Abstract
Nkx2.2 is a homeodomain-containing transcription factor essential for pancreatic islet cell specification. In this study we investigate the role of Nkx2.2 within the small intestine. We have determined that Nkx2.2 is expressed at the onset of intestinal epithelial cell differentiation in specific intestinal cell populations, including a subset of enteroendocrine cells. Similar to its role in the pancreatic islet, Nkx2.2 regulates cell fate choices within the intestinal enteroendocrine population; in the Nkx2.2 null mice, several hormone-producing enteroendocrine cell populations are absent or reduced and the ghrelin-producing cell population is upregulated. The remaining intestinal cell populations, including the paneth cells, goblet cells and enterocytes appear to be unaffected by the loss of Nkx2.2. Furthermore, similar to the pancreatic islet, Nkx2.2 appears to function upstream of Pax6 in regulating intestinal cell fates; Pax6 mRNA and protein expression is decreased in the Nkx2.2 null mice. These studies identify a novel role for Nkx2.2 in intestinal endocrine cell development and reveal the regulatory similarities between cell type specification in the pancreatic islet and in the enteroendocrine population of the intestine.
Keywords: Nkx2.2, enteroendocrine cells, intestine, ghrelin, hormones, cell type specification
Introduction
Intestinal development provides an excellent model system for understanding the molecular mechanisms that regulate cell fate decisions during embryogenesis. Numerous studies in a variety of vertebrate species have defined many of the complex developmental events that lead to the formation and differentiation of the intestinal epithelium (reviewed in de Santa Barbara et al., 2003; Hauck et al., 2005; Lee and Kaestner, 2004; Ng et al., 2005; Roberts and Pearson, 2005; Wallace et al., 2005; Walters, 2005). Remarkably, this developmental process is conserved in all vertebrates studied. These cumulative studies have demonstrated the important early patterning events that occur along the anterior-posterior axis to generate regional specification, as well as the numerous signaling pathways that pattern the remaining dorsoventral, left-right, and radial axes to direct general endodermal differentiation. Subsequent differentiation of the endoderm occurs in response to epithelial-mesenchymal interactions and gives rise to the functional epithelium, which becomes organized into villi that project into the gut lumen and the crypts that are embedded in the mesenchyme. The four major differentiated cell types in the small intestine: goblet, enteroendocrine, enterocyte and paneth differentiate from a common pluripotent stem cell, which is located in the crypt (Cheng and Leblond, 1974). As they differentiate, the goblet, enteroendocrine, and enterocyte cell populations migrate from the crypt toward the villus tip, where they eventually die and are extruded into the lumen. The paneth cells migrate downward to reside at the base of the crypt. Within the epithelium, the absorptive enterocytes are the predominant cell type. Of the remaining cell types, the goblet cells secrete mucus, the enteroendocrine cells secrete various hormones and growth factors, and the paneth cells secrete antimicrobial compounds. Although the migration, morphology and functions of each cell population have been well characterized, the molecular mechanisms that regulate the respective cell fate decisions are unknown.
The enteroendocrine population only represents approximately 1% of the gut epithelial cells (Cheng and Leblond, 1974); however this constitutes the largest population of hormone producing cells in the body. Furthermore, the intestinal enteroendocrine population is subdivided into at least 15 different cell types, which can be classified on the basis of their morphology, expression of peptide hormone, and specific marker expression. Recently several gene expression and traditional transgenic knockout studies have identified some of the signaling pathways and regulatory factors that are involved in the development and differentiation of the enteroendocrine population (reviewed in (Lee and Kaestner, 2004; Schonhoff et al., 2004). Similar to their neuroendocrine counterparts, Notch signaling plays a critical role in gut endocrine cell fate decisions in the intestine and the pancreas (Apelqvist et al., 1999; Fre et al., 2005; Jensen et al., 2000) and represses the transcription factor Hes1, to allow the initiation of cell differentiation. Subsequently, the bHLH molecules, Math1 and Neurogenin3 (Ngn3) are activated to initiate the endocrine differentiation program (Jenny et al., 2002; Lee et al., 2002; Yang et al., 2001). Further differentiation of the enteroendocrine population into the 15 different subtypes is not well understood, but mouse knockout studies have implicated several different transcription factors in this process. Pdx1, a homeobox protein that is essential for pancreatic development, is required for development of several endocrine populations in the duodenum; serotonin, secretin and cholecystokinin (CCK) are reduced and gastrin is absent in the duodenum of the Pdx1 null mice (Offield et al., 1996). The bHLH protein NeuroD1/Beta2 is essential for the formation of secretin and CCK cells (Naya et al., 1997) and the paired homeodomain factors, Pax4 and Pax6 also affect the differentiation of endocrine subtypes. Pax6 is required for pancreatic glucagon production and the intestinal GLP-1 cell formation. Pax4 is necessary for the production of most endocrine cell types in the small intestine (Hill et al., 1999; Larsson et al., 1998). Remarkably, each of these transcription factors also differentially regulates pancreatic hormone expression and the development of pancreatic hormone-producing islet cells (Miller et al., 1994; Naya et al., 1997; Offield et al., 1996; Ohlsson et al., 1993; Sosa-Pineda et al., 1997; St-Onge et al., 1997), implying a conserved regulatory pathway exists between the respective formation of intestinal and pancreatic endocrine cell populations.
Nkx2.2 is a homeodomain transcription factor that is essential for the differentiation of three of the pancreatic endocrine populations (Sussel et al., 1998). In the islets of Nkx2.2 null mice, the insulin, glucagon, and pancreatic polypeptide (PP) populations are absent or reduced, and a population of cells producing the peptide hormone ghrelin is upregulated (Prado et al., 2004). Due to the profound effect of Nkx2.2 on the specification of the islet endocrine populations and the strong regulatory connection between pancreatic and intestinal regulatory pathways, we investigated the role of Nkx2.2 in mouse intestinal development. In this study, we demonstrate that Nkx2.2 is expressed at the onset of intestinal epithelial differentiation in a subset of enteroendocrine cells. This finding is consistent with a recent study in zebrafish that demonstrates Nkx2.2 colocalization with several enteroendocrine hormones in the intestine (Ng et al., 2005).
We also determine that in the absence of Nkx2.2, most intestinal hormones in the duodenum and jejunum are absent or reduced and the ghrelin-producing cell population is correspondingly increased, similar to what is observed in the Nkx2.2 null islets. This finding further implicates Nkx2.2 as an important regulator of many endocrine cell populations in the intestine and pancreas, where it plays a critical role in modulating cell fate specification to maintain a normal ratio of endocrine cell populations. Interestingly, although the ultimate endocrine cell identities are distinct within the pancreas and the intestine, the role of Nkx2.2 in the two tissues is remarkably conserved, suggesting that additional lineage-restricted transcription factors will be required to contribute to the terminal differentiation of each endocrine cell population and regulate proper hormone production.
