Abstract
In order to search for novel components of lipid membrane microdomains involved in neural signalling pathways, mAbs (monoclonal antibodies) were raised against the detergent-insoluble membrane fraction of PC12 (pheochromocytoma) cells. Among the 22 hybrid clones, mAb PR#1 specifically detected a fucoganglioside Fuc(Gal)-GM1 [α-fucosyl(α-galactosyl)-GM1], a ganglioside homologous with GM1a (II3NeuAc,GgOse4Cer), as a novel member of microdomain components with biological functions. In the presence of mAb PR#1 in the culture medium, the outgrowth of neurites was induced in PC12 cells in a dose-dependent manner, with no effects on cell proliferation, suggesting that Fuc(Gal)-GM1 is preferentially involved in PC12 cell neuritogenesis. Effects through Fuc(Gal)-GM1 were different from those through GM1a during differentiation, e.g. under PR#1 treatment on Fuc(Gal)-GM1, round cell bodies with thinner cell processes were induced, whereas treatment with CTB (cholera toxin B subunit), a specific probe for GM1a, produced flattened cell bodies with thicker pro-cesses. Molecular analysis demonstrated that the PR#1–Fuc(Gal)-GM1 pathway was associated with Fyn and Yes of the Src family of kinases, although Src itself was not involved. No association was found with TrkA (tropomyosin receptor kinase A) and ERKs (extracellular-signal-regulated kinases), which are responsible for GM1a-induced differentiation. From these findings, it is suggested that a fucoganglioside Fuc(Gal)-GM1 provides a functional platform distinct from that of GM1a for signal transduction in PC12 cell differentiation.
Keywords: fucoganglioside, ganglioside, GM1a, lipid raft, neuritogenesis, Src family kinase
Abbreviations: CTB, cholera toxin B subunit; DIM, detergent-insoluble membrane; DMEM, Dulbecco's modified Eagle's medium; ERK, extracellular-signal-regulated kinase; FAB, fast atom bombardment; FBS, fetal bovine serum; Fuc(Gal)-GM1, IV2Fucα,IV3Galα,II3NeuAc,GgOse4Cer; Fuc-GM1, IV2Fucα,II3NeuAc,GgOse4Cer; GM1a, II3NeuAc,GgOse4Cer; GSL, glycosphingolipid; HRP, horseradish peroxidase; HS, horse serum; mAb, monoclonal antibody; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide; NF-M, neurofilament M; NGF, nerve growth factor; PBA, phenylboronate agarose; PC12, pheochromocytoma; PVP, polyvinylpyrrolidone; SFK, Src family kinase; TIP, transferrin/insulin/progesterone; TrkA, tropomyosin receptor kinase A
INTRODUCTION
Gangliosides, sialic acid-containing GSLs (glycosphingolipids), are ubiquitously expressed in eukaryotic cells and are mainly found in the outer leaflet of the plasma membrane [1]. Previous studies have proposed that they form cholesterol-containing lipid clusters, often called lipid rafts or lipid microdomains [2–4,6]. Since a variety of signalling molecules, such as the SFKs (Src family kinases) or G-proteins, are accumulated on these membrane domains, they are thought to serve as scaffolds to facilitate the association of signalling molecules, to increase the rate of their interactions and to encourage cross-talk among molecular networks [2–7].
Several ganglioside-enriched microdomains have been characterized. GM3-enriched microdomains have been separated from B16 cells [8,9]. GM1a (II3NeuAc,GgOse4Cer)-enriched membrane domains have been known to be spatially and functionally different from those enriched with sphingomyelin [10] or those enriched with phosphatidylglucoside [11]. In the nervous system, Kasahara et al. [12,13] reported that GD3 was involved in TAG-1-mediated signalling, which led to neurite outgrowth in rat cerebellar granule cells. These studies suggest that functional differentiation of the microdomains is dependent on their enriched constituents.
There exist many species of gangliosides in neurons and their progenitors [14,15]. Since their distribution and composition are altered remarkably during brain development, they are thought to play a crucial role in neuronal differentiation and/or proliferation [16]. However, the reason that various species of ganglioside are needed during this event is not readily apparent. Previous studies on lipid rafts have suggested that each ganglioside forms functionally distinct microdomains, being spatially, as well as temporally, organized throughout the cell differentiation process [8–13].
In the present study, we have identified a novel and functional constituent of gangliosides in lipid microdomains, using our mAb (monoclonal antibody) PR#1, and have proposed possible molecular mechanisms under these biological events.
MATERIALS AND METHODS
Materials
The products purchased consisted of antibodies for SFKs and phospho-SFKs (pTyr416) (Cell Signaling Technology), v-Src (Calbiochem), Fyn (Santa Cruz Biotechnology), Yes (BD Biosciences), neurofilament 160, phospho-TrkA (tropomyosin receptor kinase A) (pTyr490) and ERK1/2 (extracellular-signal-regulated kinase 1/2) (Sigma), TrkA and phospho-ERK1/2 (Upstate) and HRP (horseradish peroxidase) (Jackson ImmunoResearch Laboratories). Other products were HRP-conjugated CTB (cholera toxin B subunit) (Sigma), HRP-conjugated anti-mouse IgG (Vector Laboratories), Alexa Fluor® 488-conjugated CTB, Alexa Fluor® 488-conjugated anti-(mouse IgG) and Alexa Fluor® 594-conjugated anti-(rabbit IgG) (Molecular Probes), mouse 2.5S NGF (nerve growth factor) (Invitrogen), and protein kinase inhibitors for genistein [5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; 4′,5,6-trihydroxy-isoflavone], PP2 {4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]-pyrimidine} and TrkA specific inhibitor [17] (Calbiochem).
Cell culture
Mouse myeloma cells and hybridomas were grown in DMEM (Dulbecco's modified Eagle's medium) containing 10% (v/v) FBS (fetal bovine serum). PC12 (pheochromocytoma) cells were cultured in DMEM supplemented with 7% FBS and 7% HS (horse serum) or in TIP (transferrin/insulin/progesterone) medium (DMEM supplemented with 5 μg/ml transferrin, 5 μg/ml insulin and 0.2 μM progesterone) at 37 °C in a 10% CO2 humidified atmosphere.
Preparation of DIM (detergent-insoluble membrane) fraction
The DIM fraction was prepared as described previously [18,19], but with slight modifications. PC12 cells (4×107 cells) were lysed with 1 ml of lysis buffer consisting of 1% (v/v) Triton X-100 and TN buffer (25 mM Tris/HCl, pH 7.5, 150 mM NaCl, 0.1 mM PMSF, 1 mM NHSO3, 1 mM benzamidine, 2 mM CaCl2, 5 mM MgCl2, 0.15 mM spermine and 0.3 mM spermidine). Lysates were transferred into centrifuge tubes (14 mm×95 mm; Beckman Ultra Clear), kept on ice for 30 min, and then mixed with 1 ml of 80% sucrose in TN buffer. Sucrose solutions (5.5 ml of 30% sucrose and 3.5 ml of 5% sucrose) layered on to the lysates in 40% sucrose were centrifuged in a SW41Ti swinging rotor centrifuge (Beckman) at 4 °C and 39000 rev./min for 16 h. Light-scattering bands located at the 5% and 30% sucrose interface were collected as the DIM fraction.
