes exhibited no abnormalities when compared with wt. Furthermore, cotransfections of various fragments of Atbf1 with full-length Atbf1 and EEα reporter in 293 cells showed that none of the fragments including region of 1–963 could affect Atbf1 activation of EEα (data not shown). We thus believe that the Atbf1 gene-trap mutation generated in this study generated a hypomorphic allele of Atbf1 where posttranscriptional splicing occasionally skipped the gene trap exon, thus producing a small amount of the wt Atbf1 transcript.
Fig. 4.
Atbf1 is required for the Pit1 gene early activation. (A) Genomic structure of Atbf1 mouse gene and location of the gene-trap vector insertion at amino acid 963. It should generate a protein fusion of truncated Atbf1 (1–962) and β-geo. (B) Quantification of the dot-blot analysis of the genomic DNA isolated from gene-trap embryos, hybridized with β-geo probe. (C) q-PCR analysis of relative amounts of wt and trapped Atbf1 mRNA on total RNA extracted from the tissue of gene-trap mouse embryos showed residual presence of wt Atbf1 mRNA. (D) Immunostaining of Atbf1 and Pit1 on adjacent sections of E13.5 pituitary showed that both factors were greatly decreased in Trap mutant, whereas DAPI staining indicated that the overall morphology of mutant pituitary was intact. Pit1 RNA levels were also decreased as shown by in situ hybridization. (E) Immunostaining of cell proliferation marker Ki67 and Lhx3 showed no difference between wt and mutant pituitary glands, suggesting that early pituitary development is intact. (F) Immunostaining of POMC showed similar expression level between wt and mutant. (G) Immunostaining of GH and TSH-β showed a dramatic decrease of expression in gene-trap mutants. Error bars indicate the SEM. *, P < 0.001, the statistically significant difference between the genotype Trap/+ and Trap/Trap or +/+ (Student's t test).
Differentiation Defects in Pit1 Lineage in Atbf1 Gene-Trap Mutants Demonstrate That Atbf1 Is Epistatic to Pit1.
Knowing that low levels of wt Atbf1 transcripts were still present in Atbf1 mutant mice, we assessed Atbf1 protein expression in the pituitary gland by immunostaining with an antibody raised against the C terminus of Atbf1 protein. At E13.5, Atbf1 was barely detectable in mutant pituitary glands (Fig. 4D). A few Atbf1-positive cells could still be detected in the brain of mutant embryos, but they had a much weaker signal compared with wt embryos, in accordance to the q-PCR data. These data confirmed a residual Atbf1 expression in the mutant pituitary gland.
Despite a significant decrease of Atbf1 transcripts, the pituitary gland of the mutant animals displayed no major morphological defects during embryonic development (data not shown). Labeling with the proliferation marker Ki67 also showed a similar number of proliferating cells in Atbf1 mutants and wt embryos at E13.5 (Fig. 4E). The analysis of factors involved in the early stages of pituitary development such as Lhx3, Prop1, and Tbx19 (23) showed no alterations in expression in Atbf1 mutant mice (Fig. 4E and data not shown). Altogether it suggested that in Atbf1 mutant mice, initial pituitary organ commitment, cell proliferation, and early cells lineages specification were not altered. However, at E13.5, Pit1 mRNA levels were markedly decreased in the mutant pituitary gland (Fig. 4D), and only a few Pit1-expressing cells could be detected in the most ventral pituitary (Fig. 4D), demonstrating that Pit1 expression was affected at the transcriptional level. We next examined the differentiation of Pit1-dependent cell types. At E17.5, GH, the somatotrope marker was modestly decreased, whereas TSH-β, the thyrotrope marker, was almost absent in Atbf1 mutant mice (Fig. 4G). In contrast, the expression of POMC, marker of corticotropes and melanotropes, non-Pit1-dependent cell types, was not modified in Atbf1 mutant mice at E13.5 or E15.5 (Fig. 4F). Together, these data demonstrate that Atbf1 is directly required for early Pit1 early transcriptional activation.
Atbf1 has been reported to exert roles in control of cell proliferation (24). Although Atbf1 gene-trap mutants failed to display overt defect in cell proliferation in early pituitary development, it remains possible that a trace amount of wt Atbf1 protein may adequately function to regulate subsets of its targets, whereas Pit1 ΕΕα would be particularly sensitive to Atbf1 levels.
Thus, to date, three major regulatory elements—EEα, EEβ, and an autoregulatory enhancer (DE)—have now been identified in the Pit1 5′-flanking region. The high interspecies sequence conservation in the −10-kb/−5-kb region strongly suggests that additional regulatory sites may exist. However, of these three regions, EEα alone is able to provide efficient early activation of Pit1 transcription in vivo with only low levels of independent activation by other putative regulatory elements. Early Pit1 gene activation, therefore, appears to be subject to combinatorial regulation involving no less than three different genomic regions, including EEα, EEβ, and DE. These different enhancers each appear to recruit specific combinations of DNA-binding transcription factors and cofactors that drive the dynamic, highly regulated expression of Pit1 (Fig. 5), and they link the “giant zinc finger family” to developmental regulatory events.
