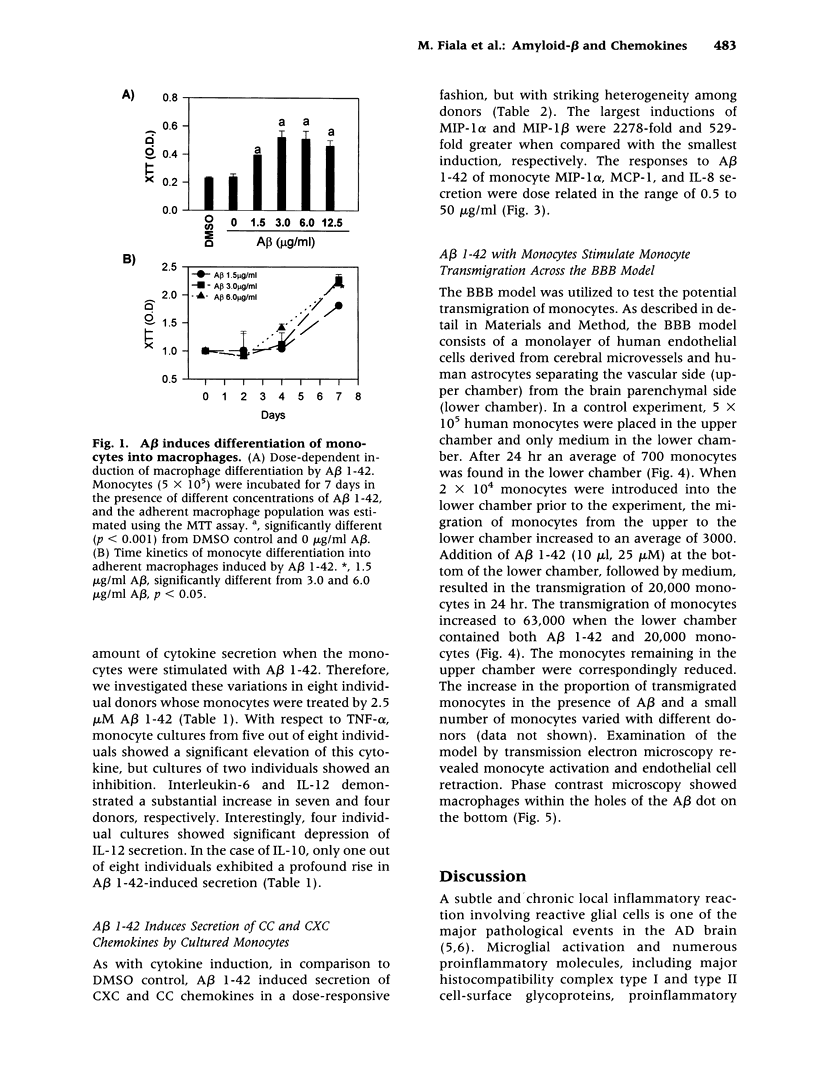
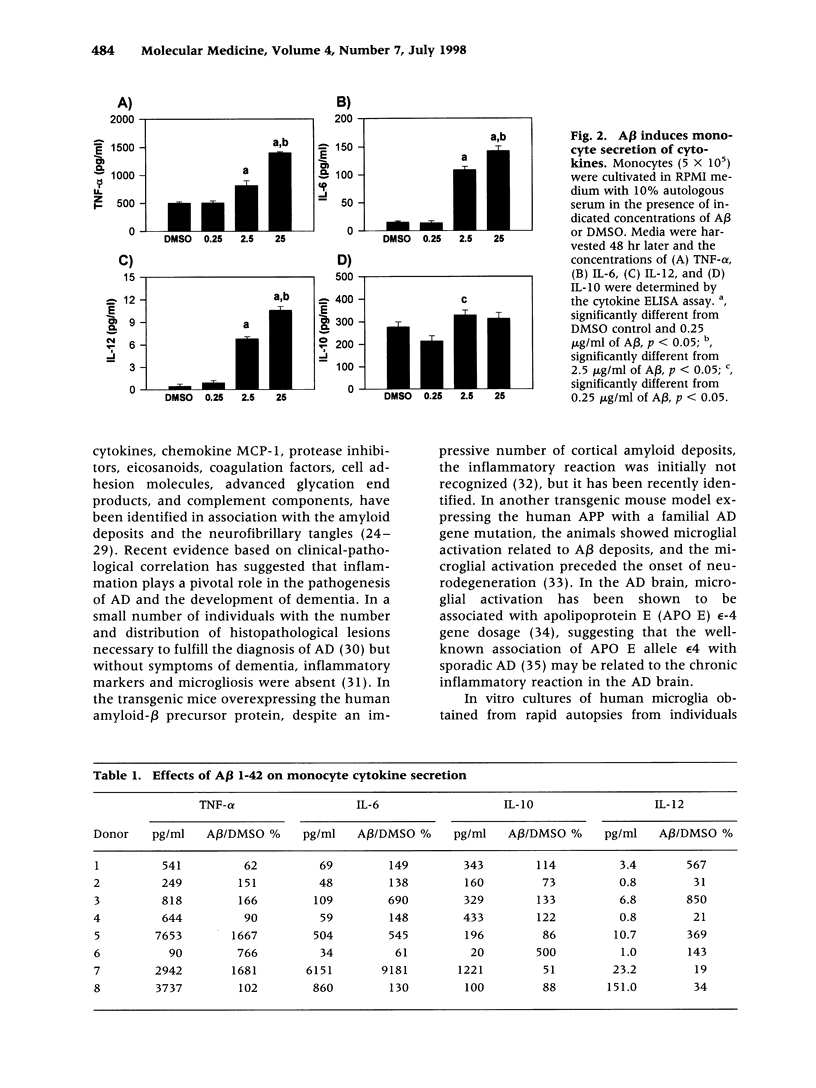
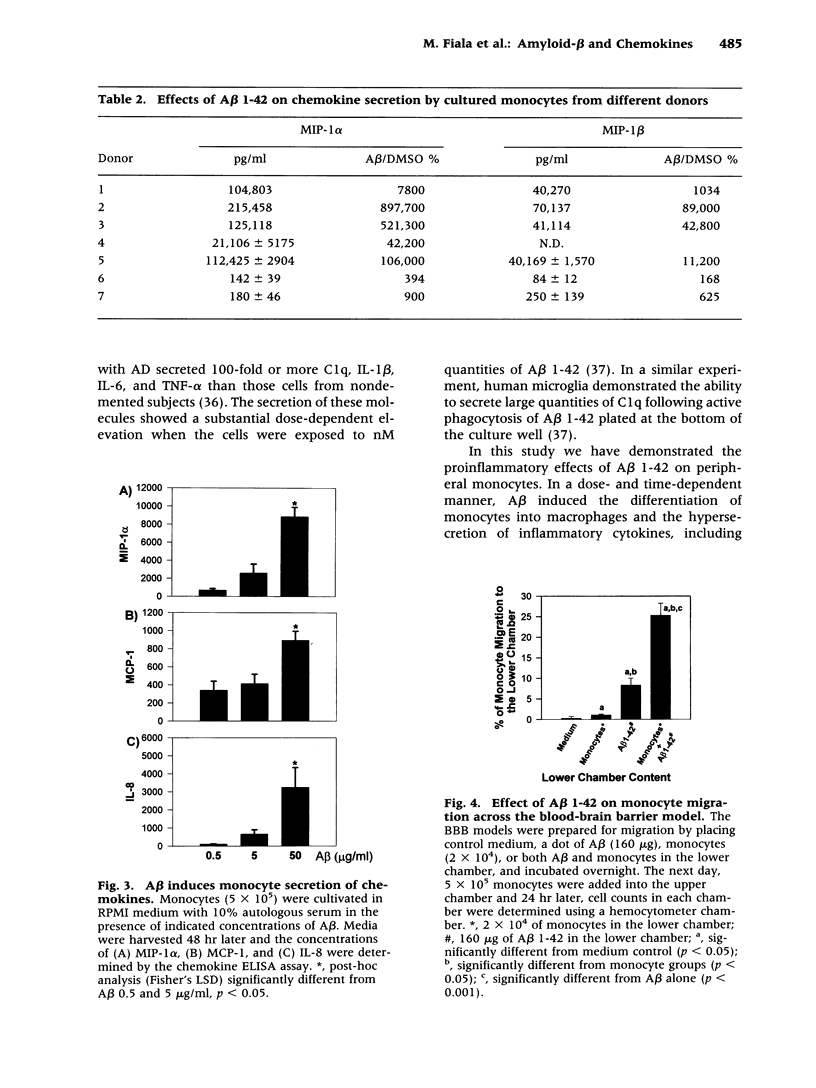
Abstract
BACKGROUND: Aside from numerous parenchymal and vascular deposits of amyloid beta (A beta) peptide, neurofibrillary tangles, and neuronal and synaptic loss, the neuropathology of Alzheimer's disease is accompanied by a subtle and chronic inflammatory reaction that manifests itself as microglial activation. However, in Alzheimer's disease, alterations in the permeability of the blood-brain barrier and chemotaxis, in part mediated by chemokines and cytokines, may permit the recruitment and transendothelial passage of peripheral cells into the brain parenchyma. MATERIALS AND METHODS: Human monocytes from different donors were tested for their capacity to differentiate into macrophages and their ability to secrete cytokines and chemokines in the presence of A beta 1-42. A paradigm of the blood-brain barrier was constructed utilizing human brain endothelial and astroglial cells with the anatomical and physiological characteristics observed in vivo. This model was used to test the ability of monocytes/macrophages to transmigrate when challenged by A beta 1-42 on the brain side of the blood-brain barrier model. RESULTS: In cultures of peripheral monocytes, A beta 1-42 induced the secretion of proinflammatory cytokines TNF-alpha, IL-6, IL-1 beta, and IL-12, as well as CC chemokines MCP-1, MIP-1 alpha, and MIP-1 beta, and CXC chemokine IL-8 in a dose-related fashion. In the blood-brain barrier model, A beta 1-42 and monocytes on the brain side potentiated monocyte transmigration from the blood side to the brain side. A beta 1-42 stimulated differentiation of monocytes into adherent macrophages in a dose-related fashion. The magnitude of these proinflammatory effects of A beta 1-42 varied dramatically with monocytes from different donors. CONCLUSION: In some individuals, circulating monocytes/macrophages, when recruited by chemokines produced by activated microglia and macrophages, could add to the inflammatory destruction of the brain in Alzheimer's disease.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barzó P., Marmarou A., Fatouros P., Corwin F., Dunbar J. G. Acute blood-brain barrier changes in experimental closed head injury as measured by MRI and Gd-DTPA. Acta Neurochir Suppl. 1997;70:243–246. doi: 10.1007/978-3-7091-6837-0_75. [DOI] [PubMed] [Google Scholar]
