Abstract
We identify the SUMO-Targeted Ubiquitin Ligase (STUbL) family of proteins and propose that STUbLs selectively ubiquitinate sumoylated proteins and proteins that contain SUMO-like domains (SLDs). STUbL recruitment to sumoylated/SLD proteins is mediated by tandem SUMO interaction motifs (SIMs) within the STUbLs N-terminus. STUbL-mediated ubiquitination maintains sumoylation pathway homeostasis by promoting target protein desumoylation and/or degradation. Thus, STUbLs establish a novel mode of communication between the sumoylation and ubiquitination pathways. STUbLs are evolutionarily conserved and include: Schizosaccharomyces pombe Slx8-Rfp (founding member), Homo sapiens RNF4, Dictyostelium discoideum MIP1 and Saccharomyces cerevisiae Slx5–Slx8. Cells lacking Slx8-Rfp accumulate sumoylated proteins, display genomic instability, and are hypersensitive to genotoxic stress. These phenotypes are suppressed by deletion of the major SUMO ligase Pli1, demonstrating the specificity of STUbLs as regulators of sumoylated proteins. Notably, human RNF4 expression restores SUMO pathway homeostasis in fission yeast lacking Slx8-Rfp, underscoring the evolutionary functional conservation of STUbLs. The DNA repair factor Rad60 and its human homolog NIP45, which contain SLDs, are candidate STUbL targets. Consistently, Rad60 and Slx8-Rfp mutants have similar DNA repair defects.
Keywords: DNA repair, desumoylation, STUbL, SUMO, ubiquitin ligase
Introduction
The post-translational modifiers SUMO and ubiquitin, together termed ubiquitin-like proteins (Ubls), modulate the activities of multiple proteins and pathways that are key to cellular survival (Ulrich, 2005; Kerscher et al, 2006). Among these key pathways, Ubls regulate DNA repair and chromosome segregation mechanisms (Tanaka et al, 1999; Gill, 2004; Nacerddine et al, 2005; Ulrich, 2005). For example, the DNA homologous recombination repair (HRR) factor RAD52 is sumoylated in both yeast and mammals (Ho et al, 2001; Sacher et al, 2006). In budding yeast, Rad52 sumoylation affects both its stability and the outcome of Rad52-dependent HRR (Sacher et al, 2006). Also, sumoylation of the budding yeast RecQ helicase, Sgs1, is proposed to contribute to the anti-recombinogenic functions of SUMO at stalled replication forks (Branzei et al, 2006). Notably, the disease related human RecQ-like helicases WRN and BLM, which act in key DNA transactions including HRR, are also sumoylated (Kawabe et al, 2000; Eladad et al, 2005). An example of ubiquitin-dependent regulation is the mono-ubiquitination of FANCD2 following genotoxic stress, which results in FANCD2 redistribution to subnuclear foci that colocalize with critical DNA repair factors (see Huang and D'Andrea, 2006). Defective FANCD2 mono-ubiquitination is observed in patients with Fanconi's anemia (see Huang and D'Andrea, 2006).
Ubls also modulate chromosome structure and therefore, accessibility to proteins involved in chromosome segregation, DNA repair, and transcription. For example, sumoylation of core histones in budding yeast generates heterochromatin, possibly through recruitment of the transcriptional corepressors HDAC1 and HP1 (Shiio and Eisenman, 2003; Nathan et al, 2006). In fission yeast, sumoylation is required for heterochromatin structure and function at centromeres and telomeres (Xhemalce et al, 2004). Critical SUMO substrates include the heterochromatin protein Swi6 and the histone methyltransferase Clr4 (Shin et al, 2005).
Ubls are covalently attached to their substrates in a multistep enzymatic process. Ubls are first processed (matured) by specific proteases, DUBs for ubiquitin and ULPs for SUMO, exposing a C-terminal di-glycine motif (Ulrich, 2005; Kerscher et al, 2006). The mature Ubl is then conjugated to the substrate via a cascade of E1 (activating), E2 (conjugating), and E3 (ligase) enzymes. The E3 ligase facilitates substrate specificity and isopeptide bond formation between the Ubl's C-terminal glycine and a lysine residue in the target protein (Ulrich, 2005; Kerscher et al, 2006). Many E3 ligases contain the RING finger motif or a variant called the SP-RING, which catalyze ubiquitination and sumoylation, respectively (Hochstrasser, 2001; Johnson and Gupta, 2001; Xhemalce et al, 2004; Kerscher et al, 2006).
To ‘read' Ubl modifications and direct the appropriate physiological responses, proteins associated with Ubl-dependent regulatory pathways contain motifs that specifically interact with either SUMO or ubiquitin (Kerscher et al, 2006). Ubiquitin-binding domains (UBDs) are found in proteins associated with degradation, ubiquitination, and DNA repair (Kerscher et al, 2006). Components of the sumoylation pathway, or proteins whose function is modulated by noncovalent SUMO interaction, contain the recently discovered SUMO-specific interaction motifs (SIMs; see Hecker et al, 2006).
Historically, unlike ubiquitination, sumoylation does not promote target protein degradation and may in fact stabilize targets by antagonizing their ubiquitination (see Ulrich, 2005). This makes our discovery of a family of E3 ubiquitin ligases that act as SUMO-Targeted Ubiquitin Ligases (STUbLs) all the more intriguing. STUbLs appear to be recruited to sumoylated proteins and proteins containing SUMO-like domains (SLDs) to mediate their ubiquitination and subsequent desumoylation/degradation. The STUbL family includes fission yeast Slx8-Rfp, human RNF4, slime mold MIP1 and budding yeast Slx5 (also known as Hex3)/Slx8 (this study and Moilanen et al, 1998; Mullen et al, 2001; Sobko et al, 2002). STUbL dysfunction causes a specific accumulation of sumoylated protein species and correlated defects in DNA repair and genetic integrity. Reducing total sumoylated species, by deleting the major SUMO E3 ligase Pli1, suppresses these phenotypes. Thus, maintenance of SUMO pathway homeostasis is critical and STUbLs are potent new regulators of this pathway. Complementation of fission yeast Slx8-Rfp mutants by human RNF4 supports the functional conservation of this pathway in humans. Importantly, our studies both identify a novel family of ubiquitin ligases, STUbLs, and, furthermore, provide a mechanistic basis for the role of this previously enigmatic protein family in genome stability.
Results
Identification of Rfp1 and Rfp2, functional homologues of budding yeast Slx5
We recently identified the Nse5-Nse6 heterodimer of fission yeast, which is required to suppress or resolve toxic DNA recombination structures (Pebernard et al, 2006). In a yeast two-hybrid screen using Nse5 as bait, we isolated an uncharacterized RING finger protein 1 (Rfp1; Figure 1A). Significantly, the budding yeast homolog of Nse5 (Pebernard et al, 2006) also interacts with a RING finger protein called Slx5 (Hazbun et al, 2003). Budding yeast Slx5 heterodimerizes with another RING finger protein, SLX8, and together they maintain genome stability through an undefined mechanism (Mullen et al, 2001). In fission yeast, an Slx8, but not an Slx5 homolog, is detectable through bioinformatics-based approaches. Since both Rfp1 and Slx5 are RING finger proteins that interact with Nse5, we tested whether they were functional homologues.
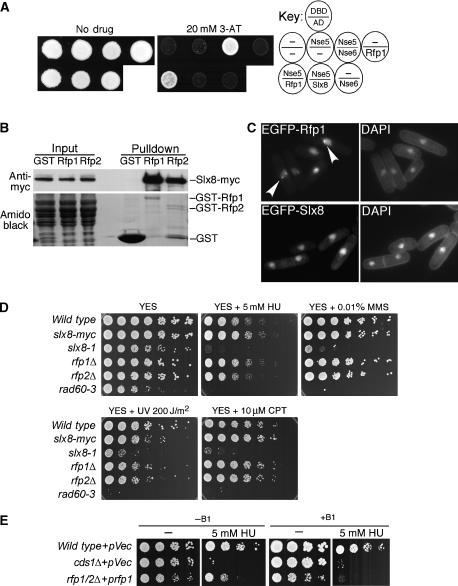
Figure 1.
