Abstract
The chaperone-like activity of α-crystallin is considered to play an important role in the maintenance of the transparency of the eye lens. However, in the case of aging and in diabetes, the chaperone function of α-crystallin is compromized, resulting in cataract formation. Several post-translational modifications, including non-enzymatic glycation, have been shown to affect the chaperone function of α-crystallin in aging and in diabetes. A variety of agents have been identified as the predominant sources for the formation of AGEs (advanced glycation end-products) in various tissues, including the lens. Nevertheless, glycation of α-crystallin with various sugars has resulted in divergent results. In the present in vitro study, we have investigated the effect of glucose, fructose, G6P (glucose 6-phosphate) and MGO (methylglyoxal), which represent the major classes of glycating agents, on the structure and chaperone function of α-crystallin. Modification of α-crystallin with all four agents resulted in the formation of glycated protein, increased AGE fluorescence, protein cross-linking and HMM (high-molecular-mass) aggregation. Interestingly, these glycation-related profiles were found to vary with different glycating agents. For instance, CML [Nϵ-(carboxymethyl)lysine] was the predominant AGE formed upon glycation of α-crystallin with these agents. Although fructose and MGO caused significant conformational changes, there were no significant structural perturbations with glucose and G6P. With the exception of MGO modification, glycation with other sugars resulted in decreased chaperone activity in aggregation assays. However, modification with all four sugars led to the loss of chaperone activity as assessed using an enzyme inactivation assay. Glycation-induced loss of α-crystallin chaperone activity was associated with decreased hydrophobicity. Furthermore, α-crystallin isolated from glycated TSP (total lens soluble protein) had also increased AGE fluorescence, CML formation and diminished chaperone activity. These results indicate the susceptibility of α-crystallin to non-enzymatic glycation by various sugars and their derivatives, whose levels are elevated in diabetes. We also describes the effects of glycation on the structure and chaperone-like activity of α-crystallin.
Keywords: advanced glycation end-product (AGE), α-crystallin, chaperone activity, Nϵ-(carboxymethyl)lysine, glucose, hydrophobicity, glucose 6-phosphate, fructose, methylglyoxal
Abbreviations: AGE, advanced glycation end-product; ANS, 8-anilinonaphthalene-1-sulfonic acid; BCA, bicinchoninic acid; CML, Nϵ-(carboxymethyl)lysine; CS, citrate synthase; G6P, glucose 6-phosphate; G6PD, G6P dehydrogenase; HMM, high-molecular-mass; MGO, methylglyoxal; TSP, total lens soluble protein
INTRODUCTION
The eye lens is a specialized structure, which provides flawless transmission of light to reach the retina for proper vision. Crystallins are the major structural proteins in the lens that account for up to 90% of the total soluble protein. There are three distinct families: α-, β- and γ-crystallins, whose structure, stability and short-range interactions are thought to contribute to lens transparency [1]. In mammalian lenses, α-crystallin can constitute up to as much as 50% of the TSP (total lens soluble protein) mass. α-Crystallin is isolated from vertebrate eye lens as a polydisperse, hetero-oligomeric complex of approx. 800 kDa, consisting of 35–40 subunits, each of which are 20 kDa, and is composed of two gene products, αA and αB, present in a ratio of 3:1 [1]. α-Crystallin had been regarded as a structural protein until its sequence similarity was established with small heat-shock proteins from Drosophila melanogaster [2–4]. Thereafter α-crystallin has been shown to function like a chaperone, binding to non-native or unfolded proteins and protecting them against aggregation induced by heat, reduction and chemical modification [2–5]. It has been shown that the chaperone-like activity of α-crystallin might play an important role in preventing the aggregation and insolubilization of other lenticular proteins, thereby maintaining the transparency of the eye lens [6]. In support for this, studies suggest that cataracts might result from the decrease in the chaperone-like activity of α-crystallin [2,4,7–10].
Owing to the unique growth pattern of the lens, there is no, or negligible, protein turnover in the differentiated fibre cells, and proteins in the nucleus of the lens are as old as the organism, thereby resulting in the accumulation of post-translational modifications such as deamidation, phosphorylation, racemization, C- or N-terminal truncation and glycation. Most of these post-translational modifications have been shown to have accumulated with aging and are accelerated in clinical conditions such as diabetes. Among them, non-enzymatic glycation has been extensively studied.
Cataracts are the leading cause of blindness worldwide, with diabetes and aging the major risk factors that accelerate cataract development [1,11–13]. Glycation of lens proteins has been considered to be one of the mechanisms responsible for both age-related and diabetic cataracts [1,14–16]. The glycation reaction occurs between the carbonyl group of sugars and a free amino group within proteins. Amadori products, the first stable product of the reaction, can be consequently transformed into AGEs (advanced glycation end-products). AGEs are generally pigmented or fluorescent adducts on proteins, and participate in the formation of protein cross-links [17]. Formation of AGEs due to glycation may alter the surface charge of the protein, leading to conformational change, which in turn may affect protein–protein and protein–water interactions, and may ultimately lead to a decrease in the transparency of the eye lens [18]. Although all the three major crystallins are susceptible to glycation, differential glycation was observed in in vitro glycation of rat lens soluble fraction, γ-crystallin being more prone to be followed by α-crystallin [19]. However, preferential glycation of α-crystallin was observed in aging and diabetic human lens [20]. Glycation-induced loss of chaperone function [21–25] and decreased chaperone-like activity of α-crystallin isolated from diabetic rat and human lens [9,26,27] emphasize that the α-crystallin chaperone function is the prime target of glycation, which in turn could result in the development of cataracts. However, very little is known about the effect of various glycating sugars on α-crystallin in terms of degree of glycation, type of AGE that is formed, oxidative damage to the protein, secondary and tertiary structure, hydrophobicity and its chaperone-like function.
Although several previous studies have investigated the effect of non-enzymatic glycation on TSP [15] and on individual crystallins [18–20], the effect of glycation on chaperone function of α-crystallin has not been reported in these studies, and a few of the more recent studies have reported mixed results on the effect of glycation on α-crystallin chaperone-like function [22,23,28]. Therefore, in the present study, we have investigated the effect of glucose, fructose, G6P (glucose 6-phosphate) and MGO (methylglyoxal) on the structure and chaperone-like activity of α-crystallin. These compounds represent four major classes of glycating agents: hexose sugars, keto sugars, sugar phosphate and dicarbonyls, whose levels are elevated in various tissues, including the lens in diabetic patients.
MATERIALS AND METHODS
Materials
Acrylamide, bis-acrylamide, ANS (8-anilinonaphthalene-1-sulfonic acid), ammonium persulfate, BSA, CS (citrate synthase), fructose, glucose, G6P, G6PD (G6P dehydrogenase), 2-mercaptoethanol, MGO, NADP, m-aminophenylboronic acid–agarose, penicillin, peroxidase-conjugated anti-rabbit IgG antibody, SDS, sodium azide, streptomycin and TEMED (N,N,N′,N′-tetramethylethylenediamine), were purchased from Sigma. The BCA (bicinchoninic acid) protein assay kit was from Pierce. Immobilon nitrocellulose membrane and disc filters were purchased from Millipore. Sephacryl S-300HR was from Amersham Biosciences, and SDS/PAGE markers were from Bio-Rad Laboratories.
Preparation of TSP and α-crystallin
Lenses were dissected from the eyeballs of young goats obtained from a local slaughterhouse. A 10% homogenate of these lenses was prepared in buffer A (0.025 M Tris, 0.1 M NaCl, 0.5 mM EDTA and 0.01% NaN3, pH 8.0) and centrifuged at 10000 g for 30 min at 4 °C to obtain the supernatant, the TSP. The TSP was applied on to a Sephacryl S-300 HR (100 cm×1.5 cm) gel-filtration column connected to a low-pressure protein purification system (Biologic-LP; Bio-Rad Laboratories). The column was equilibrated with buffer A, and crystallins were eluted using the same buffer at a flow rate of 0.25 ml/min. Fractions corresponding to α-crystallin were pooled and purity was assessed by SDS/PAGE. Protein concentration was estimated using the BCA method and stored at −80 °C until used.
Non-enzymatic glycation of α-crystallin and TSP
Stocks of glucose, fructose, G6P and MGO were prepared in 100 mM sodium phosphate buffer (pH 7.4). α-Crystallin (5 mg/ml) was incubated with glucose (0.5 M; 4 weeks), fructose (0.1 M; 3 weeks), G6P (0.05 M; 2 weeks) and MGO (0.005 M; 3 days) in 0.2 M sodium phosphate buffer (pH 7.4) containing 50 μg of penicillin and streptomycin and 0.01% sodium azide at 37 °C in the dark under sterile conditions. α-Crystallin incubated in the absence of glycating agent under similar conditions served as a control. Likewise, TSP (50 mg/ml) was incubated in the absence and presence of 0.1 M fructose for 3 weeks. At the end of the glycation period, α-crystallin and TSP preparations were dialysed against 20 mM sodium phosphate buffer (pH 7.4) to remove any unreacted sugars.
Evaluation of glycation
Non-tryptophan AGE fluorescence
Non-tryptophan AGE fluorescence was monitored using 0.15 mg/ml protein in 20 mM sodium phosphate buffer (pH 7.4), with excitation at 370 nm, and emission recorded between 400–500 nm using a spectroflorometer (Jasco FP-6500).
SDS/PAGE
The formation of HMM (high-molecular-mass) aggregates obtained as a result of protein cross-linking due to glycation was monitored using SDS/PAGE (10% gel).
Immunodetection of AGE
The formation of specific AGEs was detected with immnoblotting using anti-CML [Nϵ-(carboxymethyl)lysine], anti-MGO-BSA and anti-AGE-BSA antibodies. Polyclonal anti-CML antibodies were raised as described previously [29], anti-MGO-BSA and anti-AGE-BSA antibodies were raised according to previously reported methods [30,31]. Glycated TSP and α-crystallin preparations were resolved on SDS/PAGE (10% gel) and transferred on to a nitrocellulose membrane. The membrane was subjected to reaction with respective primary antibody (1:1000) and followed by peroxidase-conjugated goat anti-rabbit secondary antibody (1:3000) for subsequent detection using diaminobenzidine and H2O2.
Glyco-oxidative damage
The effect of modifications with different glycating agents on glyco-oxidative damage of α-crystallin was monitored by estimating total protein carbonyls according to the method of Uchida et al. [32].
Affinity chromatography
Extent of glycation in α-crystallin with various glycating agents was performed using phenylboronate affinity chromatography [33]. The ligand (m-aminophenylboronic acid–agarose beads) binds to the cis-diol groups on the sugar moiety of the glycated protein, forming a reversible five-member ring complex. These complexes were dissociated by competing with polyol such as sorbitol. In the present study, 5 mg of glycated preparations of α-crystallin were passed through a phenylboronate affinity column (8 cm×1 cm) equilibrated with buffer B [0.25 M ammonium acetate buffer (pH 8.5) containing 0.05 M MgCl2]. The unbound fraction containing non-glycated protein was washed with buffer B, while bound glycated protein was eluted using buffer C [0.1 M Tris/HCl (pH 7.5) containing 0.2 M sorbitol].
Chaperone-like activity assays
Chaperone-like activity of native and modified α-crystallin was assessed using two different types of chaperone assays: aggregation and enzyme inactivation. Aggregation assays were done by measuring the ability of α-crystallin in suppressing heat-induced aggregation of βL-crystallin (purified from control rat lenses) at 65 °C or CS at 45 °C as described previously [9]. A Cary100 spectrophotometer with a temperature-controlled cuvette holder was used to monitor the change in the absorption at 360 nm to measure protein aggregation as a function of time. In the enzyme inactivation assay, loss of G6PD activity due to heat in the presence of native and glycated α-crystallin was measured as described previously [34].
Secondary and tertiary structure
To measure secondary and tertiary structural changes due to non-enzymatic glycation, far- and near-UV CD spectra were recorded at 25 °C using a spectropolarimeter (Jasco J-810). All spectra were an average of six accumulations, and recorded using cells of 0.1 and 0.5 cm pathlength for far- and near-UV CD respectively. Protein concentration for far- and near-UV CD was 0.15 and 1.0 mg/ml respectively. Intrinsic tryptophan fluorescence was monitored using 0.15 mg/ml protein in 20 mM sodium phosphate buffer (pH 7.4), exciting at 280 nm and following the emission from 300–400 nm using a spectroflorometer.
Hydrophobicity of α-crystallin
We investigated the surface hydrophobicity as a function of ANS binding to α-crystallin. α-Crystallin (0.1 mg/ml) was incubated with 50 μM ANS for 30 min at room temperature in the dark, and the fluorescence of protein-bound dye was measured by excitation at 390 nm and measuring the emission between 450 and 550 nm using a spectroflorometer.
Gel-filtration chromatography
The gel-filtration profile of control- and fructose-glycated TSP was performed using a Superose 6 10/30 gel-filtration column connected to FPLC (AKTA-Purifier; Amersham Biosciences) at a flow rate of 0.5 ml/min.
RESULTS AND DISCUSSION
There now exists an overwhelming body of evidence indicating that non-enzymatic glycation of proteins is implicated in a number of biochemical abnormalities associated with aging and diabetes, including cataract formation [1,14–17,35–39]. A number of sugars and their metabolic derivatives are known to form AGEs upon their encounter with cellular proteins. High concentrations of these potential glycating agents were detected in the lens and their levels increase by several fold during diabetes [22,38–40]. Thus the role of these glycating agents and associated AGEs in the pathogenesis of diabetic cataracts has received considerable attention. Despite the large amount of information available on the pathological significance of AGEs in cataractogenesis, relatively little is known about the effect of AGE formation on the changes in structure and chaperone-like function of α-crystallin, in particular the relative modifications induced by different glycating agents. Although there is an increased evidence of glycation-mediated damage to lens proteins including α-crystallin, some studies have reported divergent results. For example, it has been reported that neither fructose nor G6P caused any decrease in the chaperone-like activity of α-crystallin [41]. One study indicated that glycation with galactose did not result in a loss of chaperone activity as assessed by enzyme inactivation studies [42]. Akhtar et al. [43] reported that the CML adducts of recombinant αA- and αB-crystallins formed on incubation of these proteins with glyoxalic acid in the presence of sodium borocyanate displayed greater chaperone activity than control crystallins. On the other hand, some reports state that glycation of α-crystallin is associated with decreased chaperone activity [22–25].
In the present study, we investigated alterations in the molecular chaperone-like function of α-crystallin, and the associated structural changes, upon modification with four major types of glycating agents, namely glucose, fructose, G6P and MGO. In addition, for the purpose of addressing the contribution of molecular associations between α-crystallin and other lenticular proteins on its chaperone function upon glycation, we have also performed studies with α-crystallin isolated from glycated TSP. This would help us to understand the effect of non-enzymatic glycation on the molecular chaperone function of α-crystallin in relation to cataracts.
Non-enzymatic glycation of α-crystallin
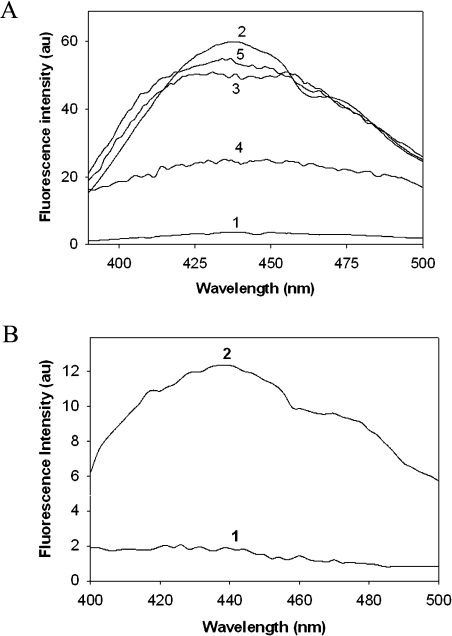
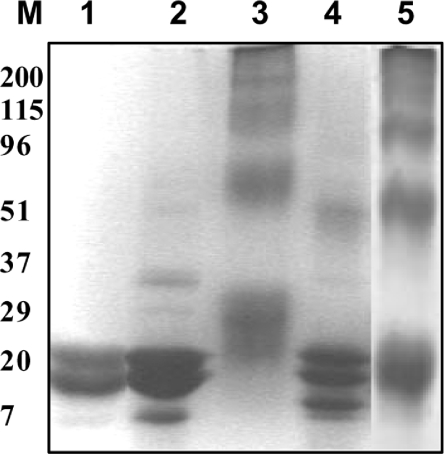
Non-tryptophan fluorescence, which represents cumulative AGE fluorescence, was monitored to assess the extent of modification by different glycating agents. AGE fluorescence increased significantly upon in vitro glycation of α-crystallin with all four glycating agents. However, glycation with G6P resulted in a relatively lower AGE fluorescence when compared with modification by glucose, fructose and MGO (Figure 1A). Increasing the concentration of G6P beyond 50 mM did not result in any further increment in AGE fluorescence (results not shown). Glycation of proteins is known to result in inter- and intra-subunit cross-linking that leads to the formation of HMM species [15]. Irrespective of glycating agent, modified α-crystallin showed formation of cross-links that may result in the formation of HMM aggregates. However, it is interesting to note that the cross-link profile formed on glycation is different for different glycating agents. Modification with glucose and MGO resulted in extensive cross-link formation compared with glycation with fructose and G6P (Figure 2).
Figure 1. Non-tryptophan AGE fluorescence of α-crystallin.
(A) Non-tryptophan AGE fluorescence of native and glycated α-crystallin. Native α-crystallin (trace 1); α-crystallin modified with 0.5 M glucose (trace 2), 0.1 M fructose (trace 3), 0.05 M G6P (trace 4) and 0.005 M MGO (trace 5). (B) Non-tryptophan AGE fluorescence of α-crystallin isolated from native TSP (trace 1) and α-crystallin isolated from TSP glycated with fructose (trace 2).
Figure 2. SDS/PAGE analysis of in-vitro-glycated α-crystallin.
Native α-crystallin (lane 1), and α-crystallin modified with 0.1 M fructose (lane 2), 0.005 M MGO (lane 3), 0.05 M G6P (lane 4) and 0.5 M glucose (lane 5).
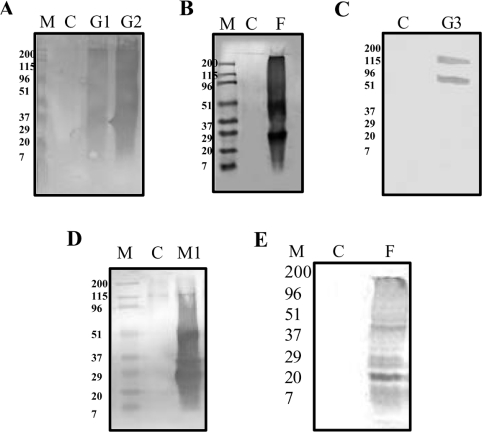
Considerable importance can be accorded to AGEs that are formed on long-lived proteins as they have been reported in the pathogenesis of several aging- and diabetes-related complications, including cataracts. Despite intensive research, very few of the AGEs have been characterized, including CML, a product of the auto-oxidation of sugar adducts on proteins [44]. Immunoblotting studies revealed that CML was the predominant AGE formed on glycation of α-crystallin with glucose and fructose (Figures 3A and 3B). Although G6P modification of α-crystallin resulted in the formation of glucose-AGE (Figure 3C), MGO modification resulted in the formation of MGO-AGE (Figure 3D). As expected, MGO-AGE could only be seen with MGO modification. In addition, formation of CML was also observed with MGO modification (results not shown). Intriguingly, glucose-AGE (which was prepared upon incubation of BSA with glucose) was not detected in the α-crystallin preparation that was incubated with glucose. These results suggest that CML could be a major AGE species formed on glycation of α-crystallin with glucose, fructose and MGO. This observation is in agreement with earlier studies which report that CML, as a major AGE, was formed on incubation of proteins with sugars [44,45].
Figure 3. Characterization of AGEs by immunoblotting.
Immunoblotting of α-crystallin with anti-CML (A, B and E), anti-AGE-BSA (C) and anti-MGO-BSA (D) antibodies. (A) Glycation of α-crystallin with glucose resulted in formation of CML. M, molecular-mass markers (in kDa); C, control; G1 and G2, α-crystallin glycated with 0.5 M and 1.0 M glucose respectively. (B) Glycation of α-crystallin with fructose resulted in formation of CML. M, molecular-mass markers; C, control; F, α-crystallin glycated with 0.1 M fructose. (C) Glycation of α-crystallin with G6P resulted in formation of AGE-BSA. C, control; G3, α-crystallin glycated with 0.05 M G6P. (D) Glycation of α-crystallin with MGO results in formation of MGO-AGE. M, molecular-mass markers; C, control; M1, modified with 0.005 M MGO. (E) α-Crystallin isolated from control TSP (C) and 0.1 M fructose-glycated TSP (F).
Accumulation of protein carbonyls by glyco-oxidative damage may contribute to protein cross-linking, and thereby protein dysfunction is evident in various age-related pathologies and is characterized by protein cross-linking. Hence we measured protein carbonyls to assess the extent of protein glycation. α-Crystallin incubated with glucose, fructose, G6P and MGO resulted in the formation of protein carbonyls (1.8 nmol/mg of protein for control, and 9.0, 12.0, 7.2 and 9.4 nmol/mg of protein for glucose-, fructose-, G6P- and MGO-modified protein respectively). Results suggests that all the agents contributed similarly to glyco-oxidation of α-crystallin.
Estimation of protein glycation
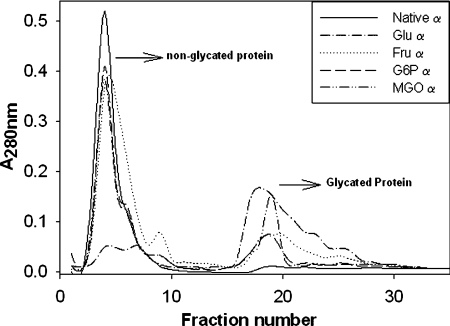
α-Crystallin modified with different sugars was subjected to affinity chromatography that selectively binds glycated proteins. As shown in Figure 4, elution of glycated protein suggests that modification by all four agents led to the formation of glycated protein, albeit at different levels. The highest level of glycated protein was observed upon modification with glucose followed by MGO, fructose and G6P (Figure 4). It is interesting to note that glycation with glucose and MGO, which resulted in extensive cross-linking (Figure 2), also resulted in the formation of protein that binds to the phenylboronate column with high affinity.
Figure 4. Phenylboronate affinity chromatogram.
α-Crystallin (5 mg), native or glycated with the various agents, was applied to column. After washing the unbound fraction with 0.25 mM ammonium acetate, the bound fraction was eluted with 0.1 M Tris/HCl (pH 7.5) containing 0.2 M sorbitol.
Effect of glycation on the chaperone-like function of α-crystallin
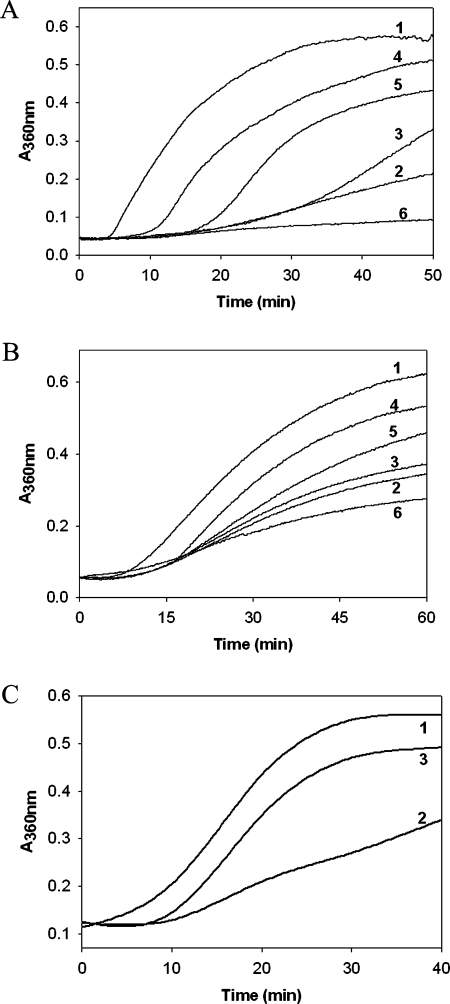
α-Crystallin modified with fructose and G6P was found to have decreased chaperone-like activity in preventing the heat-induced aggregation of βL-crystallin compared with unmodified α-crystallin (Figure 5A). There was only a modest decrease in the chaperone-like activity of α-crystallin on glycation with glucose in heat-induced aggregation of βL-crystallin, whereas MGO-modified α-crystallin had more chaperone-like activity than unmodified α-crystallin (Figure 5A). In assays that measure heat-induced aggregation of CS, fructose- and G6P-modified α-crystallin showed diminished chaperone-like activity, similar to the heat-induced βL-crystallin aggregation assay (Figure 5B). This was also true for glucose-modified α-crystallin, which again had a marginal decrease in chaperone-like activity, whereas MGO-modified α-crystallin showed an increase in chaperone-like activity in comparison with unmodified α-crystallin (Figure 5B). However, it should be noted that the agents which cause extensive cross-linking, and form glycated protein with higher affinity to phenylboronate, showed the least effect in aggregation assays. We have reported previously that an increase in the chaperone-like activity of α-crystallin upon MGO modification in aggregation assays might not be relevant to physiological situations [22]. This is because MGO modification of α-crystallin leads to extensive cross-linking and formation of very large aggregates that could provide paradoxical results in terms of its chaperone function [22]. Therefore, besides aggregation assays, we also investigated the effect of glycation on the ability of α-crystallin to prevent the heat-induced inactivation of G6PD, a non-aggregative chaperone assay. Interestingly, modification with all four glycating agents led to the loss of chaperone-like activity as measured against heat-induced inactivation of G6PD (results not shown). This includes MGO-modified α-crystallin, which also had a loss of chaperone-like activity in enzyme inactivation assays, in contrast with the aggregation assays. In fact, there was the greatest loss of α-crystallin chaperone activity with MGO-modified α-crystallin in the G6PD heat-inactivation assay, whereas fructose- and G6P-modified α-crystallin had a moderate loss of chaperone activity. There was only a partial loss of the chaperone activity of α-crystallin upon glucose modification. In general, it is believed that cross-linking and HMM aggregate formation results in the decreased chaperone activity of α-crystallin. In contrast, glucose modification resulted in HMM cross-linking but unexpectedly led to a marginal loss in chaperone function. Previously, it was reported that artificially generated CML adducts of recombinant αA- and αB-crystallins showed increased chaperone-like activity [43]. In the present study, the CML antigen was detected with all the glycating agents used, whereas CML caused decreased chaperone activity. Thus it appears that the effect of glycation on chaperone activity may vary, based on the marker used for glycation as well as the chaperone assays employed.
Figure 5. Chaperone-like activity of α-crystallin.
(A) Chaperone-like activity of α-crystallin as assessed by the suppression of heat-induced aggregation of βL-crystallin. βL-crystallin (0.2 mg/ml in 50 mM phosphate buffer pH 7.4) was incubated at 65 °C in the absence (trace 1) or in the presence of 0.025 mg/ml native α-crystallin (trace 2) or α-crystallin glycated with glucose (trace 3), fructose (trace 4), G6P (trace 5) or MGO (trace 6). The results shown were an average of three assays. (B) Chaperone-like activity of α-crystallin as assessed by the suppression of heat-induced aggregation of CS. CS (0.05 mg/ml) was incubated at 45 °C in the absence (trace 1) or presence of 0.025 mg/ml native α-crystallin (trace 2) or α-crystallin glycated with glucose (trace 3), fructose (trace 4), G6P (trace 5) or MGO (trace 6). The results shown were an average of three assays. (C) Chaperone-like activity of α-crystallin isolated from TSP as assessed by the suppression of heat-induced aggregation of βL-crystallin. βL-crystallin aggregation in the absence of α-crystallin (trace 1), in the presence of α-crystallin isolated from control TSP (trace 2) or in the presence of α-crystallin isolated from TSP glycated with fructose (trace 3).
Effect of glycation on the structural conformation of α-crystallin
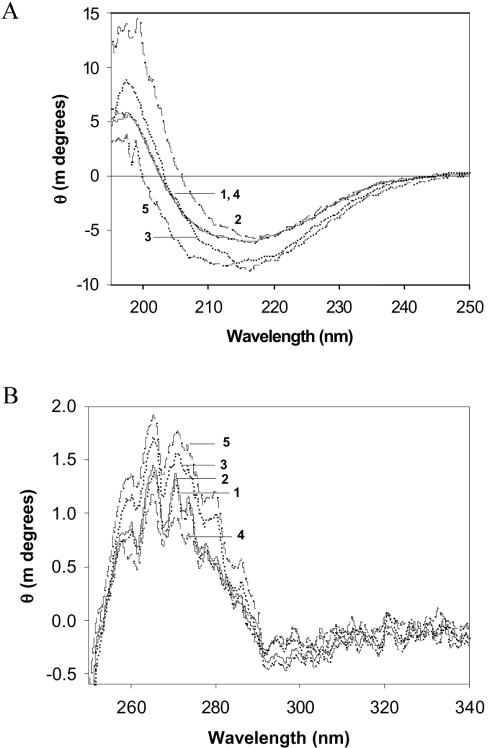
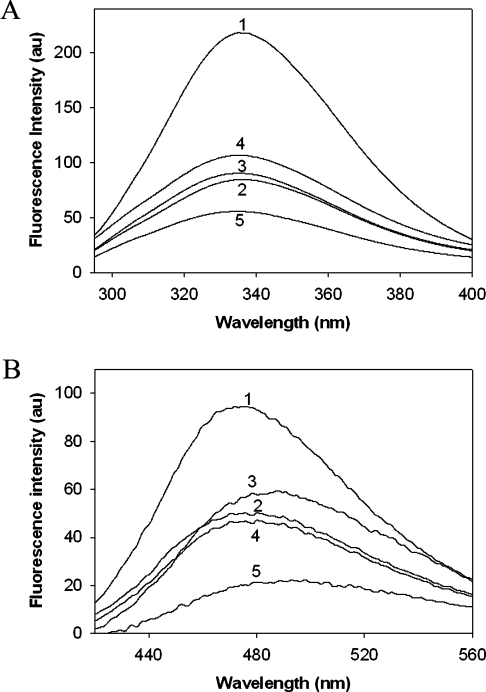
In order to understand the mechanism of loss in chaperone-like activity following non-enzymatic glycation, we have measured alterations in the secondary and tertiary structure by CD and fluorescence spectroscopy. Native α-crystallin has a maximum negative ellipticity at approx. 217 nm (Figure 6A), typical of a β-sheet structure as reported previously [9,22]. The far-UV CD signal for fructose-modified α-crystallin is increased and is shifted to lower wavelengths for MGO-modified α-crystallin (Figure 6A). These changes in the secondary structure suggest partial unfolding of α-crystallin upon fructose modification or the formation of random coil structures upon MGO modification. Surprisingly, no significant changes in the secondary structure were observed in α-crystallin modified with glucose and G6P. Furthermore, near-UV CD spectra of fructose- and MGO-modified α-crystallin demonstrated an altered signal in the aromatic region, suggesting conformational changes at a tertiary structural level due to glycation (Figure 6B). In contrast with the CD data, where glucose and G6P modification showed marginal changes (Figure 6), significant loss of tryptophan florescence was observed upon modification with all four agents (Figure 7A). This suggests that altered tryptophan fluorescence might be due to changes in the local environment upon glycation.
Figure 6. UV CD spectra of α-crystallin.
Far-UV CD spectra (A) and near-UV CD (B) spectra of α-crystallin. Trace 1, native α-crystallin; traces 2–5 correspond to α-crystallin modified with glucose, fructose, G6P and MGO respectively.
Figure 7. α-Crystallin fluorescence.
Intrinsic tryptophan fluorescence (A) and ANS fluorescence (B) of α-crystallin. Trace 1, native α-crystallin; traces 2–5 correspond to α-crystallin modified with glucose, fructose, G6P and MGO respectively.
Surface hydrophobicity
A number of studies suggest that the chaperone-like activity of α-crystallin is mediated by the presence of surface-exposed hydrophobic patches that bind, partially denaturing substrate proteins, a process mediated by hydrophobic–hydrophobic interactions [5,46–48]. We therefore measured the binding of ANS, a hydrophobic probe. Upon binding to hydrophobic sites on proteins, it displays a shift in its emission maximum that allowed us to quantify the hydrophobicity of a protein. At a saturating ANS concentration, the fluorescence intensity of ANS associated with modified α-crystallin was lower when compared with native α-crystallin, and a similar effect was observed with all four glycating agents (Figure 7B). This suggests that the decrease in the hydrophobicity of glucose-, fructose- and G6P-modified α-crystallin is associated with diminished chaperone-like activity. However, this does not correlate well in the case of MGO-modified α-crystallin in aggregation assays. In aggregation assays, MGO modification of α-crystallin led to an increase in its activity which is inversely correlated with the surface hydrophobicity. It appears that non-aggregation assays might be considered appropriate to study the chaperone-like activity, particularly for MGO-modified α-crystallin.
Non-enzymatic glycation of TSP and chaperone activity of α-crystallin
Having observed a few contrasting results upon glycation of isolated α-crystallin with different glycating agents, we investigated the effect of glycation on α-crystallin in the presence of other lens-soluble components because α-crystallin in the lens is shown to interact with various other proteins in a specific fashion to maintain the transparency of the lens [6]. Therefore it is appropriate to study the extent of glycation and its effect on α-crystallin along with other lens-soluble proteins. TSP is composed of other crystallins, mainly β- and γ-crystallin, in addition to other minor protein components and small molecules. The gel-filtration profile of glycated TSP shows an increased HMM peak and alterations in the α-, β- and γ-crystallin fractions (results not shown). Furthermore, α-crystallin isolated from TSP which was glycated with fructose also showed increased glycation, as assessed by AGE fluorescence (Figure 1B). In addition, CML was the predominant AGE in the α-crystallin fraction isolated from glycated TSP (Figure 3E). More importantly, α-crystallin isolated from fructose-modified TSP showed a decreased chaperone-like activity (Figure 5C). Thus it is evident that α-crystallin could be subjected to glycation-induced changes even in the presence of other lenticular proteins, and this could affect the protein–protein interactions of α-crystallin with other lenticular proteins. α-Crystallin isolated from lenses cultured for 2 weeks in the presence of either 55 mM glucose (P. A. Kumar and G. B. Reddy, unpublished results) or 1 mM MGO [22] also showed loss of chaperone activity. Thus these changes may play a role in the pathogenesis of cataracts in aging and diabetes.
To summarize, we propose that non-enzymatic glycation of α-crystallin by various sugars and sugar derivatives leads to alterations in secondary and tertiary structure. Glycation of α-crystallin results in the formation of HMM aggregates and AGEs. These changes may be responsible for the loss of chaperone-like activity in α-crystallin. In order to support this, α-crystallin isolated from in-vitro-modified TSP showed elevated AGE fluorescence and accumulation of CML, which was accompanied by a substantial loss in chaperone-like activity. Nevertheless, one has to be very cautious in drawing conclusions in terms of the effect of glycation on chaperone activity, which varies based on the marker used for glycation, as well the chaperone assays employed.
Acknowledgments
This work was supported by grants from the Department of Science and Technology, New Delhi, India (SP/SO/B-44/2001). P. A. K. was supported by a research fellowship from the Council of Scientific and Industrial Research, New Delhi, India.
References
- 1.Harding J. J. London: Chapman and Hall; 1991. Cataract: Biochemistry, Epidemiology and Pharmacology. [Google Scholar]
- 2.Derham B. K., Harding J. J. α-Crystallin as a molecular chaperone. Prog. Retin. Eye Res. 1999;18:463–509. doi: 10.1016/s1350-9462(98)00030-5. [DOI] [PubMed] [Google Scholar]
- 3.MacRae T. H. Structure and function of small heat shock/α-crystallin proteins: established concepts and emerging ideas. Cell. Mol. Life Sci. 2000;57:899–913. doi: 10.1007/PL00000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horwitz J. α-Crystallin. Exp. Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 5.Reddy G. B., Kumar P. A., Kumar M. S. Chaperone-like activity and hydrophobicity of α-crystallin. IUBMB Life. 2006;58:632–641. doi: 10.1080/15216540601010096. [DOI] [PubMed] [Google Scholar]
- 6.Bloemendal H., de Jong W., Jaenicke R., Lubsen N. H., Slingsby C., Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog. Biophys. Mol. Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Yan H., Harding J. J., Hui Y. N., Li M. Y. Decreased chaperone activity of α-crystallin in selenite cataract may result from selenite-induced aggregation. Eye. 2003;17:637–645. doi: 10.1038/sj.eye.6700419. [DOI] [PubMed] [Google Scholar]
- 8.Kelley M. J., David L. L., Iwasaki N., Wright J., Shearer T. R. α-Crystallin chaperone activity is reduced by calpain II in vitro and in selenite cataract. J. Biol. Chem. 1993;268:18844–18849. [PubMed] [Google Scholar]
- 9.Kumar P. A., Suryanarayana P., Reddy P. Y., Reddy G. B. Modulation of α-crystallin chaperone activity in diabetic rat lens by curcumin. Mol. Vision. 2005;11:561–568. [PubMed] [Google Scholar]
- 10.Huang F. Y., Ho Y., Shaw T. S., Chuang S. A. Functional and structural studies of α-crystallin from galactosemic rat lenses. Biochem. Biophys. Res. Commun. 2000;273:197–202. doi: 10.1006/bbrc.2000.2924. [DOI] [PubMed] [Google Scholar]
- 11.Abraham A. G., Condon N. G., West Gower E. The new epidemiology of cataract. Ophthalmol. Clin. North Am. 2000;19:415–425. doi: 10.1016/j.ohc.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Rowe N. G., Mitchell P. G., Cumming R. G., Wans J. J. Diabetes, fasting blood glucose and age-related cataract: the Blue Mountains eye study. Ophthalmic Epidemiol. 2000;7:103–114. [PubMed] [Google Scholar]
- 13.Hennis A., Wu S. Y., Nemesure B., Leske M. C. Barbados eye studies group. Risk factors for incident cortical and posterior subcapsular lens opacities in the Barbados eye studies. Arch. Ophthalmol. 2004;122:525–530. doi: 10.1001/archopht.122.4.525. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed N. Advanced glycation end products: role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 15.van Boekel M. A., Hoenders H. J. Glycation of crystallins in lenses from aging and diabetic individuals. FEBS Lett. 1992;314:1–4. doi: 10.1016/0014-5793(92)81446-s. [DOI] [PubMed] [Google Scholar]
- 16.van Boekel M. A. The role of glycation in aging and diabetes mellitus. Mol. Biol. Rep. 1991;15:57–64. doi: 10.1007/BF00364840. [DOI] [PubMed] [Google Scholar]
- 17.Monnier V. M., Nagaraj R. H., Portero-Otin M., Glomb M., Elgawish A. H., Sell D. R., Friedlander M. A. Structure of advanced Maillard reaction products and their pathological role. Nephrol. Dial. Transplant. 1996;11(Suppl. 5):20–26. doi: 10.1093/ndt/11.supp5.20. [DOI] [PubMed] [Google Scholar]
- 18.Beswick H. T., Harding J. J. Conformational changes induced in lens α- and γ-crystallins by modification with glucose 6-phosphate. Implications for cataract. Biochem J. 1987;246:761–769. doi: 10.1042/bj2460761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swamy M. S., Abraham E. C. Differential glycation of rat α-, β- and γ-crystallins. Exp. Eye Res. 1991;52:439–444. doi: 10.1016/0014-4835(91)90040-l. [DOI] [PubMed] [Google Scholar]
- 20.Swamy M. S., Abraham A., Abraham E. C. Glycation of human lens proteins: preferential glycation of αA subunits. Exp. Eye Res. 1992;54:337–345. doi: 10.1016/0014-4835(92)90046-u. [DOI] [PubMed] [Google Scholar]
- 21.Cherian M., Abraham E. C. Decreased molecular chaperone property of α-crystallins due to posttranslational modifications. Biochem. Biophys. Res. Commun. 1995;208:675–679. doi: 10.1006/bbrc.1995.1391. [DOI] [PubMed] [Google Scholar]
- 22.Kumar M. S., Reddy P. Y., Kumar P. A., Surolia I., Reddy G. B. Effect of dicarbonyl-induced browning on α-crystallin chaperone-like activity: physiological significance and caveats of in vitro aggregation assays. Biochem. J. 2004;379:273–282. doi: 10.1042/BJ20031633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derham B. K., Harding J. J. Effects of modifications of α-crystallin on its chaperone and other properties. Biochem. J. 2002;364:711–717. doi: 10.1042/BJ20011512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plater M. L., Goode D., Crabbe M. J. Ibuprofen protects α-crystallin against posttranslational modification by preventing protein cross-linking. Ophthalmic Res. 1997;29:421–428. doi: 10.1159/000268043. [DOI] [PubMed] [Google Scholar]
- 25.Yan H., Harding J. J. Carnosine inhibits modifications and decreased molecular chaperone activity of lens α-crystallin induced by ribose and fructose 6-phosphate. Mol. Vision. 2006;12:205–214. [PubMed] [Google Scholar]
- 26.Cherian M., Abraham E. C. Diabetes affects α-crystallin chaperone function. Biochem. Biophys. Res. Commun. 1995;212:184–189. doi: 10.1006/bbrc.1995.1954. [DOI] [PubMed] [Google Scholar]
- 27.Thampi P., Zarina S., Abraham E. C. α-Crystallin chaperone function in diabetic rat and human lenses. Mol. Cell. Biochem. 2002;229:113–118. doi: 10.1023/a:1017980713089. [DOI] [PubMed] [Google Scholar]
- 28.Nagaraj R. H., Oya-Ito T., Padayatti P. S., Kumar R., Mehta S., West K., Levison B., Sun J., Crabb J. W., Padival A. K. Enhancement of chaperone function of α-crystallin by methylglyoxal modification. Biochemistry. 2003;42:10746–10755. doi: 10.1021/bi034541n. [DOI] [PubMed] [Google Scholar]
- 29.Kumar P. A. PhD thesis. Hyderabad, India: Osmania University; 2007. Structure, function and expression of α-crystallin under hyper glycaemic conditions: modulation by dietary factors. [Google Scholar]
- 30.Kumar M. S. PhD Thesis. Hyderabad, India: Osmania University; 2005. Molecular chaperone function of α-crystallin. [Google Scholar]
- 31.Booth A. A., Khalifah R. G., Todd P., Hudson B. G. In vitro kinetic studies of formation of antigenic advanced glycation end products (AGEs) J. Biol. Chem. 1997;272:5430–5437. doi: 10.1074/jbc.272.9.5430. [DOI] [PubMed] [Google Scholar]
- 32.Uchida K., Kanematsu M., Sakai K., Matsuda T., Hattori N., Mizuno Y., Suzuki D., Miyata T., Noguchi N., Niki E., Osawa T. Protein-bound acrolein: potential markers for oxidative stress. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4882–4887. doi: 10.1073/pnas.95.9.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moghaddam M. S., Kumar P. A., Reddy G. B., Ghole V. S. Effect of diabecon on sugar-induced lens opacity in organ culture: mechanism of action. J. Ethanopharmacol. 2005;97:397–403. doi: 10.1016/j.jep.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 34.Reddy G. B., Reddy P. Y., Suryanarayana P. αA- and αB-crystallins protect glucose-6-phosphate dehydrogenase against UVB irradiation-induced inactivation Biochem. Biophys. Res. Commun. 2001;282:712–716. doi: 10.1006/bbrc.2001.4642. [DOI] [PubMed] [Google Scholar]
- 35.Thorpe S. R., Baynes J. W. Role of the Maillard reaction in diabetes mellitus and diseases of aging. Drugs Aging. 1996;9:69–77. doi: 10.2165/00002512-199609020-00001. [DOI] [PubMed] [Google Scholar]
- 36.Vlassara H., Brownlee M., Cerami A. Nonenzymatic glycosylation: role in the pathogenesis of diabetic complications. Clin. Chem. 1987;32(Suppl. 10):B37–B41. [PubMed] [Google Scholar]
- 37.Abraham E. C., Swamy M. S., Perry R. E. Nonenzymatic glycosylation (glycation) of lens crystallins in diabetes and aging. Prog. Clin. Biol. Res. 1989;304:123–139. [PubMed] [Google Scholar]
- 38.Lyons T. J., Silvestri G., Dunn J. A., Dyer D. G., Baynes J. W. Role of glycation in modification of lens crystallins in diabetic and nondiabetic senile cataracts. Diabetes. 1991;40:1010–1015. doi: 10.2337/diab.40.8.1010. [DOI] [PubMed] [Google Scholar]
- 39.Chellan P., Nagraj R. H. Protein crosslinking by the Maillard reaction: dicarbonyl-derived imidazolium crosslinks in aging and diabetes. Arch. Biochem. Biophys. 1999;368:98–104. doi: 10.1006/abbi.1999.1291. [DOI] [PubMed] [Google Scholar]
- 40.Thornalley P. J. The glyoxalase system in health and disease. Mol. Aspects. Med. 1993;14:287–371. doi: 10.1016/0098-2997(93)90002-u. [DOI] [PubMed] [Google Scholar]
- 41.van Boekel M. A., Hoogakker S. E., Harding J. J., de Jong W. W. The influence of some post-translational modifications on the chaperone-like activity of α-crystallin. Ophthalmic Res. 1996;28(Suppl. 1):32–38. doi: 10.1159/000267940. [DOI] [PubMed] [Google Scholar]
- 42.Blakytny R., Carver J. A., Harding J. J., Kilby G. W., Sheil M. M. A spectroscopic study of glycated bovine α-crystallin: investigation of flexibility of the C-terminal extension, chaperone activity and evidence for diglycation. Biochem. Biophys. Acta. 1997;1343:299–315. doi: 10.1016/s0167-4838(97)00145-3. [DOI] [PubMed] [Google Scholar]
- 43.Akhtar N. J., Sun T. X., Liang J. J. Conformational study of Nϵ-(carboxymethyl)lysine adducts of recombinant α-crystallins. Curr. Eye Res. 1999;18:270–276. doi: 10.1076/ceyr.18.4.270.5364. [DOI] [PubMed] [Google Scholar]
- 44.Ikeda K., Higashi T., Sano H., Jinnouchi Y., Yoshida M., Araki T., Ueda S., Horiuchi S. Nϵ-(carboxymethyl)lysine protein adduct is a major immunological epitope in proteins modified with advanced glycation end products of the Maillard reaction. Biochemistry. 1996;35:8075–8083. doi: 10.1021/bi9530550. [DOI] [PubMed] [Google Scholar]
- 45.Reddy S., Bichler J., Wells-Knecht K. J., Thorpe S. R., Baynes J. W. Nϵ-(carboxymethyl)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry. 1995;34:10872–10878. doi: 10.1021/bi00034a021. [DOI] [PubMed] [Google Scholar]
- 46.Reddy G. B., Das K. P., Petrash J. M., Surewicz W. K. Temperature-dependent chaperone activity and structural properties of human αA- and αB-crystallins. J. Biol. Chem. 2000;275:4565–4570. doi: 10.1074/jbc.275.7.4565. [DOI] [PubMed] [Google Scholar]
- 47.Das K. P., Surewicz W. K. Temperature induced exposure of hydrophobic surfaces and its effect on the chaperone activity of α-crystallin. FEBS Lett. 1995;369:321–325. doi: 10.1016/0014-5793(95)00775-5. [DOI] [PubMed] [Google Scholar]
- 48.Raman B., Rao C. M. Chaperone-like activity and quaternary structure of α-crystallin. J. Biol. Chem. 1994;269:27264–27268. [PubMed] [Google Scholar]