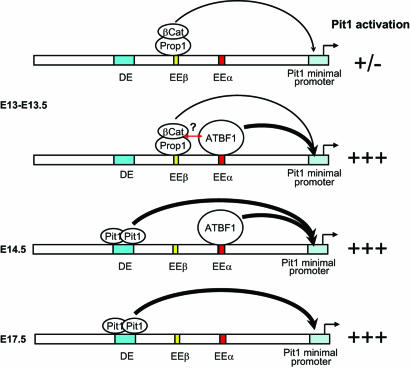
Fig. 5.
A model of early activation of the Pit1 gene. By E13–E13.5, Prop1/β-catenin complexes are recruited to EEβ, where, although they are absolutely required, they activate transcription at a barely detectable level; at the same stage, Atbf1 is recruited to EEα, where it could potentially synergize with Prop1 (red arrow), and the Pit1 gene is then highly activated. Once Pit1 is expressed at sustained levels, Prop1/β-catenin and ATBF1 are no longer required on their respective enhancers; in addition, by E17.5, Atbf1 and Prop1 are down-regulated in the pituitary gland, and Pit1 expression depends essentially on the DE.
Materials and Methods
Generation and Analysis of Transgenic Animals.
All Pit1 reporter transgenes contained Pit1 minimal promoter and hGH as reporter gene (10). A hGH probe was used to genotype the embryos by Southern blotting. Cloning information is provided in SI Materials and Methods.
DNA Affinity Purification.
DNA affinity purification is described in SI Materials and Methods.
Quantitative Proteomics Analysis by ICAT.
Proteins were labeled with cleavable ICAT reagents and analyzed as described (13).
In Situ Hybridization and Immunolabeling.
In situ hybridization and immunolabeling were done as described (10, 11). Probes are described in SI Materials and Methods. ATBF1 antibodies were a generous gift from Makoto Kawaguchi (Niigata Rosai Hospital, Johetsu, Japan).
ChIP.
ChIP assays were performed as described (25) except for the fixation of the tissue in 2% formaldehyde for 30 min. PCR primers are described in SI Materials and Methods.
Transfections and Nuclear Microinjection.
Transfection experiments were performed by using Superfect (Invitrogen) in 293 and GHFT1 cells. ATBF1 expression vector was a gift from Yutaka Miura (Nagoya Citu University Graduate School of Medical Sciences, Nagoya, Japan).
Microinjection assays were carried out as described in ref. 26 and SI Materials and Methods.
Isolation of RNA and q-PCR.
Details are described in SI Materials and Methods.
Supplementary Material
ACKNOWLEDGMENTS.
We thank M. Kawaguchi for the Atbf1A antibodies and Y. Miura for the Atbf1a cDNA. We thank C. Nelson, F. Hooshman, and H. Taylor for their technical assistance. We thank J. Hightower and M. Fisher for art and manuscript preparation. Y.Q. was supported by the U.S. Army Medical Research & Materiel Command (Grant PC40247-W81XWH-05-1-0100). C.C. is supported by the National Institutes of Health (NIH)/National Cancer Institute (Grant CA 127095) and by a Junior Faculty Development Award from Dartmouth Medical School. M.G.R. is an investigator with the Howard Hughes Medical Institute. This work was supported by National Heart, Lung and Blood Institute Grant NO1-HV-28179 (to J.A.R. and R.A.) and by NIH Grants DK018477, DK39949, and NS34934 (to M.G.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712196105/DC1.