Identification and characterization of the Slx8-Rfp complex. (A) The indicated yeast two-hybrid strains were spotted onto selective plates, which were untreated (No drug), or drug treated (20 mM 3-AT), to identify interacting proteins. Key indicates genes placed into the Gal4 DNA-binding (DBD) or the Gal4-activating (AD) domains. Empty vector (−) and positive controls (Nse5:Nse6 interaction) are shown. (B) Ectopically expressed GST-Rfp1/2 (or GST alone) were induced in an Slx8-myc strain, and subjected to GST pulldown, Inputs and Pulldowns were immunoblotted with anti-myc antisera, amido black staining is shown as a GST loading control. (C) Left panels: localization of ectopically expressed EGFP-Rfp1 or EGFP-Slx8 was detected in live cells. Right panels: DNA staining with DAPI (4′,6′-diamidino-2-phenylindole). Arrowheads indicate Rfp1 subnuclear foci. (D) Serial dilutions of the indicated strains grown at the semipermissive temperature (32°C), which were nontreated (YES), or treated with the indicated concentrations of hydroxyurea (HU), methylmethane sulfonate (MMS), camptothecin (CPT), or ultraviolet (UV) irradiated. (E) The indicated strains were serially diluted on selective media at 32°C, and either untreated, or treated with HU, under induced (−B1) or repressed (+B1) conditions.
We first tested whether Rfp1 interacts with Slx8 in vivo. Slx8 was epitope-tagged at its endogenous locus (Slx8-myc) and a GST alone, or GST-Rfp1 fusion protein was expressed in this strain. Purification of GST-Rfp1 resulted in the specific co-precipitation of Slx8-myc (Figure 1B). Furthermore, using a bacterial expression system, the direct interaction between Rfp1 and Slx8 was dependent on the Slx8 RING domain (Supplementary Figure 1). Sequence searches identified an Rfp1 paralogue in fission yeast, Rfp2, which also interacts with Slx8 (Figure 1B). The interactions between Slx8-Rfp1 and Slx8-Rfp2 were further explored using an insect cell expression system (Supplementary Figure 2). These data confirm the Slx8-Rfp1 and Slx8-Rfp2 interactions and further indicate that instead of a possible heterotrimer, Slx8 forms mutually exclusive heterodimers with Rfp1 or Rfp2 (Supplementary Figure 2). Consistent with a function in genome maintenance, both Rfp1 and Slx8 are nuclear (Figure 1C). Notably, ectopically overexpressed Rfp1, but not Slx8, forms subnuclear foci (Figure 1C). We hereafter refer to Slx8-Rfp1 and Slx8-Rfp2 complexes collectively as Slx8-Rfp.
Slx8-Rfp is critical for cell survival following genotoxic stress
We next analyzed the role of Slx8-Rfp in the cellular response to DNA damage. Fission yeast Slx8 is essential for vegetative growth and mutant cells die with an elongated morphology, caused by G2 DNA damage checkpoint activation (data not shown). Therefore, we generated a temperature-sensitive allele of slx8 (slx8-1). The slx8-1 strain was hypersensitive to hydroxyurea (HU), at a level similar to that of the rad60-3 mutant (Figure 1D). Rad60 is a DNA repair protein regulated by the replication checkpoint, and is required to prevent the formation of toxic recombination-dependent structures during replication arrest (Boddy et al, 2003; Miyabe et al, 2006; Raffa et al, 2006). The slx8-1 and rad60-3 mutants were sensitive to a similar spectrum of DNA-damaging agents, especially those that can potentially block or collapse replication forks (Figure 1D).
Unlike slx8-1, the individual rfp1Δ and rfp2Δ strains were not sensitive to any agent tested (Figure 1D). However, deletion of both rfp1 and rfp2 was lethal, resulting in the slx8Δ terminal phenotype, demonstrating the functional redundancy of Rfp1 and Rfp2. To determine the importance of the Rfps in response to replication blocks, we used a haploid rfp1Δ rfp2Δ double mutant that was rescued by inducible rfp1 expression. The pREP41 promoter controlled rfp1 expression, which is attenuated by thiamine (+B1) in the media, or fully induced in the absence of thiamine (−B1; (Maundrell, 1993)). When rfp1 was fully induced, the rfp1Δ rfp2Δ mutant displayed only mild sensitivity to HU, which is likely a result of excess rfp1 (Figure 1E; our unpublished data). However, when rfp1 expression was attenuated, but sufficient for cell viability, we observed extreme HU sensitivity (+B1; Figure 1E). Thus, Rfp depletion causes phenotypes similar to those of slx8-1, supporting their concerted action as an Slx8-Rfp heterodimer.
Genetic interactions of slx8-1 support a role in replication stress tolerance
The slx8-1 mutation causes sensitivity to replicative stress. Therefore, we tested the genetic interactions of slx8-1 with mutations in known replication fork guardians (Figure 2A). Eme1 is part of the heterodimeric Mus81-Eme1 endonuclease that cleaves recombination-dependent structures arising at stalled or collapsed replication forks (Boddy et al, 2000, 2001; Doe et al, 2002). Rqh1 is homologous to the human RecQ family helicase BLM and suppresses/resolves illegitimate recombination events at the replication fork (Doe et al, 2002). We found that slx8-1 is synthetic lethal with rad60-3, rad60-4, rqh1Δ, and eme1Δ at 34°C (Figure 2A). The genetic interaction between slx8-1 and rqh1Δ echoes that observed between deletions of the budding yeast homologues SLX8 and SGS1 (Mullen et al, 2001). However, in budding yeast, the SLX8 mutant does not depend on MUS81-EME1 (MMS4) for viability (Zhang et al, 2006), highlighting the existence of interesting differences in the functions of these complexes between the distantly related yeasts. These genetic interactions indicate that Slx8-Rfp resolves or suppresses the formation of recombination-dependent structures during replication.
Figure 2.
Analysis of the slx8-1 mutant phenotypes. (A) The indicated strains were serially diluted onto YES plates at permissive (25°C) or semipermissive (34°C) temperatures. Two independent isolates of slx8-1 rad60-4 are shown. (B) Fluorescence microscopy was used to analyze spontaneous Rad22-YFP foci formation in wild-type and slx8-1 cells, grown at the semipermissive temperature (32°C). DNA staining with DAPI is shown. Arrowheads indicate Rad22-YFP foci. At least 300 cells were scored for each strain. (C) The indicated strains were serially diluted onto YES plates at permissive (25°C) or semipermissive (34°C) temperatures.
High levels of spontaneous DNA damage in slx8-1 mutant cells
The phenotypes of slx8-1 cells suggest that they might accumulate spontaneous DNA damage. We tested this by analyzing the formation of Rad22-YFP (Rad52) foci in the slx8-1 mutant at 32°C. We observed that a large number of slx8-1 cells had at least one Rad22-YFP focus (Figure 2B). This indicates the presence of DNA damage in slx8-1 cells that is a substrate for the HRR machinery (e.g. DNA double-strand breaks). Indeed, the HRR factors rhp55 (rad55) and rhp51 (rad51) are required for the viability of slx8-1 cells at 34°C (Figure 2C).