- Belayev L., Busto R., Zhao W., Ginsberg M. D. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996 Nov 11;739(1-2):88–96. doi: 10.1016/s0006-8993(96)00815-3. [DOI] [PubMed] [Google Scholar]
- Bell M. D., Taub D. D., Perry V. H. Overriding the brain's intrinsic resistance to leukocyte recruitment with intraparenchymal injections of recombinant chemokines. Neuroscience. 1996 Sep;74(1):283–292. doi: 10.1016/0306-4522(96)00083-8. [DOI] [PubMed] [Google Scholar]
- Biere A. L., Ostaszewski B., Stimson E. R., Hyman B. T., Maggio J. E., Selkoe D. J. Amyloid beta-peptide is transported on lipoproteins and albumin in human plasma. J Biol Chem. 1996 Dec 20;271(51):32916–32922. doi: 10.1074/jbc.271.51.32916. [DOI] [PubMed] [Google Scholar]
- Buée L., Hof P. R., Delacourte A. Brain microvascular changes in Alzheimer's disease and other dementias. Ann N Y Acad Sci. 1997 Sep 26;826:7–24. doi: 10.1111/j.1749-6632.1997.tb48457.x. [DOI] [PubMed] [Google Scholar]
- Dickson D. W., Sinicropi S., Yen S. H., Ko L. W., Mattiace L. A., Bucala R., Vlassara H. Glycation and microglial reaction in lesions of Alzheimer's disease. Neurobiol Aging. 1996 Sep-Oct;17(5):733–743. doi: 10.1016/0197-4580(96)00116-9. [DOI] [PubMed] [Google Scholar]
- Eikelenboom P., Veerhuis R. The role of complement and activated microglia in the pathogenesis of Alzheimer's disease. Neurobiol Aging. 1996 Sep-Oct;17(5):673–680. doi: 10.1016/0197-4580(96)00108-x. [DOI] [PubMed] [Google Scholar]
- Fiala M., Looney D. J., Stins M., Way D. D., Zhang L., Gan X., Chiappelli F., Schweitzer E. S., Shapshak P., Weinand M. TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol Med. 1997 Aug;3(8):553–564. [PMC free article] [PubMed] [Google Scholar]
- Fiala M., Rhodes R. H., Shapshak P., Nagano I., Martinez-Maza O., Diagne A., Baldwin G., Graves M. Regulation of HIV-1 infection in astrocytes: expression of Nef, TNF-alpha and IL-6 is enhanced in coculture of astrocytes with macrophages. J Neurovirol. 1996 Jun;2(3):158–166. doi: 10.3109/13550289609146878. [DOI] [PubMed] [Google Scholar]
- Fogelman A. M., Elahi F., Sykes K., Van Lenten B. J., Territo M. C., Berliner J. A. Modification of the Recalde method for the isolation of human monocytes. J Lipid Res. 1988 Sep;29(9):1243–1247. [PubMed] [Google Scholar]
