Abstract
Several flaviviruses are important human pathogens, including dengue virus, a disease against which neither a vaccine nor specific antiviral therapies currently exist. During infection, the flavivirus RNA genome is translated into a polyprotein, which is cleaved into several components. Nonstructural protein 3 (NS3) carries out enzymatic reactions essential for viral replication, including proteolysis of the polyprotein through its serine protease N-terminal domain, with a segment of 40 residues from the NS2B protein acting as a cofactor. The ATPase/helicase domain is located at the C terminus of NS3. Atomic structures are available for these domains separately, but a molecular view of the full-length flavivirus NS3 polypeptide is still lacking. We report a crystallographic structure of a complete NS3 molecule fused to 18 residues of the NS2B cofactor at a resolution of 3.15 Å. The relative orientation between the protease and helicase domains is drastically different than the single-chain NS3-NS4A molecule from hepatitis C virus, which was caught in the act of cis cleavage at the NS3-NS4A junction. Here, the protease domain sits beneath the ATP binding site, giving the molecule an elongated shape. The domain arrangement found in the crystal structure fits nicely into an envelope determined ab initio using small-angle X-ray scattering experiments in solution, suggesting a stable molecular conformation. We propose that a basic patch located at the surface of the protease domain increases the affinity for nucleotides and could also participate in RNA binding, explaining the higher unwinding activity of the full-length enzyme compared to that of the isolated helicase domain.
Several members of the flaviviruses are important human pathogens, including yellow fever virus (YFV), Japanese encephalitis virus, tick-borne encephalitis virus, West Nile virus (WNV), and dengue virus (25). For the latter virus, neither a specific therapy nor a vaccine exists, and treatment is currently limited to the use of analgesics and fluid replacement. Since dengue virus is endemic in most tropical and subtropical areas, causing several hundreds of thousands of severe cases (dengue shock syndrome or dengue hemorrhagic fever), with approximately 30,000 deaths per year, compounds with antiviral activity are actively sought (21, 46). The positive-sense flavivirus RNA genome of 11 kb forms a single open reading frame that is translated into a polyprotein precursor of ca. 370 kDa consisting of the structural proteins C, prM, and E and seven nonstructural proteins, nonstructural protein 1 (NS1), NS2A, NS2B, NS3, NS4A, NS4B, and NS5. During viral maturation, this polyprotein is cleaved by host cell proteases in the endoplasmic reticulum and by the NS3 protein in the cytoplasm (Fig. 1A) (25). Cleavage of the polyprotein is mediated by the serine protease N-terminal domain of NS3, with a hydrophilic segment of 40 residues from the transmembrane NS2B protein acting as a cofactor necessary for this activity. The domain required for ATPase/helicase and nucleoside 5′-triphosphatase activity is located at the C terminus of NS3. Thus, the NS3 protein is a target of prime importance for antiviral therapy (24, 51, 52). Several nonstructural proteins encoded by the viral genome form a membrane-bound RNA replication complex, possibly with the participation of some host factors. The atomic structures of several individual components of this replication complex are known, including the NS3 protease domain (NS3pro) in the absence of the NS2B cofactor (30), the active NS3 protease domain (NS2B40NS3pro) (1, 12), the ATPase/helicase domain (NS3 helicase domain [NS3hel]) (44-46), the NS5 methyltransferase (10), and the RNA-dependent RNA polymerase catalytic domain (27, 49, 50). However, structural views of the complete NS3 and NS5 polypeptide chains are still lacking. Using full-length NS3 enzymes linked to 40 residues of the NS2B cofactor, elegant studies recently demonstrated that the NS3 protein undergoes autocleavage at two sites located at the NS2B-NS3 junction and within the helicase C-terminal region, respectively (4). In order to perform cis cleavage, the NS3 polypeptide substrate has to be accommodated into its own protease active site. Significant plasticity of the NS3 protein and order/disorder transitions within the NS3 polypeptide chain are likely to accompany such intramolecular proteolysis events. Structural studies of the full-length NS3 polypeptide are needed to understand the molecular basis for cis cleavage activity and to give a firm structural basis to analyze the dynamic properties of the NS3 molecule in connection to its various enzymatic activities. Moreover, an interesting comparison with the scNS3-NS4A structure from hepatitis C virus (HCV) can be drawn (48), completing the description of replicase enzymes within the family Flaviviridae. Here, we report a crystallographic structure of a complete NS3 molecule fused to 18 residues of the NS2B cofactor (hereafter named scNS2B18NS3) at 3.15-Å resolution. Small-angle X-ray scattering (SAXS) experiments suggest that this elongated conformation forms a stable molecular species that predominates in solution. We also observed a major contribution of the protease domain to increasing the affinity of the NS3 protein for ADP and ATP and attempt to correlate structural features of the full-length NS3 with this observation.
FIG. 1.
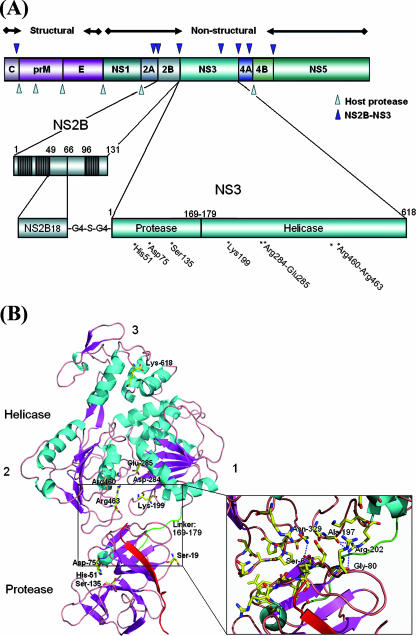
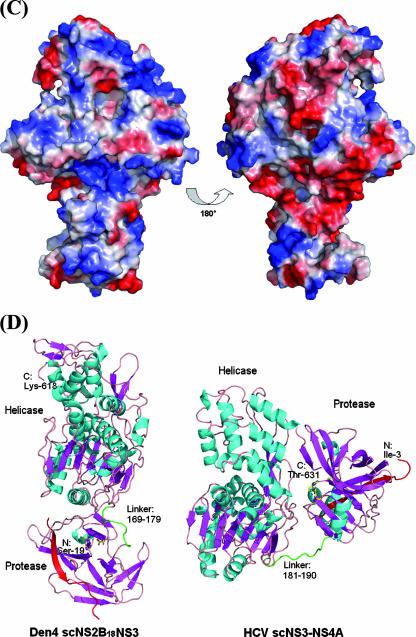
Overall structure of the scNS2B18NS3 protein from Den4. (A) Diagram of flavivirus polyprotein organization and the scNS2B18NS3 protein construct used in this work. Proteolytic sites by proteases from the host cell and by NS2B-NS3 are indicated with light and dark blue triangles, respectively. The three predicted membrane-associated regions within the NS2B proteins are represented as filled boxes. A central fragment spanning residues 49 to 66 of the NS2B protein was linked to the full-length NS3 protein through a Gly4-Ser-Gly4 linker. Evolutionarily conserved residues for NS3 enzymatic activities are indicated. (B) Ribbon representation of the scNS2B18NS3 structure. Secondary structure elements are colored in cyan (α-helix) and magenta (β-strand). The three subdomains of NS3hel are numbered. NS2B18, which forms a β-strand, is red. The region linking the protease and helicase (residues 169 to 179) is green. Key residues for NS3 enzymatic activities are shown as sticks and labeled. N-terminal residues are also labeled. A close-up view of the interface between the helicase and protease domains is also shown. (C) View of the electrostatic surface of scNS2B18NS3 with the molecule in the same orientation as above (B) (in the right panel, the molecule is rotated by 180° around a vertical axis). Positive potentials are blue, and negative potentials are red. (D) Side-by-side comparison of scNS2B18NS3 from Den4 and scNS3-NS4A from HCV (48), with their helicase domains oriented similarly. The positions of the protease domain relative to the helicase domain in the two structures are clearly different. The cofactors (NS2B18 and NS4A) are red, and the interdomain linkers are green. The N- and C-terminal residues from the two proteins are labeled.