Rfps interact with SUMO via a conserved SUMO-interaction motif
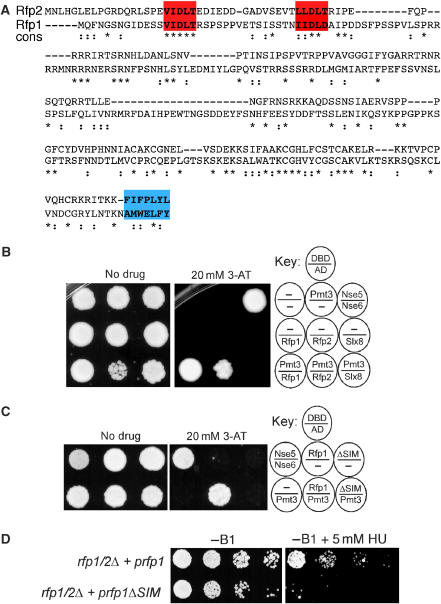
We identified potential SUMO-interacting motifs (SIMs) in Rfp1 and Rfp2, but not Slx8, which are related to the ‘core' SIM sequence found in the known SUMO interactor, PIAS1 (Hecker et al, 2006) (Figures 3A and 6A). Therefore, we compared the ability of Rfp1, Rfp2, and Slx8 to interact with SUMO in the yeast two-hybrid system. As SIM motifs promote noncovalent interaction with SUMO, we used a conjugation-defective mutant of SUMO that lacks the essential C-terminal di-glycine motif. We found that both Rfp1 and Rfp2, but not Slx8, interact in a noncovalent manner with SUMO (Figure 3B). Furthermore, deletion of the N-terminal 38 amino acids of Rfp1, which contain the SIMs, abolishes this interaction (Figure 3C). These data show that the Rfp1 and Rfp2 SIM sequences support noncovalent interaction with SUMO. To examine the in vivo importance of the Rfp SIMs, we analyzed the effect of overexpressing Rfp1ΔSIM in an rfp1Δ rfp2Δ strain. Overexpressing Rfp1ΔSIM only partially complemented the rfp1Δ rfp2Δ strain in the absence, but not presence, of HU (Figure 3D). In addition, Rfp1ΔSIM is unable to restore sumoylation homeostasis in the rfp1Δ rfp2Δ strain (discussed later and see Supplementary Figure 7). These observations underscore the in vivo importance of Rfp SIMs.
Figure 3.
SIM-dependent Rfp interactiom with SUMO. (A) S. pombe Rfp1/2 SUMO interacting motifs (SIMs; red) and C-terminal hydrophobic regions (blue) are shown. Identical (*) and conserved residues (:) are indicated. (B, C) The indicated yeast two-hybrid strains were spotted onto selective plates, which were untreated (No drug) or drug treated (20 mM 3-AT), to identify interacting proteins. Key indicates genes in the Gal4 DNA-binding (DBD) or Gal4-activating (AD) domains. Positive (Nse5:Nse6) and empty vector (−) controls are shown. (D) The indicated strains were serially diluted onto selective media at 32°C, and either untreated or HU treated. A full-colour version of this figure is available at the EMBO Journal Online.
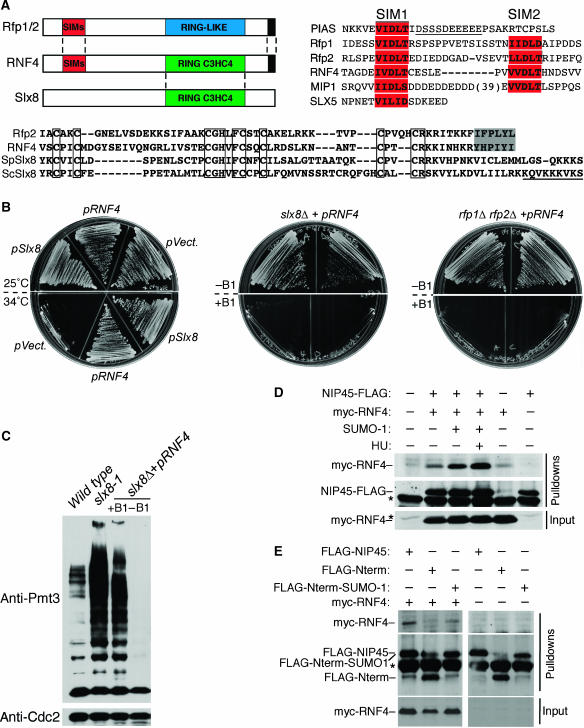
Figure 6.
Identification of a Human homolog of Slx8-Rfp. (A) Top left: the SUMO interacting motifs (SIMs; red), C3HC4 RING domain (green), RING-like domain (Blue), and C-terminal hydrophobic regions (black) are shown. Top right: alignments of the indicated proteins, depicting their SIM domains (red). An acidic stretch of amino acids in PIAS, which makes additional contacts with SUMO is underlined. Residues of each protein shown: PIAS, 454–485; Rfp1, 9–44; Rfp2, 14–48; RNF4, 41–68; MIP1, 160–231, and Slx5, 18–34. Lower Panel: C-terminal hydrophobic residues (shaded region) and RING domains (boxed residues) are shown. A region of basic residues common to the Slx8 proteins is underlined. Residues of each protein shown: Rfp2, 193–205; RNF4, 130–190; S. pombe Slx8, 204–269, and S. cerevisiae Slx8, 203–274. (B) The indicated strains were streaked on selective plates, which all contained 5 mM HU. Left panel: slx8-1 transformed with the indicated vectors, incubated at permissive (25°C; upper section) or semipermissive (34°C; lower section) temperatures. Middle and right panels: pRNF4 was either induced (−B1; upper sections) or shut off (+B1; lower sections) in two independent slx8Δ or rfp1Δ rfp2Δ strains. (C) Total lysates were immunoblotted with antisera for SUMO (Pmt3) or Cdc2 (loading control). (D, E) HEK293T cells were transfected with (+) or without (−) myc-RNF4, FLAG-NIP45, HA-SUMO1ΔC4, NIP45 mutants (FLAG-Nterm or FLAG-Nterm-SUMO1ΔC4), or treatment with 2 mM HU, as indicated. Upper panels (Pulldowns): FLAG pulldown, immunoblotted with myc or FLAG antisera. Lower panel (Input): loading controls immunoblotted with myc antisera. (*) Denotes background bands. A full-colour version of this figure is available at the EMBO Journal Online.
Slx8 but not Rfp1 or Rfp2 displays E3 ubiquitin ligase activity
The presence of RING finger domains in Slx8 and the Rfps indicates that they may possess E3 activity (Joazeiro and Weissman, 2000). Bacterially expressed GST-Slx8 but not GST-Rfps displayed robust E3 activity in vitro, catalyzing the formation of polyubiquitin chains only in the presence of E1, E2, and ATP (Figure 4A; and data not shown). GST-Slx8 appears to undergo extensive autoubiquitination, as often observed for E3s (Figure 4A). Slx8 lacking its RING domain (GST-Slx8ΔRING) was devoid of activity, demonstrating that Slx8 is a RING-dependent E3 ubiquitin ligase (Figure 4B).
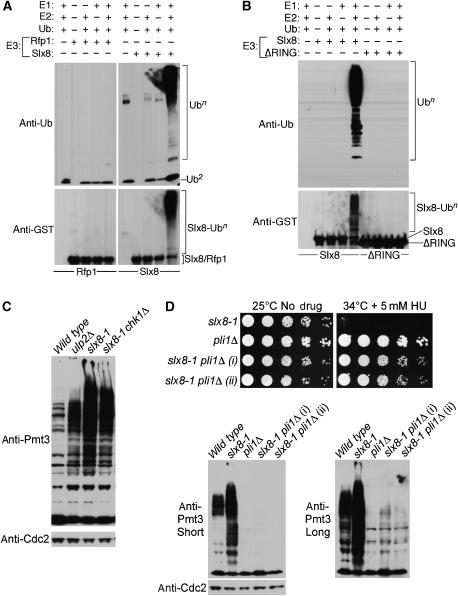
Figure 4.