Production of mAbs
A Balb/c mouse was immunized several times by intraperitoneal administration of the DIM fraction using the RIBI adjuvant system for 1.5 months and subsequently boosted with the antigen a month later. Collected spleen cells were fused with X63-Ag8.653 myeloma cells and maintained in a hypoxanthine/aminopterine/thymidine growth medium containing 10% hybridoma cloning factor (Igen) at 37 °C in a 10% CO2 chamber. The culture supernatants of the hybrid cells were screened with the DIM fraction by ELISA. The 22 hybrid clones positive for DIM were obtained and subcloned further by the limiting dilution method.
Purification of PC12 gangliosides
The total lipid extracted from the freeze-dried PC12 cells (0.85 g) or rat brain tissue (0.1 g) with chloroform/methanol (2:1, v/v) and with chloroform/methanol/water (5:8:3, by vol.) was loaded on to a PBA (phenylboronate agarose) 60 column (12 mm×60 mm) (Millipore). The column was washed with 5 vol. of chloroform/methanol (2:1, v/v) and was eluted with chloroform/methanol/water (5:4:1, by vol.). PBA eluate was loaded on to an anion-exchange column (Q-Sepharose, 6.3 mm×250 mm), washed with chloroform/methanol/water (30:60:8, by vol.), and then eluted with a gradient from chloroform/methanol/water (30:60:8, by vol.) to chloroform/methanol/2 M aqueous sodium acetate (30:60:8, by vol.). Three GSL fractions were pooled as the neutral fraction, low-acidic fraction, termed G1, and high-acidic fraction, termed G2. The G1 fraction, which contains the major immunoreactive lipid, was dialysed against water, then applied to a Senshu Pak Aquasil HPLC column (4.6 mm×250 mm) (Senshu-kagaku) and eluted with a gradient from chloroform/methanol (7:3, v/v) to chloroform/methanol/water (5:4:0.5, by vol.). Three purified gangliosides were pooled as G1-1, G1-2 and G1-3 fractions. Immunoreactive lipid was present in the G1-3 fraction.
TLC immunostaining assay
TLC immunostaining was performed according to the method of Higashi et al. [20] with slight modifications. Standard lipids were applied to a plastic TLC plate, Polygram Sil G (Macherey-Nagel), and developed with solvent, as described in the instructions. The chromatogram was soaked for 1 h at room temperature (∼24 °C) in PBS, containing 1% egg albumin and 1% PVP (polyvinylpyrrolidone) and then incubated with primary antibodies in PBS containing 3% PVP at 4 °C overnight. After washing with PBS, the chromatograms were re-incubated with HRP-conjugated anti-(mouse IgG) in PBS containing 3% PVP at room temperature for 1 h. The chromatograms were then visualized with a 4-chloro-1-naphthol/hydrogen peroxide solution.
Sugar composition analysis
Methylglycosides were obtained by methanolysis of sample GSLs with 5% anhydrous methanolic HCl at 100 °C for 3 h. Fatty acid methyl esters were extracted with hexane, and the methylglycosides remaining in the methanolic solution were concentrated, N-acetylated and trimethylsilylated. The sugar compositions were determined using a Shimadzu GC-2014 instrument equipped with a capillary column CBP-1 (0.25 mm×25 m) by raising the temperature from 100 °C to 250 °C at a rate of 5 °C/min.
FAB (fast atom bombardment) mass analysis
FAB mass spectra were obtained using a JEOL JMS-HX110 high resolution mass spectrometer equipped with an FAB ion source and XMS computer system. Xenon as an ionizing gas was used at an accelerating voltage of 6 kV neutral beam. The sample was dissolved in 30 μl of DMSO. A 1 μl aliquot of the solution was applied on to a stainless steel folder (1 mm×4 mm), followed by the addition of approx. 2 μl of triethanolamine. The samples were subsequently analysed.
Glycosidase digestion
The sample ganglioside was sonicated in 50 mM phosphate buffer (pH 6.6). Enzyme (α1-3,6-galactosidase, 200 m-units) (Calbiochem) was added and incubated for 68 h at 37 °C. The product was separated by TLC, probed with HRP-conjugated CTB, and visualized with a 4-chloro-1-naphthol/hydrogen peroxide solution.
Morphological assessment of neurite outgrowth
Collagen (Cellmatrix Type 1C; Nitta Gelatin) was diluted with HCl solution (pH 3.0) to 0.3 mg/ml and coated on to plates or dishes. PC12 cells were inoculated on to the coated plates or dishes and cultured in the TIP medium at 37 °C overnight. After treatment with or without protein kinase inhibitors at 37 °C for 30 min, PC12 cells were cultured further in the TIP medium containing NGF and/or mAb PR#1 at 37 °C for 24 h. The morphological changes of the cells were observed by phase-contrast microscopy in vivo.
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] assay
PC12 cells (5×103) were seeded with TIP medium in 96-well plates, incubated at 37 °C for 2 h, and then supplemented with PR#1 or serum. At days 0–4 of the culture, MTT assays (Chemicon) were performed, as described in the supplier's instructions. The growth of cells was quantified by assessing the reduction of MTT to formazan, measured as the absorbance at 590 nm.
Immunoblotting
Proteins were separated on polyacrylamide gels after dissolving in Tris/SDS sample buffer (Daiichi-kagaku) and then transferred on to PVDF membranes (Millipore). The blots were blocked with BlockAce (Dainihon-Seiyaku) and incubated with primary antibody and then with HRP-conjugated anti-(mouse IgG). Bands were visualized by an ECL® (enhanced chemiluminescence) system (GE Healthcare).
Immunocytochemistry
PC12 cells were fixed with 3% (w/v) paraformaldehyde in PBS for 20 min at room temperature, followed by incubation in blocking solution (2% BSA and 5% normal goat serum in PBS, pH 7.4). The cells were incubated with primary antibodies (1 μg/ml) for 1 h at room temperature, and then with fluorochrome-conjugated secondary antibodies for 1 h at room temperature. The immunolabelled cells were examined with a Zeiss LSM510 confocal microscope.
Co-immunoprecipitation analysis
PC12 cells were lysed with lysis buffer [20 mM Tris/HCl, pH 7.4, 2 mM EDTA, 2 mM Na3VO4, 2 mM NaF, 0.5% Triton X-100 and 1% protease inhibitor cocktail (Sigma)] at room temperature, then incubated with PR#1 for 1 h at 4 °C. The immunocomplexes were precipitated after incubation with Protein G–agarose beads (KPL) for 1 h at 4 °C. The beads were washed several times with PBS. The immunocomplexes were separated by SDS/PAGE and analysed by immunoblotting, as described above. With HRP-conjugated CTB and anti-HRP antibody, GM1a-associated proteins were analysed in the same way.
Analysis of tyrosine-phosphorylated proteins
PC12 cells (106) were seeded on to collagen-coated 60-mm-diameter dishes. The cells were stimulated with 20 μg/ml PR#1, 10 ng/ml NGF or 1 μg/ml CTB, and incubated at 37 °C for the indicated times. NGF was used as a positive control in this assay. After the indicated periods, the cells were rinsed once with DMEM, lysed in Tris/SDS sample buffer containing 2 mM EDTA, 2 mM Na3VO4, 2 mM NaF and 1% protease inhibitor cocktail, and immediately boiled for 5 min. The lysates were then analysed by immunoblotting, as described above.
RESULTS
Identification of a novel ganglioside involved in neuritogenesis
The 22 hybrid clones obtained were grouped into four types, based on the Western blotting patterns. mAbs in one group recognized a glycolipid band, whereas others were protein bands or nonspecific broad bands non-specifically. mAbs recognizing glycolipids in the present study were subjected to the following treatment: following PC12 cell culture (5×104 cells) for 24 h in the medium containing 10 μg/ml of each mAb from supernatant of the selected clones, mAb PR#1 (subclass IgG3) was found to have biological effects inducing neurite outgrowth on PC12 cells. No such effects were found in PC12 cells treated with other mAbs.
