Abstract
A tetrameric recombinant major histocompatibility complex (MHC) class I–peptide complex was used as a staining reagent in flow cytometric analyses to quantitate and define the phenotype of Gag-specific cytotoxic T lymphocytes (CTLs) in the peripheral blood of simian immunodeficiency virus macaque (SIVmac)-infected rhesus monkeys. The heavy chain of the rhesus monkey MHC class I molecule Mamu-A*01 and β2-microglobulin were refolded in the presence of an SIVmac Gag synthetic peptide (p11C, C–M) representing the optimal nine–amino acid peptide of Mamu-A*01–restricted predominant CTL epitope to create a tetrameric Mamu-A*01/p11C, C–M complex. Tetrameric Mamu-A*01/p11C, C–M complex bound to T cells of SIVmac-infected, Mamu-A*01+, but not uninfected, Mamu-A*01+, or infected, Mamu-A*01− rhesus monkeys. Specific staining of peripheral blood mononuclear cells (PBMC) from SIVmac-infected, Mamu-A*01+ rhesus monkeys was only found in the cluster of differentiation (CD)8α/β+ T lymphocyte subset and the percentage of CD8α/β+ T cells in the peripheral blood of four SIVmac-infected, Mamu-A*01+ rhesus monkeys staining with this complex ranged from 0.7 to 10.3%. Importantly, functional SIVmac Gag p11C-specific CTL activity was seen in sorted and expanded tetrameric Mamu-A*01/p11C, C–M complex–binding, but not nonbinding, CD8α/β+ T cells. Furthermore, the percentage of CD8α/β+ T cells binding this tetrameric Mamu-A*01/p11C, C–M complex correlated well with p11C-specific cytotoxic activity as measured in both bulk and limiting dilution effector frequency assays. Finally, phenotypic characterization of the cells binding this tetrameric complex indicated that this lymphocyte population is heterogeneous. These studies indicate the power of this approach for examining virus-specific CTLs in in vivo settings.
Cytotoxic T lymphocytes (CTLs) play an important role in containing virus spread in many viral infections. However, the activity of this cell population in vivo has proven difficult to study because its evaluation has relied on cumbersome, functional assays that require extensive cell manipulation and lengthy in vitro periods of cell cultivation. Altman et al. have recently reported that fluorescence dye-coupled tetrameric MHC class I–peptide complexes can specifically bind to subpopulations of epitope-specific cluster of differentiation (CD)18+ T cells, raising the possibility that CTLs might be studied using flow cytometric technology (1).
There is accumulating evidence for the importance of CTLs in controlling HIV-1 and simian immunodeficiency virus replication in both primary and chronic infections (2– 6). We have been studying the role of this cellular immune response in AIDS immunopathogenesis in the simian immunodeficiency virus (SIV)/macaque model of AIDS. Much of this work has focused on the evaluation of SIVmac Gag recognition by CTL in rhesus monkeys expressing the HLA-A homologue molecule Mamu-A*01. In fact, we have shown that CTL recognition of Gag in SIVmac-infected or vaccinated Mamu-A*01+ rhesus monkeys is restricted to a single epitope, 12–amino acid fragment of SIVmac 251 Gag (amino acid 179–190) (p11C), bound to Mamu-A*01 (7). Through studying the monkeys' response to this dominant CTL epitope, we have been able to evaluate efficiently a variety of novel vaccine strategies for eliciting SIVmac-specific CTL responses and assess the role of CTLs in containing the replication of SIVmac during primary and chronic infections (8–11).
In these studies, we have generated tetrameric Mamu-A*01/p11C, C–M complex using the optimal nine–amino acid fragment of SIVmac (amino acids 181–189) p11C, C-M (12) and evaluated its binding specificity in PBMCs of SIVmac-infected, Mamu-A*01+ rhesus monkeys. We demonstrate that the enumeration of CD8+ T cells that bind this complex in flow cytometric analyses correlates quantitatively with functional CTL activity and that this cell population is phenotypically heterogeneous.
Materials and Methods
Tetrameric Mamu-A*01/p11C, C–M Complex Formation.
DNA coding for the soluble domain of Mamu-A*01 with a GlySer linker at the 3′ end was amplified by PCR with the 5′ primer GTCACTGAATTCAGGAGGAATTTAAAATGGGCTCTCACTC-CATGAAG and the 3′ primer CGCACTGGATCCCGGCTCCCATTTCAGGGTGTGGGGC, using a Mamu-A*01 plasmid as the template (7). The PCR product was digested with EcoRI and BamHI, and subcloned into the expression plasmid HLA-A2/GlySer/BSP (BSP, BirA substrate peptide; reference 1), which contains the BSP (13) at the 3′ end. The expressed protein was refolded in vitro with human β2-microglobulin (β2m) in the presence of a specific peptide as described (14). The optimal nine–amino acid fragment of SIVmac 251 Gag (amino acids 181– 189; p11C, C–M) CTPYDINQM (12) was used to induce refolding of the MHC class I molecule. The Mamu-A*01/p11C, C–M monomers were purified by gel filtration on a TSK SWxl 3,000 column (TosoHaas, Montgomeryville, PA; Fig. 1 A), and further purified on a MonoQ column (Pharmacia, Piscataway, NJ) in 20 mM Tris (pH 8.0) with a gradient of NaCl from 0 to 0.5 M. The monomer was then stored in PBS with protease inhibitors: 2 μM PMSF (Sigma Chemical Co., St. Louis, MO), 1 mM dithiothreitol (Sigma Chemical Co.), 1 μg/ml aprotinin (Sigma Chemical Co.), 0.7 mg/ml pepstatin A (Sigma Chemical Co.), 1 μg/ml leupeptine (Sigma Chemical Co.), and 1 μM EDTA (Sigma Chemical Co.). The 43-kD peak from the gel filtration (Fig. 1 A) was immunoprecipitated with the anti–human MHC class I heavy chain–specific mAb BB7.7 (American Type Culture Collection, Rockville, MD) and run on a 15% reducing SDS/PAGE (Fig. 1 B). Purified Mamu-A*01/p11C, C–M monomers were biotinylated enzymatically with BirA enzyme (Avidity, Denver, CO), following the manufacturer's instructions. The efficiency of biotinylation was between 75 and 95%. A TSK SWxl 3,000 column was used to remove the free biotin from the monomers (Fig. 1 C). To generate the Mamu-A*01/p11C, C–M tetramers, the biotinylated monomers were mixed with FITC-labeled ExtrAvidin (Sigma Chemical Co.) at a molar ratio of 4:1 and were purified by gel filtration on a TSK SWxl 3,000 column (Fig. 1 D).
Figure 1.