- Frautschy S. A., Yang F., Irrizarry M., Hyman B., Saido T. C., Hsiao K., Cole G. M. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998 Jan;152(1):307–317. [PMC free article] [PubMed] [Google Scholar]
- Games D., Adams D., Alessandrini R., Barbour R., Berthelette P., Blackwell C., Carr T., Clemens J., Donaldson T., Gillespie F. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995 Feb 9;373(6514):523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Gan X. H., Robin J. P., Huerta J. M., Braquet P., Bonavida B. Inhibition of tumor necrosis factor-alpha (TNF-alpha) and interleukin-1 beta (IL-1 beta) secretion but not IL-6 from activated human peripheral blood monocytes by a new synthetic demethylpodophyllotoxin derivative. J Clin Immunol. 1994 Sep;14(5):280–288. doi: 10.1007/BF01540981. [DOI] [PubMed] [Google Scholar]
- Giulian D., Li J., Bartel S., Broker J., Li X., Kirkpatrick J. B. Cell surface morphology identifies microglia as a distinct class of mononuclear phagocyte. J Neurosci. 1995 Nov;15(11):7712–7726. doi: 10.1523/JNEUROSCI.15-11-07712.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Guo Q., Sopher B. L., Furukawa K., Pham D. G., Robinson N., Martin G. M., Mattson M. P. Alzheimer's presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: involvement of calcium and oxyradicals. J Neurosci. 1997 Jun 1;17(11):4212–4222. doi: 10.1523/JNEUROSCI.17-11-04212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga S., Akai K., Ishii T. Demonstration of microglial cells in and around senile (neuritic) plaques in the Alzheimer brain. An immunohistochemical study using a novel monoclonal antibody. Acta Neuropathol. 1989;77(6):569–575. doi: 10.1007/BF00687883. [DOI] [PubMed] [Google Scholar]
- Hardy J. A., Mann D. M., Wester P., Winblad B. An integrative hypothesis concerning the pathogenesis and progression of Alzheimer's disease. Neurobiol Aging. 1986 Nov-Dec;7(6):489–502. doi: 10.1016/0197-4580(86)90086-2. [DOI] [PubMed] [Google Scholar]
- Hickey W. F. Migration of hematogenous cells through the blood-brain barrier and the initiation of CNS inflammation. Brain Pathol. 1991 Jan;1(2):97–105. doi: 10.1111/j.1750-3639.1991.tb00646.x. [DOI] [PubMed] [Google Scholar]