Slx8, but not Rfp1/2, is a RING-dependent E3 ubiquitin ligase in vitro. (A, B) In vitro ubiquitination assays containing (+) or not (−) E1, E2, ubiquitin, and GST-Slx8, GST-Slx8 deleted for its RING motif (ΔRING) or GST-Rfp1, as indicated, were resolved by SDS–PAGE and immunoblotted with antisera for ubiquitin or GST. Polyubiquitin (Ubn) and di-ubiquitin (Ub2) chains are indicated. (C) Total lysates were prepared following 7 h incubation at 36°C, resolved by SDS–PAGE and immunoblotted with antisera for SUMO (Pmt3) or Cdc2 (loading control). (D) Upper panels: the indicated strains were serially diluted at the permissive temperature (25°C), with no drug, or semipermissive temperature (34°C) in the presence of 5 mM HU. Western blotting to determine total sumoylation levels in the indicated strains was as described in (C).
The Slx8-Rfp heterocomplex modulates sumoylation pathway homeostasis
The phenotypes caused by Slx8-Rfp mutations are similar to those caused by defects in the sumoylation pathway (al-Khodairy et al, 1995; Tanaka et al, 1999; Taylor et al, 2002). This observation, coupled with the physical interaction between the Rfps and SUMO, led us to investigate regulation of sumoylation in the slx8-1 mutant. Remarkably, a dramatic accumulation of SUMO conjugates was observed in the slx8-1 strain following incubation at the restrictive temperature (Figure 4C). This accumulation of SUMO conjugates was more extensive than that seen in cells lacking the Ulp2 SUMO isopeptidase, a rad60-3 strain, or an mts3-1 proteasome mutant (Figure 4C; also see Supplementary Figure 3A). An mts3-1 slx8-1 double mutant has greatly reduced viability at the semipermissive temperature (32°C) for each single mutant (Supplementary Figure 3B).
Since slx8-1 cells activate the DNA damage checkpoint kinase Chk1 at restrictive temperature, we tested whether the accumulation of SUMO conjugates in this background is a result of Chk1-dependent cell cycle arrest. To this end, we constructed an slx8-1 chk1Δ double mutant that no longer undergoes cell cycle arrest at 36°C. The total SUMO conjugates in the slx8-1 chk1Δ double and slx8-1 single mutants were similar, demonstrating that Slx8-Rfp dysfunction rather than cell cycle arrest accounts for this phenomenon (Figure 4C).
Suppression of Slx8 mutant phenotypes by deleting the SUMO E3 ligase Pli1
We have established that Slx8-Rfp modulates the sumoylation pathway and is required for genomic stability and DNA repair. However, it was not clear whether the accumulation of sumoylated proteins in slx8-1 mutant cells caused their phenotypes, or was a benign side effect. We hypothesized that deletion of the predominant SUMO E3 ligase Pli1, and consequent reduction in the level of SUMO conjugates, might rescue slx8-1 phenotypes (Xhemalce et al, 2004). Therefore, we constructed an slx8-1 pli1Δ double mutant strain and compared its phenotypes to those of the slx8-1 and pli1Δ single mutants (Figure 4D). The pli1Δ single mutant was viable at 34°C in the presence of HU (Figure 4D, upper panels). Strikingly, the slx8-1 pli1Δ double mutants were also viable under conditions that kill the slx8-1 single mutant (Figure 4D). In light of this rescue, we compared the levels of SUMO conjugates present in the slx8-1, pli1Δ, and slx8-1 pli1Δ double mutant strains (Figure 4D, lower panels). SUMO conjugates were barely visible in the pli1Δ single mutant, as previously observed (Figure 4D, lower panels; Xhemalce et al, 2004). Consistent with the phenotypic rescue of slx8-1 by pli1Δ, total sumoylation levels were much reduced in the slx8-1 pli1Δ double mutant strains, as compared to slx8-1 (Figure 4D, lower panels). These results demonstrate that the critical function of Slx8-Rfp is to maintain sumoylation pathway homeostasis and prevent the toxic accumulation of sumoylated proteins. In addition, deleting Pli1 also rescued the inviability of cells deleted for slx8 (Supplementary Figure 4). As observed in the slx8-1 pli1Δ double mutant, an slx8Δ pli1Δ strain no longer accumulates SUMO conjugates (Supplementary Figure 4). A weak accumulation of SUMO conjugates is observed in the slx8-1 pli1Δ and slx8Δ pli1Δ double mutants as compared to the pli1Δ single mutant (Figure 4D, lower panel long exposure; Supplementary Figure 4). Therefore, Slx8-Rfp also regulates Pli1-independent SUMO conjugates, which are presumably dependent on Nse2, the only other characterized SUMO ligase in fission yeast (Andrews et al, 2005).
Identification of DNA repair protein Rad60 as a potential Slx8-Rfp target
We performed a yeast two-hybrid screen using Rfp1 as bait and intriguingly, the screen returned 26 independent cDNAs of Rad60 and two of SUMO. The Rfp1–SUMO interaction was predictable based on the identification of SIM motifs in Rfp1. Uniquely, Rad60 and its homologues share the structural feature of tandem SLDs at their C-termini (Figure 5A; (Boddy et al, 2003; Novatchkova et al, 2005)). Therefore, we tested for interaction between Rfp1 and the Rad60 SLDs. A robust interaction was detected, which was Rfp1 SIM-dependent (Figure 5A). The interaction between Rad60 and Slx8-Rfp was then tested in vivo. GST fusion proteins of Rad60, full-length and SLDs, were expressed in a strain that also expressed Slx8-myc from its endogenous locus. Purification of either full-length Rad60 or the SLDs resulted in specific co-purification of Slx8-myc (Figure 5B). Notably, a mutation in the predicted SIM-binding pocket of the Rad60 SLD (F244A) abolishes Rfp1 interaction, as determined in the yeast two-hybrid system (Supplementary Figure 5). Furthermore, fission yeast that express rad60-F244A as the sole chromosomal copy of rad60 are HU sensitive, as observed for slx8 and rfp mutants (Supplementary Figure 5). These data support the physiological relevance of the Rfp1–Rad60 interaction and further show that the Rad60 SLDs mimic SUMO by mediating SIM-dependent interaction with Rfp1.
Figure 5.
Rfp stimulates Slx8-dependent ubiquitination of Rad60. (A) Upper panel: S. pombe Rad60 and H. Sapiens NIP45 tandem C-terminal SUMO-Like Domains (SLDs; boxed) are shown. Lower panel: the indicated yeast two-hybrid strains were spotted onto selective plates, which were either untreated (No drug), or drug treated (20 mM 3-AT), to identify interacting proteins. Key indicates the genes placed either into the Gal4 DNA-binding (DBD) or the Gal4-activating (AD) domains. (−) Denotes an empty vector. (B) Ectopically expressed GST-Full Length-Rad60 (GST-F.L.), GST-Rad60 SUMO-Like domains (GST-SLDs), or GST alone were induced in an Slx8-myc strain. Upper panel: (Pulldowns): GST pulldowns were immunoblotted with anti-myc antisera. Amido black staining is shown as a GST loading control. Lower panel (inputs): loading controls were immunoblotted with anti-myc antisera. Two independent strains expressing Rad60 SUMO-Like domains (GST-SLDs) are shown. (C, D) In vitro ubiquitination assays containing (+) or not (−) E1, E2, ubiquitin, Rad60-TAP, GST-Slx8, GST-Slx8 deleted for its RING motif (ΔRING), GST-Rfp1 or GST-Rfp1 deleted for its SIM motif (ΔSIM), as indicated, were resolved by SDS–PAGE and immunoblotted with antisera for Rad60-TAP, ubiquitin, or GST. Polyubiquitin chains (Ubn) are indicated.
