Abstract
Viral infection often activates the interferon (IFN)-γ–inducible gene, nitric oxide synthase 2 (NOS2). Expression of NOS2 can limit viral growth but may also suppress the immune system and damage tissue. This study assessed each of these effects in genetically deficient NOS2−/− mice after infection with influenza A, a virus against which IFN-γ has no known activity. At inocula sufficient to cause consolidating pneumonitis and death in wild-type control mice, NOS2−/− hosts survived with little histopathologic evidence of pneumonitis. Moreover, they cleared influenza A virus from their lungs by an IFN-γ–dependent mechanism that was not evident in wild-type mice. Even when the IFN-γ–mediated antiviral activity was blocked in NOS2−/− mice with anti–IFN-γ mAb, such mice failed to succumb to disease. Further evidence that this protection was independent of viral load was provided by treating NOS2+/+ mice with the NOS inhibitor, N ω-methyl-l-arginine (l-NMA). l-NMA prevented mortality without affecting viral growth. Thus, host NOS2 seems to contribute more significantly to the development of influenza pneumonitis in mice than the cytopathic effects of viral replication. Although NOS2 mediates some antiviral effects of IFN-γ, during influenza infection it can suppress another IFN-γ–dependent antiviral mechanism. This mechanism was observed only in the complete absence of NOS2 activity and appeared sufficient to control influenza A virus growth in the absence of changes in cytotoxic T lymphocyte activity.
Keywords: nitric oxide synthase, interferon γ, virus infection, influenza A, cytotoxic T lymphocyte
The discovery that the IFN-γ–inducible nitric oxide synthase 2 (NOS2) gene could confer potent antiviral activity (1, 2) substantially extended its role as a critical component of the innate immune system (3). Yet although a number of viruses are susceptible to the nitric oxide (NO)- derived products of NOS2, not all are inhibited (3, 4, and references therein). Moreover, as a broadly reactive, cell-permeant radical, NO has the potential to injure uninfected cells near those harboring virus (3–7, and references therein). For this reason, NOS2 has also been implicated in the pathology associated with several viral models (4, 5). That most of the studies mentioned above have relied on the use of NOS inhibitors of limited isoform specificity complicates interpretation of disparate results, since all three NOS isoforms can exhibit antiviral activity, and at least two of them (NOS2 and NOS1) can damage cells (3, 4, 8, 9).
We have resorted to NOS2 gene–targeted mice (10) in an effort to assess the contribution made by this locus towards pathogenesis and immunoregulation during viral infection. Such mice have confirmed NOS2's ability to protect the host during infection by Coxsackie B3 (11) and ectromelia viruses (11a). Here we studied infection by pneumotropic influenza A virus for two reasons. First, an earlier report with pharmacologic inhibitors implicated NOS2 in host pathology during influenza pneumonitis (6). Second, although IFN-γ has well-characterized antiviral activity and is important for NOS2 induction in vivo (1–4), control of influenza A virus does not seem to require IFN-γ, as evidenced by studies using anti–IFN-γ antibodies (12, and G. Karupiah, unpublished observations) and IFN-γ−/− mice (13, and G. Karupiah, unpublished observations). Surprisingly, treatment with neutralizing antibody revealed that IFN-γ can control influenza A virus, but only when NOS2 is absent.
Materials and Methods
Mice.
NOS2−/− mice were generated as described (10). NOS2−/− and NOS2+/+ littermates (129/SvEv × C57BL/6 F2) were bred at the specific pathogen–free unit, John Curtin School of Medical Research. NOS2 genotype was confirmed by PCR (14). Experiments were performed according to institutional guidelines for animal care and use.
Virus Infection and Enumeration.
Influenza A virus (strain A/ PR/8/34) was directly instilled in 30 μl PBS into the nasal cavity under Avertin (1.5 ml/100 g) anesthesia, and titers were thereafter determined via serial dilutions of lung homogenates plated onto MDCK cell monolayers for detection of PFU (15). The limit of assay detection was 2.0 log10 PFU.
Lung Immunocyte Isolation.
Leukocytes were isolated as outlined (12) from HBSS-perfused and aseptically excised lungs from groups of influenza virus–infected mice (n = 5 per group). Populations consisted of ∼40–50% T lymphocytes (CD3+), 20–30% B lymphocytes (CD45R/B220+), and 10–12% macrophages (F4/ 80+) as determined by flow cytometry.
Bronchoalveolar Lavage Fluid NOx Assay.
Bronchoalveolar lavage fluid (BALF) consisted of washings from intratracheal instillation of 1.0 ml PBS. Nitrite was measured by a diazotization assay (14) with a sensitivity of 4 μM after NO3 − was converted to NO2 − via nitrate reductase.
Cytotoxicity Assays.
Influenza A virus–infected and uninfected EL-4 lymphoma cells were used as targets for measurement of anti-influenza A virus–specific, class I MHC–restricted CTL activity of lung parenchymal cells using standard 51Cr-release assays (16, 17).
IFN-γ ELISA Analysis.
A sandwich ELISA (18) was used to measure IFN-γ levels in BALF and from culture supernatants of lung immunocytes (5 × 106; ∼50% CD3+) of influenza A/PR/ 8/34 virus–infected mice coincubated for 48 h with influenza virus–infected, mitomycin C–treated (50 μg/ml) genotype-matched lung parenchymal cells (5 × 105). Recombinant MuIFN-γ (Genzyme Corp., Cambridge, MA) served as the standard, with an assay detection limit of 3 U/ml. Con A was added at 4 μg/ml (Sigma Chemical Co., St. Louis, MO).
Histology.
Formalin-fixed, paraffin-embedded tissues were sectioned (5 μm) and stained with hematoxylin and eosin as outlined (18).
Neutralization of NOS2 and IFN-γ In Vivo.
N ω-methyl-l-arginine (l-NMA; Sigma Chemical Co.) or its inactive d-enantiomer (d-NMA) was administered via daily intraperitoneal injection, and efficacy was verified by BALF nitrite plus nitrate (NOx) assay. Neutralization of IFN-γ was achieved by injection of 1 mg i.p. anti–MuIFN-γ mAb (clone XMG-6, rat IgG1 [17, 18]) in 200 μl PBS on days 0, 2, and 4 after infection. Control animals received an isotype-matched mAb against bacterial β-galactosidase (clone GL 113, rat IgG1).
Statistical Analysis.
Unpaired Student's t tests at 95% confidence levels were performed using InStat software, Version 2.00 (GraphPad Software for Science, San Diego, CA).
Results and Discussion
Augmented Virus Clearance and Enhanced Survival of NOS2− /− Mice Infected with Influenza A Virus.
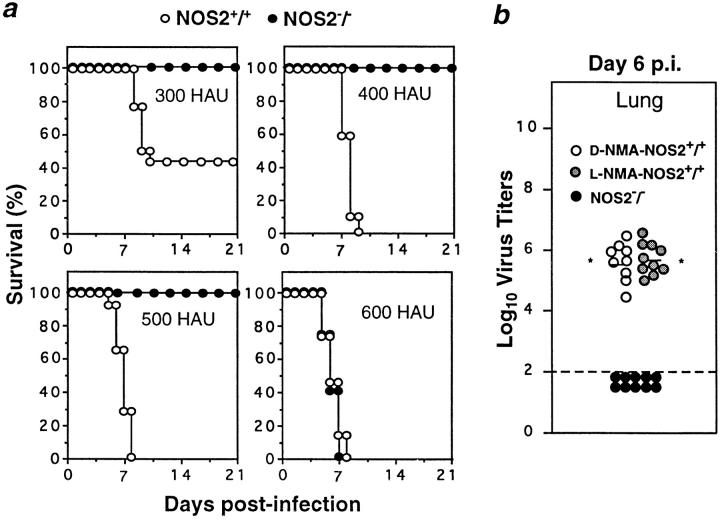
In previous work, mice treated with anti–IFN-γ antibodies (12, and G. Karupiah, unpublished observations) or lacking IFN-γ (13, and G. Karupiah, unpublished observations) responded to influenza A virus similarly to control mice, even though their capacity for NOS2 expression is known to be impaired (19, 20). Our findings in NOS2−/− mice not only corroborated the implication that NOS2 is dispensable for the control of influenza A virus in the lower respiratory tract, but also demonstrated that NOS2 is detrimental in this setting. Thus, intranasal inoculation with the virulent A/PR/8/34 strain led to rapid pneumonitis and early mortality in wild-type mice given 300, 400, or 500 hemagglutinin units (HAU) of virus. In contrast, NOS2−/− mice started to succumb only at 600 HAU (Fig. 1 a).
Figure 1.
Disruption of NOS2 confers a protective effect during influenza A virus infection. (a) Mortality in male NOS2+/+ or NOS2−/− mice (n = 20 per group at each dose) inoculated intranasally with 300, 400, 500, or 600 HAU influenza A (strain A/PR/8/34) and monitored for 21 d. Data represent four independent experiments. (b) Lung influenza A titers in d-NMA– or l-NMA–treated NOS2+/+ mice and NOS2−/− mice intranasally infected with 400 HAU of virus and determined at day 6 after infection. Dashed line, Assay detection limit. Data are derived from three experiments and expressed as log10 virus titers/gram tissue ± SEM. *P < 0.01 versus NOS2−/− group, unpaired t test.
Influenza A virus titers in the lungs of d-NMA–treated controls given 400 HAU of virus reached 5.6 ± 0.2 log10 PFU by day 6 after infection, whereas in NOS2−/− mice lung virus titers were below the limit of detection (<2.0 log10 PFU; Fig. 1 b). Surprisingly, l-NMA–treated wild-type mice harbored viral loads similar to their d-NMA– treated littermates (Fig. 1 b) but, unlike the latter, they showed no signs of morbidity and survived through the 21-d period of observation (data not shown). This outcome suggested (a) that NOS2−/− mice selectively expressed a potent antiinfluenza effector mechanism, and (b) the high output NO pathway may contribute to pathogenesis, since both l-NMA– and d-NMA–treated wild-type mice failed to eradicate the virus but only those given the inactive d-enantiomer proved susceptible, dying between days 7 and 8 after infection, like their untreated counterparts (Fig. 1 a, and data not shown).
An IFN-γ–dependent Pathway Restricts Influenza Virus Replication in NOS2− /− Mice.
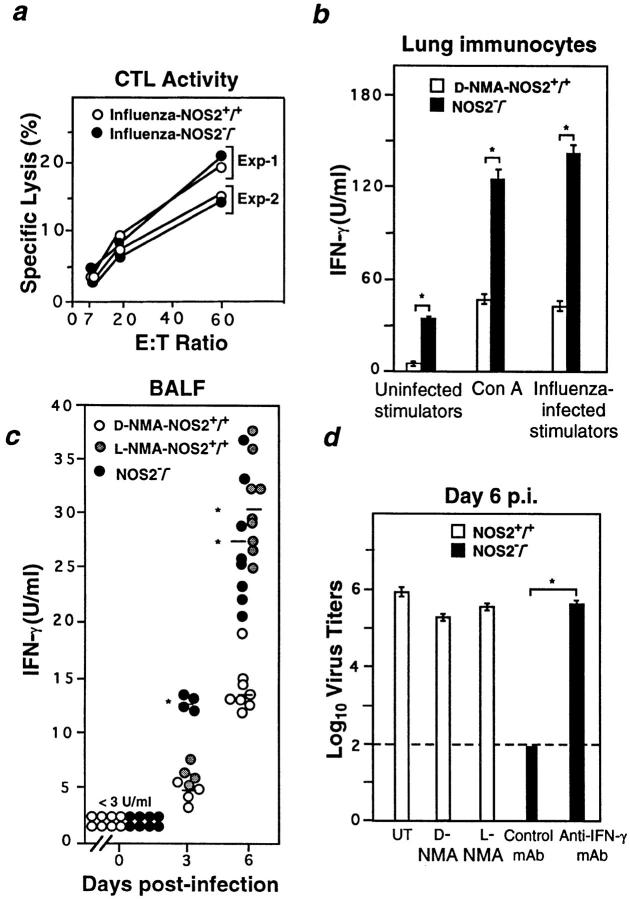
Experiments in β2-microglobulin−/− mice (21) and Fas−/− × perforin−/− chimeras (22) demonstrated the importance of CD8+ T lymphocytes for elimination of influenza A virus from the lung. To determine if this effector arm of the immune response contributed to rapid virus clearance in the NOS2−/− host, we examined influenza-specific CTL activity on day 6 after infection. Lung immunocytes lysed the influenza virus–infected targets equally well irrespective of NOS2 status (Fig. 2 a). However, measurement of local IFN-γ production by these cells (Fig. 2 b) and in BALF (Fig. 2 c) revealed that NOS2−/− mice exhibited 1.9–3.4-fold higher levels of IFN-γ than wild-type hosts; this increase was independent of antigen load (Fig. 1 b).
Figure 2.
IFN-γ–dependent antiinfluenza virus activity in NOS2−/− mice is not accompanied by changes in CTL response. (a) Ex vivo CTL activity of lung parenchymal cells (day 6 after infection) from d-NMA– or l-NMA–treated NOS2+/+ mice and NOS2−/− mice intranasally infected with 400 HAU of virus. Shown are group means (n = 4 for each of two independent experiments); SEMs fall within the symbols. (b) IFN-γ production by explanted lung parenchymal cells from influenza A virus– infected NOS2+/+ and NOS2−/− mice cultured in vitro with uninfected lung immunocytes, influenza-infected stimulators, or Con A for 48 h. Data are presented as means of triplicate cultures ± SEM (from pooled lung immunocytes of five mice per group, and represent two independent experiments). (c) IFN-γ levels in the BALF of d-NMA– or l-NMA– treated NOS2+/+ mice and NOS2−/− mice intranasally infected with 400 HAU of virus determined at day 6 after infection. Individual samples were assayed in triplicate; horizontal bars, group means. *P < 0.01 versus d-NMA–treated group, unpaired t test. (d) Influenza A virus titers in the lungs of NOS2−/− mice treated with anti–MuIFN-γ (n = 14) or control IgG1 mAb (n = 14); or in NOS2+/+ mice given d-NMA (n = 10) or l-NMA (n = 14). Untreated NOS2+/+ mice (UT) served as drug-free controls (n = 4). Dashed line, Assay detection limit. Data shown represent three experiments and are expressed as log10 virus titers/gram tissue ± SEM. *P < 0.01 versus control IgG1 mAb–treated group, unpaired t test.
Whether such an increase in IFN-γ secretion could account for the reduced viral burden in mutant animals was assessed by intraperitoneal injection with mAb to IFN-γ (18). Assay of virus infectivity levels 6 d after infection demonstrated that IFN-γ–dependent anti-influenza A virus mechanisms were operative in mutant mice as depicted by the high titers comparable to those of l-NMA or d-NMA– treated wild-type controls (Fig. 2 d). In contrast, NOS2−/− animals receiving an IgG1 isotype-matched control mAb still retained their capacity to restrict influenza virus replication within the lungs; titers were below the level of detection (Fig. 2 d).
Elevated NO Production Is Associated with Pulmonary Pathology in Influenza Virus–infected Wild-type Hosts.
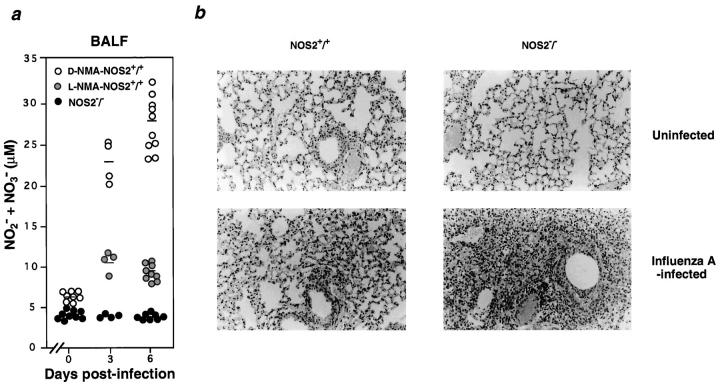
The demise of untreated or d-NMA–treated NOS2+/+ mice was not due to uncontrolled viral growth, since l-NMA– treated wild-type or anti–IFN-γ–treated NOS2−/− hosts also displayed overwhelming lung viral burdens yet showed no signs of morbidity throughout the 3-wk experimental period (Figs. 1 b, 2 d, and data not shown). Survival of the latter two groups implied that pulmonary injury in d-NMA– treated animals arose as a consequence of excessive NO production rather than by direct viral cytopathic effects. Indeed, NOx levels within the BALF of d-NMA–treated NOS2+/+ mice rose appreciably (∼3.4–4.0-fold) during the course of infection (Fig. 3 a), whereas this increase was absent in mutant mice and inhibited by 77–90% in l-NMA– treated controls (Fig. 3 a). Histopathologic examination revealed massive inflammatory foci and edema within the lungs of all d-NMA–treated wild-type mice as part of the consolidating pneumonitis observed 6 d after infection (Fig. 3 b). Neutrophils and alveolar macrophages were especially prominent. Both are capable of releasing cytotoxic oxidants besides NO (6, 23), such as the superoxide anion (O2 −), which can react with the latter to yield more damaging products like peroxynitrite (ONOO− [3]). Akaike et al. have demonstrated the local formation of both ONOO− and NO-hemoglobin in murine influenza infection using nitrotyrosine footprinting and electron spin resonance spectroscopy (6), while NO-thiol adducts also appear elevated in wild-type but not NOS2−/− mice during tuberculosis (14). Thus, it is conceivable that several NO-derived species acted in concert to cause influenza-associated pathology.
Figure 3.
Absence of inducible NO production and consolidating pneumonitis in the lungs of infected NOS2−/− mice. (a) NO2 − + NO3 − (NOx) levels in the BALF of d-NMA– or l-NMA–treated NOS2+/+ mice and NOS2−/− mice intranasally infected with 400 HAU of virus determined at day 6 after infection. Individual samples were assayed in triplicate after conversion to NO2 −; horizontal bars, group means. (b, top) Histological analyses of lungs from uninfected NOS2+/+ and NOS2−/− hosts; original magnification: ×250. (Bottom) Consolidating pneumonitis in NOS2+/+ mice which was absent in NOS2−/− mutant animals at day 6 after infection; original magnification: ×250. At least nine mice per group were examined.
Absence of Consolidating Pneumonitis in NOS2− /− Mice.
In marked contrast to the severe pneumonitis seen for all d-NMA–treated NOS2+/+ mice, the majority of mutant animals (∼70%) lacked evidence of pathology. A mild degree of inflammation was observed in the remainder; an example of the latter is depicted in Fig. 3 b. These results are consistent with the idea that NOS2 promotes the development of viral pneumonia. Additional support for this concept comes from recent studies using NOS inhibitors during infection with HSV-1 (7), murine CMV (24), or the influenza A/Kumamoto/Y5/67 (H2N2) mouse adapted strain (6). Inhibition of NOS fully protected mice from HSV-1–dependent lung injury despite a 17-fold increase in viral titers (7). That NO was necessary to control HSV-1 in vivo substantiated earlier experiments in vitro (1, 2). In contrast, in the present work, the functional loss of NOS2 alleviated pneumonitis and greatly improved survival irrespective of whether influenza virus was cleared (NOS2−/− hosts) or not (NOS2+/+ mice given l-NMA). Reduced pathology has also recently been noted in NOS2−/− mice infected with Mycobacterium avium (25) and Toxoplasma gondii (26).
IFN-γ has been implicated as the major cytokine responsible for NOS2 induction during influenza A–induced pneumonitis (6). Aside from IFN-γ's purported proinflammatory action in wild-type mice, we found that heightened IFN-γ release was both associated with and required for influenza A virus clearance in NOS2−/− animals, an effect unaccompanied by changes in antiviral CTL activity (Fig. 2, a–d). The effect was specific for mutant mice, since l-NMA– treated wild-type mice had comparable IFN-γ levels but failed to limit viral growth. IFN-γ thus appears to cooperate with immune components otherwise subject to suppression by NOS2 and not by NOS1 and/or NOS3. Complete inhibition of NOS2 by disruption of the gene encoding it, rather than the partial inhibition afforded by treatment of NOS2+/+ mice with l-NMA, also appeared necessary to permit expression of the IFN-γ–dependent antiinfluenza mechanism. Among pertinent candidates for components of this mechanism are MHC class II expression by pulmonary dendritic cells (27) and Th1 cell proliferation plus cytokine production (28, 29). Each could be compromised by NOS2, which can limit T cell responsiveness to IL-2 in the lungs of rats and mice (28) as well as inhibit the release of IL-12 and elevate the expression of TGF-β as shown in Leishmania major–infected NOS2−/− mice (30). In this manner, the high output NO pathway may regulate IFN-γ secretion via a feedback mechanism, such that once infection is cleared, autotoxic NO production is extinguished.
Beyond this, how NOS2 might influence the underlying molecular events leading to cytokine gene expression and action during antiviral immunity remains speculative. NO can activate or disable protein tyrosine kinases of the Janus kinase (JAK), extracellular signal–regulatory kinase (ERK), and src families, as well as the transcription factor nuclear factor (NF)-κB, and thus may affect cytokine signaling (31, and references therein). Indeed, NOS2−/− animals have altered levels of NF-κB and signal transducer and activator of transcription (STAT)3 activation in hemorrhage/resuscitation (32) and perturbed IFN-γ release in L. major (28, 29) and influenza A infections (Fig 2, b and c). Cytokine expression could also be regulated by NOS2 at the posttranslational level. For example, S-nitrosylation of the active site cysteine residue in caspases leads to their inactivation (33). If this inhibition were to be relieved in NOS2−/− mice, abnormally active caspase-1 might more readily promote the release of IL-18 (34, 35), a powerful inducer of IFN-γ.
During infection, NOS2 probably displays all three of its major effects to variable degrees—antimicrobial, inflammatory, and immunosuppressive. The results seen here with influenza A virus–infected mice appear to illustrate one extreme, in which the latter two effects dominate.
Acknowledgments
We thank Drs. Nicole Baumgarth and Anne Kelso for lung parenchymal cell isolation methods.
This work was supported by the National Centre for HIV Research (G. Karupiah and J.-H. Chen), and by National Institutes of Health grants HL-51967 and AI-34543 (to C.F. Nathan).
References
- 1.Karupiah G, Xie Q-w, Buller RML, Nathan C, Duarte C, MacMicking JD. Inhibition of viral replication by interferon-γ-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 2.Croen K. Evidence for an antiviral effect of nitric oxide. J Clin Invest. 1993;91:2446–2452. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacMicking J, Xie Q-w, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 4.Karupiah, G., and N. Harris. 1997. Gamma interferon- induced nitric oxide in antiviral defense and immunopathology. In Gamma Interferon in Antiviral Defense. G. Karupiah, editor. R.G. Landes Company, Austin, TX. 119–143.
- 5.Reiss CS, Komatsu T. Does nitric oxide play a critical role in viral infections? . J Virol. 1998;72:4547–4551. doi: 10.1128/jvi.72.6.4547-4551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akaike T, Noguchi Y, Ijiri S, Setoguchi K, Suga M, Zheng YM, Dietzschold B, Maeda H. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc Natl Acad Sci USA. 1996;93:2448–2453. doi: 10.1073/pnas.93.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adler H, Beland JL, Del-Pan NC, Kobzik L, Brewer JP, Martin TR, Rimm IJ. Suppression of herpes simplex virus type 1 (HSV-1)-induced pneumonia in mice by inhibition of inducible nitric oxide synthase (iNOS, NOS2) J Exp Med. 1997;185:1533–1540. doi: 10.1084/jem.185.9.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komatsu T, Bi Z, Reiss CS. Interferon-γ induced type 1 nitric oxide synthase activity inhibits viral replication in neurons. J Neuroimmunol. 1996;68:101–108. doi: 10.1016/0165-5728(96)00083-5. [DOI] [PubMed] [Google Scholar]
- 9.Barna M, Komatsu T, Reiss CS. Activation of type III nitric oxide synthase in astrocytes following a neurotropic viral infection. Virology. 1996;15:332–343. doi: 10.1006/viro.1996.0484. [DOI] [PubMed] [Google Scholar]
- 10.MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie Q-w, Sokol K, Hutchinson N, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 11.Zargoza C, Ocampo C, Suara M, Leppo M, Wei X-Q, Quick R, Moncada S, Liew FY, Lowenstein CJ. The role of inducible nitric oxide synthase in the host response to Coxsackievirus myocarditis. Proc Natl Acad Sci USA. 1998;95:2469–2474. doi: 10.1073/pnas.95.5.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Karupiah G, Chen JH, Nathan CF, Mahalingam S, MacMicking JD. Identification of nitric oxide synthase 2as an innate resistance locus against ectromelia virus infection. J Virol. 1998;72:7703–7706. doi: 10.1128/jvi.72.9.7703-7706.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumgarth N, Kelso A. In vivo blockade of gamma interferon affects the virus-induced humoral and the local cellular immune response in lung tissue. J Virol. 1996;70:4411–4418. doi: 10.1128/jvi.70.7.4411-4418.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham MB, Dalton DK, Giltinan D, Braciale VL, Stewart TA, Braciale TJ. Response to influenza infection in mice with a targeted disruption in the interferon γ gene. J Exp Med. 1993;178:1725–1732. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tannock GA, Paul JA, Barry RD. Relative immunogenicity of the cold-adapted influenza A/Ann Arbor/6/60 (A/AA/60-ca), recombinants of A/AA/6/60-ca, and parental strains with similar surface antigens. Infect Immun. 1984;43:457–462. doi: 10.1128/iai.43.2.457-462.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karupiah G, Couper BEH, Andrew ME, Boyle DB, Phillips SM, Müllbacher A, Blanden RV, Ramshaw IA. Elevated natural killer cell responses in mice infected with recombinant vaccinia virus encoding murine IL-2. J Immunol. 1990;144:290–298. [PubMed] [Google Scholar]
- 17.Karupiah G, Buller RML, Van Rooijen N, Duarte C, Chen J. Different roles for CD4+ and CD8+T lymphocytes and macrophage subsets in the control of a generalized virus infection. J Virol. 1996;70:8301–8309. doi: 10.1128/jvi.70.12.8301-8309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karupiah G, Fredrickson TN, Holmes KL, Khairallah LH, Buller RML. Importance of interferons in recovery from mousepox. J Virol. 1993;67:4214–4226. doi: 10.1128/jvi.67.7.4214-4226.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 20.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel RM, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 21.Bender BS, Croghan T, Zhang L, Small PA., Jr Transgenic mice lacking class I major histocompatibility complex–restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J Exp Med. 1992;175:1143–1145. doi: 10.1084/jem.175.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Topham DJ, Tripp RA, Doherty PC. CD8+T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 23.MacMicking JD, Willenborg DO, Weidemann MJ, Rockett KA, Cowden WB. Elevated secretion of reactive nitrogen and oxygen intermediates by inflammatory leukocytes in hyperacute autoimmune encephalomyelitis: enhancement by the soluble products of encephalitogenic T cells. J Exp Med. 1992;176:303–307. doi: 10.1084/jem.176.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka K, Nakazawa H, Okada K, Umezawa K, Fukuyama N, Koga Y. Nitric oxide mediates murine cytomegalovirus–associated pneumonitis in lungs that are free of the virus. J Clin Invest. 1997;100:1822–1830. doi: 10.1172/JCI119710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doherty TM, Sher A. Defects in cell-mediated immunity affect chronic, but not innate, resistance to Mycobacterium aviuminfection. J Immunol. 1997;158:4822–4831. [PubMed] [Google Scholar]
- 26.Khan IA, Shwartzman JD, Masuura T, Kasper LH. A dichotomous role for nitric oxide during acute Toxoplasma gondiiinfection in mice. Proc Natl Acad Sci USA. 1997;94:13955–13960. doi: 10.1073/pnas.94.25.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holt PG, Oliver J, Bilyk N, McMenamin C, McMenamin PG, Kraal G, Thepen T. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med. 1993;177:397–407. doi: 10.1084/jem.177.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Upham JW, Strickland DH, Bilyk N, Robinson BW, Holt PG. Alveolar macrophages from humans and rodents selectively inhibit T-cell proliferation but permit T-cell activation and cytokine secretion. Immunology. 1995;84:142–147. [PMC free article] [PubMed] [Google Scholar]
- 29.Wei X-Q, Charles IG, Smith A, Ure J, Feng G-j, Huang F-p, Xu D, Muller W, Moncada S, Liew FY. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 30.Diefenbach A, Schindler H, Donhauser N, Lorenz E, Laskay T, MacMicking J, Röllinghoff M, Gresser I, Bogdan C. Type 1 interferon (IFNα/β) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity. 1998;7:77–87. doi: 10.1016/s1074-7613(00)80460-4. [DOI] [PubMed] [Google Scholar]
- 31.Lander HM. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997;11:118–124. [PubMed] [Google Scholar]
- 32.Hierholzer C, Harbrecht B, Menezes J, Kane J, MacMicking JD, Nathan CF, Pietzman A, Billiar TR, Tweardy DJ. Essential role of induced nitric oxide in the initiation of the inflammatory response following hemorrhagic shock. J Exp Med. 1998;187:917–928. doi: 10.1084/jem.187.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Billiar TR, Talanian RV, Kim YM. Nitric oxide reversibly inhibits seven members of the caspase family via S-nitrosylation. Biochem Biophys Res Commun. 1997;243:419–424. doi: 10.1006/bbrc.1997.7672. [DOI] [PubMed] [Google Scholar]
- 34.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, et al. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-γ production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 35.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, et al. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science. 1997;725:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]