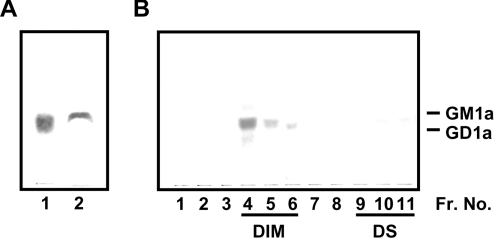
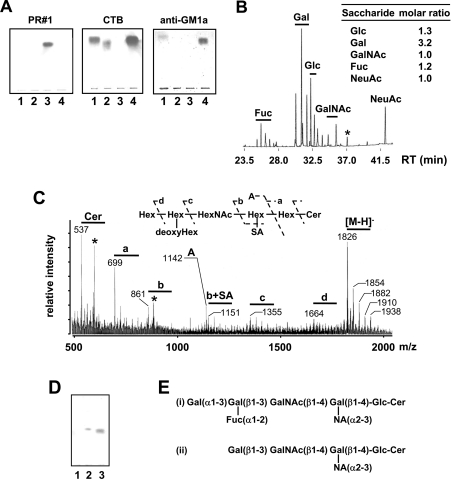
In order to identify the antigen of PR#1, a TLC immunostaining assay with total lipids extracted from PC12 cells or rat brains was carried out (Figure 1A). The immunoreactive lipid was alkali-resistant and detectable with orcinol and resorcinol reagent, indicating that the antigen of PR#1 could be a ganglioside, which was clearly localized in the DIM fraction (Figure 1B). Furthermore, the RF value of the major PR#1 immunoreactant was located between the GM1a and GD1a, a region in which three gangliosides such as GM1a, Fuc-GM1 (IV2Fucα, II3NeuAc,GgOse4Cer) and Fuc(Gal)-GM1 (IV2Fucα,IV3Galα,II3NeuAc,GgOse4Cer) have been reported to be present [21]. To specify the antigen, TLC/immunostaining with PR#1, CTB, which specifically reacted to GM1a and weakly cross-reacted to Fuc-GM1 [22], and the anti-GM1a mAb DIM24 [23] was carried out on the purified ganglioside fractions obtained from the PC12 cell extract (see the Materials and methods section and Figure 2). Since CTB reacted with the gangliosides in fractions G1-1 and G1-2 and DIM24 with fraction G1-1, gangliosides in G1-1 and G1-2 were determined to be GM1a and Fuc-GM1 respectively (Figure 2A). The ganglioside in G1-2 was confirmed as Fuc-GM1 by mass analyses as well (results not shown).
Figure 1. TLC/immunostaining with PR#1.
(A) Antigen was detected by mAb PR#1 (2 μg/ml concentration, 1 h of incubation at room temperature) in total glycolipid extraction purified through affinity columns from homogenates of PC12 cells (dry weight, 0.05 mg; lane 1) and the 14-day-old rat brain (dry weight, 25 mg; lane 2). (B) Triton X-100 extracts of PC12 cells (2×107) were subjected to sucrose density gradient centrifugation and separated into 11 fractions. PR#1 antigen was localized in DIM fractions (lanes 4–6), but not in detergent-soluble fractions (lanes 9–11). The RF values of GM1a and GD1a are indicated on the right. Fr. No., fraction number.
Figure 2. Determination of PR#1 antigen.
(A) TLC/immunostaining with mAb PR#1, CTB and anti-GM1a antibody on candidate antigens. Each lane was composed of 0.5 nmol of G1-1 (GM1a), G1-2 (Fuc-GM1), G1-3 and GM1a respectively. mAb PR#1 reacted selectively with G1-3. (B) Compositional analysis of carbohydrates of G1-3 with GC MS. Retention time (RT) is indicated. Each peak corresponds to a monosaccharide as indicated. The molar ratio was calculated based on the peak areas of standard GM1a and L-fucose. The peak indicated by an asterisk was due to the contaminated phosphatidylinositol. (C) Negative-ion FAB mass spectra and the fragmentation diagrams of G1-3 (Hex, hexose; SA, sialic acid; Cer, ceramide). Contamination with phosphatidylinositol is indicated with an asterisk. (D) Immunostaining with CTB, in order to demonstrate that G1-3 ganglioside detected by mAb PR#1 could be broken down into Fuc-GM1 by means of a digestive process through α1-3,6 galactosidase. Lane 1, G1-3; lane 2, G1-3 after treatment with α1-3,6 galactosidase; lane 3, G1-2 (Fuc-GM1). The results indicate that PR#1 antigen was Fuc(Gal)-GM1. (E) Structural comparison of (i) Fuc(Gal)-GM1 with (ii) GM1a.
The G1-3 fraction contained a dense band of antigenic gangliosides of PR#1 (Figure 2A), and it remains to be confirmed whether this band is composed of a remaining candidate ganglioside Fuc(Gal)-GM1. The hexose composition analysis showed that the ganglioside in G1-3 contains fucose, glucose, galactose, N-acetylgalactosamine and N-acetylneuraminic acid in the molar ratio 1:1:3:1:1 (Figure 2B). Figure 2(C) shows the negative-ion FAB mass spectrum and the fragmentation diagrams of the ganglioside. This spectrum showed the prominent molecular ions (M−H)− m/z 1826, 1854, 1882, 1910 and 1938, which corresponded to the molecular species of Fuc(Gal)-GM1 containing C16:0, C18:0, C20:0, C22:0 and C24:0 fatty acids respectively. The ion groups a, b, b+sialic acid, c and d, corresponding to ceramide-bearing oligosaccharide fragments (699, 861, 1151, 1355 and 1664 correspond to ceramide-bearing oligosaccharide with C16:0-sphingosine), and the ion A, corresponding to oligosaccharide residues, were also observed (Figure 2C). In addition, the digestion product of G1-3 ganglioside with α1-3,6-galactosidase was Fuc-GM1 and was detected by its specific ligand CTB (Figure 2D). These data substantiate that the antigen of PR#1 is the fucoganglioside Fuc(Gal)-GM1 (Figure 2E). Although the fraction G1-3 contained a small amount of phosphatidylinositol, PR#1 did not react with it (results not shown).