Slx8 ubiquitinates Rad60 in vitro in an Rfp1 and SIM-stimulated manner
To further test Rad60 as a target of Slx8, we purified Rad60-TAP from fission yeast and used it as a substrate for in vitro ubiquitination assays. Rad60-TAP incubated alone or in the presence of E1, E2, and ubiquitin (but no E3) showed no detectable ubiquitination (Figure 5C). Addition of GST-Slx8 resulted in the appearance of ubiquitinated Rad60-TAP, which was dependent on the Slx8 RING domain (Figure 5C). We also tested the effect of adding GST-Rfp1 to the complete reaction, as Slx8 normally heterodimerizes with Rfp1. The reaction containing both Slx8 and Rfp1 showed an increase in the ubiquitination of Rad60-TAP (Figure 5C).
We next compared the ubiquitination of Rad60 catalyzed by an Slx8-Rfp1 or Slx8-Rfp1ΔSIM heterodimer. Again, Rad60 was more efficiently ubiquitinated by the Slx8-Rfp1 heterodimer than by Slx8 alone (Figure 5D, upper panel). The Slx8-Rfp1ΔSIM mutant still undergoes robust autoubiquitination; however, it is specifically defective in the ubiquitination of Rad60 (Figure 5D). This demonstrates that Rfp1-SIM motifs recruit Slx8 ubiquitinating activity to Rad60.
Evolutionarily conserved STUbLs
Database searches with the Rfps returned human RNF4 as a possible homolog, a protein not identified in searches using budding yeast Slx5 (Figure 6A, upper left panel). We also identified Rfp homologues in all higher eukaryotes examined, including D. discoideum MIP1 (Figure 6A, upper right panel; Sobko et al, 2002).
Extensive database mining showed that there are no clear Slx8 homologues detectable in higher eukaryotes. However, upon closer inspection we found that while RNF4 shares the SIMs and C-terminal hydrophobic motifs of the Rfps, its RING finger domain is more related to that of Slx8 (Figure 6A, lower panel). This suggested the intriguing possibility that the RNF4 protein in humans performs the functions of both Slx8 and Rfp1 (Figure 6A).
Therefore, we initially tested the ability of RNF4 to rescue the temperature and HU sensitivity of the slx8-1 mutant in fission yeast. We transformed the slx8-1 strain with plasmids that express slx8, human RNF4 or an empty vector (pVect). All strains were viable at 25°C in the presence of HU (Figure 6B). Remarkably, at 34°C, the strain expressing human RNF4 grew well and was indistinguishable from that rescued by slx8 expression (Figure 6B). This result demonstrates that RNF4 is able to rescue the major slx8-1 phenotypes.
We next determined whether RNF4 could rescue an slx8Δ, or rfp1Δ rfp2Δ double mutant strain. The heterozygous diploids, slx8+/slx8Δ and rfp1+/rfp1Δ rfp2+/rfp2Δ, were transformed with pRNF4 or empty vector. Following sporulation, only haploid mutants with pRNF4 were recovered, demonstrating functional complementation of both slx8Δ and rfp1Δ rfp2Δ mutants. To extend this observation, these strains were plated on media that maintained or repressed RNF4 expression (Figure 6B). Repression of RNF4 in either mutant strain caused a phenotype comparable to that of slx8-1, including hypersensitivity to HU (Figure 6B). Moreover, expression of RNF4 maintained the viability of an slx8Δ rfp1Δ rfp2Δ triple mutant (Supplementary Figure 6). Thus, RNF4 is a bi-functional protein capable of performing the essential activities of the fission yeast heterodimeric Slx8-Rfp complex.
Expression of RNF4 modulates sumoylation pathway homeostasis in slx8Δ cells
Given the rescue of slx8-rfp mutant phenotypes by RNF4, we asked whether sumoylation pathway homeostasis was also restored. We first grew the slx8Δ+pRNF4 strain under conditions that repressed or overexpressed RNF4. When RNF4 was repressed, the slx8Δ mutant accumulated SUMO conjugates as observed in the slx8-1 mutant at restrictive temperature (Figure 6C). Strikingly, slx8Δ cells overexpressing RNF4 showed much reduced levels of SUMO conjugates, which were below wild-type levels (Figure 6C). Slx8 mutant cells overexpressing RNF4 appeared healthy, suggesting that such low levels of SUMO conjugates were not overtly toxic to cells.
We next examined sumoylation pathway homeostasis in rfp1Δ rfp2Δ strains whose viability was maintained by prfp1 or prfp1ΔSIM. When compared to slx8-1, prfp1 restored total SUMO conjugates to near wild-type levels, whether its expression was induced or repressed (Supplementary Figure 7). Notably, unlike prfp1, prfp1ΔSIM under repressed conditions displayed high levels of SUMO conjugates, similar to those seen in slx8-1 cells (Supplementary Figure 7). Even when induced, prfp1ΔSIM only slightly reduced total SUMO conjugates and was much less efficient than wild-type prfp1 (Supplementary Figure 7). This indicates that STUbLs might have weak affinity for their targets, independent of their SIM motifs. Overall, these data highlight the importance of the Rfp1-SIM motif in directing Slx8 activity towards bulk SUMO conjugates in vivo.
Interaction of RNF4 with the human Rad60 homolog NIP45
The functional interchangeability of Slx8-Rfp and RNF4 led us to test interaction between RNF4 and the human Rad60 homolog, NIP45 (Boddy et al, 2003). Epitope tagged myc-RNF4 and NIP45-FLAG were cotransfected into HEK 293T cells followed by FLAG immunoprecipitation. RNF4 was found to co-precipitate with NIP45 and interestingly, this interaction was stimulated by the coexpression of a mature form of SUMO-1 (SUMO1ΔC4; Figure 6D). Treating cells with HU further enhanced the RNF4–NIP45 interaction (Figure 6D). This is intriguing in light of the HU sensitivity caused by mutations in Slx8-Rfp and Rad60 (Figure 1D and E). Notably, the RNF4–NIP45 interaction is NIP45 SLD-dependent, as demonstrated by the lack of interaction between RNF4 and the N-terminus of NIP45 (FLAG-Nterm; Figure 6E). Furthermore, the interaction with RNF4 was reconstituted when the N-terminus of NIP45 was fused to SUMO1ΔC4 (Figure 6E). The above data suggest that the Slx8-Rfp interaction with Rad60 will be a generally conserved STUbL family function with important physiological ramifications.
Discussion
The following observations support our hypothesis that STUbLs are recruited to sumoylated target proteins or those containing SLDs to catalyze their ubiquitination and desumoylation and/or degradation (Figure 7). The prototypic STUbLs Slx8-Rfp1 (this study), Slx5–Slx8 (Hannich et al, 2005), RNF4 (Hakli et al, 2005), and MIP1 (Sobko et al, 2002) interact with SUMO/SLDs, most likely through SIMs that we have identified in each of these proteins. Furthermore, E3 ubiquitin ligase activity has been demonstrated for Slx8-Rfp, RNF4, and MIP1 (this study and see Sobko et al, 2002; Hakli et al, 2004). Sumoylated proteins accumulate to toxic levels in Slx8 mutant cells and this phenomenon is reversed by deletion of the SUMO ligase Pli1 or expression of human RNF4. Accumulation of sumoylated proteins was recently observed in Slx5–Slx8 mutants of budding yeast, although no mechanism for this was defined (Wang et al, 2006). In addition, RNF4 is a transcriptional coregulator for members of the nuclear receptor superfamily that are sumoylated within their transcriptional inhibitory domains (Moilanen et al, 1998; Poukka et al, 2000b; Abdel-Hafiz et al, 2002; Tian et al, 2002). Interestingly, the residues of RNF4 that are required for its interaction with the androgen receptor (amino acids 31–65 (Poukka et al, 2000a)) encompass the SIMs that we identified. Furthermore, the SUMO and ubiquitin-dependent regulation of D. discoideum MEK1, an MAP kinase kinase required for chemotaxis, provides particularly compelling support for our model (Sobko et al, 2002). In response to chemoattractant, MEK1 is transiently sumoylated and translocates into the cytosol to concentrate at the cell's leading edge. Following sumoylation, MEK1 undergoes MIP1-dependent ubiquitination. In an MIP1 mutant, MEK1 undergoes chemoattractant-stimulated sumoylation (indicating that STUbLs are ubiquitin and not SUMO E3 ligases) but instead of the normal transient sumoylation, the sumoylated form of MEK1 persists (Sobko et al, 2002). Again, we identified conserved SIMs in the region of MIP1 required for interaction with MEK1 (Figure 6A). Therefore, although STUbL targets are functionally diverse, they are unified through target protein sumoylation (or the presence of SUMO-like domains).