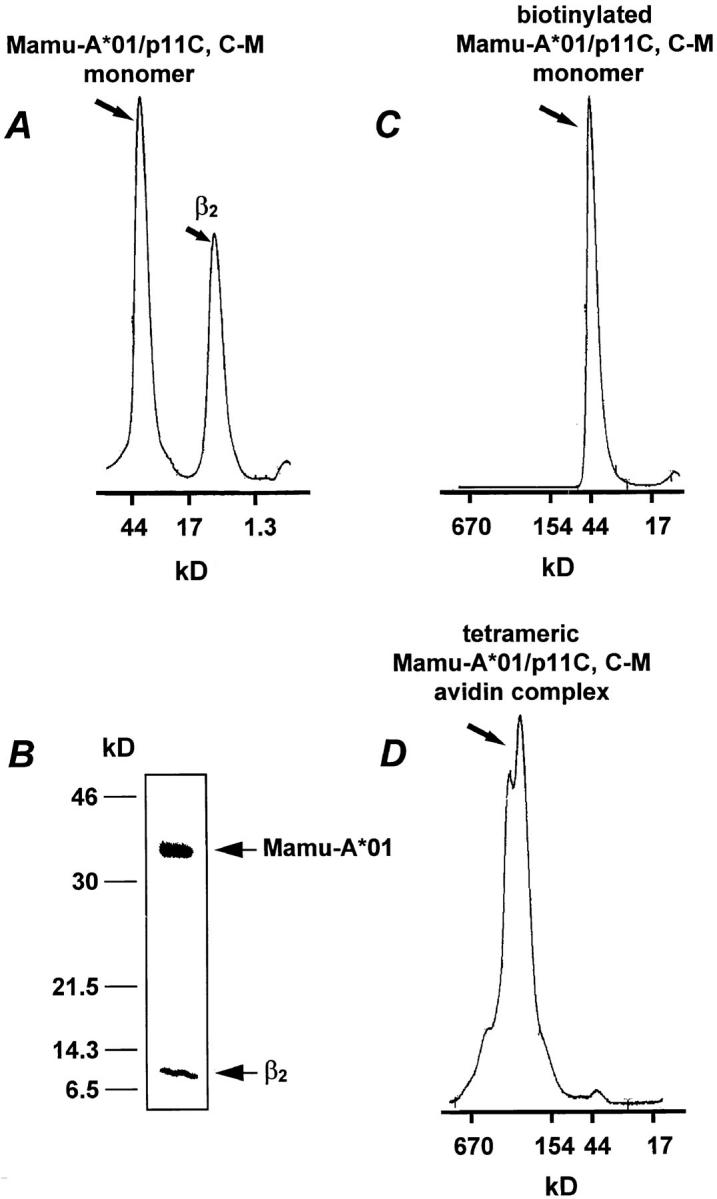
Tetrameric Mamu-A*01/p11C, C–M complex formation. (A) Gel filtration profile of Mamu-A*01 monomer refolded with β2m and p11C, C–M. (B) SDS/PAGE of the 43-kD peak shown in A immunoprecipitated with anti–human heavy chain mAb BB7.7. The positions of β2m and Mamu-A*01 are shown with arrows. (C) Gel filtration profile of biotinylated Mamu-A*01/p11C, C–M monomer. (D) Gel filtration profile of tetrameric Mamu-A*01/p11C, C–M avidin complex.
Animals.
Heparinized blood samples were obtained from rhesus monkeys (Macaca mulatta) experimentally infected with uncloned SIVmac strain 251 and healthy uninfected rhesus monkeys. These animals were maintained in accordance with the guidelines of the Committee on Animals for the Harvard Medical School (Cambridge, MA) and the "Guide for the Care and Use of Laboratory Animals" (Department of Health and Human Services Publication, National Institutes of Health, No. 82–23, revised 1985).
Selection of Mamu-A*01+ Rhesus Monkeys.
The selection of the Mamu-A*01 + rhesus monkeys was carried out by using monkey B lymphoblastoid cell lines (B-LCLs) for one-dimensional isoelectric focusing (1-D IEF; reference 15) and functional CTL assays. In brief, the monkey B-LCLs were generated by transforming PBMCs with Herpesvirus papio (16). Cells were 35S-trans-labeled for 6 h at 37°C. Pelleted cells were lysed on ice in lysis buffer, and lysates were precleared by incubating with protein A–Sepharose CL-4B beads (Sigma Chemical Co.) alone and with beads saturated with irrelevant antibodies. Immunoprecipitation was performed by incubating the precleared lysates with protein A–Sepharose CL-4B beads saturated with the mAb BB7.7. The beads were washed and then treated with neuraminidase type VIII (Sigma Chemical Co.). BB7.7 immunoprecipitates were analyzed by 1-D IEF as described previously (15). The MHC class I haplotypes of the B-LCLs selected as Mamu-A*01 + by 1-D IEF were confirmed by conventional CTL assays. The selected B-LCL, following pulsing with the peptide p11C, were assessed for susceptibility to lysis by in vitro cultured effector cells from SIVmac-infected, Mamu-A*01+ rhesus monkeys. Those animals expressing a shared band detected by 1-D IEF and whose B-LCLs were specifically lysed by effector cells from SIVmac-infected, Mamu-A*01+ monkeys were noted to be Mamu-A*01+.
Cytotoxicity Assay.
Autologous B-LCLs were used as target cells in functional CTL assays. B-LCLs were incubated with 50 μg/ml p11C (EGCTPYDINQML) or the negative control peptide p11B (ALSEGCTPYDIN) for 90 min during 51Cr labeling. For effector cells, PBMCs from monkeys chronically infected with SIVmac were cultured for 3 d at 106 cells/ml with Con A (5 μg/ml; Sigma Chemical Co.), washed, and then maintained for another 7 to 11 d in medium supplemented with recombinant human IL-2 (20 U/ml; provided by Hoffman–La Roche, Nutley, NJ). Alternatively, PBMCs were mixed with p11C-pulsed irradiated autologous PBMCs at a ratio of 1:1 and cultured for 3 d at a density of 106 cells/ml. Cells were then maintained for another 7 to 11 d in medium supplemented with recombinant human IL-2 (20 U/ml) as described above. PBMCs cultured according to one of these two protocols were then centrifuged over Ficoll-Hypaque (Ficopaque; Pharmacia) and assessed as effector cells in a standard 51Cr–release assay using U-bottomed microtiter plates containing 104 target cells with effector cells at different E/T ratios. All wells were established and assayed in duplicate. Plates were incubated in a humidified incubator at 37°C for 4 h. Specific release was calculated as [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. Spontaneous release was <20% of maximal release with detergent (1% Triton X-100; Sigma Chemical Co.) in all assays.
Limiting Dilution Assays.
Freshly isolated PBMCs from monkeys chronically infected with SIVmac were cultured at 63–8,000 cells/well in 24 replicate wells of 96-well microtiter plates. 10,000 gamma-irradiated autologous PBMCs, which had been previously pulsed with p11C for 1 h and washed two times, were added to each well. These cultures were then maintained in 100 μl of medium supplemented with the T cell growth factor Lymphocult T (Biotest AG, Dreieich, Germany) at 10 U/ml at 37°C for 14 d. Microcultures were fed at 5 and 10 d by the addition of 50 μl medium supplemented with 10 U/ml Lymphocult T. Lymphocytes from each well were tested for cytotoxicity against autologous B-LCLs labeled with peptide p11C or the control peptide p11B. Supernatants were harvested and counted for radioactivity after a 4-h incubation at 37°C. The fraction of nonresponding wells was the percentage of wells in which 51Cr-release did not exceed the mean plus three standard deviations of the spontaneous release of the 24 control wells. The specific precursor frequency of CTLs (pCTL) was estimated by the maximum likelihood method by substraction of background values of control peptide–labeled B-LCLs (17).
Staining and Phenotypic Analysis of p11C-specific CD8+ T Cells.
The mAbs used for this study were directly coupled to PE, PE–Texas red (ECD), or allophycocyanin (APC). The following mAbs were used: anti-CD8α(OKT8)-PE (Dako Corp., Carpinteria, CA), anti-CD11a(25.3.1)-PE, anti-CD14(MY4)-PE, anti-CD28(4B10)-PE, anti-CD45RA(2H4)-PE, anti-HLA-DR(I3)- PE, and anti-CD8α/β(2ST8-5H7)-ECD (Coulter Corp., Miami, FL). The mAb FN18 (gift from D.M. Neville, Jr., National Institutes of Health, Bethesda, MD), which recognizes rhesus monkey CD3, was directly coupled to APC. The three reagents: FITC-coupled tetrameric Mamu-A*01/p11C, C–M complex, anti-CD8α/ β-ECD, and anti–rhesus monkey CD3-APC were used either with anti-CD8α-PE, anti-CD11a-PE, anti-CD28-PE, anti-CD45RA-PE, or anti-HLA-DR-PE to perform four-color flow cytometric analyses. 1 μg of FITC-coupled tetrameric Mamu-A*01/p11C, C–M complex was used in conjunction with the directly labeled mAbs to stain either 100 μl of fresh whole blood or 2 × 105 lymphocytes isolated by density gradient centrifugation over Ficoll-Hypaque after in vitro culture. Whole blood samples were lysed using a COULTER® Immunoprep Reagent System and a Q-Prep Workstation (Coulter Corp.). To reduce the background level of staining, the Q-Prep procedure was modified, and lysed samples were washed with 1.0 ml PBS and centrifuged for 3 min at 300 g. Similarly, the stained lymphocyte samples were washed with 1.0 ml PBS and centrifuged for 3 min at 300 g. The supernatants were decanted, cells were resuspended in 0.5 ml PBS containing 1% paraformaldehyde, and then were maintained for 24 h at 4°C before flow cytometric analysis. Samples were analyzed on a COULTER® EPICS® Elite ESP (Coulter Corp.) equipped with argon and helium neon lasers, a gated amplifier, and a 140-μm flow cell tip. The instrument was run at high bandwidth and alignment was controlled on a daily basis using DNA-CHECK EPICS® Alignment Fluorospheres (Coulter Corp.) to maintain the same sensitivity levels during the entire study. Linear performance in each channel was controlled using the EPICS® Immuno-Brite Standards Kit (Coulter Corp.). Voltage and compensation levels were established using control samples of unstained cells for adjusting the negative/background levels of fluorescence to the first log step and single-color stained cells for adjusting spectral overlap. For analysis of whole blood specimens, a single set of control samples was prepared from whole blood of one animal on a daily basis. For analysis of in vitro cultured cells, control samples were prepared from Ficoll-Hypaque isolated lymphocytes of each culture. A total of 10,000 to 20,000 lymphocytes were analyzed in a manually set acquisition gate and positive cutoffs for fluorescence were set to the first log step to include <0.1% of nonstaining cells. Data analysis was performed using the EPICS® Elite software (version 4.02; Coulter Corp.). Data presentation was performed using WinMDI software version 2.5 (Joseph Trotter, La Jolla, CA) and Microsoft PowerPoint software version 4.0c (Microsoft Corp., Redmond, WA).
Sorting and Culture of CD8α/β+ T Cell Subsets from PBMCs of SIVmac-infected Rhesus Monkeys.
Sorting of potentially biohazardous specimens was performed on a COULTER® EPICS® Elite ESP located in a dedicated biosafety level 3 area. The enrichment level of the sorter settings were set electronically to achieve enrichments of selected subsets of >99%. PBMCs were enriched by Ficoll-Hypaque gradient centrifugation and stained with FITC-coupled tetrameric Mamu-A*01/p11C, C–M complex, anti-CD14-PE, anti-CD8α/β-ECD, and anti–rhesus monkey CD3-APC for 20 min on ice. The cells were then washed twice with PBS supplemented with 10% FCS and maintained on ice until sorting. FITC-coupled tetrameric Mamu-A*01/p11C, C–M complex positive and negative CD8α/β+ T cells, and CD14+ monocytes were electronically gated and collected in sample tubes cooled to 4°C. The sorted cells were centrifuged at 300 g for 10 min and the pellets were resuspended in RPMI 1640 containing 10% FCS, 5 μg/ml Con A, and 20 U/ml IL-2. Equal numbers of CD14+ monocytes were cultured for 10 d either with FITC-coupled tetrameric Mamu-A*01/p11C, C–M complex positive or negative CD8α/β+ T cells. The p11C-specific staining of the CD8α/β+ T cells was investigated by flow cytometric analysis and the cytotoxic activity was evaluated by performing a standard 51Cr–release assay as described above.
Results
Tetrameric Mamu-A*01/p11C, C–M Complex Binds to the CD8α/β+ Subset of T Cells from SIVmac-infected, Mamu-A*01+ Rhesus Monkeys.
We initially sought to characterize the binding specificity of the tetrameric Mamu-A*01/ p11C, C–M complex. Since the TCRs of CD8+ CTLs recognize self-MHC class I–peptide complexes, we expected the tetrameric Mamu-A*01/p11C, C–M to bind specifically to a subpopulation of CD8+ T cells from SIVmac-infected, Mamu-A*01+ rhesus monkeys, but not to CD8+ T cells from uninfected, Mamu-A*01+ or SIVmac-infected, Mamu-A*01− monkeys. PBMCs from three groups of rhesus monkeys (three monkeys per group) were assessed: SIVmac− Mamu-A*01+, SIVmac+ Mamu-A*01−, and SIVmac+ Mamu-A*01+. Whole blood specimens from these monkeys were analyzed by flow cytometry with four-color staining using FITC-coupled tetrameric Mamu-A*01/p11C, C–M complex to quantitate the percentage of p11C-specific CD8+ T cells. The mAbs used in this study included anti-CD3, anti-CD8α, and anti-CD8α/β. Because of Fc receptor expression by B cells and monocytes, we expected some nonspecific binding of the tetramer complexes to these cell populations. However, we expected binding of tetramer complexes to T cells only in infected animals expressing the appropriate MHC class I molecule. The CD8 molecule is expressed on T cells either as a CD8α/α homodimer or a CD8α/β heterodimer (18–22). Natural killer cells express the CD8 molecule only as an α/α homodimer. Since T cells expressing the CD8α/α homodimer do not necessarily interact in an MHC class I–restricted fashion (23), we expected a higher binding of the tetramer complex to CD8α/β+ T cells than to CD8α/α+ T cells.
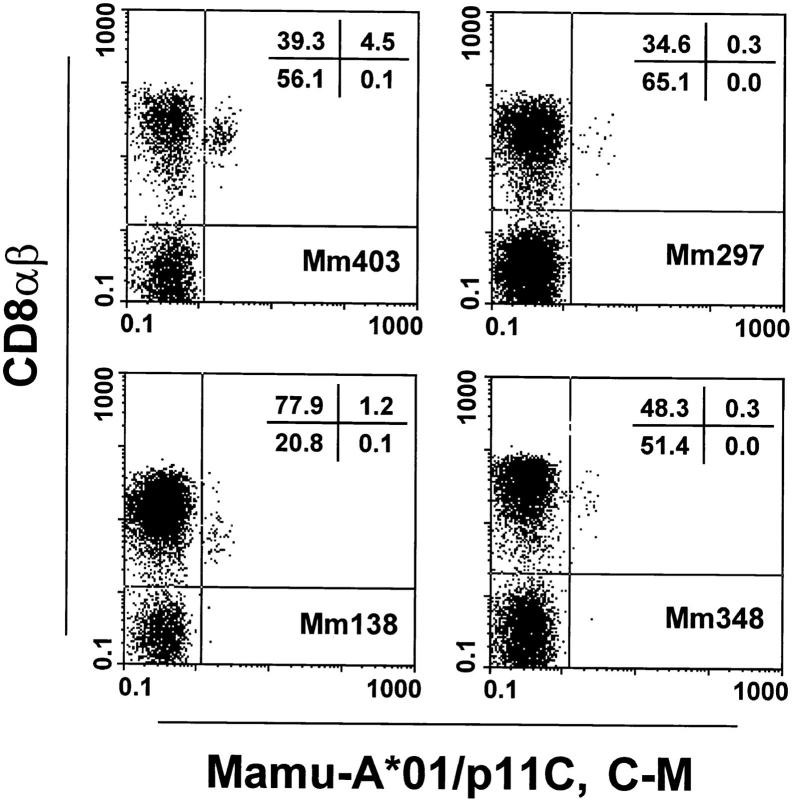
We noticed some nonspecific binding of the Mamu-A*01/p11C, C–M complex to a subset of B cells and monocytes in all the animals studied (data not shown). Although the T cells expressing CD8α/α but not CD8α/β in the peripheral blood of the monkeys represented in occasional animals up to 40% of the total CD8+ T cells (data not shown), almost all (>95%) Mamu-A*01/p11C, C–M complex–binding T cells expressed the CD8α/β heterodimer (Fig. 2). Therefore, in evaluating Mamu-A*01/ p11C, C–M complex–binding cells by flow cytometry, CD8α/β+ T cells were gated and expression of the CD3 molecule was determined as an internal control.
Figure 2.
Tetrameric Mamu-A*01/p11C, C–M complex binds only to the CD8α/β+ subset of T cells from PBMCs of four SIVmac-infected, Mamu-A*01+ rhesus monkeys. CD3+ T cells were analyzed for binding of CD8α/β and tetrameric Mamu-A*01/p11C, C–M complex.
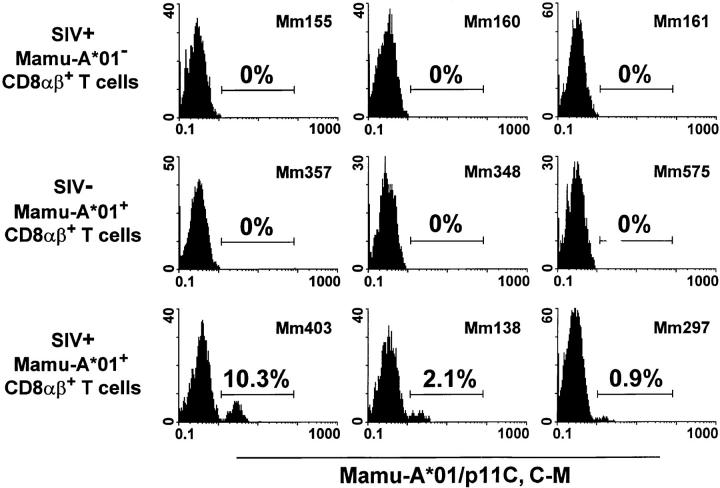
Tetrameric Mamu-A*01/p11C, C–M complex–binding CD8α/β+ T cells were detected only in PBMCs of SIVmac-infected, Mamu-A*01+ rhesus monkeys. The percentage of the positive cells ranged from 0.9 to 10.3% of CD8α/β+ T cells (Fig. 3). Relatively small changes in the percentage of tetrameric Mamu-A*01/p11C, C–M complex–binding CD8α/β+ T cells were observed performing repeated analyses on blood of individual monkeys over a period of 8 mo (data not shown). No detectable staining was seen of the CD8α/β+ T cells in PBMCs of the other two groups of animals, indicating that the Mamu-A*01/ p11C, C–M complexes bound specifically to CD8α/β+ T cells of SIVmac-infected, Mamu-A*01+ rhesus monkeys.
Figure 3.
Tetrameric Mamu-A*01/p11C, C–M complex binds specifically to CD8α/β+ T cells from PBMCs of SIVmac-infected, Mamu-A*01+ rhesus monkeys. PBMCs of three groups of monkeys, three monkeys per group, were assessed: SIVmac− Mamu-A*01+ monkeys, SIVmac+ Mamu-A*01− monkeys, and SIVmac+ Mamu-A*01+ monkeys. Flow cytometric analysis was performed on gated CD8α/β+CD3+ T cells stained with FITC-coupled tetrameric Mamu-A*01/p11C, C–M complex.
Tetrameric Mamu-A*01/p11C, C–M Complex–binding CD8α/β+ T Cells are p11C-specific CTLs.
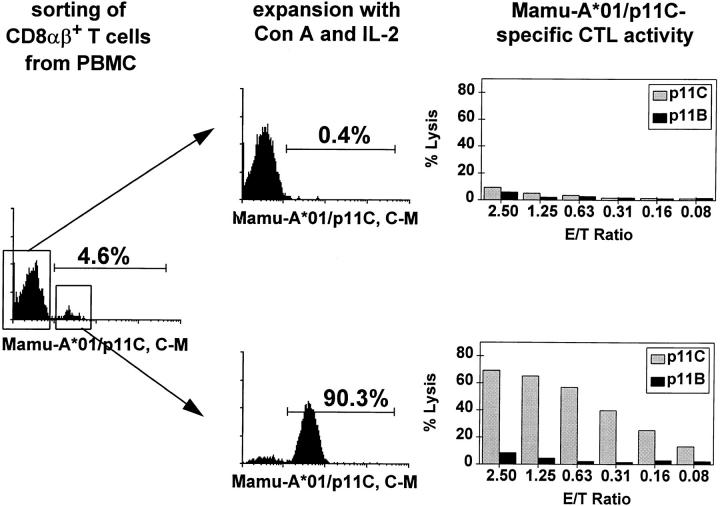
To characterize the function and specificity of the Mamu-A*01/p11C, C–M complex–binding cells, CD8α/β+ T cells of a SIVmac-infected, Mamu-A*01+ rhesus monkey were sorted by flow cytometry into cell populations that stained positively or negatively with the Mamu-A*01/p11C, C–M tetramer complex. Both cell populations were then expanded after Con A stimulation in IL-2–containing medium for 10 d, analyzed again by flow cytometry for Mamu-A*01/p11C, C–M tetramer complex binding, and assayed for p11C-specific CTL activity. Greater than 90% of the sorted tetrameric Mamu-A*01/p11C, C–M complex positive cells still bound this complex after in vitro expansion. These cells showed a high p11C-specific CTL activity, even at very low effector to target ratios (>20% specific lysis at a 0.16:1 E/T ratio; Fig. 4). On the other hand, the CD8α/ β+ T cells that initially did not bind remained tetrameric Mamu-A*01/p11C, C–M complex negative and had no p11C-specific CTL activity (Fig. 4). Thus, all expanded CD8α/β+ T cells with the potential to mediate p11C-specific lysis bound the tetrameric Mamu-A*01/p11C, C–M complex.
Figure 4.
Tetrameric Mamu-A*01/p11C, C–M complex binds p11C-specific CD8α/β+ CTLs. Freshly isolated PBMCs from SIVmac-infected, Mamu-A*01+ rhesus monkey 403 were stained with FITC-coupled tetrameric Mamu-A*01/p11C, C–M complex. CD8α/β+, Mamu-A*01/ p11C, C–M complex positive and CD8α/β+, Mamu-A*01/p11C, C–M complex negative cells were sorted by flow cytometry and expanded after Con A stimulation for 10 d in IL-2–containing medium. The cells were again stained and analyzed by flow cytometry, and the p11C-specific CTL activity of each cell population was assessed.
Tetrameric Mamu-A*01/p11C, C–M Complex Staining of T Cells Correlates with p11C-specific CTL Activity.
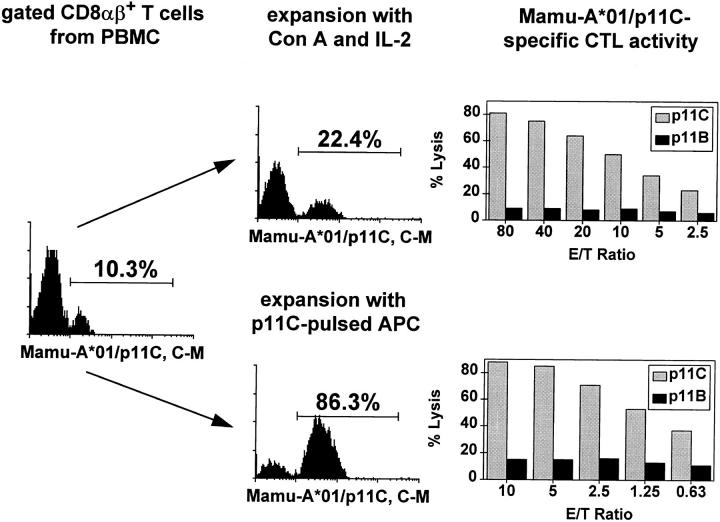
Standard methods for analyzing and quantifying antigen-specific CTL activity involve functional assays performed on lymphocytes expanded in vitro after nonspecific or antigen-specific stimulation. We sought to determine whether the enumeration of tetrameric Mamu-A*01/p11C, C–M complex–binding cells in PBMCs correlated quantitatively with functional CTL activity measured using standard functional assays. To this end we first compared tetrameric Mamu-A*01/p11C, C–M complex staining of CD8α/β+ T cells of an SIVmac-infected, Mamu-A*01+ monkey before and after in vitro expansion of PBMCs. A representative experiment is shown in Fig. 5. CD8α/β+ T cells that bound this complex increased from 10.3 to 22.4% after 12 d of culture after nonspecific stimulation and showed p11C-specific CTL activity of 73% lysis at an E/T ratio of 80:1 (Fig. 5, top right). In vitro expansion after antigen-specific stimulation with p11C-pulsed autologous PBMCs resulted in an increase of cells binding this complex from 10.3 to 86.3%. p11C-specific CTL activity of 74% lysis was detected using these lymphocytes as effector cells at an E/T ratio of 10:1 (Fig. 5, bottom right). Similar experiments were performed using PBMCs from three other SIVmac-infected, Mamu-A*01+, rhesus monkeys (Table 1).
Figure 5.
Tetrameric Mamu-A*01/p11C, C–M complex staining of a lymphocyte population correlates with its p11C-specific CTL activity. Whole blood specimen from a Mamu-A*01+, SIVmac-infected rhesus monkey 403 were stained with FITC-coupled tetrameric Mamu-A*01/p11C, C–M complex. Cells were expanded for 12 d in IL-2–containing medium, either after stimulation with Con A or with p11C-pulsed irradiated autologous PBMCs. The cells were again stained and analyzed by flow cytometry, and the p11C-specific CTL activity of each cell population was assessed.
Table 1.
Tetrameric Mamu-A*01/p11C, C–M Complex Binding to T Cells Correlates with p11C-specific CTL Activity*
| Monkey | Percent CD8α/β+ T cells stained with Mamu-A*01/p11C, C–M‡ | p11C-specific lysis§ (Percentage) | pCTL¶ (per 106 cells) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole blood | Con A/IL-2 expanded | p11C-pulsed APC expanded | Con A/IL-2 expanded (80.1)‖ | p11C-pulsed APC expanded (10.1)‖ | ||||||||
| 403 | 10.3 | 22.4 | 86.3 | 73 | 74 | 279 | ||||||
| 138 | 2.1 | 2.1 | 25.8 | 31 | 69 | 46 | ||||||
| 297 | 0.9 | 1.5 | 37.2 | 28 | 61 | <3 | ||||||
| 348 | 0.7 | 0.5 | 5.1 | 22 | 15 | <3 | ||||||
p11C, C–M represents the optimal 9–amino acid fragment of the SIV Gag 12–amino acid peptide p11C (12).
Staining with FITC-coupled tetrameric Mamu-A*01/p11C, C–M complex on gated CD8α/β T cells.
Percent p11C-specific lysis was calculated as percent specific release by p11C-pulsed MHC class I–matched target cells minus the average of the percent specific release by control peptide p11B-pulsed target cells.
E/T ratio.
CTL precursor frequency for p11C per 106 PBMCs assessed by LDA.
A limiting dilution assay (LDA) is currently accepted as the most precise method available for quantifying the precursor frequency of CTLs in PBMCs. In parallel with the studies described above, we performed LDAs on PBMCs of these four infected monkeys (Table 1). In fact, the rank ordering of the tetrameric Mamu-A*01/p11C, C–M complex staining of CD8α/β+ T cells of these monkeys was in accordance with the quantification of p11C-specific CTLs seen in both bulk and LDA functional assays. These findings suggest that the cell staining with the tetrameric complex should provide useful quantitative data in CTL analyses.
Tetrameric Mamu-A*01/p11C, C–M Complex–binding CD8α/β+ T Cells in PBMCs of SIVmac-infected, Mamu-A*01+ Rhesus Monkeys Are Phenotypically Heterogeneous.
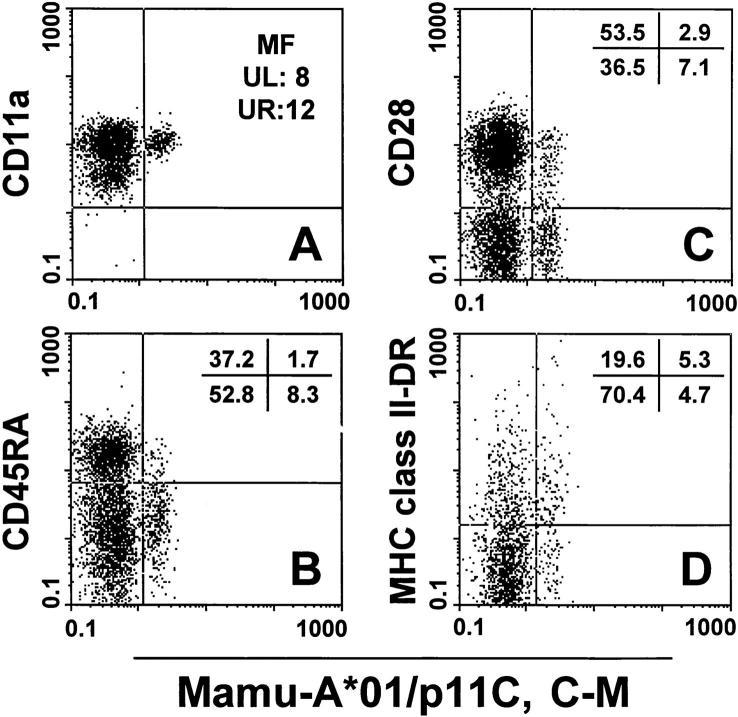
The phenotype of CD8α/β+ tetramer-binding cells of SIVmac-infected, Mamu-A*01+ rhesus monkeys was investigated by four-color flow cytometric analysis. CD8α/ β+ T cells were evaluated for binding of the tetrameric Mamu-A*01/p11C, C–M complex and expression of CD11a, CD28, CD45RA, and MHC class II DR. The tetrameric complex–binding CD8α/β+ T cells in the peripheral blood of all four animals showed a relatively high mean fluorescence in anti-CD11a staining (representative staining for CD11a is shown in Fig. 6 A) and were predominantly CD45RA− (Table 2 and Fig. 6 B). (No anti-CD45RO mAb is available that binds to rhesus monkey CD45RO.) Interestingly, a heterogeneous expression of the CD28 molecule was observed investigating tetrameric complex– binding CD8α/β+ T cells in this group of four rhesus monkeys; these cells from two animals were predominantly CD28+ and from another animal predominantly CD28− (Table 2 and Fig. 6 C). This skewing in CD28 expression on tetrameric complex–binding cells did not correlate with CD28 expression on the nonbinding CD8α/β+ T cells. MHC class II DR expression was higher on tetrameric complex binding cells from three of the four rhesus monkeys compared to their nonreactive CD8α/β+ T cells, with ≥50% of these cells expressing the MHC class II DR molecule (Table 2 and Fig. 6 D).
Figure 6.
Phenotypic characterization of tetrameric Mamu-A*01/ p11C, C–M complex–binding CD8α/β+ T cells. A whole blood specimen from a Mamu-A*01+, SIVmac-infected rhesus monkey 403 was stained with FITC-coupled tetrameric Mamu-A*01/p11C, C–M complex and four different PE-coupled mAbs (CD11a, CD28, CD45RA, and HLA-DR). (A) Mean fluorescence (MF) values are indicated for the CD11a staining. Upper left (UL) is the mean fluorescence of the CD11a positive and Mamu-A*01/p11C, C–M negative CD8α/β+ T cell population. Upper right (UR) is the mean fluorescence of the CD11a positive and Mamu-A*01/p11C, C–M positive CD8α/β+ T cell population. (B– D) Percentages of CD28, CD45RA, MHC class II DR, and tetrameric Mamu-A*01/p11C, C–M complex positive or negative CD8α/β+ T cells are indicated.
Table 2.
| Monkey MamuA*01/p11C, C–M | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mm403 | Mm138 | Mm297 | Mm348 | |||||||||||||
| − | + | − | + | − | + | − | + | |||||||||
| CD28 | ||||||||||||||||
| + | 53.5 | 2.9 | 13.6 | 1.4 | 87.6 | 0.7 | 80.2 | 0.3 | ||||||||
| − | 36.5 | 7.1 | 84.4 | 0.6 | 11.5 | 0.2 | 19.1 | 0.4 | ||||||||
| CD45RA | ||||||||||||||||
| + | 37.2 | 1.7 | 63.1 | 0.2 | 77.5 | 0.2 | 51.2 | 0.1 | ||||||||
| − | 52.8 | 8.3 | 34.9 | 1.8 | 21.6 | 0.7 | 48.1 | 0.6 | ||||||||
| MHC class II-DR | ||||||||||||||||
| + | 19.6 | 5.3 | 49.1 | 1.1 | 8.9 | 0.7 | 12.9 | 0.3 | ||||||||
| − | 70.4 | 4.7 | 48.9 | 0.9 | 90.2 | 0.2 | 86.4 | 0.4 | ||||||||
Percent of Mamu-A*01/p11C, C–M complex positive (+) and negative (−) populations staining for expression of CD28, CD45RA, and MHC class II DR (positive [+] and negative [−]) in PBMCs gated on CD8α/β+ T cells.
Discussion
The use of a tetrameric MHC class I–peptide complex was evaluated as a staining reagent for flow cytometric analysis of a virus epitope–specific CTL response in SIVmac-infected rhesus monkeys. We found that this complex bound specifically to a subset of CD8α/β+ T cells from infected monkeys of the appropriate MHC class I haplotype. Moreover, the percentage of CD8α/β+ T cells binding this complex correlated well with epitope-specific functional cytotoxic activity as measured in both bulk and limiting dilution assays.
Significantly different estimates of HIV- and SIV-specific CTL precursor cell numbers have been generated using different techniques. Functional limiting dilution analysis, a standard method for quantitating CTLs, has revealed a frequency of HIV-1–specific pCTLs between 0.001 and 0.1% of PBMCs in infected individuals (24–26). Higher levels of pCTLs, ranging from 0.2 to 1% of T cells, have been estimated when TCR complementarity determining region 3 (CDR3) sequences were used as molecular markers for individual CTL clones to estimate the frequency of these cells in PBMCs (27). Staining with tetrameric MHC class I–peptide complexes for evaluation of HIV-1 Gag- and Pol-specific CTLs has provided estimates of a similar order of magnitude (1).
In this study we estimated pCTLs in the same lymphocyte population of SIVmac-infected rhesus monkeys using both functional LDAs and tetramer staining technologies. We also found a significant discrepancy in this rhesus monkey animal model when comparing the results obtained by standard LDA and tetrameric Mamu-A*01/p11C, C–M complex staining. The p11C-specific pCTLs as determined by tetrameric Mamu-A*01/p11C, C–M complex staining PBMCs of the four SIVmac-infected, Mamu-A*01+ rhesus monkeys ranged between 0.7 and 10.3% of CD8α/β+ T cells, whereas the highest pCTL estimate arrived at by LDAs was 0.02% of all PBMCs, which is equivalent to 0.1% of CD8α/β+ T cells. A number of factors may be contributing to the substantial discrepancy between these estimates. Different culture conditions used in LDAs can generate very different precursor frequency estimates. Thus, whether an anti-CD3 antibody is used to stimulate cells, whether cells are cultured in the presence of a crude T cell growth factor, or whether the antigen used in the assay is an actively synthesized viral protein or a synthetic peptide can have a substantial impact on the estimated pCTLs. It is also possible that a cell population that can bind a single peptide–MHC class I complex is functionally heterogeneous, with only a fraction of the cells being capable of lysing virus-infected target cells at any point in time.
In fact, the phenotypic characterization of the tetrameric Mamu-A*01/p11C, C–M complex–binding cells suggests that these cells are heterogeneous in individual monkeys. Most of the Mamu-A*01/p11C, C–M–binding cells in these studies expressed relatively high levels of CD11a and did not express CD45RA, suggesting that they were memory rather than naive lymphocytes. This observation is in agreement with Altman et al. who described a homogeneous phenotypic memory type profile (CD45RO+ and CD62L−) for their MHC class I–peptide binding cells (1). Previous studies have suggested that HIV-1–specific CTLs are HLA-DR+ and CD28− (28–32). However, we found a heterogeneous expression of CD28 and MHC class II DR by the tetrameric complex–binding T cells. Heterogeneity in expression of these molecules by the Mamu-A*01/ p11C, C–M–binding cells is consistent with the possibility of heterogeneity in their function.
The percentage of circulating CD8α/β+ T cells that bound the tetrameric Mamu-A*01/p11C, C–M complex was remarkably constant over a period of months in three of the four chronically SIVmac-infected rhesus monkeys that we have studied. In the fourth animal, rhesus monkey 403, the percentage was much higher than in others, varying between 4.6 and 10.3%. However, there was considerable variability between the different animals in the percentage of CD8α/β+ peripheral blood T cells binding this complex. A number of factors may lead to this variability. A particularly high level of persistent antigenemia may contribute to a persistent clonal expansion of CD8α/β+ virus-specific CTLs. In light of the recent demonstration that vigorous CTL responses correlate with low virus load in HIV-1–infected humans (33), this is an unlikely possibility. It is possible that animals that maintain a high level of CD4+ T cell–mediated help may mount a persistently high level of virus-specific CTLs. The number of infected animals that we have studied to date is too few to allow us to assess this possibility.
The use of this approach for studying CTL responses in vivo will, in the end, be limited only by the technique's sensitivity. As currently performed, this flow cytometry– based technique can detect CTLs only if they are represented in >0.1 to 0.5% of the CD8α/β+ T cell population. A number of technical changes in this approach would probably significantly enhance its sensitivity. Changes in the dye and/or different laser configurations might increase the ability to discriminate between tetramer binding and nonbinding CD8α/β+ T cell populations. Such changes might make it possible to examine CTL responses to less dominant epitopes than the Mamu-A*01–restricted Gag peptide evaluated in this study.
The application of this novel technology in the SIVmac-infected rhesus monkey provides an important new approach for studying the role of CTLs in the immunopathogenesis of AIDS. It also provides a simple and quantitative approach for evaluating potential new vaccine strategies for preventing AIDS virus infections.
Acknowledgments
The authors thank Dr. Andrew J. McMichael for his encouragement in pursuing these studies and Dr. David N. Garboczi for the gift of the E. coli strain XA90 and advice in making the MHC class I–peptide complex.
This work was supported by National Institutes of Health grants AI-20729, AI-35166, AI-43068, AI-32426, AI-41913, RR-00168, and RR-00167, and Coulter Corp. (Miami, FL).
Abbreviations used in this paper
- β2m
β2-microglobulin
- 1-D IEF
one-dimensional isoelectric focusing
- APC
allophycocyanin
- B-LCL
B lymphoblastoid cell line
- CD
cluster of differentiation
- LDA
limiting dilution assay
- mac
macaque
- p11C
12–amino acid fragment of SIVmac 251 Gag (amino acids 179–190)
- p11C
C–M, 9–amino acid fragment of SIVmac 251 Gag (amino acids 181–189)
- pCTL
precursor frequency of CTLs
- SIV
simian immunodeficiency virus
References
- 1.Altman JD, Moss PAH, Goulder PJR, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 2.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowski W, Farthing C, Ho DD. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reimann KA, Tenner-Racz K, Racz P, Montefiori DC, Yasutomi Y, Lin W, Ransil BJ, Letvin NL. Immunopathogenic events in the acute infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Virol. 1994;68:2362–2370. doi: 10.1128/jvi.68.4.2362-2370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rinaldo C, Huang XL, Fan ZF, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M, Gupta P. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1–infected long-term nonprogressors. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsubota H, Lord CI, Watkins DI, Morimoto C, Letvin NL. A cytotoxic T lymphocyte inhibits acquired immunodeficiency syndrome virus replication in peripheral blood lymphocytes. J Exp Med. 1989;169:1421–1434. doi: 10.1084/jem.169.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker CM, Moody DJ, Stites DP, Levy JA. CD8+lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986;234:1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 7.Miller MD, Yamamoto H, Hughes AH, Watkins DI, Letvin NL. Definition of an epitope and MHC class I molecule recognized by Gag-specific cytotoxic T lymphocytes in SIVmac-infected rhesus monkeys. J Immunol. 1991;147:320–329. [PubMed] [Google Scholar]
- 8.Miller MD, Gould-Fogerite S, Shen L, Woods RM, Koenig S, Mannino RJ, Letvin NL. Vaccination of rhesus monkeys with synthetic peptide in a fusogenic proteoliposome elicits simian immunodeficiency virus-specific CD8+cytotoxic T lymphocytes. J Exp Med. 1992;176:1739–1744. doi: 10.1084/jem.176.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasutomi Y, Koenig S, Haun SS, Stover CK, Jackson RK, Conard P, Conley AJ, Emini EA, Fuerst TR, Letvin NL. Immunization with recombinant BCG-SIV elicits SIV-specific cytotoxic T lymphocytes in rhesus monkeys. J Immunol. 1993;150:3101–3107. [PubMed] [Google Scholar]
- 10.Yasutomi Y, Palker TJ, Gardner MB, Haynes BF, Letvin NL. Synthetic peptide in mineral oil adjuvant elicits simian immunodeficiency virus–specific CD8+cytotoxic T lymphocytes in rhesus monkeys. J Immunol. 1993;151:5096–5105. [PubMed] [Google Scholar]
- 11.Shen L, Chen ZW, Miller MD, Stallard V, Mazzara GP, Panicali DL, Letvin NL. Recombinant virus vaccine-induced SIV-specific CD8+cytotoxic T lymphocytes. Science. 1991;252:440–443. doi: 10.1126/science.1708168. [DOI] [PubMed] [Google Scholar]
- 12.Allen, T.M., J. Sidney, M.-F. del Guercio, R.L. Glickman, G.L. Lensmeyer, D.A. Wiebe, R. DeMars, C.D. Pauza, R.P. Johnson, A. Sette, and D.I. Watkins. 1998. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from SIV. J. Immunol. In press. [PubMed]
- 13.Shatz PJ. Use of peptide libraries to map the substrate specificity of a peptide-modifying enzyme: a 13 residue consensus peptide specifies biotinylation in Escherichia coli. . Biotechnology. 1993;11:1138–1143. doi: 10.1038/nbt1093-1138. [DOI] [PubMed] [Google Scholar]
- 14.Garboczi DN, Hung DT, Wiley DC. HLA-A2–peptide complexes: refolding and crystallization of molecules expressed in Escherichia coliand complexed with single antigenic peptides. Proc Natl Acad Sci USA. 1992;89:3429–3433. doi: 10.1073/pnas.89.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watkins DI, Kannagi M, Stone MS, Letvin NL. Major histocompatibility complex class I molecule of nonhuman primates. Eur J Immunol. 1988;18:1425–1432. doi: 10.1002/eji.1830180919. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto H, Miller MD, Tsubota H, Watkins DI, Mazzara GP, Stallard V, Panicali DL, Aldovini A, Young RA, Letvin NL. Studies of cloned simian immunodeficiency virus–specific T lymphocytes: gag-specific cytotoxic T lymphocytes exhibit a restricted epitope specificity. J Immunol. 1990;144:3385–3391. [PubMed] [Google Scholar]
- 17.Fazekas de St. Groth, S. The evaluation of limiting dilution assays. J Immunol Methods. 1982;49:R11–R23. doi: 10.1016/0022-1759(82)90269-1. [DOI] [PubMed] [Google Scholar]
- 18.Baume DM, Caligiuri MA, Manley TJ, Daley JF, Ritz J. Differential expression of CD8α and CD8β associated with MHC-restricted and non-MHC-restricted cytolytic effector cells. Cell Immunol. 1990;131:352–365. doi: 10.1016/0008-8749(90)90260-x. [DOI] [PubMed] [Google Scholar]
- 19.Moebius U, Kober G, Griscelli AL, Hercend T, Meuer SC. Expression of different CD8 isoforms on distinct human lymphocyte subpopulations. Eur J Immunol. 1991;21:1793–1800. doi: 10.1002/eji.1830210803. [DOI] [PubMed] [Google Scholar]
- 20.Norment AM, Littman DR. A second subunit of CD8 is expressed in human T cells. EMBO (Eur Mol Biol Organ) J. 1988;7:3433–3439. doi: 10.1002/j.1460-2075.1988.tb03217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terry LA, DiSanto JP, Small TN, Flomemberg N. Differential expression and regulation of human CD8α and CD8β chains. Tissue Antigens. 1990;35:82–91. doi: 10.1111/j.1399-0039.1990.tb01761.x. [DOI] [PubMed] [Google Scholar]
- 22.Alcover, A. 1995. CD8 cluster report. In Leucocyte Typing V, White Cell Differentiation Antigens. Vol. 1. S.F. Schlossman, L. Boumsell, W. Gilks, J.M. Harlan, T. Kishimoto, C. Morimoto, J. Ritz, S. Shaw, R. Siverstein, T. Springer, T.F. Tedder, and R.F. Todd, editors. Oxford University Press, Oxford. 353–354.
- 23.Rocha B, Vassalli P, Gui-Grand D. The extrathymic T-cell development pathway. Immunol Today. 1992;13:449–454. doi: 10.1016/0167-5699(92)90074-H. [DOI] [PubMed] [Google Scholar]
- 24.Gotch FM, Nixon DF, Alp N, McMichael AJ, Borysiewicz LK. High frequency of memory and effector gag specific cytotoxic T lymphocytes in HIV seropositive individuals. Int Immunol. 1990;2:707–712. doi: 10.1093/intimm/2.8.707. [DOI] [PubMed] [Google Scholar]
- 25.Koup RA, Pikora CA, Luzuriaga K, Brettler DB, Day ES, Mazzara GP, Sullivan JL. Limiting dilution analysis of cytotoxic T lymphocytes to human immunodeficiency virus gag antigens in infected persons: in vitro quantitation of effector cell populations with p17 and p24 specificities. J Exp Med. 1991;174:1593–1600. doi: 10.1084/jem.174.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)–specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J Exp Med. 1993;177:249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moss PAH, Rowland-Jones SL, Frodsham PM, McAdam S, Giangrande P, McMichael AJ, Bell JI. Persistent high frequency of human immunodeficiency virus–specific cytotoxic T cells in peripheral blood of infected donors. Proc Natl Acad Sci USA. 1995;92:5773–5777. doi: 10.1073/pnas.92.13.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ho H-N, Hultin LE, Mitsuyasu RT, Matud JL, Hausner MA, Bockstoce D, Chou C-C, O'Rourke S, Taylor JMG, Giorgi JV. Circulating HIV-specific CD8+cytotoxic T cells express CD38 and HLA-DR antigens. J Immunol. 1993;150:3070–3079. [PubMed] [Google Scholar]
- 29.Pantaleo G, De Maria A, Koenig S, Butini L, Moss B, Baseler M, Lane HC, Fauci AS. CD8+T lymphocytes of patients with AIDS maintain normal broad cytolytic function despite the loss of human immunodeficiency virus– specific cytotoxicity. Proc Natl Acad Sci USA. 1990;87:4818–4822. doi: 10.1073/pnas.87.12.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiorentino S, Dalod M, Olive D, Guillet J-G, Gomard E. Predominant involvement of CD8+CD28−lymphocytes in human immunodeficiency virus–specific cytotoxic activity. J Virol. 1996;70:2022–2026. doi: 10.1128/jvi.70.3.2022-2026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalod M, Fiorentino S, Delamare C, Rouzioux C, Sicard D, Guillet J-G, Gomard E. Delayed virus-specific CD8+ cytotoxic T lymphocyte activity in an HIV-infected individual with high CD4+cell counts: correlations with various parameters of disease progression. AIDS Res Hum Retroviruses. 1996;12:497–506. doi: 10.1089/aid.1996.12.497. [DOI] [PubMed] [Google Scholar]
- 32.Couedel-Courteille A, Le Grand R, Tulliez M, Guillet J-G, Venet A. Direct ex vivosimian immunodeficiency virus (SIV)-specific cytotoxic activity detected from small intestine intraepithelial lymphocytes of SIV-infected macaques at an advanced stage of infection. J Virol. 1997;71:1052–1057. doi: 10.1128/jvi.71.2.1052-1057.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Musey L, Hughes J, Schackter T, Shea T, Corey L, McElrath MJ. Cytotoxic–T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1306–1308. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]