Cross-linking-like effects of Fuc(Gal)-GM1 is distinct from GM1a
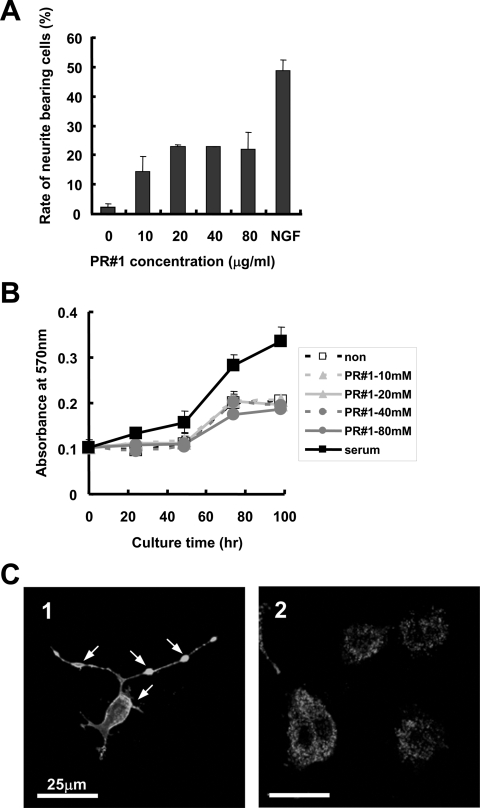
Figure 3(A) shows the dose-dependence of PR#1 for the neurite outgrowth effect. The ratio of neurite-bearing cell number to total cell number increased with the concentration of PR#1 up to 20 μg/ml. The plateau value of the ratio (approx. 25%) in PR#1 treatment was approximately half that found in the NGF treatment. Effects on cell proliferation and viability, as assayed following the MTT protocol, were almost the same between PR#1-treated and non-treated cells, even when high doses of the antibody were added (Figure 3B). In PR#1-treated cells, aggregations of antigenic lipids were ubiquitously observed at the plasma membrane of cell bodies and neurites (Figure 3C), and most cells bearing neurites possessed such aggregations. These observations suggested that the cross-linking-like effect (if not ‘cross-linking’ in a strict sense) of Fuc(Gal)-GM1 with PR#1 causes PC12 neurite outgrowth.
Figure 3. Effects of PR#1 treatment on PC12 cell differentiation.
(A) Dose-dependency of PR#1 for PC12 cell neurite outgrowth. PC12 cells were cultured in TIP medium containing various concentrations of PR#1 for 24 h, and then the number of cells with neurites longer than the diameter of the cell bodies was counted. Approx. 25% of the total number of cultured cells (means±S.D., n=3, and more than 200 cells for each condition) showed prominent neurite outgrowth following PR#1 treatment in a dose-dependent manner. The ratio was about half that found in NGF treatment. (B) Effect of PR#1 treatment on PC12 cell proliferation. PC12 cells were cultured in TIP medium with each concentration of PR#1 or serum (2% HS and 2% FBS) for the indicated time, then effects of PR#1 treatment on cell growth were analysed using the MTT assay. PR#1 did not promote cell proliferation (means±S.D., n=5, for each condition). (C) The cross-linking-like effect on Fuc(Gal)-GM1 localization in the presence of PR#1. PC12 cells were cultured in TIP medium with (panel 1) or without (panel 2) PR#1 (20 μg/ml) for 24 h, fixed with 3% (w/v) paraformaldehyde, and then immunostained with 1 μg/ml PR#1. Huge clusters of Fuc(Gal)-GM1 were frequently observed at the plasma membrane of cell bodies and neurites (indicated by arrows).
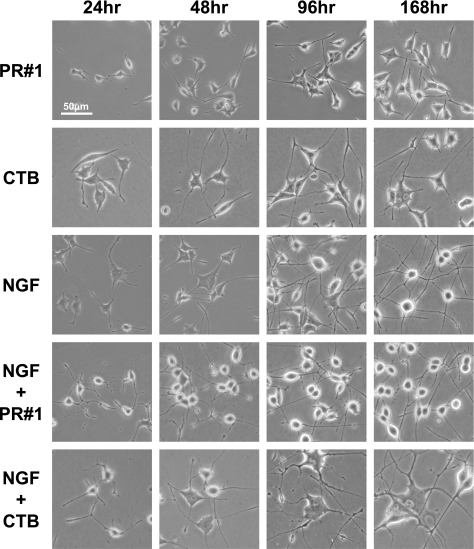
Since ganglioside GM1a is known to form microdomains at the surface of the plasma membrane [24] and is involved in neuron-like differentiation of the PC12 cells [25–31], it is worthwhile to clarify whether Fuc(Gal)-GM1-enriched domains, a homologous molecule, are similar to or distinct from GM1a-formed domains. Using PR#1 and CTB, the morphological changes of PC12 cells induced by them were compared. Under PR#1 treatment for Fuc(Gal)-GM1, the length of the cell processes continued to increase for 2–3 days, but no more elongation or morphological changes occurred thereafter (Figure 4, top row). The shape of the cell body transformed slightly into a rounded form. CTB treatment resulted in the development of cell processes longer than those generated in PR#1-treated cells and a cell soma with a flattened morphology, similar to that found with NGF treatment (Figure 4, second row from top). In both cases, the morphology of cells at later differentiation stages was distinct from that of NGF-treated cells that showed much longer processes and a round soma (Figure 4, middle row). Synergistic effects on cell differentiation by co-treatment of PR#1 and NGF were observed, i.e. much longer, multiple and profuse neurite branches in the case of addition of PR#1 (Figure 4, second row from bottom and Figure 5). In addition, this treatment led to faster development of roundness in the cell soma within 1 day, although it took at least 4 days in an ordinary NGF-induced differentiation (Figure 4, middle row), indicating that PR#1 promoted neuron-like differentiation. On the other hand, co-treatment with CTB and NGF induced mostly opposite effects in terms of the following: enlargement of cell bodies and neurites, including a subset of cells with a much more flattened form (Figure 4, bottom row).
Figure 4. Time course of the morphological alterations of PC12 cells.
PC12 cells were cultured in TIP medium with each reagent (20 μg/ml PR#1, 1 μg/ml CTB or 10 ng/ml NGF). In PR#1 treatment (first row), neurite outgrowth stopped at approx. 48 h, and no more elongation and morphological changes were observed thereafter. With CTB treatment (second row from top), cell bodies took on a flattened shape during differentiation, and neurite extension progressed beyond 48 h. In NGF treatment (middle row), most of the cell soma were flattened with elongated processes in early stages of differentiation. As differentiation progressed, cell soma tended to become rounded, and they developed a network of processes. In NGF treatment combined with PR#1 (second row from bottom), NGF-induced differentiation was promoted further, showing rapid formation of round cell soma and process networks. In NGF treatment with CTB (bottom row), enlarged and flattened cell bodies and thicker processes were formed during the time course of differentiation.
Figure 5. Synergistic effects of PR#1 on NGF-induced differentiation of PC12 cells.
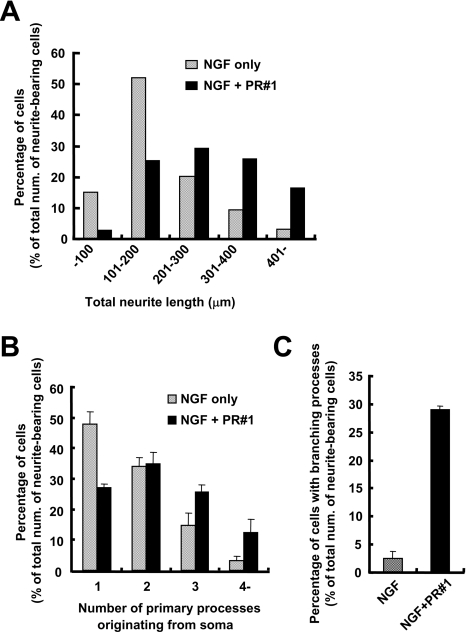
PC12 cells were cultured in TIP medium containing both 10 ng/ml NGF and 20 μg/ml PR#1 for 24 h, and then each item was calculated. (A) Neurite length of blindly selected cells (n=150 in each situation) was measured. Induction of longer neurites was promoted under the presence of both 10 ng/ml NGF and 20 μg/ml PR#1. (B) Primary processes originating from the soma, longer than its diameter, were counted in neurite-bearing cells. More primary neurites were induced by the simultaneous treatment of NGF and PR#1. (C) Branching neurites were counted in neurite-bearing cells (means±S.D., n=3, 150 or more cells in each calculation). More neurite branches were induced with additional PR#1 treatment than with NGF alone.