- Hughes S. R., Khorkova O., Goyal S., Knaeblein J., Heroux J., Riedel N. G., Sahasrabudhe S. Alpha2-macroglobulin associates with beta-amyloid peptide and prevents fibril formation. Proc Natl Acad Sci U S A. 1998 Mar 17;95(6):3275–3280. doi: 10.1073/pnas.95.6.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka K., Kimura T., Igata-yi R., Katsuragi S., Takamatsu J., Miyakawa T. Identification of monocyte chemoattractant protein-1 in senile plaques and reactive microglia of Alzheimer's disease. Psychiatry Clin Neurosci. 1997 Jun;51(3):135–138. doi: 10.1111/j.1440-1819.1997.tb02375.x. [DOI] [PubMed] [Google Scholar]
- KIDD M. Paired helical filaments in electron microscopy of Alzheimer's disease. Nature. 1963 Jan 12;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- Kalaria R. N. Cerebrovascular degeneration is related to amyloid-beta protein deposition in Alzheimer's disease. Ann N Y Acad Sci. 1997 Sep 26;826:263–271. doi: 10.1111/j.1749-6632.1997.tb48478.x. [DOI] [PubMed] [Google Scholar]
- Kalaria R. N., Harshbarger-Kelly M., Cohen D. L., Premkumar D. R. Molecular aspects of inflammatory and immune responses in Alzheimer's disease. Neurobiol Aging. 1996 Sep-Oct;17(5):687–693. doi: 10.1016/0197-4580(96)00114-5. [DOI] [PubMed] [Google Scholar]
- Lue L. F., Brachova L., Civin W. H., Rogers J. Inflammation, A beta deposition, and neurofibrillary tangle formation as correlates of Alzheimer's disease neurodegeneration. J Neuropathol Exp Neurol. 1996 Oct;55(10):1083–1088. [PubMed] [Google Scholar]
- Mackenzie I. R., Hao C., Munoz D. G. Role of microglia in senile plaque formation. Neurobiol Aging. 1995 Sep-Oct;16(5):797–804. doi: 10.1016/0197-4580(95)00092-s. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., McGeer E. G. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev. 1995 Sep;21(2):195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- Mirra S. S., Hart M. N., Terry R. D. Making the diagnosis of Alzheimer's disease. A primer for practicing pathologists. Arch Pathol Lab Med. 1993 Feb;117(2):132–144. [PubMed] [Google Scholar]
- Miyakawa T., Uehara Y. Observations of amyloid angiopathy and senile plaques by the scanning electron microscope. Acta Neuropathol. 1979 Nov;48(2):153–156. doi: 10.1007/BF00691158. [DOI] [PubMed] [Google Scholar]
- Nottet H. S., Persidsky Y., Sasseville V. G., Nukuna A. N., Bock P., Zhai Q. H., Sharer L. R., McComb R. D., Swindells S., Soderland C. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J Immunol. 1996 Feb 1;156(3):1284–1295. [PubMed] [Google Scholar]
- Perlmutter L. S., Scott S. A., Barrón E., Chui H. C. MHC class II-positive microglia in human brain: association with Alzheimer lesions. J Neurosci Res. 1992 Dec;33(4):549–558. doi: 10.1002/jnr.490330407. [DOI] [PubMed] [Google Scholar]
- Persidsky Y., Gendelman H. E. Development of laboratory and animal model systems for HIV-1 encephalitis and its associated dementia. J Leukoc Biol. 1997 Jul;62(1):100–106. doi: 10.1002/jlb.62.1.100. [DOI] [PubMed] [Google Scholar]
- Persidsky Y., Stins M., Way D., Witte M. H., Weinand M., Kim K. S., Bock P., Gendelman H. E., Fiala M. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J Immunol. 1997 Apr 1;158(7):3499–3510. [PubMed] [Google Scholar]
- Petito C. K. Early and late mechanisms of increased vascular permeability following experimental cerebral infarction. J Neuropathol Exp Neurol. 1979 May;38(3):222–234. doi: 10.1097/00005072-197905000-00003. [DOI] [PubMed] [Google Scholar]
- Plateel M., Teissier E., Cecchelli R. Hypoxia dramatically increases the nonspecific transport of blood-borne proteins to the brain. J Neurochem. 1997 Feb;68(2):874–877. doi: 10.1046/j.1471-4159.1997.68020874.x. [DOI] [PubMed] [Google Scholar]
- Roher A. E., Lowenson J. D., Clarke S., Woods A. S., Cotter R. J., Gowing E., Ball M. J. beta-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10836–10840. doi: 10.1073/pnas.90.22.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A. M., Strittmatter W. J., Schmechel D., George-Hyslop P. H., Pericak-Vance M. A., Joo S. H., Rosi B. L., Gusella J. F., Crapper-MacLachlan D. R., Alberts M. J. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993 Aug;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Schmidtmayerova H., Nottet H. S., Nuovo G., Raabe T., Flanagan C. R., Dubrovsky L., Gendelman H. E., Cerami A., Bukrinsky M., Sherry B. Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):700–704. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebach J., Bartholdi D., Frei K., Spanaus K. S., Ferrero E., Widmer U., Isenmann S., Strieter R. M., Schwab M., Pfister H. Experimental Listeria meningoencephalitis. Macrophage inflammatory protein-1 alpha and -2 are produced intrathecally and mediate chemotactic activity in cerebrospinal fluid of infected mice. J Immunol. 1995 Nov 1;155(9):4367–4375. [PubMed] [Google Scholar]