MATERIALS AND METHODS
Cloning and expression.
Dengue virus serotype 4 (Den4) viral RNA was prepared using the QIAamp viral RNA Mini kit (Qiagen). cDNA was generated using reverse transcriptase PCR (SuperScript III; Invitrogen) with the full-length NS3 reverse primer 5′-TGGTGCTCGAGTTACTTTCTTCCACTGGCAAA-3′ (the underlined sequence corresponds to the XhoI site). The fragment corresponding to amino acid residues 1 to 618 of NS3 was amplified from viral cDNA using the forward primer 5′-GGGGGCGGAGGTAGTGGTGGAGGCGGTCAGGAGCTCTGTGGGACGTC-3′ and the reverse primer described above (the nucleotide sequence encoding the Gly4-Ser-Gly4 linker sequence is in italics). The central hydrophilic fragment corresponding to amino acid residues 49 to 66 of the NS2B protein (NS2B18) was amplified from viral cDNA using forward primer 5′-GCAGACTTGTCACTAGAGAAG-3′ and reverse primer (5′-CCCGCCTCCACCACTACCTCCGCCCCCGTCCGCCATTTCATCCCA-3′(the linker sequence is in italics). A third PCR was subsequently performed to generate the complete NS2B18 Gly4-Ser-Gly4 NS3 fusion construct using the NS2B18 forward and NS3 reverse primers listed above. The fragment was digested with XhoI and cloned into a modified pET32b plasmid using T4 ligase (Roche), where the S tag and enterokinase cleavage sequence are absent. The expressed construct thus comprises the thioredoxin (Trx) protein followed by a hexahistidine tag and a thrombin cleavage site fused at the N terminus of scNS2B18NS3. Transformed Escherichia coli BL21-CodonPlus clones (Stratagene) were grown at 18°C in an autoinduction medium (34) supplemented with 100 μg ml−1 ampicillin and 50 μg ml−1 chloramphenicol. After 28 h, cells were harvested by centrifugation at 8,000 × g for 10 min at 4°C and stored at −20°C. Den4 NS3hel (residues 177 to 618) was cloned from the full-length NS3 gene by PCR using forward primer 5′-GAAGTGGATGAGGACATT-3′ and reverse primer 5′-GTGGTGCTCGAGTTACTTTCTTCCACTGGCAAA-3′ (the XhoI site is underlined). The PCR fragment was digested with XhoI and cloned into a modified pET32b plasmid using T4 ligase (Roche). The expression of the Den4 NS3hel protein was done using the same protocol as that described above for scNS2B18NS3.
Protein purification.
Cells resuspended in buffer A (20 mM Na3PO4 [pH 7.4] 0.5 M NaCl, 40 mM imidazole) were lysed by sonication, and the lysate was clarified by centrifugation at 30,000 × g for 60 min at 4°C. The supernatant was purified by metal affinity using a HisTrap HP column (Amersham Bioscience) equilibrated with buffer A. Proteins were eluted using a linear gradient of imidazole from 40 mM to 500 mM. The fraction containing Trx-His6-scNS2B18NS3 was dialyzed against buffer B (20 mM Na3PO4 [pH 7.4], 0.2 M NaCl), with the concomitant cleavage of the thioredoxin tag by thrombin digestion (substrate-to-enzyme ratio of 500:1) at 4°C for approximately 24 h. The cleavage mixture was loaded onto a HisTrap HP column equilibrated with buffer B in order to remove the Trx-His6 protein from the mixture. Proteins were further purified using anion-exchange chromatography (MonoQ HR 5/5; Amersham Bioscience) and eluted using a linear gradient ranging from 0.05 to 1.0 M NaCl in buffer C (20 mM Tris-Cl [pH 8.0], 0.05 M NaCl). Concentrated scNS2B18NS3 proteins were subjected to a final polishing step using a HiPrep Superdex-200 gel filtration column (Amersham Bioscience) in buffer D (20 mM Tris-HCl [pH 7.4], 0.2 M NaCl, 1 mM dithiothreitol, 5% glycerol). Fractions containing scNS2B18NS3 were pooled and concentrated to 20 mg ml−1. Molecular masses of the samples were determined by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (API 300 MS/MS; Applied Biosystems). Automated N-terminal amino acid sequence determination of the proteolytic fragments derived from scNS2B18NS3 was performed using an Applied Biosystems (Singapore) Procise sequencer. The purification procedure for scNS2B18NS3 was also followed for the Den4 NS3hel protein (residues 177 to 618), which was concentrated to 34 mg ml−1 before storage.
X-ray scattering experiments and data analysis of scNS2B18NS3.
X-ray scattering data were collected, according standard procedures, on the X33 beam line of the EMBL Hamburg Deutsches Elektronen Synchrotron using a MAR345 image plate (MarResearch, Norderstedt, Germany). The scattering patterns from scNS2B18NS3 at protein concentrations of 2.5 mg/ml and 10.0 mg/ml were measured using a sample detector distance of 2.4 m, covering the range of momentum transfer: 0.1 < s < 4.5 nm−1 [s = 4π sin(θ)/λ, where θ is the scattering angle and λ at 0.15 nm is the X-ray wavelength]. The data were normalized to the intensity of the transmitted beam, and the scattering of the buffer was subtracted as background. These difference curves were scaled for concentration, which allows evaluation of the molecular mass by the forward scattering, I(0), and the radius of gyration, Rg, using the Guinier (15) approximation. All data-processing steps were performed with the program package PRIMUS (22). The distance distribution function, p(r), of the particle was computed by the GNOM indirect transform package (38). For molecular mass estimation, the forward scattering of a bovine serum albumin solution was taken as a reference.
Two low-resolution models for the scNS2B18NS3 molecule were built using the programs DAMMIN (36) and GASBOR (37) as described previously (3). Both approaches start with an ensemble of densely packed dummy atoms (DAMMIN) or dummy residues (GASBOR) inside a search volume defined by a sphere of diameter, Dmax. Eleven independent GASBOR reconstructions were computed, and the models were further analyzed using the packages DAMAVER (39) and SUBCOMP, resulting in an averaged model for shape representation.
FCS experiments using scNS2B18NS3 and NS3hel.
Fluorescence correlation spectroscopy (FCS) was performed at room temperature on a ConfoCor 3 apparatus (Zeiss, Jena, Germany) using the ATP and ADP analogues EDA-ATP ATTO-647N and EDA-ADP ATTO-647N (ATTO-TEC, Siegen, Germany). The 488-nm laser line of an HeNe633 laser was attenuated to 5 mW and focused into the aqueous solution by a water immersion objective (40×/1.2-W Korr UL-VIS-IR; Zeiss). FCS was measured in 50-μl droplets of the diluted fluorescent derivatives of ATP and ADP, which were placed on a Nunc eight-well chambered cover glass. Before usage, the cover glasses were cleaned and afterwards treated with filtered and centrifuged 3% gelatin solution in H2O in order to prevent unspecific binding (17). The following filter sets were used: main beam splitter, main dichronic beam splitter 488/543/633 nm; emission filter 1, low-pass filter 655 nm; EF, none; dichronic beam splitter, none. Out-of-focus fluorescence was rejected by a 90-μm pinhole in the detection pathway, resulting in a confocal detection volume of around 0.25 fl. Fluorescence autocorrelation functions were measured for 30 s each with 10 repetitions. Solutions of Cy5 in pure water were used as references for the calibration of the confocal microscope. To analyze the autocorrelation functions of fluorescent nucleotides bound, in part, to scNS2B18NS3 or NS3hel alternatively, models with the diffusion time and the triplet state were used for fitting. Triplet lifetime and amplitudes were kept at the premeasured values in the absence of binding proteins. The diffusion times of fluorescent nucleotides and fluorescently labeled nucleotides were measured independently and were kept fixed during the fitting of the FCS data. Therefore, the determination of the binding constants required only the calculation of the relative amounts of free nucleotides with the short diffusion time and of the bound nucleotides with the diffusion time of the protein.