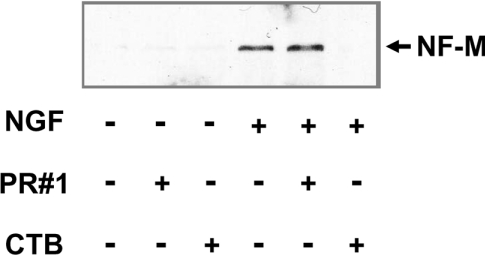
Considering some contribution of neurofilaments in the cross-linking-like effects accompanying such morphological changes, the expression levels of neurofilament proteins were examined (cf. [32]). As shown in Figure 6, neither PR#1 nor CTB could induce NF-M (neurofilament M) expression, indicating that they were responsible for cell process outgrowth, but not for neural differentiation itself. On the other hand, co-treatment with NGF made a difference in terms of NF-M expression, i.e. additional PR#1 led to a slightly stronger expression than NGF treatment alone, whereas that of CTB resulted in a cancelling out of the expression, despite enhancing cell process outgrowth. These results indicate that the mechanisms underlying the cross-linking-like effects through Fuc(Gal)-GM1 are distinct from those occurring through GM1a.
Figure 6. Expression profiles of neurofilament protein.
PC12 cells (5×105) were cultured in each condition for 4 days and then analysed (25 μg of protein/lane) by immunoblotting with an anti-NF-M antibody. Both PR#1 and CTB alone could not induce the expression of NF-M. PR#1 treatment combined with NGF revealed a slight increase of NF-M expression, whereas CTB treatment with NGF resulted in a significant decrease of NGF-induced NF-M expression.
Subcellular distribution of Fuc(Gal)-GM1 and GM1a
Using PR#1 and CTB, the distribution of Fuc(Gal)-GM1 and GM1a was compared immunocytochemically during the neuron-like differentiation of PC12 cells. There were three types of cells, i.e. GM1a-dominant cells, Fuc(Gal)-GM1-dominant cells and the cells expressing both of the gangliosides. These double-positive cells were used in the following analysis.
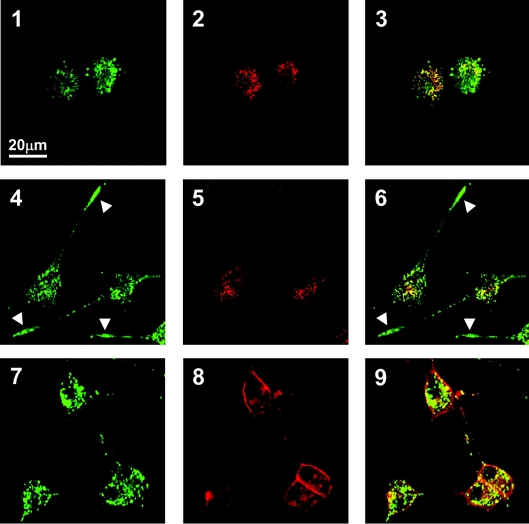
In undifferentiated cells, the majority of Fuc(Gal)-GM1 immunoreactivity appeared as punctate structures in the soma, and GM1a immunoreactivity similarly and partially overlapped with Fuc(Gal)-GM1 signals (Figure 7, panels 1–3). These ganglioside signals never overlapped with the fluorochrome signals for mitochondria or lysosomes throughout the differentiation process (results not shown). At 2 days after NGF treatment (Figure 7, panels 4–6), Fuc(Gal)-GM1 signals were found, in addition to the soma, to be concentrated at the tip portion of the extended neurites, whereas GM1a signals remained in the soma. At 10 days after NGF treatment (Figure 7, panels 7–9), Fuc(Gal)-GM1 signals at the neurite tips became less dense, but remained strongly confined to the soma. In contrast, GM1a signals tended to be accumulated mainly on the cell surface of the soma and proximal portion of neurites, rather than in the cytoplasm. The behaviour of Fuc(Gal)-GM1 in neuritogenesis was suggested to be significantly different from that of GM1a throughout the differentiation process.
Figure 7. Behaviour of Fuc(Gal)-GM1 and GM1a in neuron-like differentiation of PC12 cells by NGF treatment.
Cells fixed with paraformaldehyde were double-labelled with mAb PR#1 (panels 1, 4 and 7) and CTB (panels 2, 5 and 8) and the images merged (panels 3, 6 and 9). Partial overlapping between GM1a and Fuc(Gal)-GM1 was observed in the cytoplasmic region throughout the differentiation. In undifferentiated cells (panels 1–3), punctate signals for Fuc(Gal)-GM1 and GM1a were observed in the cytoplasmic region. At 2 days after NGF treatment (panels 4–6), Fuc(Gal)-GM1 signals were frequently observed in the tips of neurites (arrowheads), but no such behaviour was seen with the GM1a signals. At 10 days after NGF treatment (panels 7–9), Fuc(Gal)-GM1 signals were still punctate, being localized mainly to the cell soma and partially in the neurites. In contrast, GM1a signals were diffusively and strongly observed along the plasma membrane of soma and proximal portion of neurites, in addition to the punctate signals in the soma.
Certain SFKs are involved in signal transduction via the Fuc(Gal)-GM1-enriched platform
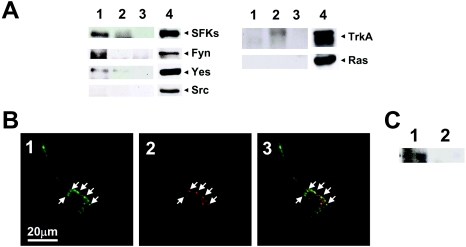
In order to search for Fuc(Gal)-GM1-associated proteins, the proteins were immunoprecipitated from Triton X-100 extracts of PC12 cells, using mAb PR#1 (Figure 8A). Identified proteins were certain SFKs, i.e. Fyn and Yes, but not Src itself. TrkA and Ras proteins, which have been known to be NGF-responsive receptors associated with GM1a-enriched platforms [27] and the TrkA-signalling pathway respectively, were not detected. Fuc(Gal)-GM1 was co-localized immunocytochemically in PC12 cells and co-precipitated with activated SFKs after 30 min of treatment with PR#1 (Figures 8B and 8C), in contrast with the TrkA with weak Yes signals in immunoprecipitates treated with CTB for 30 min (Figure 8A). These observations strongly suggest that SFKs other than Src are closely associated with Fuc(Gal)-GM1 in PC12 cells.
Figure 8. Association of the Fuc(Gal)-GM1 with SFKs.
(A) Co-immunoprecipitation analysis with PR#1. Fuc(Gal)-GM1-associated proteins were immunoprecipitated with (lanes 1) or without (lanes 3) PR#1 from Triton X-100 lysates of PC12 cells, then analysed by immunoblot analysis using the antibodies indicated on the right. With CTB–HRP and anti-HRP antibody, GM1a-associated proteins were analysed in the same way (lanes 2). Lanes 4 are Triton X-100 lysates of PC12 cells used as a positive control. SFKs Fyn and Yes were detected in the PR#1-precipitate, whereas weak signals of TrkA and Yes were found in the CTB-immunoprecipitates. (B) Fuc(Gal)-GM1 was highly co-localized with the activated form of SFKs (indicated by arrows). PC12 cells were cultured in the TIP medium containing 20 μg/ml PR#1 for 30 min, fixed with 3% (w/v) paraformaldehyde, and then double-stained with both PR#1 (green, panel 1) and anti-[Src (pTyr416)] antibody (red, panel 2), which can detect not only the phosphorylated form of Src but also that of other members. Panel 3 shows a merged image. (C) An activated form of SFKs was associated with Fuc(Gal)-GM1. Fuc(Gal)-GM1-associated proteins were immunoprecipitated with (lane 1) or without (lane 2) PR#1 from Triton X-100 lysates of PC12 cells at 30 min treatment with (lane 1) or without (lane 2) PR#1 respectively.