Materials and methods
Mice
Mice were maintained on a Swiss black (Taconic) background and were housed in the University of Colorado Health Sciences Center animal resource center. The mice were fed standard rodent chow and all animal procedures were performed according to UCHSC Institutional Review Board approved protocols.
Immunohistochemistry and in situ hybridization
Tissue was fixed overnight in 4% paraformaldehyde, cryopreserved and cryosectioned into 10 μm sections. At e18.5, n > 6 mutant embryos for each antibody and RNA in situ probe assayed; at e15.5, n >5 for each RNA in situ probe assayed. In each case, corresponding numbers of wild type littermates were simultaneously analyzed. Immunofluorescence was performed on cryo-preserved tissue with the primary antibodies listed in Table 1. Secondary antibodies include: Alexa-fluor 488, 594, or 305 (Molecular Probes, Eugene, OR; 1:800).
Table 1.
Antibodies
| Antigen | Species | Source | Dilution |
|---|---|---|---|
| CCK | Rabbit | Diasorin | 1:100 |
| ChromograninA | Rabbit | Diasorin | 1:100 |
| Gastrin | Rabbit | Phoenix peptide | 1:200 |
| GIP | Rabbit | Phoenix peptide | 1:200 |
| Glucagon | Guinea pig | Linco | 1:2000 |
| Ghrelin | Rabbit | Phoenix peptide | 1:500 |
| Ghrelin | Goat | Santa Cruz | 1:1000 |
| Neurotensin | Rabbit | Phoenix peptide | 1:200 |
| Pax6 | Rabbit | Chemicon | 1:250 |
| PH3 | Rabbit | Upstate | 1:250 |
| PYY | Guinea pig | Research Diagnostic | 1:500 |
| Secretin | Rabbit | Phoenix peptide | 1:200 |
| Serotonin | Rabbit | Diasorin | 1:1000 |
| Substance P | Rabbit | Phoenix peptide | 1:300 |
| Synaptophysin | Mouse | Chemicon | 1:10 |
RNA in situ hybridization was performed as previously described on e15.5 and e18.5 tissue (Prado et al., 2004) using antisense riboprobes transcribed from linearized plasmids. Riboprobes for all genes except Nkx2.2 were generated from full-length cDNAs, which were PCR-cloned from e17.5 intestinal RNA. Full-length Nkx2.2 was cloned from a brain cDNA library (Porteus et al., 1992). RNA in situs were performed on tissue from Nkx2.2 null embryos and a corresponding number of wild type littermates: villin (n=5), IFABP (n=4), Cryptdin1 (n=9), Cryptdin 6 (n=6), Cryptdin7 (n=10), Hes1 (n=3), Cdx1 (n=3), Cdx2 (n=3), Tcf4 (n=3), Math1 (n=4), Ngn3 (n=4), NeuroD1 (n=7), Pax6 (n=5) and ghrelin (n=8). Nkx2.2 RNA in situs were performed on e12.5, e13.5, e15.5 and e18.5 tissue. For combined Nkx2.2 RNA in situ and anti-glucagon immunohistochemical analyses, the RNA in situs were performed to completion followed by immunohistochemistry using rabbit anti-glucagon antibody and biotinylated anti-rabbit secondary antibodies (Jackson Immunoresearch). The biotinylated secondary antibodies were visualized using the Vectastain ABC kit (Vector labs). Images were obtained with a Leica DM5000 microscope and a Qimaging Evolution MP Color camera or a Nikon Eclipse 80i microscope and a Qimaging Retiga EXi-Fast 1394 camera, and were processed using ImagePro software from Media Cybernetics.
Cell counting
The total number of chromograninA, CCK, ghrelin, GIP, glucagon, PYY, secretin, serotonin, PH3 and alcian blue positive cells were individually counted and the total intestinal area was measured using standard morphometric analysis with ImagePro software on every 10th section (10 μm each, 10 sections per embryo) for wild type (ChromograninA, CCK, GIP, glucagon, PH3 and alcian blue: n=3; ghrelin, PYY, secretin, serotonin: n=7) and Nkx2.2-/- e18.5 embryos (ChromograninA, CCK, GIP, glucagon, PH3 and alcian blue: n=3; ghrelin, PYY, secretin, serotonin: n=8). The number of immunoreactive cells per cross section were counted and normalized to the total intestinal area used in the measurements. The results obtained from each embryo were averaged and statistically analyzed.
Quantitative real-time PCR
Total RNA was extracted from e18.5 wild type and Nkx2.2-/- intestine tissue (dissected between the pyloric sphincter on the anterior end to the caecum, posteriorly) and prepared using the Qiagen RNeasy Micro kit. cDNAs were prepared with random hexamer primers and Superscript III (Invitrogen). Real-time PCR was performed on the ABI 7000 using Taqman probes (ABI Assays on Demand) or individually designed Taqman probes and primers. Each gene was normalized to Gapdh. See Table 2 for the list of primers and Table 3 for the numbers of samples tested for each probe. Samples were quantified with ABI prism software.
Table 2.
Real-time PCR ABI Assays on Demand or custom probe/primer combinations.
| Mouse Gene | Probe 5’ or Abi AOD # | Forward Primer 5’-3’ | Reverse Primer 5’-3’ |
|---|---|---|---|
| Gapdh | Mm99999915_g1 | ||
| Cdx1 | Mm00438172_m1 | ||
| Cdx2 | Mm00432499_m1 | ||
| Gata4 | Mm00484689_m1 | ||
| Gata6 | Mm00802636_m1 | ||
| Hes1 | Mm00468601_m1 | ||
| Isl1 | Mm00627860_m1 | ||
| Klf4 | Mm00516104_m1 | ||
| Math1 | Mm004760355_s1 | ||
| Mucin2 | Mm004558299_m1 | ||
| NeuroD1 | Mm01280117_m1 | ||
| Ngn3 | cct gcg ctt cgc cca caa ct | gac gcc aaa ctt aca aag | gtc agt gcc cag atg t |
| Nkx2.2 | cca ttg act ctg ccc cat cgc tct | cct ccc cga gtg gca gat | gag ttc tat cct ctc caa aag ttc aaa |
| Pax4 | Mm01159043_g1 | ||
| Pax6 | Mm00443072_m1 | ||
| Pdx1 | Mm00435565_m1 | ||
| Tcf4 | Mm0043198_m1 | ||
| TFF3 | Mm00495590_m1 | ||
| Cholecystokinin (CCK) | Mm00446170_m1 | ||
| ChromograninA (ChA) | Mm0051431_m1 | ||
| Gastrin | Mm00772211_g1 | ||
| Ghrelin (Ghrl) | Mm00445450_m1 | ||
| GIP | Mm00433601_m1 | ||
| Glucagon (Gcg) | Mm00801712_m1 | ||
| Neurotensin | Mm00481140_m1 | ||
| NPY | Mm00445771_m1 | ||
| PYY | Mm00520715_m1 | ||
| Secretin (Sct) | Mm00441235_g1 | ||
| Somatostatin (Sst) | Mm00436671_m1 | ||
| Substance P | Mm00436880_m1 | ||
| Synaptophysin | Mm00436850_m1 |
Table 3.