Crystallization and data collection.
Crystals of scNS2B18NS3 were grown at 18°C by the hanging-drop vapor diffusion method over wells containing 0.1 M morpholineethanesulfonic acid (MES) (pH 6.5) and 7.5% polyethylene glycol 3350 (PEG 3350). A volume of 2 μl of precipitating solution was mixed with an equal volume of scNS2B18NS3 at a concentration of 3 to 5 mg ml−1. Crystals grew as clusters of thin elongated plates over 2 to 5 days to dimensions of approximately 0.02 by 0.30 by 0.10 mm3. For data collection, crystals were soaked in a solution containing 0.1 M MES and 20% PEG 3350 (pH 6.5) for 30 min at room temperature and were subsequently soaked for 1 min in 25% glycerol-0.1 M MES-20% PEG 3350 at pH 6.5 before being mounted and cooled to 100 K in a nitrogen gas stream (Oxford Cryosystems). Diffraction intensities were recorded on an ADSC charge-coupled device detector on the ID-23-1 beamline at the European Synchrotron Radiation Facility (Grenoble, France) using an attenuated beam with the dimensions 0.100 by 0.100 mm2. Integration, scaling, and merging of the intensities were carried out using the programs MOSFLM and SCALA from the CCP4 suite (7). The crystal parameters and data collection statistics are summarized in Table 1.
TABLE 1.
Data collection statistics
| Parameters | Native value |
|---|---|
| Wavelength (Å) | 0.9792 |
| Cell parameter (Å), P21 | a = 52.86 |
| b = 88.24 | |
| c = 76.67, β = 94.2° | |
| Resolution range (Å) | 88.3-3.15 (3.3-3.15) |
| No. of observed reflectionsa | 41,223 (6,137) |
| No. of unique reflections | 12,236 (1,786) |
| Completeness (%) | 99.8 (99.8) |
| Multiplicity | 3.4 (3.4) |
| Rmergeb | 0.14 (0.68) |
| I/σ(I) | 11 (2.1) |
| Solvent content (%) | 50.17 |
The numbers in parentheses refer to the last (highest) resolution shell.
Rmerge=ΣhΣi Ihi−<Ih> / Σh,iIhi, where Ihi is the ith observation of the reflection h, while <Ih> is its mean intensity.
Structure solution and refinement.
The crystal contains one monomer per asymmetric unit with an estimated solvent content of 50.2% based on a Matthews coefficient (Vm) value of 2.47 (29). The structure was solved by molecular replacement with the program PHASER (33) using Den2 NS3Pro (Protein Data Bank [PDB] accession number 2FOM) (12) and Den2 NS3Hel (PDB accession number 2BMF) (45) as search probes. Refinement cycles carried out using REFMAC5 (7) were interspersed with model rebuilding using Coot (11). TLS refinement was introduced in the last refinement steps. The quality of the structure was analyzed using PROCHECK (23). A summary of the refinement statistics and stereochemistry analysis is given in Table 2. Solvent-accessible surfaces areas were calculated using CCP4 program AREAIMOL with a 1.4-Å radius sphere as a probe. Superpositions of structures were carried out using the program LSQKAB from the CCP4 suite. Figures were prepared using the program Pymol (9).
TABLE 2.
Refinement statistics
| Parameter | Native value |
|---|---|
| Resolution range (Å) | 20.0-3.15 |
| Intensity cutoff [F/σ(F)] | 0 |
| Completeness (%) | 99.8 |
| No. of reflections | |
| Used for refinement | 12,166 |
| Used for Rfree calculation | 583 |
| No. of nonhydrogen atoms | |
| Proteins | 4,835 |
| Missing residues | 34 |
| Water molecules | 34 |
| Rfactora,b (%) | 20.2 (30.8) |
| Rfreec (%) | 27.7 (41.1) |
| Avg B factor (Å2) | |
| Main chain atoms | 61.1 |
| Side chain atoms | 60.9 |
| Water molecules | 40.9 |
| All atoms | 61.0 |
| RMS deviation from ideality | |
| Bond length (Å) | 0.007 |
| Bond angle (°) | 1.09 |
| Ramanchandran plot (%) | |
| Residues in most favored regions | 83.2 |
| Residues in additional allowed regions | 15.6 |
| Residues in generally allowed regions | 0.4 |
| Residues in disallowed regions | 0.8 |
| Overall G factord | −0.03 |
| PDB accession no. | 2VBC |
The numbers in parentheses refer to the last (highest) resolution shell.
Rfactor = Σ‖Fobs − Fcalc‖/ΣFobs.
Rfree was calculated with 5% of reflections excluded from the whole refinement procedure.
G factor is the overall measure of structure quality from PROCHECK (23).
Protein structure accession number.
The refined coordinates have been deposited in the PDB under accession number 2VBC.
RESULTS
Expression of scNS2B18NS3 and sensitivity to proteolysis.
The NS2B cofactor protein, which is predicted to contain three membrane-associated regions (Fig. 1A), plays an important scaffolding role for the formation of the overall protease structure. In its absence, recombinant proteins are poorly soluble. Apart from its structural role, the C-terminal region of the internal hydrophilic domain of the NS2B cofactor plays a role in catalysis but was not included in the present construct in order to obtain a more stable protein. Residues 49 to 66 were fused to the full-length NS3 protein through a flexible Gly4-Ser-Gly4 linker. This engineered molecule (scNS2B18NS3), which contains the complete NS3 polypeptide chain from Den4 but only 18 residues out of the 40 from the NS2B cofactor region that are necessary to obtain a fully proteolytically competent enzyme, was used for subsequent structural studies (Fig. 1). In spite of a well-preserved fold for its protease component, this engineered molecule is devoid of protease activity as shown previously (12). However, after purification, the recombinant scNS2B18NS3 fusion protein remains sensitive to degradation, especially upon incubation at room temperature (data not shown). The three major stable fragments obtained through limited proteolysis were subjected to N-terminal amino acid sequencing and mass spectrometry. The closest mapping onto the scNS2B18NS3 protein sequence gives the helicase domain (residues 171 to 618) as the major product. The two minor fragments comprise residues 13 to 618 and 249 to 618 of scNS2B18NS3. Traces of E. coli proteases or of thrombin, not eliminated during the purification process, are likely to be responsible for this activity.
Overall structure.
A summary of the crystallographic data collection and refinement statistics is shown in Tables 1 and 2. A total number of 615 residues is visible in the refined electron density map. Nine residues corresponding to the Gly4-Ser-Gly4 linker, 3 residues at the C terminus of the NS2B cofactor, and 18 residues at the N terminus of the protease domain are missing from the refined model. A ribbon diagram of the scNS2B18NS3 structure from Den4 is shown in Fig. 1B. The scNS2B18NS3 molecule adopts a rather elongated shape with approximate overall dimensions of 100 Å by 60 Å by 40 Å. The N-terminal protease domain resides next to the entrance of the ATPase active site between helicase subdomains 1 and 2 (Fig. 1B). The main contacts realized between the protease and helicase domains are detailed in Fig. 1B. They involve the interdomain (linker) region at approximately residues 169 to 179 and two loops that encircle the entrance to the ATP binding pocket. Of note, the rather compact arrangement adopted by the scNS2B18NS3 molecule does not preclude access to the ATP binding site by nucleotides as shown in Fig. 3B. A surface view of the protein is shown in Fig. 1C. A basic pocket is formed at the interface between the helicase and protease domains corresponding to the NTPase/RTPase active site. Conversely, if one rotates the molecule by 180° around a vertical axis, an excess of negative charges becomes visible. Hence, by abutting, the two domains create a charge distribution that is likely to optimize the diffusion of nucleotide substrates towards the ATP hydrolysis site through long-range electrostatic interactions. It is tempting to correlate this structural feature with the 10-fold increase in nucleotide binding affinities observed between scNS2B18NS3 and the isolated helicase domain (see below). Overall, the protease, the interdomain (linker) region, and subdomain 2 from the helicase appear to be the most mobile (data not shown). Mobility in subdomain 2 of the helicase has been inferred from structural comparisons with YFV helicase domains (28, 44-46) pointing to rigid-body movements of three α-helices located at the entrance of the single-stranded RNA binding tunnel during dynamic transitions of the helicase. This pattern of flexibility is also consistent with mutagenesis studies, pointing to a possible role played by residues Ile-365 and Arg-376 along the reaction pathway for strand separation (32). Comparison of the scNS2B18NS3 molecule with scNS3-NS4A from HCV (48) highlights a major difference in the relative orientations between the helicase and protease domains in the two proteins (Fig. 1D). The C-terminal end of NS3 from HCV participates actively in complex formation with the protease domain, being inserted into the active site of the protease domain, as cis cleavage occurs at the NS3-NS4A junction. On the other hand, the scNS2B18NS3 molecule from dengue virus has its C terminus located far away from the protease active site (Fig. 1D). As a result, large domain motions would be required to allow cleavage at the NS3-NS4A junction in cis by the flavivirus NS3 protein.