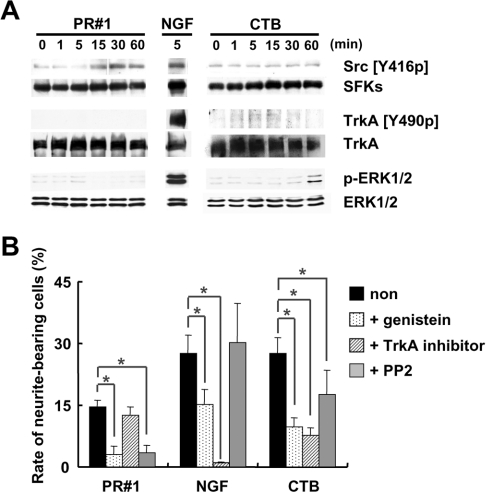
The contribution of SFKs in signal transduction via Fuc(Gal)-GM1 was confirmed by their phosphorylation specificity. Figure 9(A) shows the time course of protein tyrosine phosphorylation after PR#1, CTB or NGF treatment. In PR#1 treatment, an increase of tyrosine phosphorylation of SFKs was observed within 15 min, but was not seen with TrkA and ERKs. In CTB treatment, ERKs were activated strongly after 60 min and TrkA weakly within 5 min, but SFKs were not activated. Supportive results were obtained from examination of the effects of protein kinase inhibitors on the outgrowth of cell processes (Figure 9B). In this assay, the following inhibitors were used: (i) PP2, a specific inhibitor of SFKs; (ii) TrkA-specific inhibitor; and (iii) genistein, a wide-range tyrosine kinase inhibitor. After pre-treatment with PP2, cell process outgrowth induced by the addition of PR#1 was found to be strongly suppressed, whereas outgrowth by the addition of CTB and NGF were weakly or not at all suppressed. PP2 pre-treatment also suppressed the synergistic effect of PR#1 on neurite sprouting and branching of cells differentiated by NGF (results not shown). On the contrary, pre-treatment with the TrkA inhibitor suppressed process outgrowth attributable to CTB and NGF treatment, but no such effects were seen with PR#1 treatment. With genistein pre-treatment, process outgrowth was suppressed under all conditions. From the above findings, it was clear that PR#1-induced morphological changes were mediated exclusively by SFKs and independent of TrkA signalling pathways, which were involved in CTB and NGF activation of cell differentiation. In other words, a signal platform provided by Fuc(Gal)-GM1 was found to be specifically associated with SFKs, distinct from a GM1a platform which was associated with TrkA.
Figure 9. Comparison of the signal transduction cascades initiated by Fuc(Gal)-GM1 with that of CTB.
(A) Induction of protein tyrosine phosphorylation by PR#1 and CTB. PC12 cells were incubated with PR#1, NGF (positive control) or CTB for the indicated time (min), and their lysates were subjected to immunoblotting analysis with the antibodies indicated on the right-hand side. Treatment of PC12 cells with PR#1 led to activation of SFKs detected by Src (pTyr416) (Src [Y416p]), with no activation of TrkA and ERKs, which was detected by TrkA (pTyr490) (TrkA [Y490p]) and phospho-ERK1/2 (p-ERK1/2) respectively. On the other hand, treatment with CTB induced activation of TrkA and ERKs with no activation of SFKs. NGF could induce activation of all these molecules. (B) The effects of protein kinase inhibitors on neurite outgrowth induced by PR#1, NGF or CTB. PC12 cells were pre-treated with or without inhibitors (10 μM genistein, 10 μM TrkA inhibitor or 5 μM PP2) for 30 min, supplemented with 20 μg/ml PR#1, 10 ng/ml NGF or 1 μg/ml CTB. The proportion of neurite-bearing cells was evaluated in a similar way as in Figure 1(A) (means±S.D., n=3, some 100 cells in each case). Asterisks indicate significant differences between the cases (Student's t test, P<0.05).
DISCUSSION
It has been shown in the present study that a fucoganglioside, Fuc(Gal)-GM1, forms a distinct platform of lipid membrane microdomains that are involved in the neuron-like differentiation of PC12 cells and that signal transduction mechanisms through such domains are entirely different from those occurring through domains composed of a homologous ganglioside, GM1a. In the following, the role of Fuc(Gal)-GM1-enriched microdomains in cell differentiation will be discussed in comparison with the function of GM1a-enriched microdomains.
Fuc(Gal)-GM1 has been known as a GM1-containing blood group B determinant (BGM1) and one of the major gangliosides of PC12 cells [21,33–35]. Following NGF treatment of PC12 cells, enhancement of fucose incorporation into glycolipids and gangliosides and up-regulation of BGM1 synthase that may catalyse the synthesis of Fuc(Gal)-GM1 from Fuc-GM1 are observed, suggesting that Fuc(Gal)-GM1 is utilized in a process of PC12 cell differentiation [33,36]. However, there have been no reports that show direct evidence for the involvement of Fuc(Gal)-GM1 in cell differentiation. In the present study, we have clarified that (i) Fuc(Gal)-GM1 is localized in DIM fractions, i.e. membrane microdomains, (ii) microdomains with Fuc(Gal)-GM1 are accumulated into clusters in cells differentiated by treatment with a specific antibody PR#1, (iii) accumulation of the gangliosides is most prominent at the tip of differentiating neurite processes, and (iv) cross-linking-like effects of the gangliosides accumulated by the antibody promotes further differentiation in NGF-treated cells. Fuc(Gal)-GM1 seems to be recruited from the cytoplasm to the plasma membrane, targeted in particular at the tip of neurites at the onset of PC12 cell differentiation. They then form distinct membrane microdomains as a platform for signal transduction in neurite development.
A homologous ganglioside, GM1a, forms membrane microdomains as well [24], and it is similarly involved in neuritogenesis in PC12 cells [25–31]. As clarified in the present study, however, their behaviour is different to some extent in PC12 cell differentiation. Although both Fuc(Gal)-GM1 and GM1a in undifferentiated cells are predominantly present in the cytoplasm, some Fuc(Gal)-GM1 remained in the cytoplasm as differentiation progressed, while the majority of GM1a moves to the plasma membrane. Morphological changes induced by cross-linking-like effects of the gangliosides are also different: thin neurites and round cell soma compared with thick processes and flattened soma are effects mediated through Fuc(Gal)-GM1 and GM1a respectively. Such behavioural differences appear to reflect functional differentiation of the two gangliosides.
It is not clear in the present study whether spatial distribution of Fuc(Gal)-GM1-enriched microdomains in the plasma membrane is independent of that of GM1a-enriched microdomains, owing to the resolution limitations of the microscope. The size of individual microdomains may be too small to discriminate by conventional fluorescent microscopy [37]. However, immunocytochemical signals for Fuc(Gal)-GM1 that are partially overlapping with GM1a signals observed in the cytoplasm may indicate co-localization of the two gangliosides in situ, since it is suggested that Fuc(Gal)-GM1 can be synthesized from GM1a by a series of glycosyltransferases [33,36]. Further studies are needed to clarify this point, including identification of the corresponding glycosyltransferase for the synthesis.
As constituent proteins of Fuc(Gal)-GM1-enriched domains, Fyn and Yes of the SFKs have been extracted by immunoprecipitation with mAb PR#1 in the present study, based on the fact that SFKs are principal components of lipid microdomains and Fyn and Yes are enriched, especially in the DIM fraction of PC12 cells [38]. It appears that these kinases are functionally associated with Fuc(Gal)-GM1, as confirmed by the present findings: (i) treatment of PC12 cells with PR#1 activates SFKs; (ii) effects of PR#1 are suppressed by pre-treatment with a specific inhibitor of SFKs, PP2; and (iii) activated SFKs are highly co-localized with Fuc(Gal)-GM1 30 min after PR#1 treatment. The present study also demonstrates the distinctiveness of signal transduction mechanisms occurring through Fuc(Gal)-GM1-enriched microdomains, because of the lack of an effect from PR#1 treatment on TrkA and ERKs, which are responsible for signalling pathways through NGF [39], and the lack of influence of a TrkA-specific inhibitor [17] on PR#1-induced neurite outgrowth.
In contrast with Fuc(Gal)-GM1, GM1a is closely associated with TrkA for targeting to the plasma membrane and for its receptor functions [25,27–29,31]. However, the ‘cross-linking’ effects of GM1a in CTB-induced neurite outgrowth results in tyrosine phosphorylation of ERKs, but not of TrkA [26]. In addition to strong ERK phosphorylation, the presence of certain levels of phosphorylated TrkA in the CTB-immunoprecipitates is demonstrated in the present study, and the CTB-induced neurite outgrowth is significantly suppressed by pre-treatment with a TrkA inhibitor, the ‘cross-linking’ effects through canonical TrkA signalling seem to occur to some extent under such conditions. Association of GM1 with SFKs appears to be very weak, as judged from the present findings, i.e. no induction of SFKs phosphorylation by CTB treatment and weak suppression of neurite outgrowth by a SFK inhibitor PP2.
In conclusion, a fucoganglioside Fuc(Gal)-GM1 has been identified in the present study as a novel membrane microdomain ganglioside distinct from a homologous ganglioside, GM1a. It provides a unique platform that is responsible for SFK signal transduction cascades, through which it promotes neuritogenesis in PC12 cells. The distinctiveness of Fuc(Gal)-GM1 sheds light on the heterogeneity of the membrane microdomains, in terms of their principal component molecules, signalling mechanisms, spatial and temporal distribution, and physiological functions.
Acknowledgments
This study was supported by grants from RIKEN Brain Science Institute and Core Research for Evolutional Science and Technology (CREST) of Japan Science and Technology Corporation (JST).
References
- 1.Hakomori S. Bifunctional role of glycosphingolipids. J. Biol. Chem. 1990;265:18713–18716. [PubMed] [Google Scholar]
- 2.Simon K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 3.Brown D. A., London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 4.Brown R. E. Sphingolipid organization in biomembranes: what physical studies of model membranes reveal. J. Cell Sci. 1998;111:1–9. doi: 10.1242/jcs.111.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C., Butz S., Ying Y., Anderson R. G. W. Tyrosine kinase receptor concentrated in caveolae-like domains from neuronal plasma membrane. J. Biol. Chem. 1997;272:3554–3559. doi: 10.1074/jbc.272.6.3554. [DOI] [PubMed] [Google Scholar]
- 6.Simon K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 7.Kumanogoh H., Miyata S., Sokawa Y., Maekawa S. Biochemical and morphological analysis on the localization of Rac1 in neurons. Neurosci. Res. 2001;39:189–196. doi: 10.1016/s0168-0102(00)00211-x. [DOI] [PubMed] [Google Scholar]
- 8.Yamamura S., Handa K., Hakomori S. A close association of GM3 with c-Src and Rho in GM3-enriched microdomains at the B16 melanoma cell surface membrane: a preliminary note. Biochem. Biophys. Res. Commun. 1996;236:218–222. doi: 10.1006/bbrc.1997.6933. [DOI] [PubMed] [Google Scholar]
- 9.Iwabuchi K., Hanada K., Hakomori S. Separation of “glycosphingolipid signaling domain” from caveolin-containing membrane fraction in mouse myeloma B16 cells and its role in cell adhesion coupled with signaling. J. Biol. Chem. 1998;273:33766–33773. doi: 10.1074/jbc.273.50.33766. [DOI] [PubMed] [Google Scholar]
- 10.Kiyokawa E., Baba T., Otsuka N., Makino A., Ohno S., Kobayashi T. Spatial and functional heterogeneity of sphingolipid-rich membrane domains. J. Biol. Chem. 2005;280:24072–24084. doi: 10.1074/jbc.M502244200. [DOI] [PubMed] [Google Scholar]
- 11.Nagatsuka Y., Hara-Yokoyama M., Kasama T., Takekoshi M., Maeda F., Ihara S., Fujiwara S., Ohshima E., Ishii K., Kobayashi T., et al. Carbohydrate-dependent signaling from the phosphatidylglucoside-based microdomain induces granulocytic differentiation of HL60 cells. Proc. Natl. Acad. Sci. U.S.A. 2003;100:7454–7459. doi: 10.1073/pnas.1232503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasahara K., Watanabe Y., Yamamoto T., Sanai Y. Association of Src family kinase Lyn with ganglioside GD3 in rat brain. J. Biol. Chem. 1997;272:29947–29953. doi: 10.1074/jbc.272.47.29947. [DOI] [PubMed] [Google Scholar]
- 13.Kasahara K., Watanabe Y., Takeuchi K., Kaneko H., Oohira A., Yamamoto T., Sanai Y. Involvement of gangliosides in glycosylphosphatidylinositol-anchored neuronal cell adhesion molecule TAG-1 signaling in lipid rafts. J. Biol. Chem. 2000;275:34701–34709. doi: 10.1074/jbc.M003163200. [DOI] [PubMed] [Google Scholar]
- 14.Yu R. K., Iqbal K. Sialosylgalactosyl ceramide as a specific marker for human myelin and oligodendroglial perikarya: gangliosides of human myelin, oligodendroglia and neurons. J. Neurochem. 1979;32:293–300. doi: 10.1111/j.1471-4159.1979.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 15.Yu R. K., Yanagisawa M. Glycobiology of neural stem cells. CNS Neurol. Disord. 2006;5:415–423. doi: 10.2174/187152706777950675. [DOI] [PubMed] [Google Scholar]
- 16.Hirabayashi Y., Hirota M., Matsumoto M., Tanaka H., Obata K., Ando S. Developmental changes of C-series polysialogangliosides in chick brains revealed by mouse monoclonal antibodies M6704 and M7103 with different epitope specificities. J. Biochem. (Tokyo) 1988;104:973–979. doi: 10.1093/oxfordjournals.jbchem.a122593. [DOI] [PubMed] [Google Scholar]