References
- 1.Carriere C, Gleiberman A, Lin CR, Rosenfeld MG. From panhypopituitarism to combined pituitary deficiencies: Do we need the anterior pituitary? Rev Endocr Metab Disord. 2004;5:5–13. doi: 10.1023/B:REMD.0000016120.84792.54. [DOI] [PubMed] [Google Scholar]
- 2.Sheng HZ, et al. Multistep control of pituitary organogenesis. Science. 1997;278:1809–1812. doi: 10.1126/science.278.5344.1809. [DOI] [PubMed] [Google Scholar]
- 3.Scully KM, Rosenfeld MG. Pituitary development: Regulatory codes in mammalian organogenesis. Science. 2002;295:2231–2235. doi: 10.1126/science.1062736. [DOI] [PubMed] [Google Scholar]
- 4.Herzog W, et al. Adenohypophysis formation in the zebrafish and its dependence on sonic hedgehog. Dev Biol. 2003;254:36–49. doi: 10.1016/s0012-1606(02)00124-0. [DOI] [PubMed] [Google Scholar]
- 5.Liu NA, et al. Pituitary corticotroph ontogeny and regulation in transgenic zebrafish. Mol Endocrinol. 2003;17:959–966. doi: 10.1210/me.2002-0392. [DOI] [PubMed] [Google Scholar]
- 6.Cushman LJ, Camper SA. Molecular basis of pituitary dysfunction in mouse and human. Mamm Genome. 2001;7:485–494. doi: 10.1007/s003350040002. [DOI] [PubMed] [Google Scholar]
- 7.Li S, et al. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990;347:528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- 8.Scully KM, et al. Allosteric effects of Pit-1 DNA sites on long-term repression in cell types specification. Science. 2000;290:1127–1131. doi: 10.1126/science.290.5494.1127. [DOI] [PubMed] [Google Scholar]
- 9.Rhodes SJ, et al. A tissue-specific enhancer confers Pit-1-dependent morphogen inducibility and autoregulation on the Pit-1 gene. Genes Dev. 1993;7:913–932. doi: 10.1101/gad.7.6.913. [DOI] [PubMed] [Google Scholar]
- 10.DiMattia GE, et al. The Pit-1 gene is regulated by distinct early and late pituitary-specific enhancers. Dev Biol. 1997;182:180–190. doi: 10.1006/dbio.1996.8472. [DOI] [PubMed] [Google Scholar]
- 11.Olson LE, et al. Homeodomain-mediated β-catenin-dependent switching events dictate cell-lineage determination. Cell. 2006;125:593–605. doi: 10.1016/j.cell.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 12.Lew D, et al. GHF-1-promoter-targeted immortalization of a somatotropic progenitor cell results in dwarfism in transgenic mice. Genes Dev. 1993;7:683–693. doi: 10.1101/gad.7.4.683. [DOI] [PubMed] [Google Scholar]
- 13.Ranish JA, et al. The study of macromolecular complexes by quantitative proteomics. Nat Genet. 2003;33:349–355. doi: 10.1038/ng1101. [DOI] [PubMed] [Google Scholar]
- 14.Himeda CL, et al. Quantitative proteomic identification of six4 as the trex-binding factor in the muscle creatine kinase enhancer. Mol Cell Biol. 2004;24:2132–2143. doi: 10.1128/MCB.24.5.2132-2143.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morinaga T, Yasuda H, Hashimoto T, Higashio K, Tamaoki T. A human α-fetoprotein enhancer-binding protein, ATBF1, contains four homeodomains and seventeen zinc fingers. Mol Cell Biol. 1991;11:6041–6049. doi: 10.1128/mcb.11.12.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miura Y, et al. Cloning and characterization of an ATBF1 isoform that expresses in a neuronal differentiation-dependent manner. J Biol Chem. 1995;270:26840–26848. doi: 10.1074/jbc.270.45.26840. [DOI] [PubMed] [Google Scholar]
- 17.Ishii Y, et al. ATBF1-A protein, but not ATBF1-B, is preferentially expressed in developing rat brain. J Comp Neurol. 2003;465:57–71. doi: 10.1002/cne.10807. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe M, et al. Developmental changes in expression of the ATBF1 transcription factor gene. Brain Res Mol Brain Res. 1996;42:344–349. doi: 10.1016/s0169-328x(96)00204-5. [DOI] [PubMed] [Google Scholar]
- 19.Iida M, et al. Alteration of the AT motif binding factor-1 expression in α-fetoprotein producing gastric cancer: Is it an event for differentiation and proliferation of the tumors? Oncol Rep. 2004;11:3–7. [PubMed] [Google Scholar]
- 20.Sun X, et al. Frequent somatic mutations of the transcription factor ATBF1 in human prostate cancer. Nat Genet. 2005;37:407–412. doi: 10.1038/ng1528. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, et al. ATBF1-a messenger RNA expression is correlated with better prognosis in breast cancer. Clin Cancer Res. 2005;11:193–198. [PubMed] [Google Scholar]
- 22.Kawaguchi M, et al. DNA/RNA-dependent ATPase activity is associated with ATBF1, a multiple homeodomain-zinc finger protein. Biochim Biophys Acta. 2001;1550:164–174. doi: 10.1016/s0167-4838(01)00284-9. [DOI] [PubMed] [Google Scholar]
- 23.Kelberman D, Dattani MT. The role of transcription factors implicated in anterior pituitary development in the aetiology of congenital hypopituitarism. Ann Med. 2006;38:560–577. doi: 10.1080/07853890600994963. [DOI] [PubMed] [Google Scholar]
- 24.Jung CG, et al. Homeotic factor ATBF1 induces the cell cycle arrest associated with neuronal differentiation. Development. 2005;132:5137–5145. doi: 10.1242/dev.02098. [DOI] [PubMed] [Google Scholar]
- 25.Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell. 2000;103:843–852. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 26.Jepsen K, et al. Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell. 2000;102:753–763. doi: 10.1016/s0092-8674(00)00064-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.