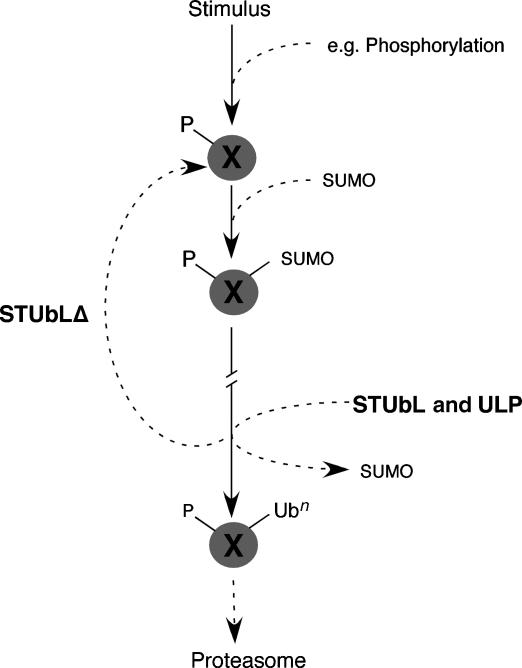
Figure 7.
Model for SUMO-directed ubiquitination by the conserved STUbL family. Following the appropriate stimulus and priming modification, e.g. phosphorylation, a target protein X is subject to sumoylation. Upon completion of the function associated with sumoylated target X (// on arrow indicates variable time elapsed), it is subject to simultaneous desumoylation by the Ulp isopeptidases and STUbL-dependent ubiquitination. STUbL-dependent ubiquitination of X may also require a priming modification, e.g. phosphorylation/dephosphorylation of X. STUbL-dependent ubiquitination may directly target X to the proteasome for degradation, thus clearing the pool of ‘sumoylation competent' target X (e.g. phosphorylated X). In the absence of STUbLs (STUbLΔ), target X may be locked in a cycle of sumoylation and desumoylation, resulting in a net increase in sumoylated proteins in the cell. In this model, ubiquitin and SUMO do not coexist on the target. Indeed, ubiquitination by STUbLs could be on the same (or proximal) lysine that was sumoylated, thus blocking resumoylation.
STUbLs may counteract the accumulation of sumoylated proteins in multiple ways. For example, they could direct sumoylated targets to the desumoylating activities of ULP isopeptidases, or mark sumoylated proteins for degradation. Given the apparently global effect of STUbL overproduction or attenuation on the abundance of SUMO conjugates, we tested whether SUMO itself was a critical acceptor of STUbL-mediated ubiquitination. Fission yeast SUMO (Pmt3) contains nine lysine residues, all of which could be acceptors for ubiquitination (among other modifiers). Therefore, to prevent ubiquitination of SUMO, we generated a fission yeast strain expressing a SUMO (Pmt3) mutant that has all nine lysine residues substituted by arginine (pmt3-allR) as the sole source of SUMO in the cell. Strikingly, fission yeast pmt3-allR cells are healthy and are wild type for growth at 36°C, unlike pmt3Δ cells (Supplementary Figure 8). This result indicates that the essential function of Slx8-Rfp is not direct ubiquitination of SUMO. Instead, together with the observation that the normally essential slx8 gene can be deleted when the pli1 SUMO ligase is also deleted (Supplementary Figure 4A), these data indicate that the proteins to which SUMO is conjugated are the essential Slx8-Rfp targets. Among these targets might be components of the sumoylation machinery, i.e. E1-3, as they are known to be SUMO modified (Wohlschlegel et al, 2004). This may partially explain the global deregulation of sumoylation in STUbL mutants. Our experiments to test the ubiquitination of sumoylated proteins in vivo have not detected the coexistence of these modifiers on a single protein. While we cannot exclude the comodification of targets with SUMO and ubiquitin on a cursory level, this observation is consistent with the kinetics of MEK1 ubiquitination by the Dictyostelium STUbL MIP1 (Sobko et al, 2002). This is because the transient sumoylated form of MEK1 becomes undetectable before MEK1 ubiquitination is maximal. Thus our model of STUbL function incorporates the possibility that target proteins are desumoylated concomitantly with their ubiquitination (Figure 7). Importantly, as shown in our model, we are not proposing that sumoylation of target proteins leads directly to their degradation. Instead, we propose that STUbL-dependent downregulation of sumoylated proteins (desumoylation/degradation) is only engaged upon completion of their function. Thus, depending on the process involved, sumoylation may actually increase the observed half-life of the SUMO-modified protein. Notably, STUbLs might degrade and clear sumoylated proteins that may otherwise inhibit critical downstream events in various processes. For example, sumoylation of the HRR protein Rad52 could direct it to DNA double-strand breaks, where it binds with high affinity. Once it has executed its role in HRR, for the ordered progression of the recombination reaction to occur, it must be removed. It is possible that desumoylation alone would be insufficient to override the affinity of Rad52 for DNA ends and therefore, STUbLs might promote degradation of this pool of Rad52 to allow rapid downregulation of its activities.
Rad60 and NIP45 are evolutionarily conserved STUbL interactors and potential targets. Both contain SLDs and those of Rad60 support SIM-dependent interaction with Rfps. Rad60 is ubiquitinated by Slx8 in an Rfp SIM-dependent manner, as predicted by our model. Three observations support the physiological relevance of the interaction between Rfp1 and Rad60. First, Rad60 and Slx8-Rfp mutants display a very similar spectrum of DNA damage sensitivities. Second, mutations that compromise the activity of Rad60 and its budding yeast homolog Esc2 are lethal in the absence of the RecQ helicases Rqh1 and Sgs1, respectively (Mullen et al, 2001; Tong et al, 2001; Boddy et al, 2003). Likewise, the RecQ helicases are required for viability when the STUbLs of either yeast are compromised. Thus, STUbLs and Rad60 family proteins share similar genetic interactions and DNA repair defects. Third, and perhaps most compelling, a specific mutation in the SIM-binding site of the Rad60 SLDs, rad60-F244A, abolishes Rad60–Rfp1 interaction and renders cells HU sensitive. Notably, human RNF4 interacts with the Rad60 homolog NIP45, in an SLD-dependent manner, demonstrating that this interaction is conserved and likely to be physiologically important.
We have shown that Slx8-Rfp is a critical guardian of genomic integrity and that the Slx8-Rfp mutant phenotypes result from the aberrant accumulation of sumoylated proteins. Given the observed functional conservation, it is likely that RNF4, which has been implicated in tumor suppression, will also play important roles in mammalian genome maintenance (Pero et al, 2001; Hirvonen-Santti et al, 2003).
In conclusion, we have identified major crosstalk between the ubiquitin and SUMO pathways by defining the STUbL family. STUbLs recognize sumoylated and SLD-containing proteins, which is a novel means of target recognition by ubiquitin ligases. Moreover, by demonstrating specific deregulation of the sumoylation pathway, our studies provide a mechanistic basis for the genome-destabilizing effects of STUbL inactivation.
Materials and methods


S. pombe protein preparation and in vitro ubiquitination assays
GST-pulldown experiments were as described previously (Raffa et al, 2006). Co-precipitated proteins were resolved on 12% SDS–PAGE and immunoblotted for the myc and GST epitopes using anti-myc (9E10, Covance) and anti-GST (rabbit polyclonal) antibodies.