- 17.Wood E. R., Kuyper L., Petrov K. G., Hunter R. N., Harris P. A., Lackey K. Discovery and in vitro evaluation of potent TrkA kinase inhibitors: oxindole and aza-oxindoles. Bioorg. Med. Chem. Lett. 2004;14:953–957. doi: 10.1016/j.bmcl.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Brown D. K., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 19.Katsumata O., Yokoyama M. H., Fridman C. S., Nagatsuka Y., Katada T., Hirabayashi Y., Shimizu K., Yoshigaki J. F., Sugiya H., Furuyama S. Association of FcγRII with low-density detergent-resistant membrane is important for cross-linking-dependent initiation of the tyrosine phosphorylation pathway and superoxide generation. J. Immunol. 2001;167:5814–5823. doi: 10.4049/jimmunol.167.10.5814. [DOI] [PubMed] [Google Scholar]
- 20.Higashi H., Fukui Y., Ueda S., Kato S., Hirabayashi Y., Matsumoto N., Naiki M. Sensitive enzyme-immunostaining and densitometric determination on thin-layer chromatography of N-glycolylneuraminic acid-containing glycosphingolipids, Hanganutziu-Deicher antigens. J. Biochem. (Tokyo) 1987;95:1517–1520. doi: 10.1093/oxfordjournals.jbchem.a134760. [DOI] [PubMed] [Google Scholar]
- 21.Ariga T., Kobayashi K., Kuroda Y., Yu R. K., Suzuki M., Kitagawa H., Inagaki F., Miyata T. Characterization of tumor-associated fucogangliosides from PC12 pheochromocytoma cells. J. Biol. Chem. 1987;262:14146–14153. [PubMed] [Google Scholar]
- 22.Masserini M., Freire E., Palestini P., Calappi E., Tettamanti G. Fuc-GM1 ganglioside mimics the receptor function of GM1 for cholera toxin. Biochemistry. 1992;31:2422–2426. doi: 10.1021/bi00123a030. [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki Y., Nagatsuka Y., Oshima E., Suzuki Y., Hirabayashi Y., Hashikawa T. Comprehensive analysis of monoclonal antibodies against detergent-insoluble membrane/lipid rafts of HL60 cells. J. Immunol. Methods. 2006;311:106–116. doi: 10.1016/j.jim.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Dietrich C., Volovyk Z. N., Levi M., Thompson N. L., Jacobson K. Partitioning of Thy-1, GM1, and cross-linked phospholipid analogs into lipid rafts reconstituted in supported model membrane monolayers. Proc. Natl. Acad. Sci. U.S.A. 2001;98:10642–10647. doi: 10.1073/pnas.191168698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mutoh T., Tokuda A., Guroff G., Fujiki N. The effect of the B subunit of cholera toxin on the action of nerve growth factor on PC12 cells. J. Neurochem. 1993;60:1540–1547. doi: 10.1111/j.1471-4159.1993.tb03319.x. [DOI] [PubMed] [Google Scholar]
- 26.Kimura M., Hidari K., Suzuki T., Miyamoto D., Suzuki Y. Engagement of endogenous ganglioside GM1a induces tyrosine phosphorylation involved in neuron-like differentiation of PC12 cells. Glycobiology. 2001;11:335–343. doi: 10.1093/glycob/11.4.335. [DOI] [PubMed] [Google Scholar]
- 27.Mutoh T., Tokuda A., Miyadai T., Hamaguchi M., Fujiki N. Ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc. Natl. Acad. Sci. U.S.A. 1995;92:5087–5091. doi: 10.1073/pnas.92.11.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mutoh T., Tokuda A., Inokuchi J., Kuriyama M. Glucosylceramide synthase inhibitor inhibits the action of nerve growth factor in PC12 cells. J. Biol. Chem. 1998;273:26001–26007. doi: 10.1074/jbc.273.40.26001. [DOI] [PubMed] [Google Scholar]
- 29.Mutoh T., Hamano T., Yano S., Koga H., Yamamoto H., Furukawa K., Ledeen R. W. Stable transfection of GM1 synthase gene into GM1-deficient NG108–15 cells, CR-72 cells, rescues the responsiveness of Trk-neurotrophin receptor to its ligand, NGF. Neurochem. Res. 2002;27:801–806. doi: 10.1023/a:1020209008169. [DOI] [PubMed] [Google Scholar]
- 30.O'Hanlon G. M., Hirst T. R., Willison H. J. Ganglioside GM1 binding toxins and human neuropathy-associated IgM antibodies differentially promote neuritogenesis in a PC12 assay. Neurosci. Res. 2003;47:383–390. doi: 10.1016/s0168-0102(03)00239-6. [DOI] [PubMed] [Google Scholar]
- 31.Nishio M., Fukumoto S., Furukawa K., Ichimura A., Miyazaki H., Kusunoki S., Urano T., Furukawa K. Overexpressed GM1 suppresses nerve growth factor (NGF) signals by modulating the intracellular localization of NGF receptors and membrane fluidity in PC12 cells. J. Biol. Chem. 2004;279:33368–33378. doi: 10.1074/jbc.M403816200. [DOI] [PubMed] [Google Scholar]
- 32.Lindenbaum M. H., Carbonetto S., Grosveld F., Flavell D., Mushynski W. E. Transcriptional and post-transcriptional effects of nerve growth factor on expression of the three neurofilament subunits in PC-12 cells. J. Biol. Chem. 1988;263:5662–5667. [PubMed] [Google Scholar]
- 33.Margolis R. K., Salton S. R. J., Margolis R. U. Complex carbohydrates of cultured PC12 pheochromocytoma cells. J. Biol. Chem. 1983;258:4110–4117. [PubMed] [Google Scholar]
- 34.Margolis R. U., Mazzulla M., Greene L. A., Margolis R. K. Fucosyl gangliosides of PC12 pheochromocytoma cells. FEBS Lett. 1984;172:339–342. doi: 10.1016/0014-5793(84)81153-9. [DOI] [PubMed] [Google Scholar]
- 35.Schwarting G. A., Gajewski A., Barbero L., Tischler A. S., Costopoulos D. Complex glycosphingolipids of the pheochromocytoma cell line PC12: enhanced fucosylglycolipid synthesis following nerve growth factor treatment. Neuroscience. 1986;19:647–656. doi: 10.1016/0306-4522(86)90287-3. [DOI] [PubMed] [Google Scholar]
- 36.Kanda T., Ariga T., Yamawaki M., Pal S., Katoh-Semba R., Yu R. K. Effect of nerve growth factor and forskolin on glycosyltransferase activities and expression of a globo-series glycosphingolipid in PC12D pheochromocytoma cells. J. Neurochem. 1995;64:810–817. doi: 10.1046/j.1471-4159.1995.64020810.x. [DOI] [PubMed] [Google Scholar]
- 37.Shaw S. S. Lipid rafts: now you see them, now you don't. Nat. Immunol. 2006;7:1139–1142. doi: 10.1038/ni1405. [DOI] [PubMed] [Google Scholar]
- 38.Kasai A., Shima T., Okada M. Role of Src family tyrosine kinases in the down-regulation of epidermal growth factor signaling in PC12 cells. Genes Cells. 2005;10:1175–1187. doi: 10.1111/j.1365-2443.2005.00909.x. [DOI] [PubMed] [Google Scholar]
- 39.Vaudry D., Stork P. J. S., Lazarovici P., Eiden L. E. Signaling pathways for PC12 cell differentiation: making the right connections. Science. 2002;296:1648–1649. doi: 10.1126/science.1071552. [DOI] [PubMed] [Google Scholar]
- 40.IUPAC-IUB Commission on Biochemical Nomenclature. The nomenclature of lipids. Lipids. 1977;12:455–463. [PubMed] [Google Scholar]
- 41.Svennerholm L. The gangliosides. J. Lipid Res. 1964;5:145–153. [PubMed] [Google Scholar]