FIG. 3.
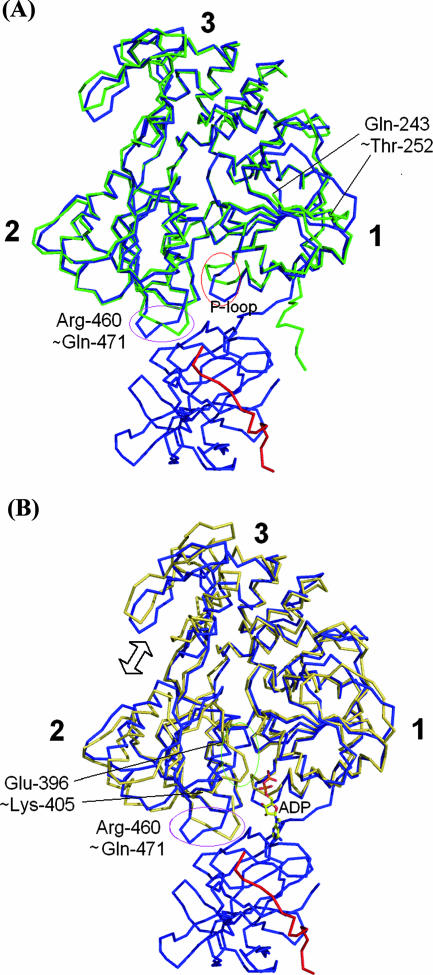
The helicase domain. (A) Cα traces for Den4 scNS2B18NS3 (in blue, with the NS2B18 cofactor in red), with Den2 NS3hel in green (PDB accession number 2BMF) (45). The P loop and the loop connecting Arg-460 to Gln-471 in subdomain 2, which are involved in contacts with the protease domain, adopt different conformations. (B) Superposition with YFV NS3hel (in yellow) (PDB accession number 1YMF) (44). A large difference occurs within subdomain 2: residues between Glu-396 and Lys-405 of the YFV helicase are disordered, while the equivalent residues in scNS2B18NS3 fold into an α-helix. The ADP moiety, which was bound to the YFV helicase, is shown as sticks.
The protease domain.
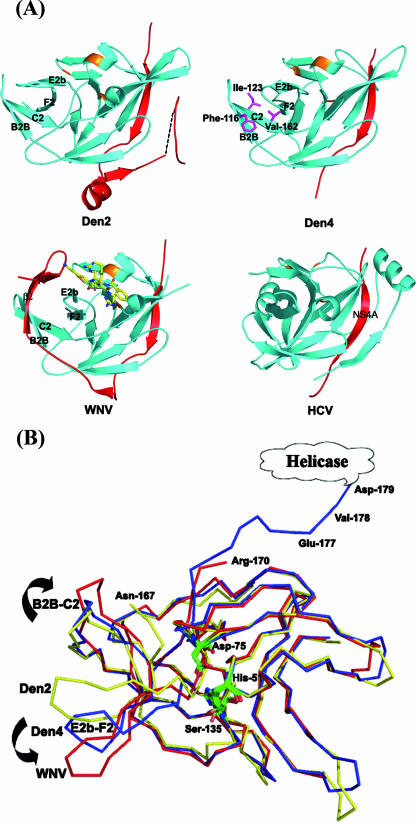
The Den4 protease domain from the scNS2B18NS3 molecule can be superimposed with the corresponding isolated domain from Den2 (NS2B40NS3Pro) (12), with a root mean square (RMS) deviation of 0.86 Å for 125 Cα atoms (Fig. 2A). Thus, the present protease core domain, which is inactive, retains a conformation similar to that of a fully active flaviviral protease. At a resolution of 3.15 Å, its catalytic triad (residues His-51, Asp-75, and Ser-135) appears to adopt the same orientation, and residues from the NS2B cofactor (residues 49 to 66) follow the same path (Fig. 2). This confirms the crucial role for the protease activity played by residues 67 to 80 from the NS2B cofactor as Erbel and colleagues inferred from the structure of the WNV protease in complex with a substrate-based inhibitor (12). This segment contributes to the formation of the P2 residue binding site through a large movement induced upon substrate binding (12). Comparison with the NS3 protease from HCV reveals a similar overall conformation, including that for its NS4A cofactor, which contributes one strand to the amino-terminal β-barrel of NS3 (Fig. 2A). A significant departure from the isolated protease structure is observed in its C-terminal region, with a large reorientation of two β-hairpins (Fig. 2B). In the Den4 scNS2B18NS3 molecule, this movement projects residues C terminal from Thr-166 towards the helicase domain. Instead, in Den2 NS2B40NS3Pro, a hydrophobic core is formed by the clustering of the same β-hairpin B2B-C2 (residues Ile-132 and Phe-116) and E2b-F2 (residue Val-162), with no space left to accommodate the NS2B β2-β3 hairpin, which occupies this space during activation of the protease (Fig. 2A) (1, 12). In our structure, loop E2b-F2 adopts a slightly more open conformation, possibly mimicking an intermediate state during the protease activation pathway (Fig. 2B). Several residues including Phe-116, Ile-123, Tyr-161, and Val-162 are evolutionarily conserved in flaviviruses. This pocket could thus be targeted for the design of an antiviral “allosteric” drug that would hinder this dynamic transition.
FIG. 2.
Comparison of protease domains from the Flaviviridae. (A) Ribbon representations of Den2 NS2B40NS3 (PDB accession number 2FOM), Den4 scNS2B18NS3 (this work) (PDB accession number 2VBC), WNV NS2B40NS3 (PDB accession number 2FP7), and HCV NS3-NS4A (PDB accession number 1CU1) protease domains. The colors used for the protease domains and their cofactors are cyan and red, respectively. The bound WNV NS3 protease inhibitor (Bz-Nle-Lys-Arg-Arg-H) is shown as yellow sticks. Two β-hairpins (B2B-C2 and E2b-F2) at the C-terminal NS3 protease domain and hairpin (β2 to β3) from the NS2B cofactor are labeled. The side chain of conserved Phe-116, Ile-123, and Val-162 are labeled and presented as purple sticks. The backbone positions of the three catalytic residues (His-51, Asp-75, and Ser-135 in Den4) are orange. (B) Superimposition of the Cα traces of Den4 scNS2B18NS3 (blue), Den2 NS2B40NS3pro (yellow), and WNV NS2B40NS3 (red) (12). NS2B cofactors were removed for clarity. The relative position of the catalytic triad is strictly conserved (green sticks). The main deviation is in the C-terminal region of the protease domain, where two β-hairpins (B2B-C2 and E2b-F2) adopt an open conformation in Den4 compared to the closed conformation observed in Den2.
The helicase domain.
A comparison of the helicase domain with the isolated Den2 helicase structure (45) returns a value of 0.78 Å for 400 equivalent Cα atoms (Fig. 3A). Structural variations are limited to two segments that form the interface with the protease in the full-length NS3: the phosphate binding P loop at the ATPase active site and a segment comprising residues Arg-460 to Gln-471 in subdomain 2 (Fig. 3). Within subdomain 1 of the helicase, a segment of 10 residues, Gln-243 to Thr-252, which were disordered in the isolated Den2 helicase structure, are now visible. These residues form a β-strand that runs antiparallel to a β-strand from the helicase subdomain 2 of a neighboring molecule. Superimposition of the Cα trace of scNS2B18NS3 with YFV NS3hel (PDB accession number 1YMF) gave RMS deviations of 1.63 Å for 388 Cα atoms (Fig. 3B). Within subdomain 2, approximately residues Glu-396 to Lys-405 are disordered in the YFV helicase (44), while the equivalent residues in scNS2B18NS3 fold into an α-helix (Fig. 3B). The bound ADP molecule seen in the YFV helicase structure (44) can also be accommodated in the ATPase active site of our full-length NS3 without steric hindrance, suggesting that the conformation observed in the crystal structure is compatible with ATP hydrolysis.
Nucleotide binding properties of scNS2B18NS3 and of its isolated helicase domain.
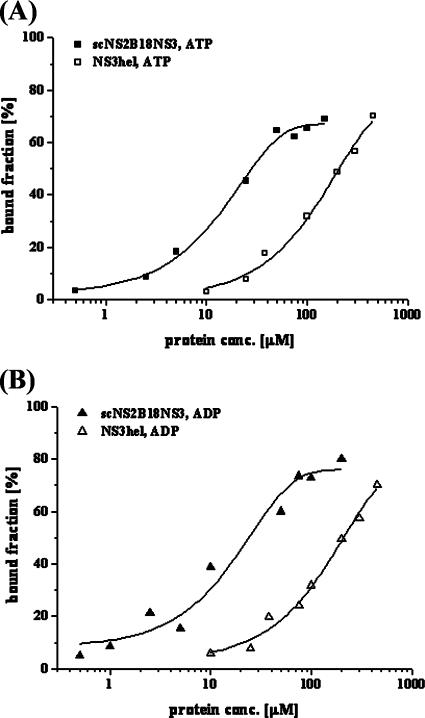
Using FCS, we measured ATP and ADP binding by scNS2B18NS3 and NS3hel, respectively. The FCS technique is a highly sensitive tool to determine binding/dissociation equilibrium constants in the nanomolar range. The characteristic diffusion time, τD, was measured by binding labeled nucleotides to nonlabeled proteins. The addition of scNS2B18NS3 resulted in a significant change of τD. This increase is due to the larger mass of the diffusing particle once ATP ATTO-647N is bound to scNS2B18NS3. This confirmed that nucleotides bound to scNS2B18NS3 in the presence of Mg2+. The percentage of bound nucleotides was analyzed using a two-component binding scheme, [E] + [S] ↔ [ES]. The concentration-dependent binding of scNS2B18NS3 and NS3hel to ATP ATTO-647N and ADP ATTO-647N is shown in Fig. 4. The dissociation constant for the protein-substrate complexes of scNS2B18NS3:Mg-ADP ATTO-647N was ∼10 to 20 μM, that for scNS2B18NS3:Mg-ATP ATTO-647N was ∼20 to 30 μM, that for NS3hel:Mg-ADP ATTO-647N was ∼100 to 200 μM, and that for NS3hel S3hel:Mg-ATP ATTO-647N was ∼200 to 300 μM (the dissociation constants were estimated based on data shown in Fig. 4; the calculation was done with FCS software [AxioVision; Zeiss], Microsoft Excel, and a nonlinear curve fit via Origin V.7.5). The data indicate that the affinity of scNS2B18NS3 for both nucleotides is approximately 10-fold higher than that of NS3hel. Interestingly, in the case of the HCV NS3 protein, where the contact between the domains is different, the full-length NS3 protein binds ATP more weakly than the truncated helicase domain (14).
FIG. 4.
Fluorescence correlation spectroscopy of scNS2B18NS3 and the fluorescent nucleotide analogues in the presence of 2 mM Mg2+. Concentration-dependent binding of scNS2B18NS3 (▪) and NS3hel (□) to ATP ATTO-647N (A) and Mg-ADP ATTO-647N linking to scNS2B18NS3 (▴) and NS3hel (▵) (B) are shown. The percentage of bound nucleotides was analyzed using a two-component binding scheme, [E] + [S] ↔ [ES]. Best fits yielding the binding constants are represented as continuous lines (see the text).
Since the helicase activity of the full-length dengue virus NS3 enzyme is about 30-fold higher than that of the isolated helicase domain (45), this supports a major role played by the protease domain to maximize chemical energy derived from ATP hydrolysis for the strand separation activity of dengue virus NS3. This also illustrates how viral evolution can operate through the fusion of protein modules (here, a chymotrypsin-like protease fold and a helicase domain containing two β-α-β modules) to produce an original molecular solution for a multifunctional enzyme involved in replication.
Relative orientation between the helicase and protease domains.
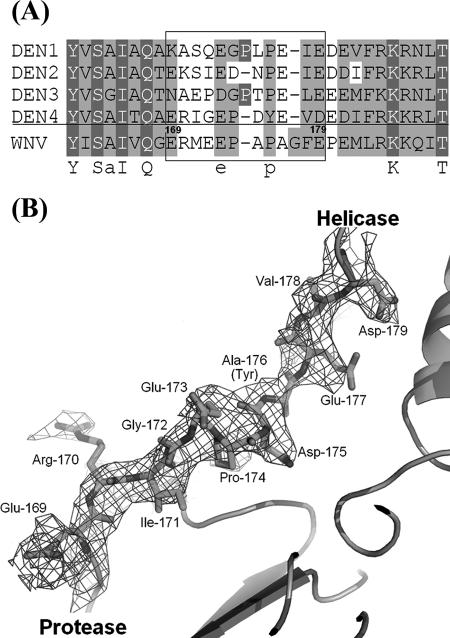
A single polypeptide segment spanning approximately residues 169 to 179 of the NS3 sequence connects the protease and the helicase domains, with most of its residues well defined in the final electron density map (Fig. 5). This interdomain (“linker”) region adopts an extended conformation (Fig. 5). A sequence alignment of the NS3 protein from the four dengue virus serotypes and WNV for the individual protease and helicase domains shows average sequence identities of 66% and 76%, respectively. By contrast, amino acid residues from the linker are less conserved, suggesting limited functional constraints on this region. An exception is the presence of an acidic residue (Glu-173 in Den4) in its middle followed by a small amino acid (Pro or Gly), which are found in the four dengue virus serotypes, in WNV, in Japanese encephalitis virus, and in YFV (Fig. 5A) (1). Attributing the linker region to NS3hel, one obtains a value of 1,320 Å2 for the total solvent-accessible surface area buried between the two domains of the scNS2B18NS3 molecule. This value is comparable to those observed for the formation of stable protein-protein interfaces (e.g., antibody-antigen complexes) (6, 19). Omitting residues from the interdomain region, the buried surface area between the protease and helicase domain is reduced to 380 Å2. Hydrogen bonds involving residues Ser-62 with Asn-329 and Gly-80 with Arg-202 and Ala-197 are established between the protease and subdomains 1 and 2 of the helicase (Fig. 1B). Thus, the interdomain region plays an important role in the association of the protease and helicase domains of NS3. This mode of interaction appears to form a rather stable molecular species yielding the crystals analyzed in this report. A complete analysis of the dynamics of the NS3 protein is beyond the scope of this report. However, several potential interdomain motions can be predicted using a simple normal mode analysis (data not shown) (35).
FIG. 5.
Interdomain linker between the protease and the helicase domains. (A) Sequence alignment of dengue virus serotypes and WNV scNS2B18NS3 in the linker region (in a rectangle box). Conserved residues are shaded. (B) 2Fo-Fc electron density map for the linker region between the protease and helicase domains. The map is contoured at 1σ for residues 169 to 179, which are shown as sticks and labeled.
SAXS experiments of scNS2B18NS3 in solution.
We used SAXS experiments in solution to assess which quaternary conformations are likely to be present in solution for the scNS2B18NS3 molecule (Fig. 6). SAXS patterns from scNS2B18NS3 were recorded and processed as described in Materials and Methods to yield the final composite scattering curve shown in Fig. 6B. The Rg and Dmax values of scNS2B18NS3 are 3.9 ± 0.2 nm and 10.1 ± 0.3 nm, respectively, suggestive of a rather elongated particle. Comparison with the scattering curve from reference solutions of bovine serum albumin yields an estimate for the molecular mass of 74 ± 4 kDa, indicating that scNS2B18NS3 is monomeric at the concentrations used. A qualitative analysis of the distance distribution function suggests that scNS2B18NS3 consists of a major part yielding a principal maximum in the p(r) function at around 3.3 nm (Fig. 6C), whereas the separated protuberance domain gives rise to a shoulder from 6.1 nm to 10.1 ± 0.2 nm. The gross structure of scNS2B18NS3 was restored ab initio from the scattering pattern shown in Fig. 6A using the shape determination program DAMMIN and the dummy residue modeling program GASBOR as described in Materials and Methods. The two approaches yielded similar results, but the models provided by DAMMIN could fit the data only up to an s of 0.29 nm−1. Below, the models obtained with GASBOR are presented, which yield good fits to the experimental data in the entire scattering range (a typical fit as displayed in Fig. 6B, curve 2, has a discrepancy, χ, of 1.35). Eleven independent reconstructions yielded reproducible models, and the average model is superimposed on the crystallographic structure of scNS2B18NS3 (Fig. 6A). In solution, the scNS2B18NS3 molecule appears elongated with two distinct domains, a main globular domain with a length of about 6.1 nm and a hook-like domain of about 4.0 nm in length. The current atomic model for scNS2B18NS3 is well accommodated within the shape of the same protein in solution. The NS3hel and the protease domain lie in the main globular and the hook-like domains, respectively. A very similar elongated SAXS shape for a full-length NS3 protein from the WNV Kunjin virus was recently proposed (28). However, the fits of individual domains differ significantly from those of our model. Interestingly, these results suggest that NS3 proteins from different flaviviruses adopt a similar configuration in solution that differs from that of the NS3 protease-helicase from HCV.
FIG. 6.
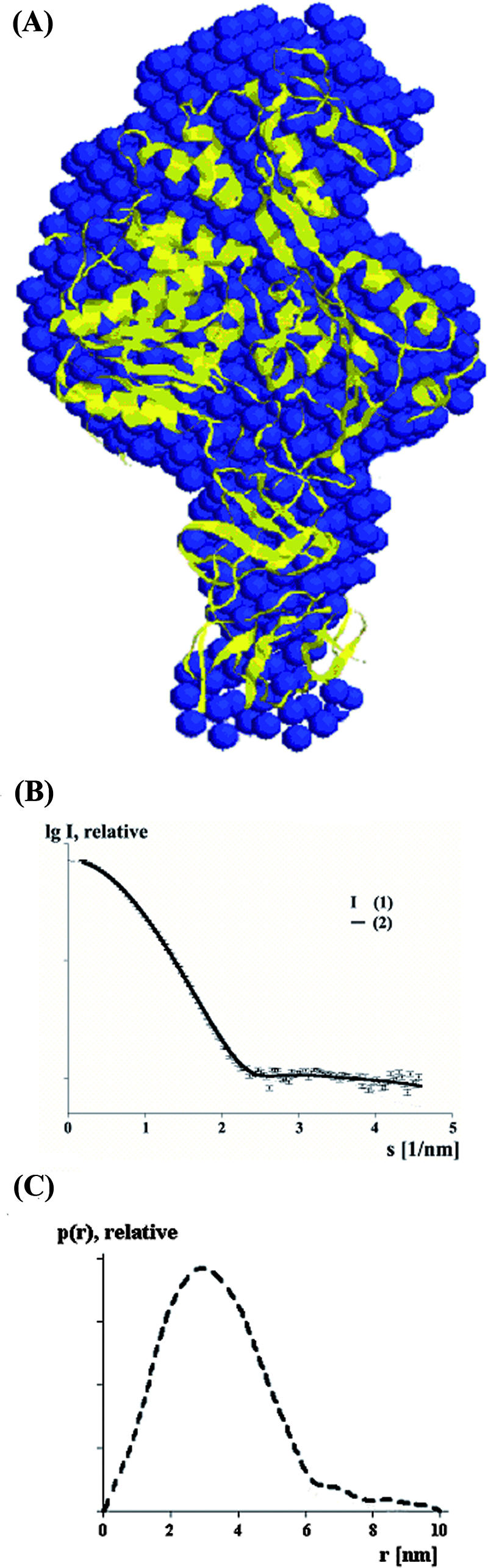
Agreement between the envelope derived from SAXS and the X-ray structure for scNS2B18NS3. (A) Superposition of the low-resolution structure (blue) of scNS2B18NS3 derived from solution X-ray scattering with the crystallographic model (ribbon diagram) (yellow). (B) Experimental SAXS curve from scNS2B18NS3 (1) and scattering from a typical ab initio model of scNS2B18NS3 (2) (computed by use of the program GASBOR) (38). (C) The distance distribution function of scNS2B18NS3 was computed from the experimental data by the program GNOM.
DISCUSSION
The NS2B cofactor.
Residues N terminal from residue 49 and C terminal to residue 96 of NS2B are predicted to be anchored into the endoplasmic reticulum membrane (8, 16). It is possible that the exposed hydrophobic loop (Gly-29∼Leu-30∼Phe-31∼Gly-32) from the NS3 protease domain points towards the membrane. This loop, which is conserved among flaviviruses, was proposed to play a role analogous to that of the hydrophobic N-terminal helix of the HCV protease (1, 47). The fact that NS2B40 could form a “belt-like” structure that wraps around the protease domain to form the active site (12) was intriguing. The observation of a similar conformation for a flaviviral NS2B40NS3pro complex containing a trypsin inhibitor aprotinin at the active site (1) has confirmed this unique mode of interaction between a protease and its cofactor. Available structures clearly indicate that the central hydrophilic region of the NS2B cofactor can adopt at least two distinct orientations representing the substrate-bound state and the substrate-free state, respectively. Residues 65 to 67 on NS2B constitute the hinge around which the C-terminal region (residues 68 to 95) swings around to form the active site upon substrate/inhibitor binding. Alternatively, the same residues fold back to leave the site accessible for the substrate.
Implications for polyprotein processing and formation of the replication complex.
cis protease cleavage sites have been identified recently (4). For several flaviviruses, a specific site of internal cleavage of NS3 by the viral protease corresponding to Arg-458 to Gly-459 in helicase subdomain 2 has been observed. Since Arg-458 is buried inside the protein and is inaccessible to proteases, cleavage at this position would require large conformational changes, reorientations of the protein domains, and even partial unfolding of the helicase domain. These might be the rate-limiting steps that are consistent with the fact that this internal cleavage is less efficient than the cleavage at the NS2B-NS3 junction. The biological implications of the internal cleavage of NS3 in the flavivirus life cycle are not known, although this site is also cleaved in virus-infected cells (2). In vitro studies have shown that the dengue virus NS2B-NS3 protease could also efficiently cleave in cis at the NS2A-NS2B junction, while the NS3-NS4A junction is probably cleaved in trans (5, 13, 31). It was shown that the conformation of the nonstructural polyprotein precursors NS1 to NS5 affects NS3-NS4A cleavage efficiency in Den2 (54). Thus, an additional level of regulation for viral polyprotein processing could derive from protein-protein interactions and membrane anchorage via the NS2B protein. Other nonstructural viral proteins are known to interact with NS3 and affect NS3 helicase/NTPase activity, such as NS4B (40) and NS5 (18, 20, 53). As part of the viral replication complex, NS3 interacts with the RNA-dependent RNA polymerase NS5. The region for the NS5 interaction was mapped to subdomains 2 and 3 of the helicase domain of NS3 (18, 20, 41). It is possible that several arrangements are adopted during the replication cycle, with one configuration reflecting the protein-membrane association state adopted during viral polyprotein processing and another during viral genome replication. Supporting evidences come from colocalization studies of nonstructural proteins and viral RNAs inside infected cells. The NS2B and NS3 polypeptides colocalize with convoluted membranes, whereas NS3 together with other membrane-associated NS proteins and nascent viral RNA replication intermediates are associated with perinuclear vesicle membranes (26, 42, 43).
More studies are now required to understand how these various proteins assemble to form the molecular machinery required for polyprotein processing and viral RNA replication. The structure of the NS3 protein from Den4 should provide an impetus for further dynamics and structural studies and offer additional possibilities for the design of compounds that are active against flaviviruses.
Acknowledgments
We thank M. Kotaka, A. Sampath, and S. P. Lim for help and discussions. We thank the European Synchrotron Radiation Facility staff, especially E. P. Mitchell and S. Monaco, for expert help with data collection. We acknowledge the EMBL Outstation, Hamburg, Germany, for provision of the synchrotron radiation facility, and we thank M. Roessle for his great help in collecting SAXS data.
Financial support via grants from the Singapore Biomedical Research Council (06/1/22/19/447) and the Singapore Ministry of Education (ARC5/07) to the laboratory of J.L. is acknowledged.
Footnotes
Published ahead of print on 17 October 2007.
REFERENCES
- 1.Aleshin, A. E., S. A. Shiryaev, A. Y. Strongin, and R. C. Liddington. 2007. Structural evidence for regulation and specificity of flaviviral proteases and evolution of the Flaviviridae fold. Protein Sci. 16795-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias, C. F., F. Preugschat, and J. H. Strauss. 1993. Dengue 2 virus NS2B and NS3 form a stable complex that can cleave NS3 within the helicase domain. Virology 193888-899. [DOI] [PubMed] [Google Scholar]
- 3.Armbrüster, A., D. I. Svergun, U. Coskun, S. Juliano, S. M. Bailer, and G. Grüber. 2004. Structural analysis of the stalk subunit Vma5p of the yeast V-ATPase in solution. FEBS Lett. 570119-125. [DOI] [PubMed] [Google Scholar]
- 4.Bera, A. K., R. J. Kuhn, and J. L. Smith. 2007. Functional characterization of cis and trans activity of the flavivirus NS2B-NS3 protease. J. Biol. Chem. 28212883-12892. [DOI] [PubMed] [Google Scholar]
- 5.Chambers, T. J., C. S. Hahn, R. Galler, and C. M. Rice. 1990. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 44649-688. [DOI] [PubMed] [Google Scholar]
- 6.Chitarra, V., P. M. Alzari, G. A. Bentley, T. N. Bhat, J. L. Eisele, A. Houdusse, J. Lescar, H. Souchon, and R. J. Poljak. 1993. Three-dimensional structure of a heteroclitic antigen-antibody cross-reaction complex. Proc. Natl. Acad. Sci. USA 907711-7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collaborative Computational Project, Number 4. 1994. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50760-763. [DOI] [PubMed] [Google Scholar]
- 8.Cserzo, M., E. Wallin, I. Simon, G. von Heijne, and A. Elofsson. 1997. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 10673-676. [DOI] [PubMed] [Google Scholar]
- 9.DeLano, W. L. 2002. The PyMOL user's manual. DeLano Scientific, Palo Alto, CA.
- 10.Egloff, M. P., D. Benarroch, B. Selisko, J. L. Romette, and B. Canard. 2002. An RNA cap (nucleoside-2′-O-)-methyltransferase in the flavivirus RNA polymerase NS5: crystal structure and functional characterization. EMBO J. 212757-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emsley, P., and K. Cowtan. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 602126-2132. [DOI] [PubMed] [Google Scholar]
- 12.Erbel, P., N. Schiering, A. D'Arcy, M. Renatus, M. Kroemer, S. P. Lim, Z. Yin, T. H. Keller, S. G. Vasudevan, and U. Hommel. 2006. Structural basis for the activation of flaviviral NS3 proteases from dengue and West Nile virus. Nat. Struct. Mol. Biol. 13372-373. [DOI] [PubMed] [Google Scholar]
- 13.Falgout, B., M. Pethel, Y. M. Zhang, and C. J. Lai. 1991. Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J. Virol. 652467-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frick, D. N., R. S. Rypma, A. M. Lam, and B. Gu. 2004. The nonstructural protein 3 protease/helicase requires an intact protease domain to unwind duplex RNA efficiently. J. Biol. Chem. 2791269-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guinier, A., and G. Fournet. 1955. Small angle scattering of X-rays. Wiley, New York, NY.
- 16.Hirokawa, T., S. Boon-Chieng, and S. Mitaku. 1998. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics 14378-379. [DOI] [PubMed] [Google Scholar]
- 17.Hunke, C., W. J. Chen, H. J. Schafer, and G. Grüber. 2007. Cloning, purification, and nucleotide-binding traits of the catalytic subunit A of the V1VO ATPase from Aedes albopictus. Protein Expr. Purif. 53378-383. [DOI] [PubMed] [Google Scholar]
- 18.Johansson, M., A. J. Brooks, D. A. Jans, and S. G. Vasudevan. 2001. A small region of the dengue virus-encoded RNA-dependent RNA polymerase, NS5, confers interaction with both the nuclear transport receptor importin-beta and the viral helicase, NS3. J. Gen. Virol. 82735-745. [DOI] [PubMed] [Google Scholar]
- 19.Jones, S., and J. M. Thornton. 1996. Principles of protein-protein interactions. Proc. Natl. Acad. Sci. USA 9313-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapoor, M., L. Zhang, M. Ramachandra, J. Kusukawa, K. E. Ebner, and R. Padmanabhan. 1995. Association between NS3 and NS5 proteins of dengue virus type 2 in the putative RNA replicase is linked to differential phosphorylation of NS5. J. Biol. Chem. 27019100-19106. [DOI] [PubMed] [Google Scholar]
- 21.Keller, T. H., Y. L. Chen, J. E. Knox, S. P. Lim, N. L. Ma, S. J. Patel, A. Sampath, Q. Y. Wang, Z. Yin, and S. G. Vasudevan. 2006. Finding new medicines for flaviviral targets. Novartis Found. Symp. 277102-114, 114-119, 251-253. [PubMed] [Google Scholar]
- 22.Konarev, P. V., M. V. Petoukhov, and D. I. Svergun. 2001. MASSHA—a graphics system for rigid-body modelling of macromolecular complexes against solution scattering data. J. Appl. Crystallogr. 34527-532. [Google Scholar]
- 23.Laskowski, R. A., M. W. Macarthur, D. S. Moss, and J. M. Thornton. 1993. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26283-291. [Google Scholar]
- 24.Li, J., S. P. Lim, D. Beer, V. Patel, D. Wen, C. Tumanut, D. C. Tully, J. A. Williams, J. Jiricek, J. P. Priestle, J. L. Harris, and S. G. Vasudevan. 2005. Functional profiling of recombinant NS3 proteases from all four serotypes of dengue virus using tetrapeptide and octapeptide substrate libraries. J. Biol. Chem. 28028766-28774. [DOI] [PubMed] [Google Scholar]
- 25.Lindenbach, B. D., H. J. Thiel, and C. M. Rice. 2007. Flaviviridae: the viruses and their replication, p. 1101-1152. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed., vol. 1. Lippincott-Raven Publishers, Philadelphia, PA. [Google Scholar]
- 26.Mackenzie, J. M., A. A. Khromykh, M. K. Jones, and E. G. Westaway. 1998. Subcellular localization and some biochemical properties of the flavivirus Kunjin nonstructural proteins NS2A and NS4A. Virology 245203-215. [DOI] [PubMed] [Google Scholar]
- 27.Malet, H., M. P. Egloff, B. Selisko, R. E. Butcher, P. J. Wright, M. Roberts, A. Gruez, G. Sulzenbacher, C. Vonrhein, G. Bricogne, J. M. Mackenzie, A. A. Khromykh, A. D. Davidson, and B. Canard. 2007. Crystal structure of the RNA polymerase domain of the West Nile virus non-structural protein 5. J. Biol. Chem. 28210678-10689. [DOI] [PubMed] [Google Scholar]
- 28.Mastrangelo, E., M. Milani, M. Bollati, B. Selisko, F. Peyrane, V. Pandini, G. Sorrentino, B. Canard, P. V. Konarev, D. I. Svergun, X. de Lamballerie, B. Coutard, A. A. Khromykh, and M. Bolognesi. 2007. Crystal structure and activity of Kunjin virus NS3 helicase; protease and helicase domain assembly in the full length NS3 protein. J. Mol. Biol. 372444-455. [DOI] [PubMed] [Google Scholar]
- 29.Matthews, B. W. 1968. Solvent content of protein crystals. J. Mol. Biol. 33491-497. [DOI] [PubMed] [Google Scholar]
- 30.Murthy, H. M., S. Clum, and R. Padmanabhan. 1999. Dengue virus NS3 serine protease. Crystal structure and insights into interaction of the active site with substrates by molecular modeling and structural analysis of mutational effects. J. Biol. Chem. 2745573-5580. [DOI] [PubMed] [Google Scholar]
- 31.Preugschat, F., C. W. Yao, and J. H. Strauss. 1990. In vitro processing of dengue virus type 2 nonstructural proteins NS2A, NS2B, and NS3. J. Virol. 644364-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sampath, A., T. Xu, A. Chao, D. Luo, J. Lescar, and S. G. Vasudevan. 2006. Structure-based mutational analysis of the NS3 helicase from dengue virus. J. Virol. 806686-6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Storoni, L. C., A. J. McCoy, and R. J. Read. 2004. Likelihood-enhanced fast rotation functions. Acta Crystallogr. D Biol. Crystallogr. 60432-438. [DOI] [PubMed] [Google Scholar]
- 34.Studier, F. W. 2005. Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 41207-234. [DOI] [PubMed] [Google Scholar]
- 35.Suhre, K., and Y. H. Sanejouand. 2004. ElNemo: a normal mode web server for protein movement analysis and the generation of templates for molecular replacement. Nucleic Acids Res. 32W610-W614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svergun, D. I. 1992. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Crystallogr. 25495-503. [Google Scholar]
- 37.Svergun, D. I. 1997. Restoring three-dimensional structure of biopolymers from solution scattering. J. Appl. Crystallogr. 30792-797. [Google Scholar]
- 38.Svergun, D. I. 1994. Solution scattering from biopolymers: advanced contrast-variation data analysis. Acta Crystallogr. A 50391-402. [Google Scholar]
- 39.Svergun, D. I., M. V. Petoukhov, and M. H. J. Koch. 2001. Determination of domain structure of proteins from X-ray solution scattering. Biophys. J. 802946-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Umareddy, I., A. Chao, A. Sampath, F. Gu, and S. G. Vasudevan. 2006. Dengue virus NS4B interacts with NS3 and dissociates it from single-stranded RNA. J. Gen. Virol. 872605-2614. [DOI] [PubMed] [Google Scholar]
- 41.Vasudevan, S. G., M. Johansson, A. J. Brooks, L. E. Llewellyn, and D. A. Jans. 2001. Characterisation of inter- and intra-molecular interactions of the dengue virus RNA dependent RNA polymerase as potential drug targets. Farmaco 5633-36. [DOI] [PubMed] [Google Scholar]
- 42.Westaway, E. G., A. A. Khromykh, and J. M. Mackenzie. 1999. Nascent flavivirus RNA colocalized in situ with double-stranded RNA in stable replication complexes. Virology 258108-117. [DOI] [PubMed] [Google Scholar]
- 43.Westaway, E. G., J. M. Mackenzie, M. T. Kenney, M. K. Jones, and A. A. Khromykh. 1997. Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J. Virol. 716650-6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu, J., A. K. Bera, R. J. Kuhn, and J. L. Smith. 2005. Structure of the flavivirus helicase: implications for catalytic activity, protein interactions, and proteolytic processing. J. Virol. 7910268-10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu, T., A. Sampath, A. Chao, D. Wen, M. Nanao, P. Chene, S. G. Vasudevan, and J. Lescar. 2005. Structure of the dengue virus helicase/nucleoside triphosphatase catalytic domain at a resolution of 2.4 Å. J. Virol. 7910278-10288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu, T., A. Sampath, A. Chao, D. Wen, M. Nanao, D. Luo, P. Chene, S. G. Vasudevan, and J. Lescar. 2006. Towards the design of flavivirus helicase/NTPase inhibitors: crystallographic and mutagenesis studies of the dengue virus NS3 helicase catalytic domain. Novartis Found. Symp. 27787-97, 97-101, 251-253. [DOI] [PubMed] [Google Scholar]
- 47.Yan, Y., Y. Li, S. Munshi, V. Sardana, J. L. Cole, M. Sardana, C. Steinkuehler, L. Tomei, R. De Francesco, L. C. Kuo, and Z. Chen. 1998. Complex of NS3 protease and NS4A peptide of BK strain hepatitis C virus: a 2.2 A resolution structure in a hexagonal crystal form. Protein Sci. 7837-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao, N., P. Reichert, S. S. Taremi, W. W. Prosise, and P. C. Weber. 1999. Molecular views of viral polyprotein processing revealed by the crystal structure of the hepatitis C virus bifunctional protease-helicase. Structure 71353-1363. [DOI] [PubMed] [Google Scholar]
- 49.Yap, T. L., Y. L. Chen, T. Xu, D. Wen, S. G. Vasudevan, and J. Lescar. 2007. A multi-step strategy to obtain crystals of the dengue virus RNA-dependent RNA polymerase that diffract to high resolution. Acta. Crystallogr. F Struct. Biol. Cryst. Commun. 6378-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yap, T. L., T. Xu, Y. L. Chen, H. Malet, M. P. Egloff, B. Canard, S. G. Vasudevan, and J. Lescar. 2007. Crystal structure of the dengue virus RNA-dependent RNA polymerase catalytic domain at 1.85-angstrom resolution. J. Virol. 814753-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yin, Z., S. J. Patel, W. L. Wang, W. L. Chan, K. R. Ranga Rao, G. Wang, X. Ngew, V. Patel, D. Beer, J. E. Knox, N. L. Ma, C. Ehrhardt, S. P. Lim, S. G. Vasudevan, and T. H. Keller. 2006. Peptide inhibitors of dengue virus NS3 protease. Part 2: SAR study of tetrapeptide aldehyde inhibitors. Bioorg. Med. Chem. Lett. 1640-43. [DOI] [PubMed] [Google Scholar]
- 52.Yin, Z., S. J. Patel, W. L. Wang, G. Wang, W. L. Chan, K. R. Rao, J. Alam, D. A. Jeyaraj, X. Ngew, V. Patel, D. Beer, S. P. Lim, S. G. Vasudevan, and T. H. Keller. 2006. Peptide inhibitors of dengue virus NS3 protease. Part 1: Warhead. Bioorg. Med. Chem. Lett. 1636-39. [DOI] [PubMed] [Google Scholar]
- 53.Yon, C., T. Teramoto, N. Mueller, J. Phelan, V. K. Ganesh, K. H. Murthy, and R. Padmanabhan. 2005. Modulation of the nucleoside triphosphatase/RNA helicase and 5′-RNA triphosphatase activities of dengue virus type 2 nonstructural protein 3 (NS3) by interaction with NS5, the RNA-dependent RNA polymerase. J. Biol. Chem. 28027412-27419. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, L., and R. Padmanabhan. 1993. Role of protein conformation in the processing of dengue virus type 2 nonstructural polyprotein precursor. Gene 129197-205. [DOI] [PubMed] [Google Scholar]