To test whether Slx8, Rfp1, and Rfp2 displayed E3 activity, bacterially expressed recombinant GST-tagged versions of these proteins were used for in vitro ubiquitination assays. Rfp1 (SPAC19A8.10), Rfp1ΔSIM (aa 37–254), Rfp2 (SPAC343.18), Slx8 (SPBC3D6.11C), and Slx8ΔRING (aa 1–190) were cloned into pGEX6-P1 (GE Healthcare), transformed and induced in BL21 DE3 (Novagen, Madison, WI), lysed in 1 × PBS, 1% Triton-X 100, 1 mM 1,4-dithiothreitol (DTT; Roche, Indianapolis, IN), 1 × Complete protease inhibitor mix EDTA-free (Roche, Indianapolis, IN), and 2 mM phenylmethylsulfonyl fluoride (PMSF), bound to Glutathione 4B Sepharose beads (GE Healthcare), washed 2 × with 150 mM NaCl, 50 mM Tris–HCl pH7.5, 1 mM DTT, and 10% glycerol, with elution in the same buffer plus 10 mM glutathione. In vitro ubiquitination assays were performed in a 20 μl final volume with 50–125 nM yeast E1 (Boston Biochem), 500nM–1μM human UbcH5a (E2; Boston Biochem), and 25–37.5 μg bovine erythrocyte ubiquitin (Sigma-Aldrich), in 1 × Reaction Buffer (50 mM Tris–HCl pH 7.5, 10 mM MgCl2, 0.2 mM CaCl2, 4 mM ATP, and 1 mM DTT), incubated at 30°C for 30 min, and stopped by the addition of 20 μl 2 × SDS sample buffer with β-mercaptoethanol. These reactions were resolved on 4–20% gradient Tris-Glycine gels (Invitrogen, Carlsbad, CA) and immunoblotted using antisera raised against ubiquitin (Mouse anti-ubiquitin, Zymed, Invitrogen) or GST (rabbit polyclonal).
Endogenous Rad60-TAP-tagged protein purifications were performed as described previously (Raffa et al, 2006). Levels of polyubiquitinated Rad60-TAP were assessed by immunoblotting with the peroxidase-anti-peroxidase (PAP) antibody (Sigma-Aldrich). To assay total levels of sumoylated proteins, cells were harvested in the appropriate media/conditions and lysed in denaturing buffer (8 M urea, 0.5% Nonidet P-40, 50 mM sodium phosphate, and 50 mM Tris–HCL pH 8.0) with 1 × Complete protease inhibitor mix EDTA-free, equal quantities of protein were then resolved on 4–20% gradient Tris-Glycine gels and immunoblotted using antisera raised against Pmt3 (Felicity Watts, Sussex University, UK). Anti-cdc2 was used as a loading control (Steve Reed, TSRI). Mammalian cell transfections and methods are included in the Supplementary data.
Supplementary Material
Supplementary Figures
Acknowledgments
We thank Paul Russell, Benoit Arcangioli, Steve Reed, Felicity Watts, Frauke Melchior, and Stephane Coulon for strains, SUMO constructs, and antibodies. We are grateful to Peter Wright and The Scripps Cell Cycle Group for support and encouragement. This work was funded in part by NIH grant GM068608 awarded to MNB and NCI grant CA095114 to CHMcG.
References
- Abdel-Hafiz H, Takimoto GS, Tung L, Horwitz KB (2002) The inhibitory function in human progesterone receptor N termini binds SUMO-1 protein to regulate autoinhibition and transrepression. J Biol Chem 277: 33950–33956 [DOI] [PubMed] [Google Scholar]
- al-Khodairy F, Enoch T, Hagan IM, Carr AM (1995) The Schizosaccharomyces pombe hus5 gene encodes a ubiquitin conjugating enzyme required for normal mitosis. J Cell Sci 108 (Part 2): 475–486 [DOI] [PubMed] [Google Scholar]
- Andrews EA, Palecek J, Sergeant J, Taylor E, Lehmann AR, Watts FZ (2005) Nse2, a component of the Smc5-6 complex, is a SUMO ligase required for the response to DNA damage. Mol Cell Biol 25: 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy MN, Furnari B, Mondesert O, Russell P (1998) Replication checkpoint enforced by kinases Cds1 and Chk1. Science 280: 909–912 [DOI] [PubMed] [Google Scholar]
- Boddy MN, Gaillard PH, McDonald WH, Shanahan P, Yates JR III, Russell P (2001) Mus81-Eme1 are essential components of a Holliday junction resolvase. Cell 107: 537–548 [DOI] [PubMed] [Google Scholar]
- Boddy MN, Lopez-Girona A, Shanahan P, Interthal H, Heyer WD, Russell P (2000) Damage tolerance protein Mus81 associates with the FHA1 domain of checkpoint kinase Cds1. Mol Cell Biol 20: 8758–8766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy MN, Shanahan P, McDonald WH, Lopez-Girona A, Noguchi E, Yates IJ, Russell P (2003) Replication checkpoint kinase Cds1 regulates recombinational repair protein Rad60. Mol Cell Biol 23: 5939–5946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branzei D, Sollier J, Liberi G, Zhao X, Maeda D, Seki M, Enomoto T, Ohta K, Foiani M (2006) Ubc9- and mms21-mediated sumoylation counteracts recombinogenic events at damaged replication forks. Cell 127: 509–522 [DOI] [PubMed] [Google Scholar]
- Doe CL, Ahn JS, Dixon J, Whitby MC (2002) Mus81-Eme1 and Rqh1 involvement in processing stalled and collapsed replication forks. J Biol Chem 277: 32753–32759 [DOI] [PubMed] [Google Scholar]
- Eladad S, Ye TZ, Hu P, Leversha M, Beresten S, Matunis MJ, Ellis NA (2005) Intra-nuclear trafficking of the BLM helicase to DNA damage-induced foci is regulated by SUMO modification. Hum Mol Genet 14: 1351–1365 [DOI] [PubMed] [Google Scholar]
- Gill G (2004) SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev 18: 2046–2059 [DOI] [PubMed] [Google Scholar]
- Hakli M, Karvonen U, Janne OA, Palvimo JJ (2005) SUMO-1 promotes association of SNURF (RNF4) with PML nuclear bodies. Exp Cell Res 304: 224–233 [DOI] [PubMed] [Google Scholar]
- Hakli M, Lorick KL, Weissman AM, Janne OA, Palvimo JJ (2004) Transcriptional coregulator SNURF (RNF4) possesses ubiquitin E3 ligase activity. FEBS Lett 560: 56–62 [DOI] [PubMed] [Google Scholar]
- Hannich JT, Lewis A, Kroetz MB, Li SJ, Heide H, Emili A, Hochstrasser M (2005) Defining the SUMO-modified proteome by multiple approaches in Saccharomyces cerevisiae. J Biol Chem 280: 4102–4110 [DOI] [PubMed] [Google Scholar]
- Hazbun TR, Malmstrom L, Anderson S, Graczyk BJ, Fox B, Riffle M, Sundin BA, Aranda JD, McDonald WH, Chiu CH, Snydsman BE, Bradley P, Muller EG, Fields S, Baker D, Yates JR III, Davis TN (2003) Assigning function to yeast proteins by integration of technologies. Mol Cell 12: 1353–1365 [DOI] [PubMed] [Google Scholar]
- Hecker CM, Rabiller M, Haglund K, Bayer P, Dikic I (2006) Specification of SUMO1- and SUMO2-interacting motifs. J Biol Chem 281: 16117–16127 [DOI] [PubMed] [Google Scholar]
- Hirvonen-Santti SJ, Rannikko A, Santti H, Savolainen S, Nyberg M, Janne OA, Palvimo JJ (2003) Down-regulation of estrogen receptor beta and transcriptional coregulator SNURF/RNF4 in testicular germ cell cancer. Eur Urol 44: 742–747; discussion 747 [DOI] [PubMed] [Google Scholar]
- Ho JC, Warr NJ, Shimizu H, Watts FZ (2001) SUMO modification of Rad22, the Schizosaccharomyces pombe homologue of the recombination protein Rad52. Nucleic Acids Res 29: 4179–4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstrasser M (2001) SP-RING for SUMO: new functions bloom for a ubiquitin-like protein. Cell 107: 5–8 [DOI] [PubMed] [Google Scholar]