Real time PCR: number of samples screened
| Gene of Interest | Number of WT | Number of mutants |
|---|---|---|
| CCK | 7 | 7 |
| Cdx1 | 6 | 5 |
| Cdx2 | 6 | 5 |
| chromogranin A | 6 | 4 |
| gastrin | 7 | 7 |
| Gata4 | 6 | 5 |
| Gata6 | 6 | 5 |
| ghrelin | 6 | 5 |
| GIP | 7 | 7 |
| glucagon | 13 | 11 |
| Ngn3 | 4 | 3 |
| Hes1 | 6 | 5 |
| Isl1 | 4 | 3 |
| Klf4 | 7 | 7 |
| Math1 | 6 | 5 |
| Muc2 | 6 | 5 |
| NeuroD1 | 9 | 9 |
| Ngn3 | 4 | 3 |
| neurotensin | 7 | 7 |
| Nkx2.2 | 19 | 16 |
| NPY | 7 | 7 |
| Pax4 | 6 | 4 |
| Pax6 | 6 | 4 |
| Pdx1 | 6 | 4 |
| PYY | 13 | 11 |
| secretin | 7 | 7 |
| somatostatin | 7 | 7 |
| substance P | 7 | 7 |
| synaptophysin | 6 | 4 |
| TCF4 | 6 | 5 |
| TFF3 | 6 | 5 |
Results
Nkx2.2 is expressed in scattered cell populations throughout the intestine during embryonic development
To determine when and where Nkx2.2 is expressed in the developing mouse intestine, we performed RNA in situ analysis to assess Nkx2.2 mRNA expression patterns throughout embryogenesis. At e12.5, Nkx2.2 is expressed robustly in the dorsal pancreas and in the emerging ventral pancreas, but cannot be detected in the developing intestine (Figure 1a). By e13.5, a small number of Nkx2.2-producing cells can be detected in a few cells of the proximal forestomach (Figure 1b), but cannot be detected in the developing proximal intestine (Figure 1c and data not shown). Between e14 and e15, cytodifferentiation of the intestinal epithelium begins, and the four epithelial cell types (goblet, enteroendocrine, enterocyte, and paneth) develop shortly after. At this critical e15.5 time point, Nkx2.2 is first detected in scattered cells throughout the duodenum and small intestine, and expression is maintained through birth (Figure 1d and e). At perinatal stages, the position, spatial distribution and shape of Nkx2.2 positive cells in the intestinal epithelium is similar to that of the enteroendocrine population. We confirmed that Nkx2.2 was expressed in a subpopulation of enteroendocrine cells by performing RNA in situ hybridization for Nkx2.2 combined with anti-glucagon immunohistochemical staining. As shown in Figure 2, Nkx2.2 is expressed in glucagon-positive cells of the intestine. Because it can be difficult to visualize the co-stained cells, representative coexpression of Nkx2.2 mRNA and glucagon protein in the pancreas (where Nkx2.2 is known to be co-expressed with glucagon (Sussel et al., 1998)) is also shown in Figure 2 e and f. Consistent with Nkx2.2 being expressed in the enteroendocrine cells, analysis of a transgenic line of zebrafish, Tg(nkx2.2a:mEGFP) demonstrated co-expression of nkx2.2a with the endocrine hormones, glucagon and somatostatin in the intestinal epithelium (Ng et al., 2005).
Figure 1. Nkx2.2 is expressed in the developing intestine.

RNA in situ analyses of Nkx2.2 expression at different embryonic stages. a) e12.5; b and c) e13.5; and c) e15.5. The boxed area is shown in higher magnification in d. d) higher magnification of box shown in c. dp=dorsal pancreas, vp=ventral pancreas, s=stomach, i=intestine.
Figure 2. Nkx2.2 is expressed in the glucagon-producing enteroendocrine cells.

RNA in situ analysis of Nkx2.2 (purple) combined with immunoperoxidase staining of glucagon-expressing cells (brown) at e18.5. a) Co-staining of Nkx2.2 and glucagon in enteroendocrine cells of the small intestine. The boxed areas are shown in higher magnification in b-d. b and c) Nkx2.2-positive, glucagon-positive enteroendocrine cells (arrowheads). d) Nkx2.2-positive, glucagon-negative intestinal cell (arrows). e) Co-staining of Nkx2.2 and glucagon in the pancreatic islet. The boxed area is shown in higher magnification in f.
Nkx2.2 is specifically required for appropriate cell type specification of the enteroendocrine lineages
With the discovery that Nkx2.2 is expressed in specific cells of the developing intestine, we wanted to determine whether Nkx2.2 plays a role in intestinal epithelial development and cell type differentiation, similar to its role in the pancreatic islet. For this study, we analyzed mice that had been deleted for Nkx2.2 (Sussel et al., 1998). Because the Nkx2.2 null mice die shortly after birth with pancreatic islet and neural defects (Briscoe et al., 1999; Sussel et al., 1998), our analysis of the intestine was restricted to the late embryonic stages, shortly after differentiation of the intestinal epithelium is initiated. The Nkx2.2 null mice appear to have no gross alterations in their intestinal morphology when assessed by hemotoxylin and eosin (H&E) staining at any stage during embryonic development (Figure 5a and b). Since Nkx2.2 has a known role in regulating endocrine cell fate decisions in the pancreatic islet, the apparent expression of Nkx2.2 in intestinal enteroendocrine cells led us to analyze the enteroendocrine lineages of the gut epithelium in the Nkx2.2 knockout mice. Immunofluorescence analysis of several enteroendocrine hormones that are normally present in the proximal small intestine demonstrated that many of these cell populations were missing or reduced in the Nkx2.2 null mice (Figure 3 and data not shown). Quantification of serotonin, CCK, GIP and gastrin confirmed that there was a significant reduction in these hormone-producing endocrine populations in the absence of Nkx2.2 (Table 4). We confirmed that these and additional hormones were also down-regulated at the mRNA level by performing real-time PCR analysis on mRNA collected from the small intestine of wild type and Nkx2.2 null animals. These studies showed that the expression of CCK, gastrin, glucagon, GIP, neurotensin, and somatostatin is significantly lower in the Nkx2.2 null mice than in their wild type littermates. Interestingly, substance P and secretin expression are only moderately reduced. The numbers of PYY-producing endocrine cells of the distal small intestine were also unchanged in the Nkx2.2 null mice (Table 4) and PYY and NPY were expressed at similar levels in the wild type and Nkx2.2 null mice, suggesting that these more distal cell populations are unaffected by the loss of Nkx2.2.
Figure 5. Nkx2.2 does not regulate the formation of non-enteroendocrine cell populations in the small intestine.
