Abstract
Rationale: Tissue fibrosis is considered a dysregulated wound-healing response. Fibronectin containing extra type III domain A (EDA) is implicated in the regulation of wound healing. EDA-containing fibronectin is deposited during wound repair, and its presence precedes that of collagen.
Objectives: To investigate the role of EDA-containing fibronectin in lung fibrogenesis.
Methods: Primary lung fibroblasts from patients with idiopathic pulmonary fibrosis or from patients undergoing resection for lung cancer were assessed for EDA-containing fibronectin and α-smooth muscle actin (α-SMA) expression. Mice lacking the EDA domain of fibronectin and their wild-type littermates were challenged with the bleomycin model of lung fibrosis. Primary lung fibroblasts from these mice were assayed in vitro to determine the contribution of EDA-containing fibronectin to fibroblast phenotypes.
Measurements and Main Results: Idiopathic pulmonary fibrosis lung fibroblasts produced markedly more EDA-containing fibronectin and α-SMA than control fibroblasts. EDA-null mice failed to develop significant fibrosis 21 days after bleomycin challenge, whereas wild-type controls developed the expected increase in total lung collagen. Histologic analysis of EDA-null lungs after bleomycin showed less collagen and fewer α-SMA–expressing myofibroblasts compared with that observed in wild-type mice. Failure to develop lung fibrosis in EDA-null mice correlated with diminished activation of latent transforming growth factor (TGF)-β and decreased lung fibroblast responsiveness to active TGF-β in vitro.
Conclusions: The data show that EDA-containing fibronectin is essential for the fibrotic resolution of lung injury through TGF-β activation and responsiveness, and suggest that EDA-containing fibronectin plays a critical role in tissue fibrogenesis.
Keywords: fibrosis, fibronectin, TGF-β, myofibroblast
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Extra type III domain A (EDA) fibronectin is important in wound healing, where it is necessary for fibroblast activation. Pulmonary fibrosis reflects a dysregulated wound-healing response.
What This Study Adds to the Field
In this study, EDA fibronectin was necessary for pulmonary fibrosis to develop. In the absence of EDA fibronectin, fibroblasts were unable to differentiate into myofibroblasts or to fully activate latent transforming growth factor-β.
Fibroproliferative disorders, characterized by the increased production and deposition of extracellular matrix (ECM) proteins in tissues, are not well understood despite major efforts to elucidate pathogenic mechanisms. Recent attention has focused on ECM components and mesenchymal cell phenotypes as being critical to the development of fibrosis (1, 2). The ECM is a highly dynamic complex that varies in composition according to its tissue localization and physiologic circumstances. Collagens are the predominant ECM proteins identified in fibrotic lesions and are the hallmark of fibroproliferative diseases, but fibronectins (FNs) are also present in abnormally large quantities and localize to areas of active fibrogenesis (3, 4).
FNs are multifunctional glycoproteins found in the ECM of tissues and plasma. Two main forms of FN exist: plasma FN, a dimeric and soluble form produced by hepatocytes that lacks the EDA and EDB sequences, and cellular FN (cFN), a multimeric form synthesized by mesenchymal, epithelial, and inflammatory cells, which is deposited in ECM fibrils and that contains variable proportions of the extra type III domains A and B (EDA and EDB) (5). Although the function or functions of these domains have not been fully elucidated, attention has focused on the contribution of EDA-containing FN (EDA cFN) in fibroblast activation (6) and in wound healing (7).
Physiologic (or pathologic) factors that induce EDA cFN production are poorly defined, although the molecular mechanisms regulating alternative EDA exon splicing have been well described (8, 9). Interestingly, one known factor that promotes EDA inclusion into FN is transforming growth factor (TGF)-β1, a cytokine implicated in the pathogenesis of fibroproliferative disorders (10). Treatment of normal human fibroblasts with picomolar quantities of TGF-β results in a two- to threefold increase of EDA cFN relative to total FN (11). In addition, EDA cFN is necessary for the induction of the myofibroblastic phenotype by TGF-β (6). Therefore, because TGF-β is present at sites of fibrosis (12) and is a profibrotic growth factor, one of the pathways by which TGF-β promotes fibrogenesis could be via the induction of EDA cFN, which in turn promotes differentiation of mesenchymal cells into fibrogenic myofibroblasts.
Numerous lines of evidence support a role for EDA in tissue fibrosis. In surgical lung biopsy specimens from patients with idiopathic pulmonary fibrosis (IPF), a progressive fibroproliferative disorder of the lung, EDA cFN can be identified in fibroblastic foci (the presumed site of active fibrogenesis) interspersed between fibroblasts and collagens (13). In experimental models, EDA cFN expression occurs early after lung injury, preceding collagen deposition (3). Importantly, mice lacking the ability to generate EDA cFN (EDA−/−) do not heal cutaneous wounds appropriately, and instead develop ulcerated lesions that undergo abnormal, disordered reepithelialization (7). Despite these data, the in vivo requirement for EDA cFN in the development and progression of tissue fibrosis has not yet been demonstrated.
METHODS
Primary Lung Fibroblast Isolation
Primary human lung fibroblasts and lung fibroblasts from EDA−/− and wild-type (WT) mice were isolated as we have previously described (14). Written, informed consent was obtained from all subjects in accordance with the University of Michigan Institutional Review Board. Cells were maintained in Dulbecco's modified Eagle medium with 10% fetal calf serum, antibiotics, glutamine, and N-2-hydroxyethylpiperazone-N′-ethane sulfonic acid.
FN Purification
For experiments using purified EDA-containing FN, IMR-90 (Institute for Medical Research, Camden, NJ) human lung fibroblasts were cultured in growth media containing TGF-β1 (2 ng/ml) for 5 days after achieving confluence. Cell surface–associated FN was purified using affinity chromatography as described elsewhere (15). Purity was confirmed by Coomassie staining and by Western blot for EDA FN (not shown).