- Huang TT, D'Andrea AD (2006) Regulation of DNA repair by ubiquitylation. Nat Rev Mol Cell Biol 7: 323–334 [DOI] [PubMed] [Google Scholar]
- Joazeiro CA, Weissman AM (2000) RING finger proteins: mediators of ubiquitin ligase activity. Cell 102: 549–552 [DOI] [PubMed] [Google Scholar]
- Johnson ES, Gupta AA (2001) An E3-like factor that promotes SUMO conjugation to the yeast septins. Cell 106: 735–744 [DOI] [PubMed] [Google Scholar]
- Kawabe Y, Seki M, Seki T, Wang WS, Imamura O, Furuichi Y, Saitoh H, Enomoto T (2000) Covalent modification of the Werner's syndrome gene product with the ubiquitin-related protein, SUMO-1. J Biol Chem 275: 20963–20966 [DOI] [PubMed] [Google Scholar]
- Kerscher O, Felberbaum R, Hochstrasser M (2006) Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol 22: 159–180 [DOI] [PubMed] [Google Scholar]
- Maundrell K (1993) Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123: 127–130 [DOI] [PubMed] [Google Scholar]
- Miyabe I, Morishita T, Hishida T, Yonei S, Shinagawa H (2006) Rhp51-dependent recombination intermediates that do not generate checkpoint signal are accumulated in Schizosaccharomyces pombe rad60 and smc5/6 mutants after release from replication arrest. Mol Cell Biol 26: 343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moilanen AM, Poukka H, Karvonen U, Hakli M, Janne OA, Palvimo JJ (1998) Identification of a novel RING finger protein as a coregulator in steroid receptor-mediated gene transcription. Mol Cell Biol 18: 5128–5139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JR, Kaliraman V, Ibrahim SS, Brill SJ (2001) Requirement for three novel protein complexes in the absence of the Sgs1 DNA helicase in Saccharomyces cerevisiae. Genetics 157: 103–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacerddine K, Lehembre F, Bhaumik M, Artus J, Cohen-Tannoudji M, Babinet C, Pandolfi PP, Dejean A (2005) The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Dev Cell 9: 769–779 [DOI] [PubMed] [Google Scholar]
- Nathan D, Ingvarsdottir K, Sterner DE, Bylebyl GR, Dokmanovic M, Dorsey JA, Whelan KA, Krsmanovic M, Lane WS, Meluh PB, Johnson ES, Berger SL (2006) Histone sumoylation is a negative regulator in Saccharomyces cerevisiae and shows dynamic interplay with positive-acting histone modifications. Genes Dev 20: 966–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novatchkova M, Bachmair A, Eisenhaber B, Eisenhaber F (2005) Proteins with two SUMO-like domains in chromatin-associated complexes: the RENi (Rad60-Esc2-NIP45) family. BMC Bioinformatics 6: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pebernard S, Wohlschlegel J, McDonald WH, Yates JR III, Boddy MN (2006) The Nse5-Nse6 dimer mediates DNA repair roles of the Smc5–Smc6 complex. Mol Cell Biol 26: 1617–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pero R, Lembo F, Di Vizio D, Boccia A, Chieffi P, Fedele M, Pierantoni GM, Rossi P, Iuliano R, Santoro M, Viglietto G, Bruni CB, Fusco A, Chiariotti L (2001) RNF4 is a growth inhibitor expressed in germ cells but not in human testicular tumors. Am J Pathol 159: 1225–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poukka H, Aarnisalo P, Santti H, Janne OA, Palvimo JJ (2000a) Coregulator small nuclear RING finger protein (SNURF) enhances Sp1- and steroid receptor-mediated transcription by different mechanisms. J Biol Chem 275: 571–579 [DOI] [PubMed] [Google Scholar]
- Poukka H, Karvonen U, Janne OA, Palvimo JJ (2000b) Covalent modification of the androgen receptor by small ubiquitin-like modifier 1 (SUMO-1). Proc Natl Acad Sci USA 97: 14145–14150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa GD, Wohlschlegel J, Yates JR III, Boddy MN (2006) SUMO-binding motifs mediate the Rad60-dependent response to replicative stress and self-association. J Biol Chem 281: 27973–27981 [DOI] [PubMed] [Google Scholar]
- Sacher M, Pfander B, Hoege C, Jentsch S (2006) Control of Rad52 recombination activity by double-strand break-induced SUMO modification. Nat Cell Biol 8: 1284–1290 [DOI] [PubMed] [Google Scholar]
- Shiio Y, Eisenman RN (2003) Histone sumoylation is associated with transcriptional repression. Proc Natl Acad Sci USA 100: 13225–13230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JA, Choi ES, Kim HS, Ho JC, Watts FZ, Park SD, Jang YK (2005) SUMO modification is involved in the maintenance of heterochromatin stability in fission yeast. Mol Cell 19: 817–828 [DOI] [PubMed] [Google Scholar]
- Sobko A, Ma H, Firtel RA (2002) Regulated SUMOylation and ubiquitination of DdMEK1 is required for proper chemotaxis. Dev Cell 2: 745–756 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nishide J, Okazaki K, Kato H, Niwa O, Nakagawa T, Matsuda H, Kawamukai M, Murakami Y (1999) Characterization of a fission yeast SUMO-1 homologue, pmt3p, required for multiple nuclear events, including the control of telomere length and chromosome segregation. Mol Cell Biol 19: 8660–8672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DL, Ho JC, Oliver A, Watts FZ (2002) Cell-cycle-dependent localisation of Ulp1, a Schizosaccharomyces pombe Pmt3 (SUMO)-specific protease. J Cell Sci 115: 1113–1122 [DOI] [PubMed] [Google Scholar]
- Tian S, Poukka H, Palvimo JJ, Janne OA (2002) Small ubiquitin-related modifier-1 (SUMO-1) modification of the glucocorticoid receptor. Biochem J 367: 907–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong AH, Evangelista M, Parsons AB, Xu H, Bader GD, Page N, Robinson M, Raghibizadeh S, Hogue CW, Bussey H, Andrews B, Tyers M, Boone C (2001) Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294: 2364–2368 [DOI] [PubMed] [Google Scholar]
- Ulrich HD (2005) Mutual interactions between the SUMO and ubiquitin systems: a plea of no contest. Trends Cell Biol 15: 525–532 [DOI] [PubMed] [Google Scholar]
- Wang Z, Jones GM, Prelich G (2006) Genetic analysis connects SLX5 and SLX8 to the SUMO pathway in Saccharomyces cerevisiae. Genetics 172: 1499–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlschlegel JA, Johnson ES, Reed SI, Yates JR III (2004) Global analysis of protein sumoylation in Saccharomyces cerevisiae. J Biol Chem 279: 45662–45668 [DOI] [PubMed] [Google Scholar]
- Xhemalce B, Seeler JS, Thon G, Dejean A, Arcangioli B (2004) Role of the fission yeast SUMO E3 ligase Pli1p in centromere and telomere maintenance. EMBO J 23: 3844–3853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Roberts TM, Yang J, Desai R, Brown GW (2006) Suppression of genomic instability by SLX5 and SLX8 in Saccharomyces cerevisiae. DNA Repair (Amst) 5: 336–346 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures