Abstract
GATA-2 is an essential transcription factor in the hematopoietic system that is expressed in hematopoietic stem cells (HSCs) and progenitors. Complete deficiency of GATA-2 in the mouse leads to severe anemia and embryonic lethality. The role of GATA-2 and dosage effects of this transcription factor in HSC development within the embryo and adult are largely unexplored. Here we examined the effects of GATA-2 gene dosage on the generation and expansion of HSCs in several hematopoietic sites throughout mouse development. We show that a haploid dose of GATA-2 severely reduces production and expansion of HSCs specifically in the aorta-gonad-mesonephros region (which autonomously generates the first HSCs), whereas quantitative reduction of HSCs is minimal or unchanged in yolk sac, fetal liver, and adult bone marrow. However, HSCs in all these ontogenically distinct anatomical sites are qualitatively defective in serial or competitive transplantation assays. Also, cytotoxic drug-induced regeneration studies show a clear GATA-2 dose–related proliferation defect in adult bone marrow. Thus, GATA-2 plays at least two functionally distinct roles during ontogeny of HSCs: the production and expansion of HSCs in the aorta-gonad-mesonephros and the proliferation of HSCs in the adult bone marrow.
Keywords: GATA-2, hematopoietic stem cells, AGM, haploinsufficiency, gene dosage
Introduction
Hematopoietic stem cells (HSCs) at the foundation of the adult hematopoietic differentiation hierarchy have the ability to self-renew and produce all the distinct blood cell lineages (1, 2). HSCs capable of complete long-term hematopoietic repopulation of irradiated adult recipients are first generated in the aorta-gonads-mesonephros (AGM) region at mid–embryonic day (E)10.5 (3, 4) and localize to the dorsal aorta, vitelline, and umbilical arteries (5). Subsequently, at mid/late E11, HSC activity is also found in the yolk sac (YS) and fetal liver (FL). HSC activity increases significantly in E11 AGM and E12 YS when they are cultured as whole tissue explants for 3 d (3, 6). Although the spatial and temporal appearance of HSCs during development has been described and quantitated, the molecular mechanisms underlying HSC generation, expansion, and maintenance are not well explored.
One molecule important during hematopoietic ontogeny is GATA-2, a member of the GATA family of zinc finger transcription factors (7, 8). RT-PCR analysis shows high expression of GATA-2 in adult hematopoietic progenitor cells and HSCs (9, 10). Furthermore, immunohistochemistry, in situ hybridization, and transgenic analyses show GATA-2 expression as early as E8 in the para-aortic splanchnopleura (precursor tissue to AGM) and subsequently in the AGM (11, 12). In the E11.5 AGM, GATA-2 is expressed in the aortic endothelium and neighboring mesenchymal cells, which are both considered putative hemogenic cell populations. Embryos lacking GATA-2 are anemic, have moderately reduced numbers of primitive erythroid cells and hematopoietic progenitor cells (13), and die at E10.5, the time of HSC induction and expansion. Due to this lethality, the role of GATA-2 has been examined mainly by in vitro colony-forming assays of cells from early embryonic tissues and hematopoietic cultures of GATA-2 −/− embryonic stem (ES) cells. In both cases, hematopoietic progenitor numbers are severely reduced. Further studies in chimeric mice produced with GATA-2 −/− ES cells show no contribution of the mutant cells to any hematopoietic tissues. Together, these data indicate that GATA-2 is crucial for the maintenance, proliferation, and/or survival of immature hematopoietic progenitors (13).
The function of GATA-2 had also been studied through enforced overexpression achieved by retroviral transduction or transfection of genes encoding either a wild-type GATA-2 or an inducible GATA-2–estrogen receptor (ER) fusion protein (12, 14–19). Irrespective of these attempts, a simple conclusion on the function of GATA-2 in the HSCs or progenitor cells is difficult. On one hand, expression of the inducible form of GATA-2–ER fusion protein in the multipotent hematopoietic progenitor cell line FDCP promotes differentiation (17). On the other hand, constitutive expression of GATA-2 in murine BM cells blocked progenitor-derived colony formation (14). The opposing results may be due to the cell types chosen for these experiments. However, it is also suggested that the biochemical behavior of the artificially generated GATA-2–ER fusion protein may not be the same as the wild-type unmodified GATA-2 protein (18). Thus, the most relevant data on GATA-2 dose effects on hematopoiesis may be best obtained within the physiological context of the whole organism wherein GATA-2 is expressed under the endogenous regulatory machinery in the appropriate cell types.
The study of transcription factor dose and function at the earliest stages of hematopoietic development is of particular interest for an understanding of HSC generation. Studies on the runx1 transcription factor have shown that a haploid dose results in changes in HSC induction, expansion, and distribution in the midgestation mouse embryo (20). Moreover, haploinsufficiency of human Runx1 (AML-1) results in thromobocytopenia and a propensity to develop myeloid leukemia (21). Recently, a correlation between a reduction in GATA-2 expression and aplastic anemia (22) has been demonstrated. Hence, to further understand the role of GATA-2 in the ontogeny of HSCs we examined the effects of GATA-2 haploinsufficiency on induction and expansion of HSCs during development by in vivo hematopoietic transplantation assays and phenotypic analysis of compound transgenic embryos (GATA-2 +/− –Ly-6A GFP) (23).
Here we present data showing that the numbers of hematopoietic progenitors in GATA-2 +/− embryos are reduced. More importantly, we observe a dramatic quantitative reduction in HSC activity specifically in GATA-2 +/− AGMs and a further reduction in the serial repopulating ability of these HSCs. In contrast, GATA-2 +/− HSC numbers appear quantitatively normal in the adult BM but are qualitatively defective in the setting of competitive transplantation. In addition, GATA-2 +/− HSCs exhibit a delay in regeneration of the hematopoietic system after cytotoxic drug challenge, suggesting that GATA-2 levels play a role in HSC proliferation. Thus, GATA-2 plays functionally distinct roles in the production of HSCs in the AGM region and the proliferation of HSCs throughout ontogeny.
Materials and Methods
GATA-2 Mutant Mice and Embryos.
GATA-2 mutant mice (13) were backcrossed onto the C57BL/6 background for over 10 generations and were housed in the Erasmus Medical Center Animal unit according to the institution guidelines with food and water provided ad libitum. The day of vaginal plug discovery from overnight matings (GATA-2 +/− male × C57BL/6 GATA-2 +/+ or GATA-2 +/− female) was counted as day 0. Pregnant dams were killed and embryos isolated from the uterus as described previously (24). Embryos (E10–E11) were staged by counting somite pairs (25). Genotyping was performed by PCR as described previously (13). Compound transgenic embryos were obtained by mating Ly-6A GFP hemizygous (23) and GATA-2 +/− mice.
Dissection, Explant Culture, Cell Preparation, and In Vivo Transplantation.
Dissections, tissue explants, and cell preparation were performed as described previously (24). Recipient mice (C57BL/6 or [129Sv × C57BL/6] F1 females, 8–16 wk old) received a split dose of 1,000 rad (for colony-forming unit–spleen [CFU-S11]), 900 rad (for HSCs), or 640 rad (for competitive repopulation assay) at a 3-h interval from a 137Cs source on the day of donor cell injection. Cells were injected i.v. into the tail veins. Except for CFU-S, serial, and competitive transplantation assays, 2 × 105 female spleen cells from the recipient strain were coinjected to provide short-term survival. Secondary transplantations were performed with 3 × 106 BM cells from the primary recipients. Cell dose for competitive repopulation assays was 3 × 105–3 × 107. Injected animals were provided with 0.16% Neomycin (Sigma-Aldrich)-supplemented water. For CFU-S11, recipients were killed at 11 d posttransplantation by cervical dislocation, spleens isolated, and microscopic colonies scored after fixing with Teleynesnicki's solution overnight.
Semiquantitative PCR for Donor Contribution.
Blood, tissue, or specific cell lineage DNA (100 ng) was used for semiquantitative PCR to detect the donor HSC contribution to the recipient. For male-derived donor cells, YMT-specific PCR (350-bp product) was used together with myogenin-specific PCR (250 bp) for DNA normalization. The detection of GATA-2 mutant–derived donor cells was performed with GATA-2/NEO (950 bp) and GATA-2 wild-type (600 bp) PCR. Primers and PCR conditions were as previously described (4, 13).
Cell Sorting and Flow Cytometry Analysis.
FACS was performed on a FACS Vantage SE (Becton Dickinson) (23), and flow cytometric analyses were performed on a FACSCalibur dual laser instrument (Becton Dickinson) with CellQuest software (BD Bioscience). Staining of embryonic tissue cell suspensions was performed in PBS supplemented with 10% FCS, and 2 μg/ml 7AAD (Molecular Probes) was added for dead cell exclusion. Staining of adult HSCs was performed in PBS supplemented with 0.5% BSA. Biotin-conjugated anti–Gr-1 (ER-MP20) was a gift from Dr. P.J.M. Leenen (Erasmus MC, Rotterdam, The Netherlands). All other antibodies were obtained from BD Biosciences including APC-conjugated anti–c-kit (clone 2B8), PE-conjugated anti–Sca-1 (clone D7), PerCP-Cy5.5–conjugated anti-CD8a (clone 53–6.7), anti-B220 (clone RA3-6B2), anti-CD19 (1D3), anti-CD11b (anti–Mac-1, clone M1/70), and biotin-conjugated anti-CD3 (145-2C11), anti-CD4 (H129.19), Ly-76 (TER-119), anti-IgM (II/41), and anti-NK1.1 (PK136). A secondary step was sometimes performed with streptavidin-conjugated PerCP-Cy5.5 (BD Biosciences).
Immunohistochemistry.
Embryos were fixed for 30 min with 2% paraformaldehyde/PBS at 4°C and equilibrated in 20% sucrose/PBS overnight at 4°C. They were immersed in Tissue Tek, quick frozen on dry ice, and stored at −80°C until ready for use. Serial cryosections (10 μm) were treated in 100% cold acetone for 10 min, washed three times with PBS (0.05% Tween), blocked with 0.5% BSA and 50% vol/vol Avidin D block solution (Vector Laboratories) for 15 min, washed three times, blocked with Biotin blocking solution (Vector Laboratories) for 15 min, and washed three times. Subsequently, sections were incubated with a biotin-conjugated anti-CD34 (clone RAM34; BD Biosciences) diluted 1:50 in 1% BSA/0.05% Tween/PBS at room temperature for 1 h, washed three times, incubated with the detection reagent Streptavidin-Cy5 (Rockland) diluted 1:500 in 1% BSA/0.05% Tween/PBS at room temperature for 30 min, washed three times, dehydrated in ethanol (from 70 to 100%), and mounted with vectashield (Vector Laboratories).
Confocal images were taken of every tenth section starting caudally at the point where the urogenital ridges first appeared up to the rostral bifurcation of the dorsal aorta.
5-Fluorouracil Treatment.
GATA-2 +/+ and GATA-2 +/− mice (9–10 wk old) were i.v. injected with 150 mg 5-fluorouracil (5-FU; Sigma-Aldrich) per 10 g body weight. Treated mice were then killed at 4, 8, 12, and 16 d posttreatment, and BM cells were isolated and analyzed by flow cytometry and in vitro culture assay.
Progenitor Colony Assay.
BM cells were plated in triplicates from 2 × 104 to 5 × 105 cells per plate in methylcellulose medium (Methocult GF M3434; StemCell Technologies Inc.) supplemented with stem cell factor (SCF), IL-3, IL-6, and Epo. All cultures were incubated at 37°C in a humidified chamber under 5% CO2. Colony-forming unit–granulocyte macrophage (CFU-GM) were scored with an inverted microscope at day 7 of the culture.
Results
GATA-2+/− AGM and YS Explants Contain Fewer CFU-S11.
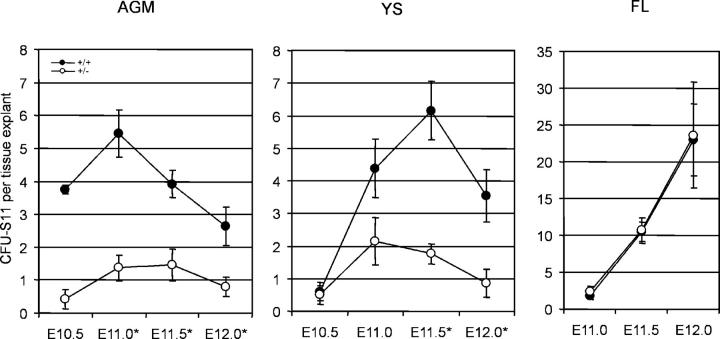
To investigate if GATA-2 gene dosage affects the production of hematopoietic progenitors during development, colony-forming unit spleen activity was assayed at 11 d postinjection (CFU-S11) so as to measure the more immature erythro-myeloid progenitors. AGM, YS, and FL were isolated from GATA-2 +/+ and GATA-2 +/− embryos at E10.5–E12, explant cultured, and cells transplanted into irradiated adult recipients (Fig. 1). At all time points tested, CFU-S11 activity was detected both in GATA-2 +/+ and GATA-2 +/− AGM, YS, and FL explants (Fig. 2). As expected from previous data (3), high numbers of E10.5 CFU-S11 are first detected in the GATA-2 +/+ AGM explants, they increase at E11, and thereafter decline in number. In GATA-2 +/− AGM and YS explants, CFU-S11 were reduced by three- to ninefold and one- to fourfold, respectively, compared with wild-type tissues. In contrast, CFU-S11 activity in GATA-2 +/− FL explants was normal. No reductions in FL CFU-S11 numbers were observed at any time. Therefore, GATA-2 gene dosage affects the generation and/or proliferation of immature hematopoietic progenitor cells in the YS and AGM of the midgestation embryo.
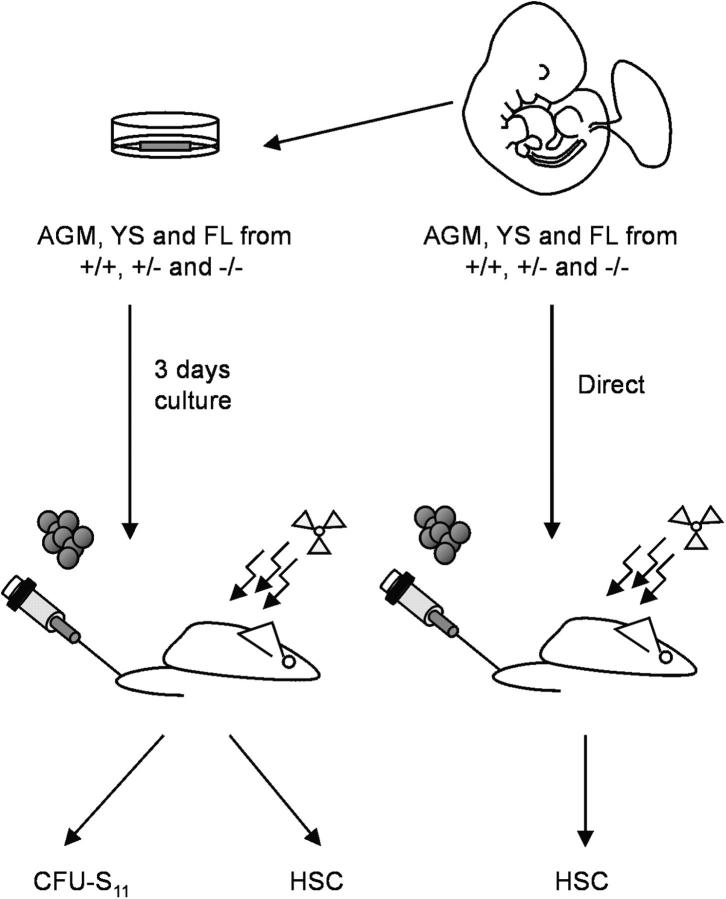
Figure 1.
Strategy for studying HSCs and hematopoietic progenitor cells in GATA-2 mutant embryonic tissues. GATA-2 +/+, GATA-2 +/−, and GATA-2 −/− AGM regions, YS, and FL were harvested from midgestation mouse embryos. In some cases (left) they were then cultured for 3 d as whole tissue explants before preparation of single cell suspensions and injection into irradiated adult recipients to assay for CFU-S11 or HSCs. In some cases (right), single cell suspensions were prepared directly from freshly isolated tissues and injected into irradiated adult recipients for HSC activity.
Figure 2.
CFU-S11 activity in GATA-2 mutant tissue explants. CFU-S11 were assayed from AGM, YS, and FL explants (E10.5–E12.0) after 3 d of culture. Each point represents the average CFU-S11 number per embryo tissue equivalent ± SEM detected in the corresponding tissue and genotype. 3–14 independent experiments were performed with 0.2–4.5 tissue equivalents injected per recipient. +/+, GATA-2 +/+; +/−, GATA-2 +/−. The total number of injected AGM explants: E10.5 +/+ = 6, E11.0 +/+ = 21, E11.5 +/+ = 14, E12.0 +/+ = 12, E10.5 +/− = 7, E11 +/− = 22, E11.5 +/− = 12, E12.0 +/− = 12; YS explants E10.5 +/+ = 8, E11.0 +/+ = 8, E11.5 +/+ = 11, E12.0 +/+ = 10.75, E10.5 +/− = 8, E11.0 +/− = 11.5, E11.5 +/− = 15.5, E12.0 +/− = 9.5; FL explants E11.0 +/+ = 17, E11.5 +/+ = 18, E12.0 +/+ = 8.7, E11.0 +/− = 23, E11.5 +/− = 19.5, E12.0 +/− = 8. Embryonic tissues from E10.5 ranged from 36 to 40 somite pairs (sp), E11.0 ranged from 41–47 sp, E11.5 contain >48 sp and E12 contained >60 sp. *Significant difference in the CFU-S11 number between GATA-2 +/+ and GATA-2 +/− tissue explants: AGM E11.0, P < 0.001; E11.5, P < 0.01; E12.0, P < 0.05; YS E11.5, P < 0.001; E12, P < 0.05. Note that fewer CFU-S11 are detected in both the GATA-2 +/− AGM and YS explants in comparison to the GATA-2 +/+ explants, whereas the FL CFU-S11 numbers are unaffected.
HSC Activity Is Severely Reduced in the GATA-2+/− AGMs.
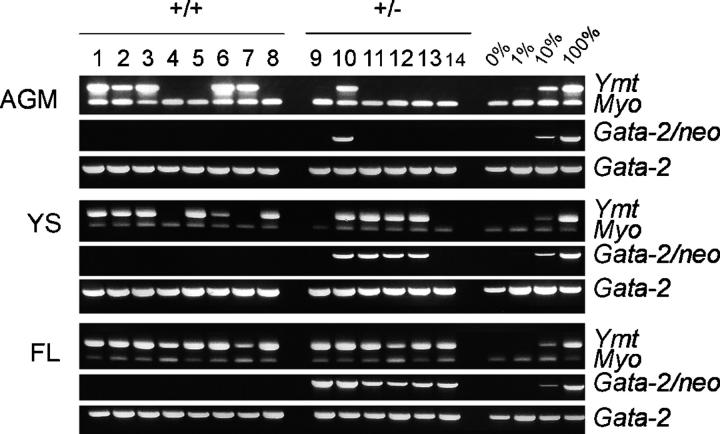
To investigate if GATA-2 dose also affects midgestation HSC development, we performed the most stringent functional HSC test: the long-term, high level, multilineage repopulation of irradiated adult mouse recipients. E11 and E12 GATA-2 +/+ and GATA-2 +/− AGM, YS, and FL cells were transplanted directly into irradiated adult recipients (Fig. 1). Engraftment by GATA-2 +/+ and GATA-2 +/− cells was assayed by semiquantitative PCR of the male Y chromosome–specific marker Ymt and the GATA-2/NEO mutant allele (respectively) in recipient peripheral blood DNA at 4 mo posttransplantation. Only those recipients showing >10% engraftment with donor-marked cells were considered positive for high level repopulation. PCR results of one representative experiment are shown in Fig. 3. Briefly, each recipient received one-third of the cells obtained from an individually prepared E12 tissue (0.33 tissue equivalents). At 4 mo postinjection, progeny of transplanted GATA-2 +/+ AGM cells were found in the peripheral blood of five out of eight recipients (Ymt PCR). In contrast, reduced HSC activity was found in E12 GATA-2 +/− AGMs. Only one out of six recipients was highly engrafted (GATA-2 mutant and Ymt PCR). Similarly, recipients transplanted with YS cells revealed some reduction of HSC activity in GATA-2 +/− embryos. However, no reduction in HSC activity was found in GATA-2 +/− FLs. Further analysis of recipients repopulated with GATA-2 +/− cells revealed high level multilineage engraftment within all hematopoietic tissues (blood, thymus, LNs, BM, and spleen) and subsets tested (splenic T and B lymphocytes, erythroid and myeloid cells) (not depicted).
Figure 3.
Detection of donor hematopoietic cell contribution in transplantation recipients by peripheral blood DNA PCR analysis. A representative PCR analysis for donor cell contribution to the peripheral blood of transplantation recipients. DNA was isolated from the corresponding recipients (at >4 mo posttransplantation) of GATA-2 +/+, GATA-2 +/−, and GATA-2 −/− AGM, YS, and FL. Lanes 1–8 and 9–14 are blood DNA samples isolated from recipients receiving cells from E12 GATA-2 +/+ and +/− tissues, respectively. Each sample was analyzed with primers specific for Y chromosome (ymt), and GATA-2 (GATA-2/NEO for targeted allele). DNA samples were normalized by PCR with two endogenous gene controls (myo, myogenin; GATA-2, wild-type allele). Control DNA: 0, 1, 10, and 100% represents percentage of the male GATA-2 +/− DNA mixed with female DNA. Only when the donor marker-specific PCR product was >10%, compared with controls, was the recipient considered to be positive.
The cumulative results of all transplantation experiments are shown in Table I and reveal that at both E11 and E12, HSC activity is severely reduced in GATA-2 +/− AGMs. The percentage of recipients repopulated with E11 GATA-2 +/− AGM cells is only 6%, whereas 25% of recipients are repopulated with E11 GATA-2 +/+ AGM cells. This represents a greater than fourfold decrease in HSC activity in E11 GATA-2 +/− AGMs. Furthermore, at E12 GATA-2 +/− AGMs are ninefold reduced in HSC activity compared with GATA-2 +/+ AGMs. Reductions in the HSC activity of GATA-2 +/− YS and FL tissues are less severe and stage dependent. The percentage of mice repopulated by E11 GATA-2 +/− YS (23%) is comparable to that of GATA-2 +/+ YS (28%), and the FL at this stage contains only limited HSC activity. However, at E12 slight reductions in HSC activity are observed for both GATA-2 +/− YS and FL (1.6- and 1.3-fold, respectively) compared with GATA-2 +/+ tissues. Thus, two copies of the GATA-2 gene are required for the normal generation, expansion, and/or survival of HSCs in the AGM region.
Table I.
HSC Activity in GATA-2 Mutant Embryonic Tissues
| AGM
|
YS
|
FL
|
||||
|---|---|---|---|---|---|---|
| Stage | +/+ | +/− | +/+ | +/− | +/+ | +/− |
| E11.0 | 1a/4b (25)c | 1/16 (6) | 2/7 (28) | 4/17 (23) | 0/6 (0) | 1/19 (5) |
| E12.0 | 11/16 (69) | 1/13 (8) | 11/15 (73) | 6/13 (46) | 19/19 (100) | 11/14 (78) |
| 2°E12.0 | 6/6 (100) | 0/3 (0) | 6/6 (100) | 4/9 (44) | 6/6 (100) | 4/9 (44) |
E11 and E12 AGM, YS, and FL tissues were made into a single cell suspension and injected into irradiated adult recipients. Each result represents
the number of recipient mice showing donor cells in peripheral blood (DNA) at >4 mo posttransplantation,
the total number of mice transplanted, and
the percentage of repopulated recipients.
Only when the donor cells represented >10% was the recipient considered to be positive. Three and two independent experiments, respectively, were performed for E11 (41–47 somite pairs) and E12 tissues (>60 somite pairs). 1 and 0.33 tissue equivalents transplanted for E11.0 and E12.0, respectively. 2°E12, secondary transplantation with 3 × 106 BM cells isolated from high level repopulated primary recipients that received cells from E12 tissues (two independent experiments). +/+, GATA-2+/+; +/−, GATA-2+/−.
Ex Vivo Expansion and Maintenance of AGM HSC Activity Is Sensitive to GATA-2 Dose.
Since it was shown previously that HSC activity generated in the AGM can be amplified (either by induction or proliferation) when whole tissues are cultured for 3 d (3), we examined the effects of GATA-2 gene dosage on HSCs in such explant cultures of AGM, YS, and FL from GATA-2 +/+ and GATA-2 +/− embryos. Tissues (E10.5–E12) were dissected, cultured as explants for 3 d, made into a single cell suspension, and injected into irradiated adult recipients (Fig. 1). Repopulation was measured at 4 mo posttransplantation, and only those recipients showing >10% donor cell multilineage hematopoietic repopulation (measured in several hematopoietic tissues and lineages) were considered positive. The results are summarized in Table II.
Table II.
HSC Activity in GATA-2 Mutant Tissues after Explant Culture
| AGM explants | YS explants | FL explants | ||||||
|---|---|---|---|---|---|---|---|---|
| Stage | +/+ | +/− | +/+ | +/− | +/+ | +/− | ||
| E10.5 | 2a/3b (66)c | 1/12 (8) | 0/3 (0) | 0/11 (0) | ND | 0/2 (0) | ||
| E11.0 | 1/2 (50) | 0/4 (0) | 1/4 (25) | 1/5 (20) | 0/3 (0) | 0/5 (0) | ||
| E11.5 | 11/12 (50) | 0/27 (0) | 7/25 (28) | 7/29 (24) | 6/25 (24) | 3/28 (11) | ||
| E12.0 | 7/12 (58) | 0/13 (0) | 4/16 (25) | 2/14 (14) | 11/12 (92) | 10/19 (53) | ||
| +/+ | +/− | −/− | +/+ | +/− | −/− | +/+ | +/− | |
| E10.0 | 0/7 (0) | 0/8 (0) | 0/3 (0) | 0/7 (0) | 0/8 (0) | 0/3 (0) | ND | ND |
E10 to E12 AGM, YS, and FL explants were cultured for 3 d, made into a single cell suspension, and injected into irradiated adult recipients. Each result represents
the number of recipient mice showing donor cells in the peripheral blood (DNA) isolated at >4 mo posttransplantation,
the total number of mice transplanted, and
the percentage of repopulated recipients.
ND, transplantation not performed. Only when donor contribution was >10% was the recipient considered to be positive. Two to four independent experiments performed for each stage and tissue type. For E10, one to five tissue explant equivalents were transplanted per recipient. 1 tissue explant equivalent was transplanted per recipient for E10.5 and 0.33 tissue explant equivalents for E11.0, E11.5, and E12.0. +/+, GATA-2+/+ and +/−, GATA-2+/−. E10, 31–35 somite pairs (sp); E10.5, 36–40 sp; E11.0, 41–47 sp; E11.5 > 48 sp; E12.0 > 60 sp.
Compared with the results of the direct transplantation experiments (Table I), GATA-2 +/− AGM explants were even more severely reduced in the HSC activity. At E10.5, only 8% of recipients receiving GATA-2 +/− AGM cells were repopulated, representing an eightfold decrease in HSC activity from GATA-2 +/+ AGM cells. The GATA-2 +/− cell contribution to the various hematopoietic organs (thymus, spleen, LN, and BM) and purified cell lineages (B and T lymphocytes, myeloid and erythroid) was tested and found to be multipotent, thus demonstrating that GATA-2 +/− AGMs do generate functional HSCs, albeit at much reduced levels. At later developmental time points (E11, E11.5, and E12), HSC activity, although increasing in GATA-2 +/+ AGM explants, is completely absent from GATA-2 +/− AGM explants. As seen in the GATA-2 +/+ AGM explants, HSC generation and expansion occurs from E10.5 to E11.5, with HSC numbers maintained thereafter (E12). Thus, the severely reduced HSC activity in GATA-2 +/− AGM explants can be attributed to reduced HSC expansion, survival, and/or homing in the irradiated recipient.
The HSC activity of GATA-2 +/− YS and FL explants was also reduced in comparison to the GATA-2 +/+ explants. However, this reduction was only slight compared with the AGM. At the first appearance of HSCs in the YS on E11, GATA-2 +/− YS HSC activity begins to decrease and by E12 is decreased by 1.8-fold from GATA-2 +/+ YS. The decrease in YS explant HSC activity at E12 corresponds with that seen in the directly transplanted YS, suggesting that the expansion but not the maintenance of YS HSC activity is sensitive to GATA-2 dose.
Similarly, we observed slight reductions in the HSC activity of GATA-2 +/− FL explants. At the first appearance of HSCs in FL explants on E11.5, HSC activity is reduced from 24% of recipients repopulated with GATA-2 +/+ cells to 11% repopulated with GATA-2 +/− cells, representing a 2.2-fold decrease in HSC activity. At E12, GATA-2 +/− FL explants show a 1.7-fold decrease in HSC activity. The changes in FL HSC activity with time are most likely related to the numbers of incoming HSCs from the AGM and YS. Thus, these findings suggest GATA-2 dose affects the expansion but not the survival of HSCs in the FL.
The Onset of HSC Activity in GATA-2+/− Embryos Is Normal.
Previously, we reported CFU-S11 and HSC deficiencies in embryos with a haploid dose of the runx1 transcription factor (20). The spatial distribution of HSC activity was altered and an unexpected early appearance of HSC activity was found in runx1 +/− AGM and YS. To examine if there was also a premature appearance of HSC activity in GATA-2 mutant embryos, AGM and YS explants from early E10 (31–35 somite pairs) GATA-2 +/+, GATA-2 +/−, and GATA-2 −/− embryos were isolated, cultured for 3 d, and cells were transplanted into irradiated adult recipients. As shown in Table II, although high tissue equivalents (up to five) of cells from GATA-2 mutant (+/− and −/−) AGM and YS explants were injected, HSC activity was not detected in any of the recipients. Also, GATA-2 +/− E10.5 YS and E10.5 and E11 FL explants showed no HSC activity. However, HSC activity initiates normally in GATA-2 +/− AGM explants at E10.5 at the same stage as in the GATA-2 +/+ AGM. HSCs also appear at normal time points in GATA-2 +/− YS and FL explants (E11 and E11.5, respectively). Therefore, we conclude that HSC induction initiates on schedule and that there is no early onset of HSC activity in GATA-2 +/− AGM, YS, or FL.
Serial Transplantation Potential of Midgestation HSCs Is Severely Reduced.
HSC self-renewal can be tested by serial transplantation of HSCs from primary to secondary recipients. Since we found that GATA-2 +/− AGM HSCs are severely reduced in their expansion, we examined whether GATA-2 +/− embryo-derived HSCs are as potent in their serial repopulation ability as wild-type HSCs. Whole BM cells from primary recipient mice showing high donor contribution from transplanted E12 GATA-2 +/+ or GATA-2 +/− AGM, YS, and FL cells were injected into irradiated secondary adult recipients. Consistent with previous published results, GATA-2 +/+ AGM-, YS-, and FL-derived HSCs can successfully reconstitute secondary recipients; 100% of secondary recipients were repopulated with HSCs from primary recipients of these midgestation tissues (Table I). In contrast, HSCs from a primary GATA-2 +/− AGM recipient failed to repopulate any of the secondary recipients analyzed (0%; zero out of three). Reduced HSC activity was also observed in the secondary recipients receiving BM cells from GATA-2 +/− YS and FL primary recipients (44% compared with 100% recipient repopulation with GATA-2 +/+ primary BM cells). These results demonstrate that GATA-2 dose affects HSC serial repopulation ability and suggests a defect in HSC self-renewal.
GATA-2+/− BM HSCs Are at a Competitive Disadvantage.
The decreased serial repopulation ability of embryo-derived HSCs prompted us to investigate if adult BM HSCs are also affected by a reduction in GATA-2 dose. Initially, we injected limiting doses of GATA-2 +/+ and GATA-2 +/− BM cells into lethally irradiated adult recipients but found no quantitative differences in repopulation. Hence, we performed reciprocal competitive transplantations in which different concentrations of unmanipulated GATA-2 +/+ and GATA-2 +/− BM cells were injected into sublethally irradiated GATA-2 +/+ and GATA-2 +/− adult recipients.
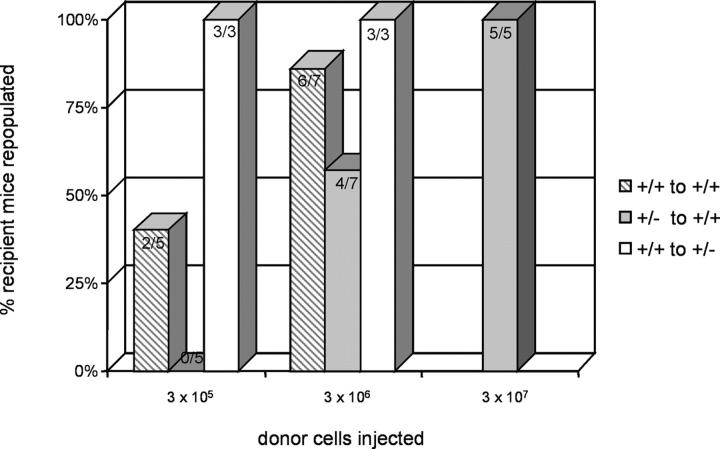
When 3 × 105 whole GATA-2 +/+ BM cells were transplanted into GATA-2 +/+ adult recipients, long-term high level donor contribution was found in two out of five (40%) recipients, whereas GATA-2 +/− cells at this dose provided no repopulation (zero out of five recipients; 0%) (Fig. 4). Only at a 10-fold higher cell dose were the GATA-2 +/− cells able to repopulate four out of seven (57%) recipients. A dose of 3 × 106 GATA-2 +/+ cells repopulated almost all recipients (six out of seven; 86%), whereas 3 × 107 GATA-2 +/− cells were required for repopulation of all recipients (five out of five; 100%). To further examine the competitive abilities of GATA-2 +/− cells, GATA-2 +/+ cells were injected into sublethally irradiated GATA-2 +/− recipients. Only 3 × 105 (or fewer) wild-type cells were required to fully out-compete all the GATA-2 +/− HSCs in the recipients. Thus, GATA-2 +/+ HSCs compete more effectively against GATA-2 +/+ HSCs than do GATA-2 +/− HSCs, demonstrating that GATA-2 +/− adult BM contains fewer HSCs or that these HSCs are qualitatively less potent.
Figure 4.
Competitive transplantation of GATA-2 +/+ and GATA-2 +/− BM in sublethally irradiated adult recipients. Varying concentrations (3 × 105–3 × 107) of GATA-2 +/+ or GATA-2 +/− BM cells were transplanted into sublethally irradiated GATA-2 +/+ or GATA-2 +/− recipients to test for HSC competition in repopulation. The y axis shows the percentage of recipient animals engrafted with >10% donor cells in hematopoietic tissues. Engraftment results are shown in gray striped bars for GATA-2 +/+ donor cells transplanted into GATA-2 +/+ recipients, in gray bars for GATA-2 +/− donor cells transplanted into GATA-2 +/+ recipients, and in white bars for GATA-2 +/+ donor cells transplanted into GATA-2 +/−. The results show that GATA-2 +/+ HSCs out-compete GATA-2 +/− HSCs.
GATA-2 Dose Affects the Number of Phenotypically Defined HSCs in the Embryo But Not the Adult.
Our in vivo transplantation results clearly show that GATA-2 +/− HSC activity is affected throughout development. To more specifically investigate the cell types that are affected in the GATA-2 +/− mice, we crossed the GATA-2 mutant allele into Ly-6A GFP transgenic mice, in which HSCs can be detected by the expression of the green fluorescent protein (GFP) reporter under the transcriptional control of Ly-6A regulatory sequences (26). Ly-6A encodes the Sca-1 surface glycoprotein that is expressed on HSCs. Previously, we have shown that all AGM, FL, and adult BM HSCs express the Ly-6A GFP transgene and that GFP expression is highly restricted in the AGM region to a few aortic endothelial cells and hematopoietic clusters (23, 27).
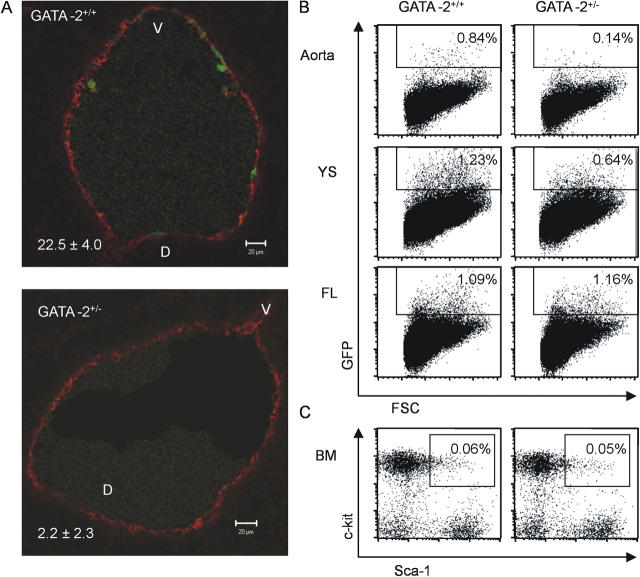
To determine whether GATA-2 dose affects these cells, we examined transverse sections through the E11 dorsal aorta from compound transgenics (Ly-6A GFP–GATA-2 +/+ and Ly-6A GFP–GATA-2 +/−). As shown in representative sections in Fig. 5 A, GFP-positive cells are decreased in number in the GATA-2 +/− aorta compared with the GATA-2 +/+ aorta. Quantitations were performed by counting GFP-positive and CD34-positive cells in 37 aorta sections from each genotype (CD34 immunostaining of endothelial cells provided a normalization control). GFP-positive cells were present but decreased by a factor of 10 or more in the GATA-2 +/− aortas. Hence, GATA-2 haploinsufficiency leads to a significant decrease in HSCs and/or HSC precursors in the AGM.
Figure 5.
Phenotypic analysis of HSCs in GATA-2 +/− embryos and adults. GATA-2–Ly-6A GFP compound transgenic embryos were generated by timed pluggings. (A) Representative transverse sections through the E11 dorsal aorta of a GATA-2 + / + –Ly-6A GFP embryo (45 somite pairs; top) and a GATA-2 + / − –Ly-6A GFP embryo (43 somite pairs; bottom). Sections were taken from the caudal end of the AGM, at the height of the hindgut, and stained with anti-CD34 antibody. In total, four embryos were analyzed (2 embryos and a total of 37 sections from each genotype) and cells counted in the aortic endothelium throughout the levels containing the gonads and mesonephroi. CD34+ endothelial cells served as a control for section quality and normalization. Red fluorescence (CD34) and green fluorescence (GFP). The percentage of GFP+/CD34+ endothelial cells ± SEM is shown on the bottom left and is significantly reduced in the GATA-2 +/− embryos; P < 0.05. Flow cytometric analysis of phenotypically defined HSCs was performed on (B) embryonic hematopoietic tissues and (C) adult BM. Expression of the Ly-6A GFP HSC marker was analyzed on E11 aorta, YS, and FL cells. Adult BM cells were analyzed for the percentage of cells in the Lin− fraction that are Sca-1+c-kit+. Percentages of GFP+ cells in the embryonic tissue and Sca-1+c-kit+ cells enclosed in each gate are shown.
Flow cytometric analyses were performed to verify these results and to examine if the phenotypic HSC content of the other hematopoietic tissues was also changed. As shown in Fig. 5 B, in such compound transgenic embryos a sixfold decrease in GFP-positive cells in the E11 GATA-2 +/− aorta was found compared with GATA-2 +/+ aorta. E11 GATA-2 +/− YS showed a 1.9-fold decrease in GFP-positive cells. However, no decrease was found in E11 GATA-2 +/− FL. Similarly, no difference in the percentages of HSCs as defined by the Lin−Sca-1+c-kit+ (LSK) phenotype was found when GATA-2 +/+ and GATA-2 +/− adult BM was analyzed (Fig. 5 C). Together, these phenotypic data support the transplantation data in showing that HSCs are quantitatively decreased in the AGM but that HSCs increase to normal numbers in the FL and adult BM.
Cytotoxic Drug Treatment Reveals a Proliferation Defect in GATA-2+/− BM HSCs.
To test if the qualitative defect in GATA-2 +/− HSCs is related to proliferation, GATA-2 +/+ and GATA-2 +/− mice were treated with the cytotoxic drug 5-FU (28). At 0, 4, 8, 12, and 16 d after treatment, BM cells were tested in in vitro assays and analyzed by flow cytometry for evidence of hematopoietic regeneration. As shown in Fig. 6 A, both GATA-2 +/+ and GATA-2 +/− mice showed similar reductions in total BM cell number at 4 d post 5-FU. Total BM cell numbers increased to starting numbers by 16 d post–5-FU. No significant differences were observed between the number of total GATA-2 +/+ and GATA-2 +/− BM cells at any time point. In addition, no defect in hematopoietic differentiation was observed in GATA-2 +/− BM cells, as flow cytometric analysis showed the presence of all three lineages, lymphoid, myeloid, and erythroid at similar levels in GATA-2 +/+ and GATA-2 +/− BM (not depicted).
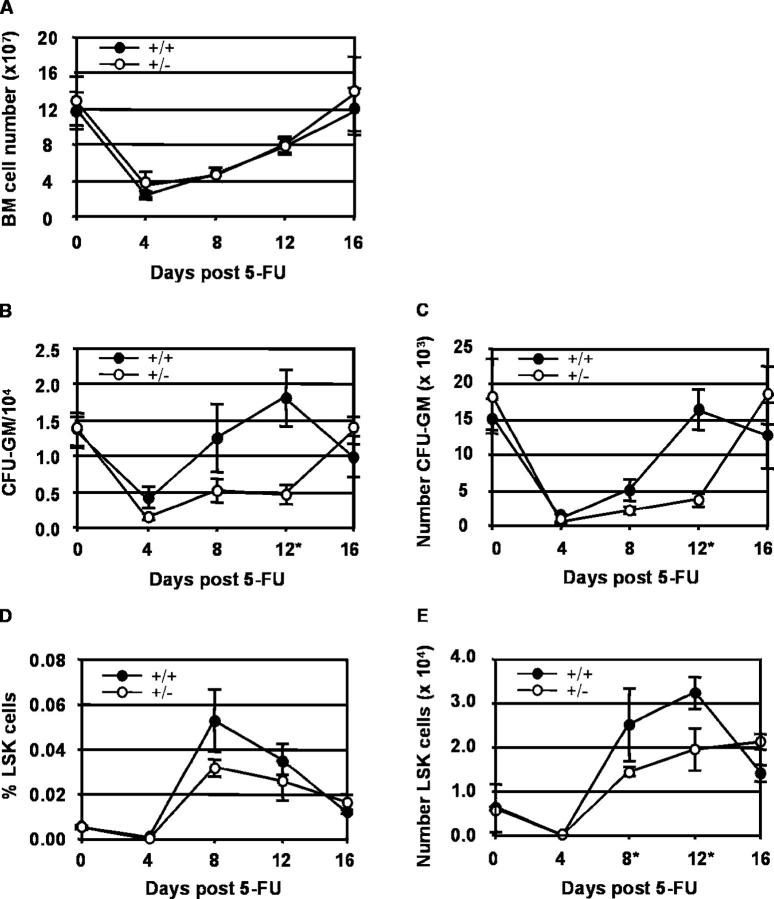
Figure 6.
Hematopoietic regeneration after 5-FU treatment. The temporal regeneration of the hematopoietic system within the BM compartment of GATA-2 +/+ and GATA-2 +/− mice was examined at days 0, 4, 8, 12, and 16 after 5-FU injection for (A) total BM cell numbers, (B) the frequency of CFU-GM in 104 BM cells, and (C) the total number of CFU-GM in the BM (two tibias plus two femurs). The regeneration of BM HSCs was examined by quantification of the Sca-1+ and c-kit+ cells within the lin− population by flow cytometry. (D) Percentage and (E) absolute number of LSK BM cells was examined at days 0, 4, 8, 12, and 16 after 5-FU injection. GATA-2 +/+ samples are represented by •, and GATA-2 +/− samples are represented by ○. Two complete time course experiments were performed. Each point represents an average of two to three animals with SEM. *Significant difference between the GATA-2 +/+ and GATA-2 +/−. (B) CFU-GM frequency day 12, P < 0.008. (C) CFU-GM numbers day 12, P < 0.002. (E) LSK cell number day 8 and 12, P < 0.04.
To investigate whether specific immature hematopoietic progenitors were affected in 5-FU–treated GATA-2 +/− mice, we performed in vitro colony assays for CFU-GM. As shown in Fig. 6, B and C, the starting frequency and number of CFU-GM were the same in GATA-2 +/+ and GATA-2 +/− BM. At 4 d post 5-FU treatment, frequency and number of CFU-GM reached a similar low point in both GATA-2 +/+ and GATA-2 +/− BM. However, at 12 d after 5-FU treatment, CFU-GM frequency and number in GATA-2 +/+ BM reached higher or the same levels as the untreated BM, whereas the GATA-2 +/− BM CFU-GM frequency and number remained low (P < 0.008 and 0.002, respectively). Only at day 16 after 5-FU treatment did GATA-2 +/− CFU-GM frequency and number reach the same levels as in untreated BM. Thus, the 4 d delay in the regeneration of CFU-GM in GATA-2 +/− BM suggests a GATA-2 dose–related proliferation defect acting within these progenitors. Alternatively, a GATA-2 dose–related proliferation defect acts within HSCs and only secondarily influences CFU-GM.
To test this, we analyzed the regeneration of HSCs. We performed flow cytometric analysis for LSK BM cells at 0, 4, 8, 12, and 16 d post–5-FU treatment. In both GATA-2 +/+ and GATA-2 +/− BM, HSC percentages and absolute numbers were similarly reduced at 4 d post–5-FU treatment (Fig. 6, D and E). Both GATA-2 +/+ and GATA-2 +/− BM HSC percentages and numbers began recovering at day 8 post–5-FU, when they surpassed the initial percentages and numbers. However, GATA-2 +/− BM HSC numbers remained significantly lower than in GATA-2 +/+ BM at both day 8 and 12 post–5-FU treatment (p < 0.04). Furthermore, whereas GATA-2 +/+ HSC numbers peaked at day 12 posttreatment and declined thereafter, GATA-2 +/− HSC numbers increased slowly up to day 16 post–5-FU treatment. The finding that HSC expansion in 5-FU–treated GATA-2 +/− mice is delayed by at least 4 d strongly suggests a GATA-2 dose–dependent proliferation defect in HSCs.
Discussion
The data presented here show for the first time that a full dose of GATA-2 is required during embryonic and adult stages for quantitatively and qualitatively normal HSC activity in vivo. Although HSCs are most likely not produced in GATA-2 −/− embryos, the effects of GATA-2 haploinsufficiency had been largely unexplored because such GATA-2 +/− animals grow normally and present an overtly normal adult hematologic profile. Here we have shown that with only half the dose of GATA-2, HSC numbers are severely and specifically reduced in the AGM region, where the first induction and expansion of HSCs is occurring during midgestation. Moreover, AGM HSC quality is compromised. Thereafter, in the other tissues harboring HSCs, quantitative deficiencies in GATA-2 +/− HSCs appear to be compensated through normal (albeit delayed) expansion of HSCs, but qualitative deficiencies are retained through to adulthood. Therefore, given that only a few HSCs out of the whole cohort of HSCs are actively contributing to the hematopoietic system at any one time (29, 30) and that GATA-2 +/− cells are not defective in differentiation, no general hematologic defects would be expected in haploinsufficient adults. Only through stringent in vivo transplantations or cytotoxic stress are HSC functional defects observable. The results of these experiments strongly suggest an essential role for GATA-2 in the induction and expansion of the first HSCs in the AGM and an additional, distinctive role for GATA-2 in the proliferation of HSCs.
HSC Quantitative Processes Are Altered in GATA-2+/− Mice.
In in vivo transplantation experiments we show quantitatively reduced HSC activity in GATA-2 +/− embryos. The four- to ninefold decrease in HSC activity in E11/E12 GATA-2 +/− AGMs compared with GATA-2 +/+ AGMs is the consequence of fewer HSCs, as aorta sections and flow cytometric analysis show a sixfold decrease in phenotypically defined HSCs. Hence, GATA-2 +/− AGMs can neither expand nor maintain HSCs compared with GATA-2 +/+ AGMs. In contrast, HSCs are expanded and maintained in E11/E12 GATA-2 +/− YS (at a slightly decreased number), with the fold decrease in phenotypically defined HSCs in the YS corresponding closely to the fold decrease in HSC activity. Considering the fact that HSCs are first detected in the AGM region and then in the YS and FL, the reduced HSC content of the GATA-2 +/− YS and FL may well be a secondary effect of the reduction in the GATA-2 +/− AGM region.
Our in vivo analyses for hematopoietic progenitor cells in the AGM region and the YS show that CFU-S11 are also GATA-2 dose dependent. These data are consistent with previous in vitro studies on GATA-2 −/− YS and ES cells (13, 31), showing much reduced hematopoietic progenitor activity. The reduced CFU-S11 activity in GATA-2 +/− AGMs and YSs could further be a consequence of the reduced HSC activity we detected in these tissues. However, the source of cells providing the CFU-S11 activity in the embryo is not clear. Whereas in the adult, hematopoietic progenitor cells are derived from HSCs, in the preE10.5 AGM region and the YS they may be derived from hemangioblasts and/or hemogenic endothelium rather than via a HSC ancestor. Hence, GATA-2 may act directly on the in vivo generation, survival, and/or expansion of the hematopoietic progenitor cells, HSCs, and/or their direct precursors in the AGM and YS.
The YS as a Compensatory Generator of HSCs Independent of GATA-2 Dose.
For over three decades, the origins of adult HSCs have been a focus of research. The view that the mammalian YS is able to provide hematopoietic cells that migrate and colonize the FL and then the BM during the neonatal/adult stages has been altered by the finding that the first fully functional adult HSCs are autonomously generated in the AGM region. Shortly thereafter, the YS contains HSCs, but due to the experimental constraints of mammalian embryos, it is difficult to definitively demonstrate whether these HSCs are AGM derived or autonomously generated in the YS. Recent data suggest that indeed YS can autonomously generate and expand HSCs (6) and putative pre-HSCs (32). Since we observe a dramatic reduction of HSCs in GATA-2 +/− AGMs but only a slight reduction of HSCs in GATA-2 +/− YSs, our transplantation data support the notion of YS HSC generation potential (albeit in a GATA-2 +/− embryo). However, since HSCs are still generated in the GATA-2 +/− AGM 1 d earlier than in the YS, it remains possible that YS HSCs are AGM derived. Interestingly, the reduced HSC activity in the GATA-2 +/− YS can be expanded to a magnitude comparable to that of the GATA-2 +/+ YS in explant cultures, suggesting that at least some of the reduced activity in the GATA-2 +/− YS is a secondary effect of the reduction of HSCs in the AGM. Furthermore, GATA-2 +/− HSC numbers are compensated to normal levels in the adult, possibly due to HSC generation and expansion in the YS and the further expansion in the FL and BM. Notwithstanding, these data imply that the underlying molecular mechanisms in which the AGM generates, maintains, and expands HSCs are different from that of the YS. The AGM region is exquisitely sensitive to the level of the GATA-2 dose, whereas the YS is much less sensitive. Hence, the HSC defects in GATA-2 +/− AGMs do not result in severe anemia in adults since GATA-2 +/− YS can generate and/or expand sufficient HSCs irrespective of the haploinsufficiency.
HSC Qualitative Processes Are Altered in GATA-2+/− Mice.
The results of adult BM competitive transplantation experiments clearly demonstrate a qualitative difference between the GATA-2 +/+ and GATA-2 +/− HSCs. The high percentage of GATA-2 +/− mice engrafted with GATA-2 +/+ cells, even at low donor cell numbers, demonstrate that GATA-2 +/+ BM HSCs have a proliferative advantage over the GATA-2 +/− BM HSCs. In the reciprocal transplantation in which GATA-2 +/− BM cells were transplanted into GATA-2 +/+ recipients, high numbers of cells were needed to obtain a high percentage of donor-engrafted recipient mice and thus imply that: (a) the number of HSCs in GATA-2 +/− BM is quantitatively reduced; (b) the GATA-2 +/− HSCs have a lower proliferative advantage over the GATA-2 +/+ HSCs; and/or (c) the homing efficiency is lower for GATA-2 +/−–derived HSCs. The fact that no significant difference in the percentage or absolute number of LSK BM cells was found between GATA-2 +/− and wild-type BM indicates that the decreased HSC activity is not due to a quantitative decrease in GATA-2 +/− BM HSCs. However, the delayed expansion of HSCs in the 5-FU recovery experiments does strongly suggest that the major GATA-2 dose-dependent defect is in HSC proliferation. Although homing of HSCs is not required in this experimental scenario, we cannot exclude an additional GATA-2 dose–dependent defect in homing.
How Does GATA-2 Dose Affect the Quantitative and Qualitative Development of HSCs?
We propose here that the GATA-2 dose effects we observe in the AGM act at the level of the hemogenic cells that differentiate into HSCs. Normally, a full dose of GATA-2 is required for the generation, maintenance, and/or expansion of these precursor cells. In the haploinsufficient AGM region, these hemogenic cells fail to differentiate, survive, and/or divide. However, owing to the stochastic nature of gene expression, some hemogenic cells still achieve a threshold level of GATA-2 protein, and therefore, the target genes (which are needed for the differentiation, survival and/or division of the precursor cells) can be activated at some low frequency in the E10.5 AGM. The outcome of GATA-2 haploinsufficiency is then a small production of HSCs followed by an overall reduction in the absolute number of AGM HSCs that we can detect functionally in our transplantation assay and phenotypically in immunostained sections and flow cytometry analysis.
Recent GATA-2 expression data in the AGM support the notion of a role for GATA-2 in hemogenic precursors. Transgenic embryos with a GFP marker under the control of GATA-2 transcriptional regulatory sequences show high levels of GATA-2 expression in CD45− AGM cells with hemogenic potential and a significant decrease in the percentage of CD45+ cells in GATA-2 +/− E11.5 AGMs (12). Moreover, during midgestation, at the time of the first induction of HSCs, GATA-2 is expressed in the endothelial cells lining the dorsal aorta and some underlying mesenchymal cells. Hence, high GATA-2 expression in hemogenic cells of the AGM suggests that GATA-2 is acting on the cells just immediately preceding the induction of HSCs.
Since GATA-2 is a transcription factor, its target genes within hemogenic AGM cells are of particular interest. Several markers of AGM HSCs and aortic hemogenic cells have been recently described: the Ly-6A (Sca-1) cell surface glycoprotein (23, 33) and Runx-1 transcription factor (27). These molecules are overlapping with GATA-2 in their expression patterns in hemogenic cells of the dorsal aorta. Targeted mutation of these genes results in qualitative and/or quantitative defects in HSCs. Whereas Ly-6A −/− embryos thrive into adulthood with no or little effect on HSC generation in the embryo, functional analyses of HSCs derived from Ly-6A −/− mutant BM show defects in their self-renewal ability (34), similar to our findings in GATA-2 +/− BM. In contrast, runx-1 −/− embryos are completely devoid of HSCs and exhibit FL anemia leading to lethality at E12 (35–37). Moreover, runx-1 haploinsufficiency leads to a premature extinction of AGM HSCs (20). Hence, the Ly-6A and runx-1 genes could be targets of GATA-2 or, alternatively, contribute to the activation of the same pathways for HSC self-renewal and/or HSC generation. At present, although many GATA consensus-binding sites appear in the sequences surrounding these genes, there is no in vivo data showing that any of these sites are functional.
Nonetheless, two bona fide target genes of GATA-2 have been proposed. These are SCL/tal-1, an essential early hematopoietic transcription factor and E4bp4, a transcription factor implicated in cell survival. In vivo studies show that GATA-2 forms a multiprotein complex with Fli-1 and Elf-1 that binds the SCL enhancer and activates the expression in HSCs, endothelial cells, and their bipotent progenitor, the hemangioblast (38). However, in vivo mutation analysis on the HSC-specific GATA sites within the SCL locus affects SCL expression not only in the AGM but also in YS and FL (38). Therefore, it is unlikely that the selective defect in the GATA-2 + / − AGM HSCs can be attributed to defective SCL expression. Chromatin immunoprecipitation studies on BaF3 cell line stimulated with IL-3 show that GATA-2 binds to a sequence downstream of the transcriptional start site of E4bp4 and is necessary for transcriptional activation of this gene (39). Considering that IL-3 is a survival factor for HSCs, it is plausible that GATA-2 is involved in the activation of this pathway.
In conclusion, GATA-2 dosage is important in regulation of HSC production and expansion. Haploinsufficiency of GATA-2 results in quantitative decreases in HSCs in the AGM and qualitative defects in HSCs in both the embryonic-derived and adult BM HSCs. The pivotal importance of GATA-2 in these processes within HSCs now awaits the identification of the relevant target genes and the functional cascades that GATA-2 activates, most likely in concert with other factors in multiprotein complexes.
Acknowledgments
The authors thank all the members of the laboratory for assistance in embryo dissection and transplantation and for helpful discussions, especially Drs. Kirsty Harvey, Catherine Robin, and Marian Peeters. We also thank the Experimental Dieren Centrum, especially Yasmine Allen, for upkeep of transgenic mice and transplant recipients.
This work was supported by the National Institutes of Health (RO1 DK51077), Dutch Cancer Society (2001-2442 and 2002-2699), and Association of International Cancer Research (02-243). S.H. Orkin is an Investigator of the Howard Hughes Medical Institute.
Abbreviations used in this paper: AGM, aorta-gonads-mesonephros; CFU-GM, colony-forming unit–granulocyte macrophage; CFU-S11, colony-forming unit–spleen; E, embryonic day; ER, estrogen receptor; ES, embryonic stem; FL, fetal liver; 5-FU, 5-fluorouracil; GFP, green fluorescent protein; HSC, hematopoietic stem cell; LSK, Lin−Sca-1+c-kit+; YS, yolk sac.
References
- 1.Spangrude, G.J., L. Smith, N. Uchida, K. Ikuta, S. Heimfeld, J. Friedman, and I.L. Weissman. 1991. Mouse hematopoietic stem cells. Blood. 78:1395–1402. [PubMed] [Google Scholar]
- 2.Lemischka, I.R. 1991. Clonal, in vivo behavior of the totipotent hematopoietic stem cell. Semin. Immunol. 3:349–355. [PubMed] [Google Scholar]
- 3.Medvinsky, A., and E. Dzierzak. 1996. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 86:897–906. [DOI] [PubMed] [Google Scholar]
- 4.Muller, A.M., A. Medvinsky, J. Strouboulis, F. Grosveld, and E. Dzierzak. 1994. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1:291–301. [DOI] [PubMed] [Google Scholar]
- 5.de Bruijn, M.F., N.A. Speck, M.C. Peeters, and E. Dzierzak. 2000. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 19:2465–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumaravelu, P., L. Hook, A.M. Morrison, J. Ure, S. Zhao, S. Zuyev, J. Ansell, and A. Medvinsky. 2002. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 129:4891–4899. [DOI] [PubMed] [Google Scholar]
- 7.Ko, L.J., and J.D. Engel. 1993. DNA-binding specificities of the GATA transcription factor family. Mol. Cell. Biol. 13:4011–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merika, M., and S.H. Orkin. 1993. DNA-binding specificity of GATA family transcription factors. Mol. Cell. Biol. 13:3999–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orlic, D., S. Anderson, L.G. Biesecker, B.P. Sorrentino, and D.M. Bodine. 1995. Pluripotent hematopoietic stem cells contain high levels of mRNA for c-kit, GATA-2, p45 NF-E2, and c-myb and low levels or no mRNA for c-fms and the receptors for granulocyte colony-stimulating factor and interleukins 5 and 7. Proc. Natl. Acad. Sci. USA. 92:4601–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minegishi, N., J. Ohta, N. Suwabe, H. Nakauchi, H. Ishihara, N. Hayashi, and M. Yamamoto. 1998. Alternative promoters regulate transcription of the mouse GATA-2 gene. J. Biol. Chem. 273:3625–3634. [DOI] [PubMed] [Google Scholar]
- 11.Minegishi, N., J. Ohta, H. Yamagiwa, N. Suzuki, S. Kawauchi, Y. Zhou, S. Takahashi, N. Hayashi, J.D. Engel, and M. Yamamoto. 1999. The mouse GATA-2 gene is expressed in the para-aortic splanchnopleura and aorta-gonads and mesonephros region. Blood. 93:4196–4207. [PubMed] [Google Scholar]
- 12.Minegishi, N., N. Suzuki, T. Yokomizo, X. Pan, T. Fujimoto, S. Takahashi, T. Hara, A. Miyajima, S.I. Nishikawa, and M. Yamamoto. 2003. Expression and domain-specific function of GATA-2 during differentiation of the hematopoietic precursor cells in midgestation mouse embryos. Blood. 102:896–905. [DOI] [PubMed] [Google Scholar]
- 13.Tsai, F.Y., G. Keller, F.C. Kuo, M. Weiss, J. Chen, M. Rosenblatt, F.W. Alt, and S.H. Orkin. 1994. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 371:221–226. [DOI] [PubMed] [Google Scholar]
- 14.Persons, D.A., J.A. Allay, E.R. Allay, R.A. Ashmun, D. Orlic, S.M. Jane, J.M. Cunningham, and A.W. Nienhuis. 1999. Enforced expression of the GATA-2 transcription factor blocks normal hematopoiesis. Blood. 93:488–499. [PubMed] [Google Scholar]
- 15.Ezoe, S., I. Matsumura, S. Nakata, K. Gale, K. Ishihara, N. Minegishi, T. Machii, T. Kitamura, M. Yamamoto, T. Enver, and Y. Kanakura. 2002. GATA-2/estrogen receptor chimera regulates cytokine-dependent growth of hematopoietic cells through accumulation of p21(WAF1) and p27(Kip1) proteins. Blood. 100:3512–3520. [DOI] [PubMed] [Google Scholar]
- 16.Briegel, K., K.C. Lim, C. Plank, H. Beug, J.D. Engel, and M. Zenke. 1993. Ectopic expression of a conditional GATA-2/estrogen receptor chimera arrests erythroid differentiation in a hormone-dependent manner. Genes Dev. 7:1097–1109. [DOI] [PubMed] [Google Scholar]
- 17.Heyworth, C., K. Gale, M. Dexter, G. May, and T. Enver. 1999. A GATA-2/estrogen receptor chimera functions as a ligand-dependent negative regulator of self-renewal. Genes Dev. 13:1847–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitajima, K., M. Masuhara, T. Era, T. Enver, and T. Nakano. 2002. GATA-2 and GATA-2/ER display opposing activities in the development and differentiation of blood progenitors. EMBO J. 21:3060–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikonomi, P., C.E. Rivera, M. Riordan, G. Washington, A.N. Schechter, and C.T. Noguchi. 2000. Overexpression of GATA-2 inhibits erythroid and promotes megakaryocyte differentiation. Exp. Hematol. 28:1423–1431. [DOI] [PubMed] [Google Scholar]
- 20.Cai, Z., M. de Bruijn, X. Ma, B. Dortland, T. Luteijn, R.J. Downing, and E. Dzierzak. 2000. Haploinsufficiency of AML1 affects the temporal and spatial generation of hematopoietic stem cells in the mouse embryo. Immunity. 13:423–431. [DOI] [PubMed] [Google Scholar]
- 21.Song, W.J., M.G. Sullivan, R.D. Legare, S. Hutchings, X. Tan, D. Kufrin, J. Ratajczak, I.C. Resende, C. Haworth, R. Hock, et al. 1999. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat. Genet. 23:166–175. [DOI] [PubMed] [Google Scholar]
- 22.Fujimaki, S., H. Harigae, T. Sugawara, N. Takasawa, T. Sasaki, and M. Kaku. 2001. Decreased expression of transcription factor GATA-2 in haematopoietic stem cells in patients with aplastic anaemia. Br. J. Haematol. 113:52–57. [DOI] [PubMed] [Google Scholar]
- 23.de Bruijn, M.F., X. Ma, C. Robin, K. Ottersbach, M.J. Sanchez, and E. Dzierzak. 2002. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity. 16:673–683. [DOI] [PubMed] [Google Scholar]
- 24.Dzierzak, E., and M. de Bruijn. 2002. Isolation and analysis of hematopoetic stem cells from mouse embryos. Methods in Molecular Medicine: Hematopoietic Stem Cell Protocols. C. Klug and C. Jordan, editors. The Humana Press Inc., Totowa, NJ. 1–14. [DOI] [PubMed]
- 25.Kaufman, M.H. 1992. The Atlas of Mouse Development. Academic Press, Limited, London.
- 26.Ma, X., K.W. Ling, and E. Dzierzak. 2001. Cloning of the Ly-6A (Sca-1) gene locus and identification of a 3′ distal fragment responsible for high-level gamma-interferon-induced expression in vitro. Br. J. Haematol. 114:724–730. [DOI] [PubMed] [Google Scholar]
- 27.North, T.E., M.F. de Bruijn, T. Stacy, L. Talebian, E. Lind, C. Robin, M. Binder, E. Dzierzak, and N.A. Speck. 2002. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 16:661–672. [DOI] [PubMed] [Google Scholar]
- 28.Van Zant, G. 1984. Studies of hematopoietic stem cells spared by 5-fluorouracil. J. Exp. Med. 159:679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordan, C.T., and I.R. Lemischka. 1990. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 4:220–232. [DOI] [PubMed] [Google Scholar]
- 30.Keller, G., and R. Snodgrass. 1990. Life span of multipotential hematopoietic stem cells in vivo. J. Exp. Med. 171:1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai, F.Y., and S.H. Orkin. 1997. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood. 89:3636–3643. [PubMed] [Google Scholar]
- 32.Yoder, M.C., K. Hiatt, P. Dutt, P. Mukherjee, D.M. Bodine, and D. Orlic. 1997. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 7:335–344. [DOI] [PubMed] [Google Scholar]
- 33.Hanson, P., V. Mathews, S.H. Marrus, and T.A. Graubert. 2003. Enhanced green fluorescent protein targeted to the Sca-1 (Ly-6A) locus in transgenic mice results in efficient marking of hematopoietic stem cells in vivo. Exp. Hematol. 31:159–167. [DOI] [PubMed] [Google Scholar]
- 34.Ito, C.Y., C.Y. Li, A. Bernstein, J.E. Dick, and W.L. Stanford. 2003. Hematopoietic stem cell and progenitor defects in Sca-1/Ly-6A-null mice. Blood. 101:517–523. [DOI] [PubMed] [Google Scholar]
- 35.Okuda, T., J. van Deursen, S.W. Hiebert, G. Grosveld, and J.R. Downing. 1996. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 84:321–330. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki, K., H. Yagi, R.T. Bronson, K. Tominaga, T. Matsunashi, K. Deguchi, Y. Tani, T. Kishimoto, and T. Komori. 1996. Absence of fetal liver hematopoiesis in mice deficient in transcriptional coactivator core binding factor beta. Proc. Natl. Acad. Sci. USA. 93:12359–12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, Q., T. Stacy, M. Binder, M. Marin-Padilla, A.H. Sharpe, and N.A. Speck. 1996. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc. Natl. Acad. Sci. USA. 93:3444–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gottgens, B., A. Nastos, S. Kinston, S. Piltz, E.C. Delabesse, M. Stanley, M.J. Sanchez, A. Ciau-Uitz, R. Patient, and A.R. Green. 2002. Establishing the transcriptional programme for blood: the SCL stem cell enhancer is regulated by a multiprotein complex containing Ets and GATA factors. EMBO J. 21:3039–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu, Y.L., Y.J. Chiang, and J.J. Yen. 2002. GATA factors are essential for transcription of the survival gene E4bp4 and the viability response of interleukin-3 in Ba/F3 hematopoietic cells. J. Biol. Chem. 277:27144–27153. [DOI] [PubMed] [Google Scholar]