EDA-Transgenic Mice, Intratracheal Bleomycin Administration, and Bronchoalveolar Lavage
EDA−/− mice have been described previously (7). WT littermates were used as controls. Animals were bred and housed at the International Centre for Genetic Engineering and Biotechnology (ICGEB) (Trieste, Italy) and the University of Michigan (Ann Arbor, MI). Experimental procedures were approved by the Animal Care and Use Committees of each institution. Bleomycin experiments were performed as we have previously described (16). Bronchoalveolar lavage (BAL) was performed by tracheal cannulation, and lungs were lavaged twice with 750 μl phosphate-buffered saline (PBS)/ethylenediaminetetraacetic acid. Cytospin preparations of equal numbers of cells were stained for differential cell count determination.
Immunohistochemistry
Immunohistochemistry on paraffin-embedded sections was performed as previously described (16) with Masson's trichrome, or with anti–α-smooth muscle actin (anti–α-SMA) antibody (clone 1A4; Dako Corporation, Carpinteria, CA) or anti–phospho-Smad2 antibody (catalog no. 3101; Cell Signaling Technology, Beverly, MA) followed by color development with DAB. A Nikon Eclipse 50i microscope (Nikon Instruments, Melville, NY) was used to visualize and photograph sections. Immunohistochemistry for β6-integrins was performed as previously described (17).
Hydroxyproline Assay
Collagen content was measured in lungs via a conventional hydroxyproline assay (18). Experimental results were quantitated by comparison to a standard curve of known hydroxyproline concentrations.
Western Blot
Western blot analysis of cellular lysates was performed as previously described (16). Antibody against the EDA of FN (clone 3E2) was from Sigma (St. Louis, MO).
Sircol Assay
Sircol assay (Biocolor Ltd., Carrickfergus, UK), measuring soluble collagens, was performed following the manufacturer's instructions as previously described (16).
Semiquantitative Reverse Transcriptase–Polymerase Chain Reaction
Semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) was performed as previously described (16). Primer and probe sequences are reported in the online supplement (Table E1).
TGF-β Activation Assay
TGF-β activation was assessed using the method of Munger and colleagues (17) with minor modifications. Briefly, 2 × 105 EDA−/− or WT lung fibroblasts were cocultured overnight with equal numbers of mink lung epithelial cells (MLECs) stably expressing luciferase coupled to the plasminogen activator inhibitor (PAI)-1 promoter. Cells were assessed for luminescence using a standard reporter assay (Promega, Madison, WI). Luminescence from cocultures was normalized to untreated MLECs alone. MLECs treated with escalating concentrations of active TGF-β1 were used to generate a luminescence standard curve against which raw data were plotted. Results were expressed as relative TGF-β activity.
RESULTS
Lung Fibroblasts from Patients with IPF Express More EDA cFN and α-SMA Than Those from Control Patients
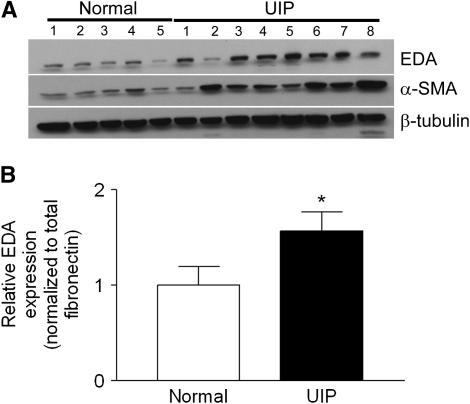
EDA cFN is expressed in the fibroblastic foci of IPF (Reference 13 and Figure E1); thus, we postulated that EDA cFN might be an important contributor to lung fibrosis. To assess this possibility, we determined EDA cFN expression by Western blot in primary human IPF and normal lung fibroblasts. We found that IPF lung fibroblasts expressed markedly more EDA cFN than control lung fibroblasts (Figure 1A). When the membrane was stripped and reprobed for α-SMA, a marker of fibroblast activation, we observed that increased α-SMA expression in IPF fibroblasts accompanied the increase in EDA cFN (Figure 1A). To ensure that the increase in EDA cFN expression was not due solely to increased total FN production, we performed quantitative RT-PCR for both EDA cFN and total FN on RNA harvested from IPF lung fibroblasts (n = 8) and normal lung fibroblasts (n = 8). Although we observed an increase in total FN expression from IPF lung fibroblasts (not shown), the proportion of transcripts including the EDA domain had also increased to approximately 1.5 times that of normal lung fibroblasts (Figure 1B).
Figure 1.
Extra type III domain A (EDA) cellular fibronectin (cFN) regulation in fibroblasts derived from patients with usual interstitial pneumonia (UIP). (A) EDA cFN and α-smooth muscle actin (α-SMA) expression are markedly increased by Western blot in lung fibroblasts from patients with idiopathic pulmonary fibrosis compared with control lung fibroblasts. The membrane was stripped and reprobed for β-tubulin as a loading control. The blot is representative of two similar experiments of separately derived lysates from each patient. (B) Relative expression of EDA-containing mRNA to total fibronectin transcripts in UIP (n = 8) and normal (n = 8) lung fibroblasts as assessed by quantitative reverse transcriptase–polymerase chain reaction. Data are presented as relative EDA inclusion into fibronectin normalized to total fibronectin expression. *P < 0.05.
EDA−/− Mice Are Protected from the Development of Experimentally Induced Fibrosis
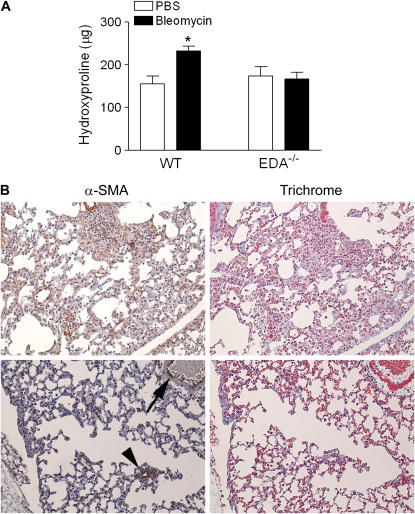
We have previously shown that EDA−/− mice are incapable of adequately healing cutaneous wounds (7) due to ulceration of the newly formed epidermis, abnormal reepithelialization, and ongoing proliferation of infiltrating inflammatory cells. To explore the active role of EDA cFN in lung repair and fibrosis, EDA−/− and WT littermate control mice received a standard dose of intratracheal bleomycin (0.025 U/mouse) to induce lung injury. We observed that WT mice developed a significant degree of fibrosis, as evidenced by elevated levels of lung collagen 21 days after injury (Figure 2A). However, EDA−/− mice failed to develop significant increases in lung collagen (Figure 2A). Histologic evaluation of trichrome-stained lung tissue revealed significant patchy fibrosis after bleomycin in WT animals, which is typical of this model. Conversely, trichrome-stained lung sections from EDA−/− mice demonstrated variable thickening of alveolar walls with a conspicuous lack of collagen deposition but with continued interstitial inflammation (Figure 2B). WT and EDA−/− mice receiving diluent alone (PBS) demonstrated no inflammation or fibrosis 21 days after challenge, and no differences in lung architecture were observed (not shown). We next assessed lung tissue sections for the presence of α-SMA–expressing myofibroblasts. As expected, fibrotic regions of lung in WT mice expressed clusters of α-SMA–expressing myofibroblasts. However, thickened alveolar septae and perivascular thickened areas lacking collagen in EDA−/− mice appeared devoid of such cells (Figure 2B), although airway smooth muscle cells stained positive for α-SMA, providing evidence for the in vivo necessity of EDA cFN for fibroblast α-SMA expression but not for smooth muscle α-SMA expression. To ensure that differences in proliferative potential of the fibroblasts did not account for the relative lack of α-SMA–expressing cells in EDA−/− lungs, we assessed [3H]thymidine incorporation rates in WT and EDA−/− fibroblasts. We found no difference between the two genotypes with respect to thymidine incorporation (Figure E2).
Figure 2.
Extra type III domain A–null (EDA−/−) mice fail to develop significant lung fibrosis after bleomycin-induced lung injury. (A) Lung tissue from EDA−/− and wild-type (WT) controls 21 days after bleomycin injury was assessed for total collagen content by hydroxyproline assay. Data are representative of four independently performed experiments. *P < 0.005. (B) Lung sections of WT (top panels) and EDA−/− (bottom panels) mice 21 d after intratracheal bleomycin stained with trichrome to evaluate collagen deposition and anti–α-smooth muscle actin (α-SMA) to identify myofibroblasts. WT mice displayed nests of α-SMA–positive cells corresponding to areas of collagen deposition (blue on trichrome stain), whereas EDA−/− mice demonstrate minimal α-SMA or collagen staining. In EDA−/− mice, α-SMA expression could be observed in airway (arrowhead) and vascular (arrow) smooth muscle cells. Three mice in each group were examined, with similar findings. PBS = phosphate-buffered saline.
The bleomycin-induced inflammatory pattern in EDA−/− mice is similar to that observed in bleomycin-treated β6-integrin–null mice (17). β6-integrin is an epithelium-restricted integrin that binds TGF-β latency-associated peptide (LAP) complexes and, through conformational changes, activates TGF-β1. Thus, to determine whether differences in β6-integrin expression might account for differences in development of lung fibrosis, histologic sections of PBS- and bleomycin-treated WT and EDA−/− mice were assessed for β6-integrin by immunohistochemistry. We did not detect substantial differences in β6-integrin expression between strains in either condition (Figure E3).
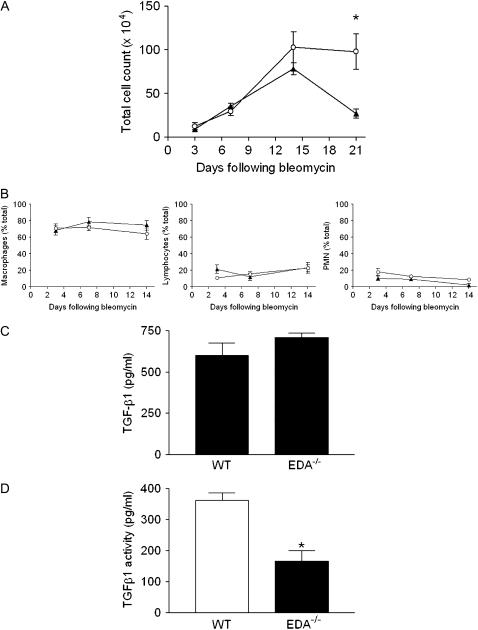
Kinetic Analysis of Inflammatory Cell Recruitment after Intratracheal Bleomycin Reveals No Differences between EDA−/− and WT Animals
Intratracheal bleomycin instillation typically induces a brisk inflammatory response in the lung beginning within 3 days of injury (19). Examination of the inflammatory response in BAL fluid revealed no difference in total cell counts between EDA−/− and WT mice 3, 7, and 14 days after bleomycin-induced lung injury (Figure 3A). However, by Day 21 after bleomycin instillation, WT mice showed marked resolution of the inflammatory response as assessed by decreasing BAL fluid cell count. In contrast, BAL fluid cell counts from EDA−/− mice remained elevated and significantly higher than WT mice, indicating a persistent inflammatory response. Determination of inflammatory cell subtypes by differential counting revealed equivalent recruitment of macrophages, lymphocytes, and polymorphonuclear leukocytes (Figure 3B). These data suggest that lack of fibrosis after bleomycin in EDA−/− mice may be due to a failure to transition from an inflammatory response to a fibrotic one, a role typically ascribed to the effects of TGF-β1. Thus, we next sought to determine the ability of EDA−/− cells to produce and activate latent TGF-β. Activation of TGF-β requires conformational changes that allow TGF-β receptors to access the mature TGF-β, which can occur under acid conditions, via αvβ6 or other integrins (17) or via plasmin (20). To assess TGF-β production, lung fibroblasts from untreated EDA−/− and WT mice were cultured for 24 hours in serum-free media. Conditioned media from these cells were harvested and TGF-β was measured using a commercially available ELISA assay (Assay Designs, Ann Arbor, MI). This kit requires acid treatment of conditioned media and, as such, measures both latent and active forms of TGF-β1. We observed no significant difference in production of TGF-β1 between the two genotypes (Figure 3C). Next, EDA−/− or WT lung fibroblasts were cocultured in FN-depleted media overnight with MLECs stably expressing a TGF-β–responsive portion of the PAI-1 promoter, followed by lysis for assessment of luciferase activity as previously described (17). Results were calculated based on a standard curve of luciferase activity in reporter cells after stimulation with known concentrations of active TGF-β1. We noted that EDA−/− fibroblasts were significantly less able to activate TGF-β1 than WT fibroblasts in vitro (Figure 3D).
Figure 3.
Persistent inflammation in the lungs of extra type III domain A–null (EDA−/−) mice after intratracheal bleomycin challenge. (A) Bronchoalveolar lavage (BAL) fluid was collected on the indicated days and total cells counted. Results are expressed as mean number of cells (×104). A minimum of seven mice were tested in each group at each time point. *P = 0.009. Open circles represent wild-type (WT) mice, solid triangles represent EDA−/− mice. (B) Differential cell counting in BAL fluid isolated as in (A). Results are expressed as percentage of total cellular infiltrate. PMN = polymorphonuclear leukocytes. Open circles represent WT mice, solid triangles represent EDA−/− mice. EDA cellular fibronectin is dispensable for transforming growth factor (TGF)-β1 production, but necessary for activation. (C) Twenty-four-hour conditioned media from WT and EDA−/− lung fibroblasts were assayed for TGF-β1 by ELISA. No difference in 24-hour TGF-β1 production was observed. (D) Activation of TGF-β is impaired in EDA−/− fibroblasts. WT and EDA−/− fibroblasts were cocultured overnight with mink lung epithelial cells expressing a portion of the plasminogen activator inhibitor-1 promoter fused to luciferase. Reporter constructs cultured alone were a negative control. EDA−/− cells possessed significantly less TGF-β activity than WT cells. *P = 0.0023.
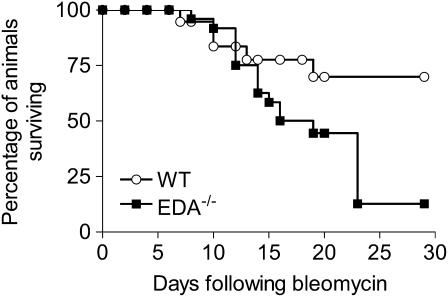
Increased Mortality in EDA−/− Mice after High-Dose Bleomycin Injury
To test whether EDA−/− mice would generate lung fibrosis after any inflammatory insult, we used a model in which EDA−/− and WT mice received a single intratracheal bleomycin injection of 0.075 U/mouse (“high dose,” corresponding to three times the “standard” dose), a dose expected to induce mortality rates of 35–40% (21). Surprisingly, we observed an increased mortality of EDA−/− mice compared with WT mice (Figure 4). Histologic examination revealed profound and ongoing inflammatory changes in EDA−/− mice at 12, 19, 23, and 29 days postinjury, whereas WT mice at similar time points showed progressive architectural distortion consistent with lung fibrosis (Figure E4). These data supported our hypothesis that EDA cFN might be necessary for transitioning from an acute inflammatory response to a chronic fibrotic response, and showed a functional significance to our data that EDA−/− mice were less capable of activating TGF-β1.
Figure 4.
Increased mortality in extra type III domain A–null (EDA−/−) mice after high-dose intratracheal bleomycin. By 29 days, 69.9% of wild-type (WT) mice but only 12.7% of EDA−/− mice had survived (P < 0.01 by the log-rank test). Data are pooled from three experiments encompassing 28 EDA−/− mice and 22 WT mice.
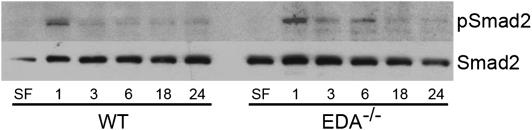
Smad Signaling Is Similar between EDA−/− and WT Fibroblasts
Although our observations suggested a defect in TGF-β1 activation in EDA−/− animals, we wanted to also explore potential differential TGF-β signaling between WT and EDA−/− mice; thus, we next evaluated functional TGF-β1 signaling in lung fibroblasts from each genotype. Serum-starved EDA−/− and WT lung fibroblasts were cultured in the absence or presence of active TGF-β1 (2 ng/ml) for the indicated time points, lysed, and assessed for Smad2 phosphorylation. Blots were stripped and reprobed for total Smad2 as a loading control. Smad2 phosphorylation after TGF-β was similar between the WT and mutant cells, with peak phosphorylation occurring 1 hour after stimulation (Figure 5), suggesting that early TGF-β signaling was intact in both EDA−/− and WT fibroblasts. To assess this effect in vivo, we next performed immunohistochemistry for phospho-Smad2 in mouse lung samples 21 days after bleomycin administration. Smad2 phosphorylation could be observed in both EDA−/− and WT mice, although to a much greater degree in WT mice (Figure E5). This finding suggests that, although EDA−/− cells in vitro are capable of phosphorylating Smad2 equally after active TGF-β1 stimulation, in vivo they do not appear to phosphorylate Smad2 in fibrotic lung injury, consistent with an inability to activate TGF-β in vivo.
Figure 5.
Extra type III domain A (EDA) cellular fibronectin (cFN) is dispensable for early transforming growth factor (TGF)-β1 signaling. Lung fibroblasts from EDA−/− and wild-type (WT) mice were serum starved, treated without (SF) or with TGF-β1 (2 ng/ml) for the indicated time points, and assayed for phosphorylated Smad2 (pSmad2) and total Smad2 by Western blot. EDA−/− and WT fibroblasts equally demonstrate robust Smad2 phosphorylation by 1 hour after TGF-β1 treatment.
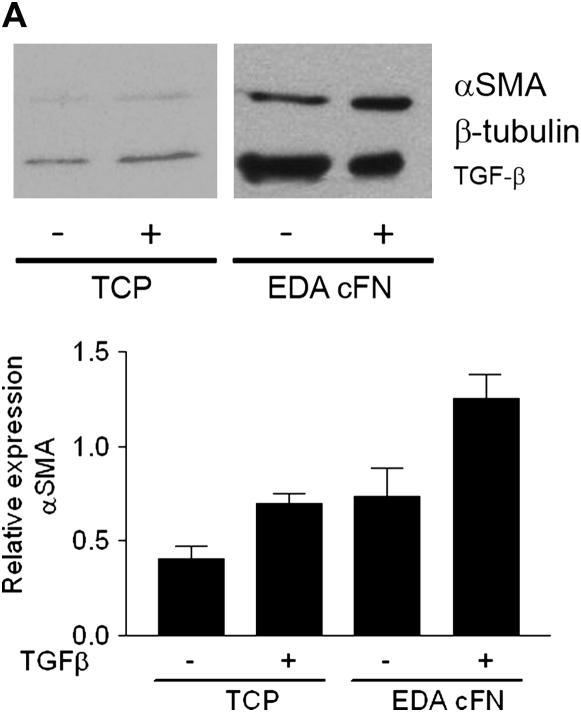
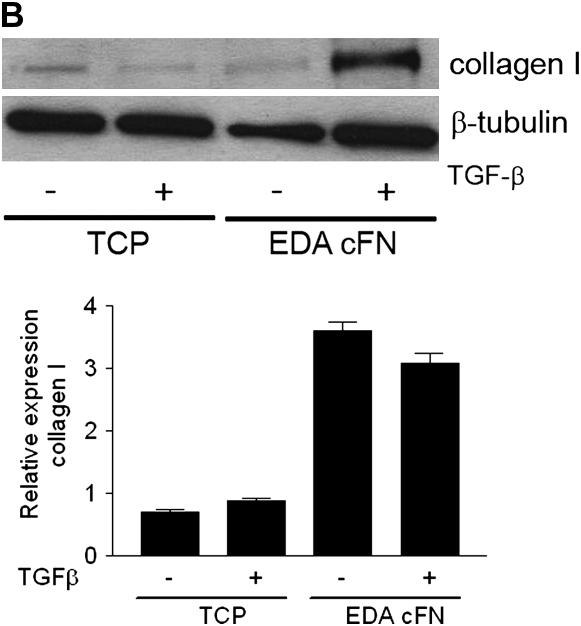
Culture of EDA−/− Fibroblasts on EDA-containing FN Restores TGF-β1–stimulated Increases in α-SMA and Collagen Expression
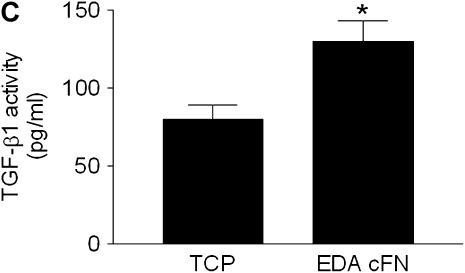
As confirmation of the critical role of EDA FN in TGF-β1–stimulated α-SMA and collagen induction, we next assessed whether plating EDA−/− lung fibroblasts on plates coated with EDA cFN (50 μg/ml) was capable of restoring the observed deficiencies. Consistent with previous data (6), TGF-β1 failed to induce α-SMA expression in EDA−/− cells plated on plastic (Figure 6A). However, if EDA−/− cells were cultured on EDA-containing cFN, α-SMA induction in response to TGF-β1 stimulation at the gene and protein level was restored (Figure 6A). Similarly, collagen expression in EDA−/− fibroblasts grown on plastic after TGF-β1 (2 ng/ml) stimulation was not significantly augmented (Figure 6B). Yet again, culture of EDA−/− fibroblasts on EDA-containing cFN resulted in restoration of TGF-β1 responsiveness as evidenced by increased collagen production at the gene and protein level (Figure 6B). Interestingly, at the gene level, collagen I was markedly induced in EDA−/− cells plated on EDA cFN, regardless of the presence of TGF-β1 (Figure 6B), suggesting that post-translational regulation of collagen was also influenced by plating cells on EDA cFN. Finally, we observed that EDA−/− cells plated on EDA cFN significantly enhanced TGF-β1 activation (Figure 6C).
Figure 6.
Extra type III domain A (EDA)–containing fibronectin rescues EDA−/− cell phenotypic deficiencies in transforming growth factor (TGF)-β1 responsiveness and activation. (A) Serum-starved EDA−/− lung fibroblasts were cultured in the absence (−) or presence (+) of TGF-β1 (2 ng/ml) on tissue culture plastic (TCP) or EDA-containing cellular fibronectin (EDA cFN) for 24 hours. Cell lysates and RNA were collected for (A) α-smooth muscle actin (α-SMA) or (B) collagen I by Western blot (top panel) and semiquantitative reverse transcriptase–polymerase chain reaction (bottom panel). (C) EDA−/− fibroblasts were cocultured overnight on TCP (−) or EDA-containing cFN (+) with mink lung epithelial cells expressing a portion of the plasminogen activator inhibitor-1 promoter fused to luciferase. The next day, lysates were assessed for luciferase activity. *P = 0.0069.
We conclude that EDA−/− mice are protected from the development of experimental lung fibrosis due to a crucial role of EDA cFN in resolution of inflammation, fibroblast activation, and subsequent tissue fibrosis. Mechanistically, EDA cFN is necessary in vitro for TGF-β activation and myofibroblast differentiation. In aggregate, these data suggest that EDA cFN plays an essential role in fibrogenesis.
DISCUSSION
The pathogenesis of progressive tissue fibrosis is incompletely understood, but has been likened to an exuberant and dysregulated wound-healing response (22). Therefore, understanding the means by which tissue injury is limited and normal wound healing is triggered is of critical importance. Here we present evidence of a critical role of EDA cFN in tissue repair/progressive fibrosis after lung injury. We found that, in the absence of EDA cFN, mice fail to develop lung fibrosis after a fibrogenic insult. The histopathologic observations in EDA−/− mice were conspicuously different from WT mice, showing a prolonged inflammatory response instead of a resolving fibrotic response. Furthermore, mutant mice succumbed to overwhelming lung injury after high-dose bleomycin challenge. Notably, EDA−/− mice produced equivalent amounts of TGF-β1 as their WT counterparts, and were similarly capable of signaling responses to exogenous active TGF-β1. However, they were less capable of activating latent TGF-β and producing collagen and α-SMA after active TGF-β1 stimulation. Our results suggest the following: (1) EDA cFN has a critical role in the development of lung fibrosis, (2) EDA cFN is necessary for TGF-β–induced myofibroblast differentiation but is not sufficient to initiate the differentiation process, and (3) EDA cFN may have a fundamental role in activation of latent TGF-β.
FN alternative splicing occurs in three distinct regions of the molecule, resulting in potentially 20 separate isoforms in humans (5). Our work focuses solely on the EDA splice isoform in wound healing and tissue fibrosis, and we did not address alternative splicing of the EDB domain. Primarily, EDB FN appears to be expressed in fetal tissue and in newly generated vasculature of certain malignancies (i.e., breast and liver) (23, 24). Although it is possible that lack of EDA FN in EDA−/− mice resulted in an overexpression of the EDB and subsequent phenotypic differences, our group has previously shown that EDA and EDB alternative splicing are completely autonomous (25), thus diminishing the likelihood of this occurring.
The most surprising finding of our study was that EDA−/− mice experienced ongoing lung inflammation after low-dose bleomycin instillation, and augmented mortality after high-dose bleomycin lung injury. We postulated that an inability to develop significant fibrosis after inflammatory injury would have a protective effect in animals. Indeed, this was our observation in animals receiving standard-dose bleomycin. However, we noted exaggerated lung inflammation in EDA−/− mice, resulting in increased mortality after high-dose bleomycin. Although these data appear to be contradictory to our initial hypothesis, they are in line with previously published work invoking the antiinflammatory, profibrotic role of TGF-β1. Notably, Munger and colleagues have previously demonstrated that mice lacking the β6-integrin develop inflammation in the lung after bleomycin, which does not transition to a fibrotic response (17). Similar findings are observed to occur spontaneously in the lungs and hearts of mice with a targeted deletion of the TGF-β1 gene (26). Interestingly, genetic knockout of CD44, a membrane receptor for the ECM protein hyaluronan, also results in an exaggerated inflammatory response without resultant fibrosis in the lung after bleomycin (27); evidence suggests that this may be in part due to CD44 regulation of TGF-β1 production (28), again highlighting the antiinflammatory properties of TGF-β1. Given our novel finding that EDA−/− fibroblasts are less capable of activating latent TGF-β, as well as responding to active TGF-β1, we conclude that EDA cFN influences wound healing and fibrosis via a TGF-β1–dependent effect.
Our data show that Smad2 phosphorylation in response to active TGF-β1 in vitro is largely intact in WT and EDA−/− lung fibroblasts. However, in these same cells, collagen production at the gene and protein level are markedly decreased compared with WT cells. Although the reasons for this are not entirely clear, one can speculate that other influences on collagen metabolism are likely to be involved. For example, EDA−/− cells, in response to TGF-β1 stimulation, may be capable of greater production of antifibrotic molecules, such as prostaglandin E2 or IFN-γ, which would have a net effect of preventing collagen up-regulation. In support of these possibilities, Riquet and colleagues (29) and our own group (30) have shown that prostaglandin E2 is a potent suppressor of both α-SMA and collagen I expression at the gene and protein level, even in the face of active TGF-β1 stimulation. Similarly, Jaffe and colleagues have shown that murine fibroblasts infected with adenovirus encoding murine IFN-γ results in a selective inhibition of procollagen I mRNA and secretion of total collagen compared with uninfected cells (31). In addition, it is possible that EDA−/− cells have greater capacity to prevent intracellular decreases in glutathione after TGF-β1 stimulation, which would also serve to prevent collagen expression at the gene level (32). In vivo, Smad2 phosphorylation was not as robust in EDA−/− mice as in WT mice, suggesting that a deficiency in TGF-β1 activation is likely to be the primary reason EDA−/− mice do not develop significant fibrosis in the bleomycin model. However, the other possibilities mentioned above may also contribute to the protection from fibrosis observed in EDA−/− mice.
Our data demonstrate that lung fibroblasts derived from patients with usual interstitial pneumonia, a progressive fibroproliferative lung disorder, express markedly greater quantities and proportions of EDA cFN than normal control lung fibroblasts, and are in line with previous work (13). This correlates with greater overall α-SMA expression in the same cells, suggesting an autocrine feedback loop that results in (myo)fibroblast activation. Although EDA cFN was shown to be necessary for TGF-β–induced α-SMA augmentation in myofibroblasts (6), a previous report in EDA−/− mice demonstrated no defect in α-SMA induction in pericytes during tumor angiogenesis (33). This observation, while seemingly contradictory to our own findings, is intriguing but may be accounted for by alternative explanations. First, it is possible that α-SMA expression in pericytes surrounding angiogenic vessels is mediated by another factor that does not require EDA cFN. Notably, Ball and colleagues recently reported the ability of platelet-derived growth factor (PDGF) receptor to directly up-regulate mesenchymal stem cell α-SMA expression by an RhoA/ROCK/cofilin-dependent mechanism (34), and PDGF has been shown to be secreted by certain neoplasms as a means to promote pericyte recruitment (35). Alternatively, it is possible that the requirement for certain cofactors governing α-SMA expression is cell-type dependent. As an example, whereas we do not observe α-SMA–expressing myofibroblasts in bleomycin-injured areas of EDA−/− mice (Figure 2B), α-SMA–expressing airway and vascular smooth muscle is readily observed in these same animals (Figure 2B). Because TGF-β is likely involved in the pathogenesis of bleomycin-induced pulmonary fibrosis (36), our data appear to bolster the contention that EDA cFN is a requisite factor for TGF-β–induced α-SMA expression in vivo.
Another important finding in our study was that plating EDA−/− cells on matrices containing EDA cFN was capable of reversing the defect in myofibroblast differentiation and TGF-β activation in EDA−/− cells. Previous studies have shown that blockade of EDA cFN attenuates TGF-β1–induced myofibroblast differentiation (6). Coupled with our data, we conclude that EDA cFN, whether cell derived or ECM associated, is critical for influencing wound healing through its effects on fibroblast phenotypes. Recently, our group has shown that a substantial portion of extracellular FN is derived from plasma (37). Because plasma FN is hepatocyte derived and lacks the EDA, it stands to reason that locally produced and deposited EDA cFN is a key regulator of tissue repair.
The concept that alternative splicing of FN in general, and EDA cFN in particular, is essential in wound healing has been well established by our group and others (7, 38, 39). EDA cFN (also termed oncofetal or embryonic FN) is an important substrate for cell migration (40), adhesion (41), and myofibroblast differentiation (Reference 6 and data presented here). These phenotypic features of cells repairing wounded tissues are also seen, in great abundance, in both embryonic development and fibroproliferative disorders, thereby cementing the relationships among organ development, physiologic wound healing, and pathologic tissue fibrosis. Therefore, we speculate that physiologic wound healing is an attempt by the organism to not only limit ongoing injury but to restore normal function by reverting to a developmental stage (characterized by increased EDA cFN secretion) in which normal tissue is generated. In pathologic tissue fibrosis, the organism mounts an appropriate fibrotic response that then progresses abnormally. On the basis of data presented in this report, we propose that EDA cFN production is instrumental in the initiation of tissue fibrosis, and that blocking EDA cFN might be a plausible target in the treatment of pathologic fibroproliferative disorders.
Supplementary Material
Supported by National Institutes of Health grants HL070990 and HL085083, a T. Franklin Williams Geriatrics Research Development Award from the Association of Specialty Professors, the CHEST Foundation, and the Martin E. Galvin Fund and Quest for Breath Foundation (all to E.S.W.); and a Specialized Center of Research grant HL56402 (to F.J.M. and G.B.T.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200708-1291OC on December 20, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Matsuyama W, Watanabe M, Shirahama Y, Mitsuyama H, Higashimoto I, Osame M, Arimura K. Discoidin domain receptor 1 contributes to the survival of lung fibroblast in idiopathic pulmonary fibrosis. Am J Pathol 2006;168:866–877. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Gharaee-Kermani M, Gyetko MR, Hu B, Phan SH. New insights into the pathogenesis and treatment of idiopathic pulmonary fibrosis: a potential role for stem cells in the lung parenchyma and implications for therapy. Pharm Res 2007;24:819–841. [DOI] [PubMed] [Google Scholar]
- 3.Hernnas J, Nettelbladt O, Bjermer L, Sarnstrand B, Malmstrom A, Hallgren R. Alveolar accumulation of fibronectin and hyaluronan precedes bleomycin-induced pulmonary fibrosis in the rat. Eur Respir J 1992;5:404–410. [PubMed] [Google Scholar]
- 4.Kuhn C III, Boldt J, King TE Jr, Crouch E, Vartio T, McDonald JA. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis 1989;140:1693–1703. [DOI] [PubMed] [Google Scholar]
- 5.Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci 2002;115:3861–3863. [DOI] [PubMed] [Google Scholar]
- 6.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol 1998;142:873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, Stanta G, Baralle FE. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J Cell Biol 2003;162:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muro AF, Caputi M, Pariyarath R, Pagani F, Buratti E, Baralle FE. Regulation of fibronectin EDA exon alternative splicing: possible role of RNA secondary structure for enhancer display. Mol Cell Biol 1999;19:2657–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buratti E, Baralle FE. Influence of RNA secondary structure on the pre-mRNA splicing process. Mol Cell Biol 2004;24:10505–10514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgess HA, Daugherty LE, Thatcher TH, Lakatos HF, Ray DM, Redonnet M, Phipps RP, Sime PJ. PPAR-γ agonists inhibit TGF-β induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol 2005;288:L1146–L1153. [DOI] [PubMed] [Google Scholar]
- 11.Borsi L, Castellani P, Risso AM, Leprini A, Zardi L. Transforming growth factor-beta regulates the splicing pattern of fibronectin messenger RNA precursor. FEBS Lett 1990;261:175–178. [DOI] [PubMed] [Google Scholar]
- 12.Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci USA 1991;88:6642–6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhn C, McDonald JA. The roles of the myofibroblast in idiopathic pulmonary fibrosis: ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol 1991;138:1257–1265. [PMC free article] [PubMed] [Google Scholar]
- 14.White ES, Thannickal VJ, Carskadon SL, Dickie EG, Livant DL, Markwart S, Toews GB, Arenberg DA. Integrin α4β1 regulates migration across basement membranes by lung fibroblasts: a role for phosphatase and tensin homologue deleted on chromosome 10. Am J Respir Crit Care Med 2003;168:436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akiyama SK. Purification of fibronectin. In: Bonifacino JS, Dasso M, Harford JB, Lippincott-Schwartz J, Yamada KM, editors. Current protocols in cell biology. Hoboken, NJ: Wiley; 1999. pp. 10.15.11–10.15.13.
- 16.White ES, Atrasz RG, Hu B, Phan SH, Stambolic V, Mak TW, Hogaboam CM, Flaherty KR, Martinez FJ, Kontos CD, et al. Negative regulation of myofibroblast differentiation by pten (phosphatase and tensin homolog deleted on chromosome 10). Am J Respir Crit Care Med 2006;173:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999;96:319–328. [DOI] [PubMed] [Google Scholar]
- 18.Sisson TH, Hattori N, Xu Y, Simon RH. Treatment of bleomycin-induced pulmonary fibrosis by transfer of urokinase-type plasminogen activator genes. Hum Gene Ther 1999;10:2315–2323. [DOI] [PubMed] [Google Scholar]
- 19.Adamson IY, Bowden DH. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am J Pathol 1974;77:185–197. [PMC free article] [PubMed] [Google Scholar]
- 20.Lyons RM, Gentry LE, Purchio AF, Moses HL. Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J Cell Biol 1990;110:1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hattori N, Degen JL, Sisson TH, Liu H, Moore BB, Pandrangi RG, Simon RH, Drew AF. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest 2000;106:1341–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mutsaers SE, Bishop JE, McGrouther G, Laurent GJ. Mechanisms of tissue repair: from wound healing to fibrosis. Int J Biochem Cell Biol 1997;29:5–17. [DOI] [PubMed] [Google Scholar]
- 23.Koukoulis GK, Howeedy AA, Korhonen M, Virtanen I, Gould VE. Distribution of tenascin, cellular fibronectins and integrins in the normal, hyperplastic and neoplastic breast. J Submicrosc Cytol Pathol 1993;25:285–295. [PubMed] [Google Scholar]
- 24.Koukoulis GK, Shen J, Virtanen I, Gould VE. Immunolocalization of cellular fibronectins in the normal liver, cirrhosis, and hepatocellular carcinoma. Ultrastruct Pathol 1995;19:37–43. [DOI] [PubMed] [Google Scholar]
- 25.Chauhan AK, Iaconcig A, Baralle FE, Muro AF. Alternative splicing of fibronectin: a mouse model demonstrates the identity of in vitro and in vivo systems and the processing autonomy of regulated exons in adult mice. Gene 2004;324:55–63. [DOI] [PubMed] [Google Scholar]
- 26.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA 1993;90:770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science 2002;296:155–158. [DOI] [PubMed] [Google Scholar]
- 28.Rameshwar P, Chang VT, Gascon P. Implication of CD44 in adhesion-mediated overproduction of TGF-beta and IL-1 in monocytes from patients with bone marrow fibrosis. Br J Haematol 1996;93:22–29. [DOI] [PubMed] [Google Scholar]
- 29.Riquet FB, Lai WF, Birkhead JR, Suen LF, Karsenty G, Goldring MB. Suppression of type I collagen gene expression by prostaglandins in fibroblasts is mediated at the transcriptional level. Mol Med 2000;6:705–719. [PMC free article] [PubMed] [Google Scholar]
- 30.Kolodsick JE, Peters-Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol 2003;29:537–544. [DOI] [PubMed] [Google Scholar]
- 31.Jaffe HA, Gao Z, Mori Y, Li L, Varga J. Selective inhibition of collagen gene expression in fibroblasts by an interferon-gamma transgene. Exp Lung Res 1999;25:199–215. [DOI] [PubMed] [Google Scholar]
- 32.Liu RM, Liu Y, Forman HJ, Olman M, Tarpey MM. Glutathione regulates transforming growth factor-beta-stimulated collagen production in fibroblasts. Am J Physiol Lung Cell Mol Physiol 2004;286:L121–L128. [DOI] [PubMed] [Google Scholar]
- 33.Astrof S, Crowley D, George EL, Fukuda T, Sekiguchi K, Hanahan D, Hynes RO. Direct test of potential roles of EIIIA and EIIIB alternatively spliced segments of fibronectin in physiological and tumor angiogenesis. Mol Cell Biol 2004;24:8662–8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ball SG, Shuttleworth CA, Kielty CM. Platelet-derived growth factor receptor-α is a key determinant of smooth muscle α-actin filaments in bone marrow-derived mesenchymal stem cells. Int J Biochem Cell Biol 2007;39:379–391. [DOI] [PubMed] [Google Scholar]
- 35.Furuhashi M, Sjoblom T, Abramsson A, Ellingsen J, Micke P, Li H, Bergsten-Folestad E, Eriksson U, Heuchel R, Betsholtz C, et al. Platelet-derived growth factor production by B16 melanoma cells leads to increased pericyte abundance in tumors and an associated increase in tumor growth rate. Cancer Res 2004;64:2725–2733. [DOI] [PubMed] [Google Scholar]
- 36.Nakao A, Fujii M, Matsumura R, Kumano K, Saito Y, Miyazono K, Iwamoto I. Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J Clin Invest 1999;104:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moretti FA, Chauhan AK, Iaconcig A, Porro F, Baralle FE, Muro AF. A major fraction of fibronectin present in the extracellular matrix of tissues is plasma-derived. J Biol Chem 2007;282:28057–28062. [DOI] [PubMed] [Google Scholar]
- 38.ffrench-Constant C, Van de Water L, Dvorak HF, Hynes RO. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol 1989;109:903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathews GA, ffrench-Constant C. Embryonic fibronectins are up-regulated following peripheral nerve injury in rats. J Neurobiol 1995;26:171–188. [DOI] [PubMed] [Google Scholar]
- 40.Inoue T, Nabeshima K, Shimao Y, Meng JY, Koono M. Regulation of fibronectin expression and splicing in migrating epithelial cells: migrating MDCK cells produce a lesser amount of, but more active, fibronectin. Biochem Biophys Res Commun 2001;280:1262–1268. [DOI] [PubMed] [Google Scholar]
- 41.Manabe R, Ohe N, Maeda T, Fukuda T, Sekiguchi K. Modulation of cell-adhesive activity of fibronectin by the alternatively spliced EDA segment. J Cell Biol 1997;139:295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.