- Shah G. N., Mooradian A. D. Age-related changes in the blood-brain barrier. Exp Gerontol. 1997 Jul-Oct;32(4-5):501–519. doi: 10.1016/s0531-5565(96)00158-1. [DOI] [PubMed] [Google Scholar]
- Shaver S. W., Wall K. M., Wainman D. S., Gross P. M. Regional quantitative permeability of blood-brain barrier lesions in rats with chronic renal hypertension. Brain Res. 1992 May 1;579(1):99–106. doi: 10.1016/0006-8993(92)90747-w. [DOI] [PubMed] [Google Scholar]
- Sievers J., Parwaresch R., Wottge H. U. Blood monocytes and spleen macrophages differentiate into microglia-like cells on monolayers of astrocytes: morphology. Glia. 1994 Dec;12(4):245–258. doi: 10.1002/glia.440120402. [DOI] [PubMed] [Google Scholar]
- Tsuzuki K., Fukatsu R., Hayashi Y., Yoshida T., Sasaki N., Takamaru Y., Yamaguchi H., Tateno M., Fujii N., Takahata N. Amyloid beta protein and transthyretin, sequestrating protein colocalize in normal human kidney. Neurosci Lett. 1997 Feb 7;222(3):163–166. doi: 10.1016/s0304-3940(97)13369-9. [DOI] [PubMed] [Google Scholar]
- Uchihara T., Akiyama H., Kondo H., Ikeda K. Activated microglial cells are colocalized with perivascular deposits of amyloid-beta protein in Alzheimer's disease brain. Stroke. 1997 Oct;28(10):1948–1950. doi: 10.1161/01.str.28.10.1948. [DOI] [PubMed] [Google Scholar]
- Wisniewski H. M., Wegiel J., Wang K. C., Kujawa M., Lach B. Ultrastructural studies of the cells forming amyloid fibers in classical plaques. Can J Neurol Sci. 1989 Nov;16(4 Suppl):535–542. doi: 10.1017/s0317167100029887. [DOI] [PubMed] [Google Scholar]
- Zlokovic B. V. Cerebrovascular transport of Alzheimer's amyloid beta and apolipoproteins J and E: possible anti-amyloidogenic role of the blood-brain barrier. Life Sci. 1996;59(18):1483–1497. doi: 10.1016/0024-3205(96)00310-4. [DOI] [PubMed] [Google Scholar]
- Zlokovic B. V., Martel C. L., Matsubara E., McComb J. G., Zheng G., McCluskey R. T., Frangione B., Ghiso J. Glycoprotein 330/megalin: probable role in receptor-mediated transport of apolipoprotein J alone and in a complex with Alzheimer disease amyloid beta at the blood-brain and blood-cerebrospinal fluid barriers. Proc Natl Acad Sci U S A. 1996 Apr 30;93(9):4229–4234. doi: 10.1073/pnas.93.9.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre J. C., Mussivand T. Can disturbed brain microcirculation cause Alzheimer's disease? Neurol Res. 1993 Jun;15(3):146–153. doi: 10.1080/01616412.1993.11740127. [DOI] [PubMed] [Google Scholar]