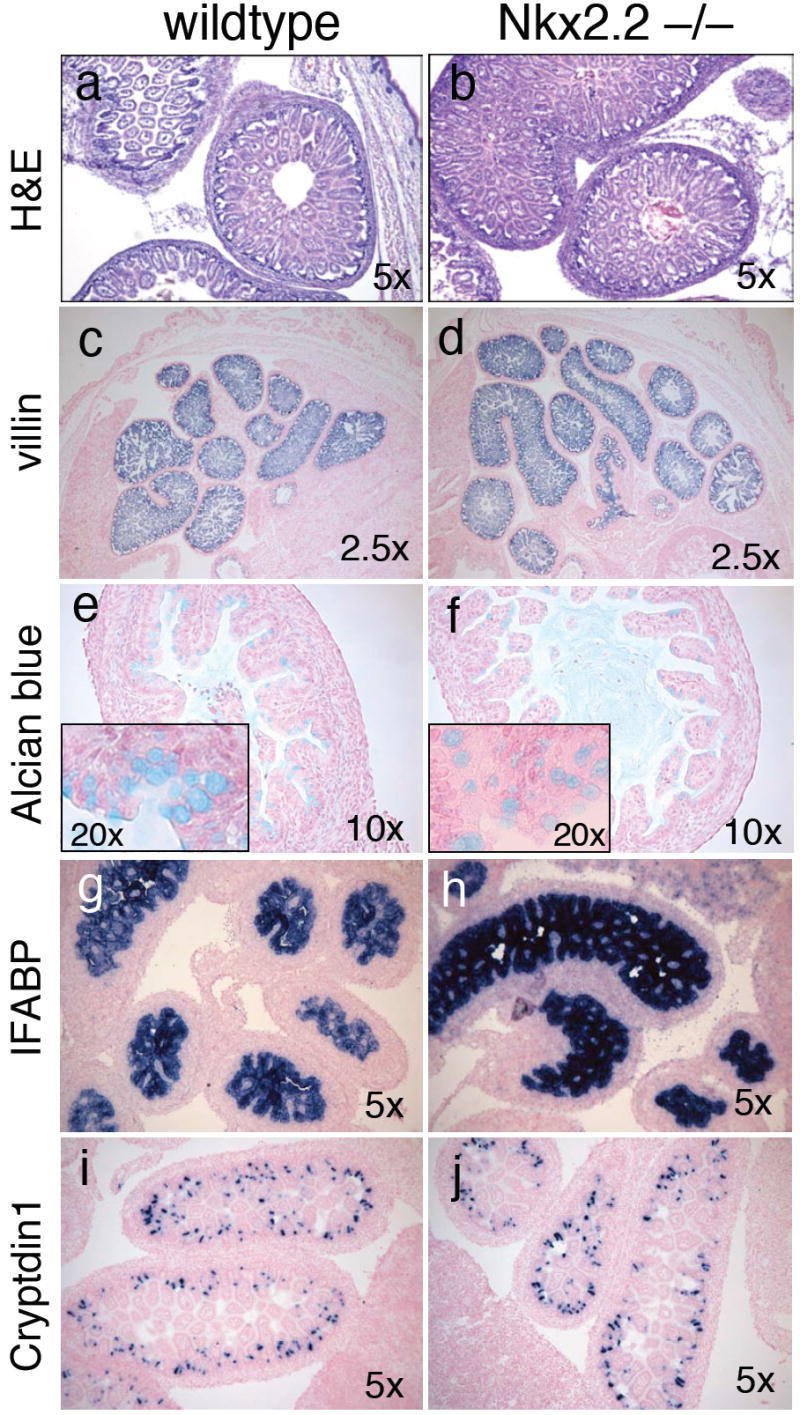
Analysis of intestinal cell populations in e18.5 embryos. a,b) H&E staining of wild type and mutant tissue. c,d) RNA in situ of villin-expressing intestinal epithelial cells. e,f) Alcian blue staining of intestinal goblet cells. g,h) RNA in situ analysis of IFABP-expressing enterocytes. i,j) RNA in situ analysis of cryptdin1-producing paneth cells.
Figure 3. In the Nkx2.2 null mice, many intestinal hormone-producing enteroendocrine populations are reduced and ghrelin-producing cells are significantly upregulated.
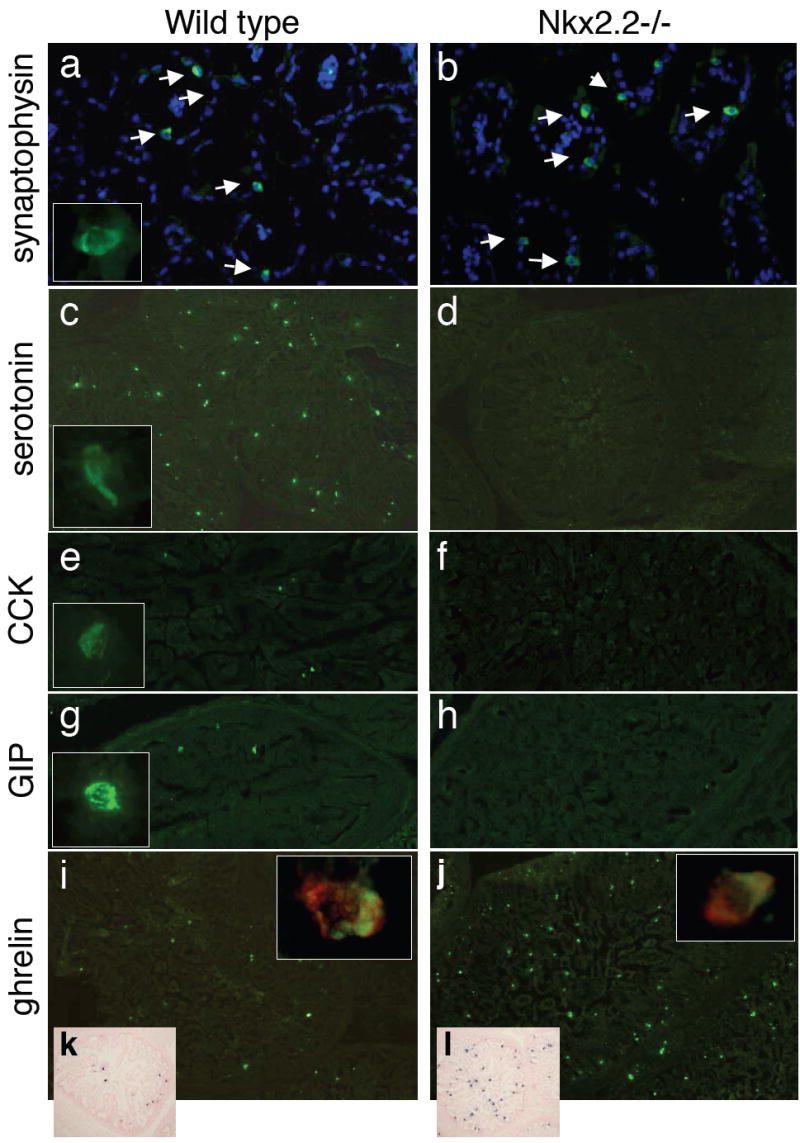
a-h) Immunofluorescence analysis of e18.5 wild type (a, c, e, g and i) and Nkx2.2 null (b, d, f, h, j) mice. a,b) Synaptophysin (green) costained with DAPI (blue). Inset contains a higher magnification of a synaptophysin-positive cell. c,d) Serotonin (green) with a higher magnification inset of a serotonin-positive cell. e,f) CCK (green) with a higher magnification inset of a CCK-positive cell. ; g, h) GIP (green) with a higher magnification inset of a GIP-positive cell. i,j) Ghrelin expression (green) with higher magnifcation insets of ghrelin-positive cells (green) costained with synaptophysin (red). k, l) RNA in situ analysis of ghrelin expression at e18.5.
Table 4.
Enteroendocrine cells in wild type and Nkx2.2-/- gastrointestinal tract
| ChA | CCK | Ghrelin | GIP | Gcg | PYY | Sec | Ser | |
|---|---|---|---|---|---|---|---|---|
| Wild type | 57.1±5.6 | 29.5±2.1 | 12.4±4.1 | 47.9±4.3 | 15.2±3.4 | 24.4±2.3 | 27.6±1.4 | 65±5.1 |
| Nkx2.2 -/- | 60.9±13.6 | 9.9±0.8** | 44.6±13.3** | 4.2±1.1* | 4.7±1.7* | 21.6±13.4 | 14.2±7.8 | 1.5±0.6** |
Although there is a reduction in many of the hormone-producing cells in the Nkx2.2 null mice, immunohistochemical analysis of the pan-endocrine markers chromograninA and synaptophysin suggested that the total numbers of enteroendocrine cells is unchanged (Figure 3 and data not shown). Quantification of the chromograninA immunoreactive cells confirmed that there were similar numbers of enteroendocrine cells in the wild type and Nkx2.2 null small intestine (Table 4). ChromograninA and synaptophysin mRNA expression was also unchanged in the Nkx2.2 null mice (Figure 4). In contrast, however, the number of cells expressing the ghrelin hormone is significantly increased throughout the proximal small intestine in the Nkx2.2 null embryos (Figure 3 and Table 4) and ghrelin mRNA expression is correspondingly elevated (Figure 4). The ghrelin-producing cells in the Nkx2.2 null mice appear to retain the characteristics of endocrine cells as they continue to express the endocrine marker synaptophysin (Figure 3 inset and Supplemental figure 1). This analysis suggests that, similar to its role in the pancreatic islet, Nkx2.2 functions to regulate cell fate specification of the enteroendocrine populations in the intestine.
Figure 4. Quantification of hormone expression in e18.5 Nkx2.2 null small intestine.
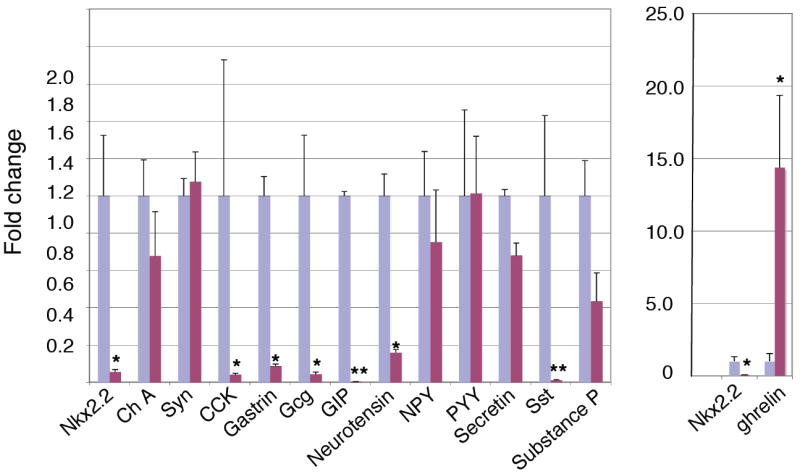
Real time PCR analysis of relative hormone expression in the Nkx2.2 wild type and mutant small intestine. Note the change of scale for the comparative ghrelin expression. See Table 3 for numbers tested. Fold change normalized to wild type; * p < 0.01; ** p < 0.001.
The loss of several hormone-producing populations and the simultaneous increase in ghrelin-producing cells suggests that the ghrelin cell population may be specified at the expense of the other endocrine cell lineages. However, it remains possible that the loss of Nkx2.2 activity increases the proliferation of the existing ghrelin cell population, independently of losing the other hormone-producing cells. To test this possibility, we analyzed the expression of phospho-Histone H3 to assess the mitotic status of the ghrelin-positive cells. Similar to our results in the pancreatic islet, we could not detect any proliferating ghrelin-positive cells in the intestine of wild-type or Nkx2.2 null embryos (data not shown) and the total number of proliferating cells in the intestine is not significantly altered when we quantified the total number of phospo-histone H3 positive cells (wild type: 216.2±18.6 (n=3); Nkx2.2: 169±11.1 (n=3). Therefore, we hypothesize that in the absence of Nkx2.2 several of the endocrine populations appear to be replaced by ghrelin-producing endocrine cells.
Nkx2.2 does not affect the differentiation of the other intestinal cell populations
The loss of several hormone-producing enteroendocrine populations and corresponding upregulation of ghrelin cells in the absence of Nkx2.2 activity confirms the importance of Nkx2.2 in intestinal enteroendocrine cell specification. However, because we could not perform co-staining analysis of Nkx2.2 with the other intestinal epithelial cell populations, we could not rule out that Nkx2.2 was either directly or indirectly involved in the development and differentiation of other intestinal cell populations. To determine whether Nkx2.2 affected the development of these remaining intestinal cell types we assessed the expression of characteristic cell-type specific markers during intestinal development (Figure 4). Villin is one of the first genes to be expressed in the intestine and is located globally throughout the intestinal epithelium (Braunstein et al., 2002). We do not detect any change in villin expression in the Nkx2.2 knockout mice suggesting that the early intestinal patterning events are not perturbed (Figure 5c and d). We were also unable to detect any defects in the development of the mature enterocyte, goblet, or paneth cell populations by assessing the expression of markers characteristic of each cell type (Figure 5). For this analysis we assessed the expression of the enterocyte marker IFABP, an intracellular protein abundantly expressed in the differentiated enterocyte population, which represents the largest cell population in the intestine (Figure 5g and h,); Alcian blue labeling, which characteristically stains goblet cells (Figure 5e and f), and quantitative PCR analysis of two goblet cell specific genes, TFF3 and mucin2 (n=5; Figure 5k); and cryptdin 1, 6 and 7 as markers of paneth cell differentiation (Figure 5i and j; data not shown). According to this marker analysis, there were no discernable differences in the non-endocrine cell populations between the developing wild type and Nkx2.2 null intestine. Furthermore, quantification of the alcian blue stained cells revealed that total goblet cell numbers were unchanged in the Nkx2.2 null mice (wild type: 182.9±5.6 (n=3); Nkx2.2-/-: 184.1±11.1 (n=3) and expression of Klf4, a zinc finger transcription factor that is essential for the terminal differentiation of goblet cells (Katz et al., 2002) is also maintained. These studies indicate that Nkx2.2 is predominantly involved in the differentiation of the enteroendocrine intestinal cells and, similar to its role in the pancreatic islet, plays an important role in cell specification decisions within this population.
Positioning Nkx2.2 in the intestinal regulatory pathway
The increase in ghrelin cells at the expense of the several intestinal endocrine populations has not been reported in other mouse knockout models known to affect intestinal enteroendocrine cell differentiation. To better understand the molecular basis of the defects observed in the Nkx2.2 knockout and to position Nkx2.2 in the hierarchy of transcription factors regulating intestinal cell fates, we assessed expression of many of the regulatory factors known to be involved in intestinal cell differentiation. Several different transcription factors have been shown to regulate the cell type differentiation process of all intestinal cell populations or the enteroendocrine lineages, specifically. We used RNA in situ analysis and quantitative real-time PCR to determine which, if any, of the transcription factors had altered expression in the Nkx2.2 knockout mice (Figure 6 a-l, Supplemental figure 2). Consistent with the Nkx2.2 mutant phenotype, which appears to be restricted to the enteroendocrine lineages, expression of transcription factors such as Cdx1 and Cdx2, which are involved in early gut patterning are not affected by the loss of Nkx2.2 (Figure 6a,b, Supplemental figure 2). In addition, expression of the stem cell factor Tcf4, the progenitor cell regulator Hes1, and the early cell lineage regulator Math1 are also not affected in the mutant mice (Figure 6c-f, Supplemental figure 2). We also assessed the expression of the Gata4 and Gata6 transcription factors, which have recently been shown to be differentially expressed in the enterocyte and enteroendocrine populations of the gastrointestinal tract and the endocrine cells of the pancreas (Bosse et al., 2006; Decker et al., 2006; Dusing and Wiginton, 2005). Both by RNA in situ analysis and quantitative PCR, we could not detect an alteration in Gata4 or Gata6 expression in the Nkx2.2 null intestine (Supplemental figure 2 and data not shown).
Figure 6. Intestinal transcription factor expression in Nkx2.2 wild type and mutant small intestine.

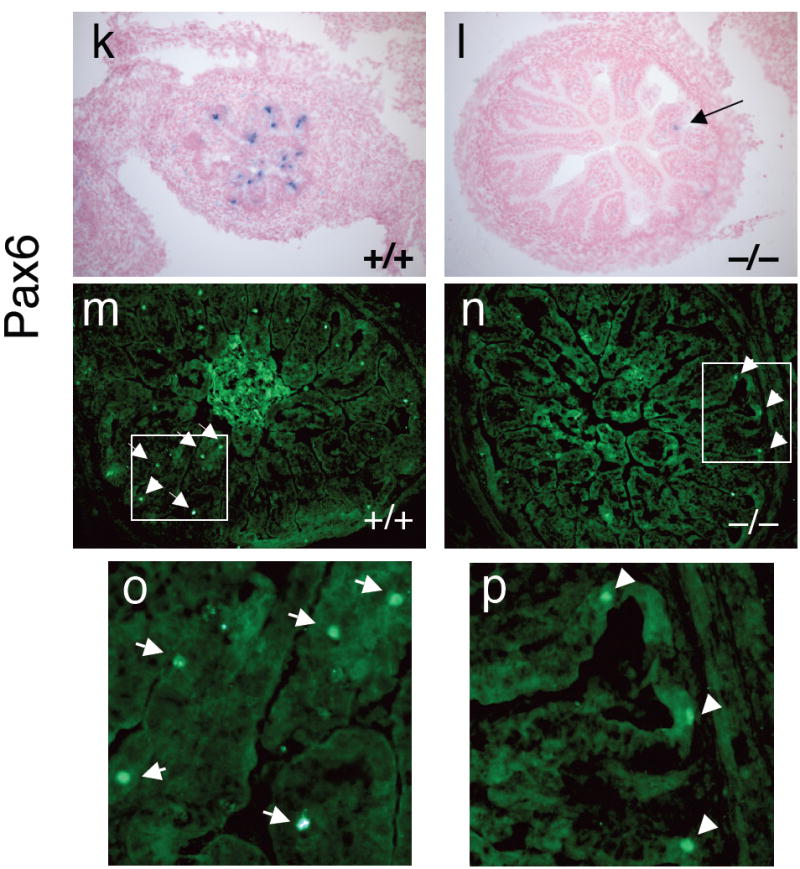
RNA in situ analysis of transcription factor expression in wild type (a,c,e,g, i and k) and Nkx2.2 null (b,d,f,h,j, and l) embryos. a and b) tissue derived from e18.5 embryos; c-l) tissue derived from e15.5 embryos. m and n) Immunofluorescent staining of Pax6 protein in wild type and Nkx2.2 null e18.5 embryos. Boxed area is shown in higher magnification in o and p. Arrows point to Pax6-immunoreactive cells.
Many additional transcription factors are thought to be responsible for the correct specification and/or development of several enteroendocrine lineages. Ngn3 and NeuroD1/Beta2 (hereafter referred to as NeuroD1) are basic helix-loop-helix transcription factors that are also involved in the endocrine cell differentiation process. Ngn3 is required for the differentiation of all endocrine cells in the gastrointestinal tract and pancreatic islet; Ngn3 null mice fail to develop these populations. Ngn3 expression is not altered in the Nkx2.2 knockout mice (Figure 6g-h, Supplemental figure 2), which is consistent with the observation that there are normal numbers of enteroendocrine cells in the Nkx2.2 null embryos. NeuroD1 has been shown to function downstream of Ngn3 and appears to regulate the formation of CCK and secretin cells (Huang et al., 2000; Jenny et al., 2002; Naya et al., 1997). NeuroD1 mRNA expression is modestly, but consistently downregulated in the intestine of Nkx2.2 null mice (Supplemental figure 2), however RNA in situ analysis does not reflect a two-fold reduction in the area of intestine staining for NeuroD1 (Figure 6i-j). The discrepancy between the PCR results and the RNA in situ analysis may be due to the increased sensitivity of real time PCR assay or may reflect a decrease in the amount of NeuroD1 expressed in each cell. The non-quantitative nature of RNA in situ assays makes this issue difficult to resolve, however a reduction in NeuroD1 expression the Nkx2.2 null mice is consistent with the observed reduction in the numbers of CCK and secretin cell populations.
Islet1 (Isl1) and Pdx1 play important regulatory roles in the differentiation of islet and intestinal endocrine populations. It has been suggested that the LIM homeodomain protein, Isl1 is involved in the early differentiation of gastrin and somatostatin cells, however the early lethality associated with the Isl1 knockout mice precludes confirmation of an Isl1 intestinal function (Ahlgren et al., 1997; Larsson, 2000). A deletion of the homeodomain protein, Pdx1, however, results in a loss or reduction of several endocrine cell populations in the duodenum (Larsson et al., 1996). Although the Nkx2.2 knockout affects many of the same endocrine cell populations, the expression of both these genes is unaffected by the loss of Nkx2.2 (Supplemental figure 2).
Pax4 and Pax6 are also critical endocrine regulatory factors that, similar to Nkx2.2, have been implicated in both pancreatic and intestinal cell fate determination (Larsson et al., 1998). In the intestine, Pax4 is essential for the formation of serotonin, secretin, CCK, GIP, and PYY cells and Pax6 is required for GIP cell differentiation. Interestingly, although it appears that Pax4 and Nkx2.2 regulate an overlapping set of intestinal cell types, Pax4 expression is not affected in the Nkx2.2 knockout mice (Supplemental figure 2). This would suggest that Pax4 functions upstream of Nkx2.2 or in a parallel pathway for the formation of several different enteroendocrine cell types. This result is consistent with what we observe with corresponding Nkx2.2 and Pax4 functions in the pancreatic islet. Pax6, on the other hand, is down-regulated in the absence of Nkx2.2 (Figure 6k-p), suggesting that it functions downstream of Nkx2.2 to specify GIP cells. Again, this parallels the corresponding functions of Nkx2.2 and Pax6 in the islet.
Discussion
Nkx2.2 has previously been shown to be essential for the appropriate differentiation of ventral spinal cord neurons, oligodendrocytes, and pancreatic islet cells (Briscoe et al., 2000; Prado et al., 2004; Qi et al., 2001; Sussel et al., 1998; Zhou et al., 2001). In this study, we demonstrate that Nkx2.2 is also critical for the appropriate differentiation of enteroendocrine cells in the small intestine. We assessed the protein and/or mRNA expression of many of the hormones produced in the developing small intestine and found that several of endocrine cell populations that populate the proximal small intestine were reduced. Interestingly, these endocrine populations were not completely eliminated, suggesting that Nkx2.2-independent differentiation programs exist, or compensatory mechanisms are sometimes able to operate. Another Nkx family member, Nkx6.1 is also expressed in the small intestine, although analysis of Nkx6.1 in the intestine has not yet been performed.
In addition to the reduction of several intestinal hormone-producing populations in the Nkx2.2 knockout, we observed a significant increase in the number of cells expressing ghrelin, suggesting that some enteroendocrine populations may be replaced by the ghrelin-producing cells. This is similar to what is observed in the Nkx2.2 knockout pancreatic islet, where the insulin-producing β cell population and most glucagon-producing α cells are also replaced by the ghrelin cells. This cell type conversion implies that Nkx2.2 plays an early role in specifying endocrine cell identities and, in its absence, the differentiation pathway either defaults or is diverted to the ghrelin cell fate. Alternatively, it is possible that cell fate changes have not occurred in the intestine of Nkx2.2 null mice, but instead Nkx2.2 differentially regulates hormone expression at the transcriptional level; the hormones that are lost would be activated by Nkx2.2 and ghrelin would be repressed by Nkx2.2. While we had additional cell-specific markers to demonstrate that the ghrelin-producing cells in the Nkx2.2 null islet were not molecularly identical to α or β cells, similar molecular tools are not yet available for the intestinal endocrine cell populations. We have, however, determined that Nkx2.2 does not repress transcription of the mouse or human ghrelin promoter in αTC and HCT8 cell lines, which both express ghrelin endogenously (Christina Chao and Lori Sussel, unpublished data). Further analysis of the individual enteroendocrine populations affected in the Nkx2.2 null mice will be necessary to determine the mechanism by which Nkx2.2 functions to regulate endocrine cell fates and hormone expression in the intestine.
Similar to many of the other intestinal transcription factors, which predominantly regulate the development of small subsets of enteroendocine cell populations, deletion of Nkx2.2 does not affect all hormone-producing endocrine populations in the small intestine. Furthermore, the affected endocrine cell populations are variably reduced. This is similar to what is observed in the pancreatic islet, where Nkx2.2 is required for the formation of all insulin-producing β cells, and only subsets of the glucagon-producing α cells and PP cells. This would suggest that unlike Ngn3, Nkx2.2 is not a global regulator of the endocrine cell populations, but functions a later stage to specify the differentiation of specific endocrine cell fates. Interestingly, Nkx2.2 is not required for the formation of the somatostatin-expressing δ cell population in the islet, whereas it is required for the expression of somatostatin in the intestine. This further underscores that while there are obvious similarities between the endocrine cell populations in the intestine and pancreas, fundamental regulatory differences exist in each cell type as well.
It is interesting, however, that the ghrelin cell population is upregulated in both the islet and small intestine when Nkx2.2 is deleted. This suggests that mechanism by which Nkx2.2 controls some of the early general fate decisions in the pancreas and intestine are conserved. Consistent with this, several other transcription factors commonly regulate cell fate decisions in the islet and intestine (reviewed in Lee and Kaestner, 2004; Schonhoff et al., 2004). Furthermore, our study has demonstrated that portions of the transcriptional regulatory pathway may also be conserved between the two tissues: in Nkx2.2-deficient pancreas and intestine, Pdx1, Ngn3 and Pax4 expression is unaffected, while Pax6 and NeuroD1 expression is reduced. What is remarkable about this conservation is that the ultimate cell fates and hormone products of the islet and gut endocrine cells are different. We propose that although these common regulatory factors may contribute to the final cell fate decisions, unique combinations of additional tissue and cell type specific proteins will be required to initiate the different terminal differentiation pathways and differential hormone expression. Currently there is limited information regarding the lineage relationships between the many enteroendocrine populations; these types of analyses will be essential to unraveling the regulatory pathways and cell fate decision processes that distinguish islet and intestinal endocrine populations, as well as the events that regulate differentiation of each endocrine cell subpopulation. However, the similarities between early islet and intestinal endocrine differentiation regulatory pathways also suggests that the well-characterized intestinal stem cell population could be harnessed to produce alternative sources of islet cells for therapeutic purposes.
Supplementary Material
Co-immunofluorescent staining of ghrelin-positive cells (green) and the pan-endocrine marker, synaptophysin (red) in the small intestine of wild type and Nkx2.2 null embryos at e18.5.
Real time PCR analysis of transcription factor mRNA isolated from wild type and Nkx2.2 null tissue. See Table 2 for the probe sets. See Table 3 for of embryonic samples tested. Fold change normalized to wild type; * p < 0.01.
Number of immuno-positive cells per cross-section (x±SEM). To quanitify the cells expressing each hormone, 10 cross sections of an embryo were examined for each hormone. The number of immunoreactive cells per cross section was counted and the results averaged.
Acknowledgments
We would like to thank the Dr. Kristin Artinger and the Sussel lab for critical reading of this manuscript. This research was supported by the NIH NIDDK and the American Diabetes Association (L.S.), and a T32 training award to MD. Additional support was provided by the University of Colorado Cancer Center Transgenic/Knockout Core and the Diabetes and Endocrinology Research Center (NIH P30 DK57516).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlgren U, et al. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–60. doi: 10.1038/385257a0. [DOI] [PubMed] [Google Scholar]
- Apelqvist A, et al. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–81. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- Bosse T, et al. Gata4 is essential for the maintenance of jejunal-ileal identities in the adult mouse small intestine. Mol Cell Biol. 2006 doi: 10.1128/MCB.00124-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein EM, et al. Villin: A marker for development of the epithelial pyloric border. Dev Dyn. 2002;224:90–102. doi: 10.1002/dvdy.10091. [DOI] [PubMed] [Google Scholar]
- Briscoe J, et al. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–45. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Briscoe J, et al. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398:622–7. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. III. Entero-endocrine cells. Am J Anat. 1974;141:503–19. doi: 10.1002/aja.1001410405. [DOI] [PubMed] [Google Scholar]
- de Santa Barbara P, et al. Development and differentiation of the intestinal epithelium. Cell Mol Life Sci. 2003;60:1322–32. doi: 10.1007/s00018-003-2289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker K, et al. Gata6 is an important regulator of mouse pancreas development. Dev Biol. 2006;298:415–29. doi: 10.1016/j.ydbio.2006.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusing MR, Wiginton DA. Epithelial lineages of the small intestine have unique patterns of GATA expression. J Mol Histol. 2005;36:15–24. doi: 10.1007/s10735-004-2908-9. [DOI] [PubMed] [Google Scholar]
- Fre S, et al. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–8. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- Hauck AL, et al. Twists and turns in the development and maintenance of the mammalian small intestine epithelium. Birth Defects Res C Embryo Today. 2005;75:58–71. doi: 10.1002/bdrc.20032. [DOI] [PubMed] [Google Scholar]
- Hill ME, et al. Essential requirement for Pax6 in control of enteroendocrine proglucagon gene transcription. Mol Endocrinol. 1999;13:1474–86. doi: 10.1210/mend.13.9.0340. [DOI] [PubMed] [Google Scholar]
- Huang HP, et al. Regulation of the pancreatic islet-specific gene BETA2 (neuroD) by neurogenin 3. Mol Cell Biol. 2000;20:3292–307. doi: 10.1128/mcb.20.9.3292-3307.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny M, et al. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. Embo J. 2002;21:6338–47. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, et al. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Katz JP, et al. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–28. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson LI. Developmental biology of gastrin and somatostatin cells in the antropyloric mucosa of the stomach. Microsc Res Tech. 2000;48:272–81. doi: 10.1002/(SICI)1097-0029(20000301)48:5<272::AID-JEMT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Larsson LI, et al. Pancreatic-duodenal homeobox 1 -role in gastric endocrine patterning. Mech Dev. 1996;60:175–84. doi: 10.1016/s0925-4773(96)00609-0. [DOI] [PubMed] [Google Scholar]
- Larsson LI, et al. Pax 4 and 6 regulate gastrointestinal endocrine cell development. Mech Dev. 1998;79:153–9. doi: 10.1016/s0925-4773(98)00182-8. [DOI] [PubMed] [Google Scholar]
- Lee CS, Kaestner KH. Clinical endocrinology and metabolism. Development of gut endocrine cells. Best Pract Res Clin Endocrinol Metab. 2004;18:453–62. doi: 10.1016/j.beem.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Lee CS, et al. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 2002;16:1488–97. doi: 10.1101/gad.985002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CP, et al. IDX-1: a new homeodomain transcription factor expressed in rat pancreatic islets and duodenum that transactivates the somatostatin gene. Embo J. 1994;13:1145–56. doi: 10.1002/j.1460-2075.1994.tb06363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya FJ, et al. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev. 1997;11:2323–34. doi: 10.1101/gad.11.18.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng AN, et al. Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Dev Biol. 2005;286:114–35. doi: 10.1016/j.ydbio.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Offield MF, et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–95. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- Ohlsson H, et al. IPF1, a homeodomain-containing transactivator of the insulin gene. Embo J. 1993;12:4251–9. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteus MH, et al. Isolation and characterization of a library of cDNA clones that are preferentially expressed in the embryonic telencephalon. Brain Res Mol Brain Res. 1992;12:7–22. doi: 10.1016/0169-328x(92)90063-h. [DOI] [PubMed] [Google Scholar]
- Prado CL, et al. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc Natl Acad Sci U S A. 2004;101:2924–9. doi: 10.1073/pnas.0308604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, et al. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development. 2001;128:2723–33. doi: 10.1242/dev.128.14.2723. [DOI] [PubMed] [Google Scholar]
- Roberts RJ, Pearson MD. Infectious pancreatic necrosis in Atlantic salmon, Salmo salar L. J Fish Dis. 2005;28:383–90. doi: 10.1111/j.1365-2761.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- Schonhoff SE, et al. Minireview: Development and differentiation of gut endocrine cells. Endocrinology. 2004;145:2639–44. doi: 10.1210/en.2004-0051. [DOI] [PubMed] [Google Scholar]
- Sosa-Pineda B, et al. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- St-Onge L, et al. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature. 1997;387:406–9. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- Sussel L, et al. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 1998;125:2213–21. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- Wallace KN, et al. Intestinal growth and differentiation in zebrafish. Mech Dev. 2005;122:157–73. doi: 10.1016/j.mod.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Walters JR. Recent findings in the cell and molecular biology of the small intestine. Curr Opin Gastroenterol. 2005;21:135–40. doi: 10.1097/01.mog.0000153309.13080.8b. [DOI] [PubMed] [Google Scholar]
- Yang Q, et al. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–8. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- Zhou Q, et al. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron. 2001;31:791–807. doi: 10.1016/s0896-6273(01)00414-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Co-immunofluorescent staining of ghrelin-positive cells (green) and the pan-endocrine marker, synaptophysin (red) in the small intestine of wild type and Nkx2.2 null embryos at e18.5.
Real time PCR analysis of transcription factor mRNA isolated from wild type and Nkx2.2 null tissue. See Table 2 for the probe sets. See Table 3 for of embryonic samples tested. Fold change normalized to wild type; * p < 0.01.
Number of immuno-positive cells per cross-section (x±SEM). To quanitify the cells expressing each hormone, 10 cross sections of an embryo were examined for each hormone. The number of immunoreactive cells per cross section was counted and the results averaged.


