Abstract
It is currently well established that HIV-1 Vpr augments viral replication in primary human macrophages. In its virion-associated form, Vpr has been suggested to aid efficient translocation of the proviral DNA into the cell nucleus. Although Vpr growth-arrests dividing T cells, the relevance of this biological activity in nondividing macrophages is unclear. Here we use Vpr-mutants to demonstrate that the molecular determinants involved in G2-arresting T cells are also involved in increasing viral transcription in macrophages, even though these cells are refractive to the diploid DNA status typical of G2 phase. Our results suggest that the two phenotypes, namely the nuclear localization and the G2-arrest activity of the protein, segregate functionally among the late and early functions of Vpr. The nuclear localization property of Vpr correlates with its ability to effectively target the proviral DNA to the cell nucleus early in the infection, whereas the G2-arrest phenotype correlates with its ability to activate viral transcription after establishment of an infection. These two functions may render Vpr's role essential and not accessory under infection conditions that closely mimic the in vivo situation, that is, primary cells being infected at low viral inputs.
The Vpr protein is one of the regulatory gene products coded by HIV-1, the etiological agent of AIDS, and is expressed late in the infection cycle (1, 2). All primate lentiviruses including HIV-1 code for a Vpr-like product. However, some lentiviruses such as HIV-2 and most simian immunodeficiency viruses (SIVs)1 code not only for Vpr but also for a second protein named Vpx, which shares considerable sequence homology with Vpr (3, 4). Phenotypically, several studies demonstrate that viruses that code for a Vpr protein replicate to much higher levels in macrophages than do their Vpr-negative counterparts (5–9). However, exactly how the protein contributes to this effect is not clearly understood.
The fact that Vpr is packaged in the progeny virions at high copy numbers strongly suggests that the protein could play a role early in the infection (10). Experimental support for an early functional role comes from studies in which Vpr contributed to the nuclear import of proviral DNA in nondividing cells such as macrophages (11). In addition, a late function is also suggested by experiments in which excluding the late de novo expression of Vpr effectively abolished the augmented replication even though the protein was made available in the virion early in the infection (7). However, the ability of Vpr to target the proviral DNA to the nuclear compartment early in the infection was not monitored in this study, and therefore the authors could not formally preclude an additional role for the protein early in the infection (7).
Hence, several issues remain unclear about our understanding of the ability of Vpr to increase viral replication in macrophages. Does Vpr play a role late in the viral life cycle that can be segregated from its function early in the infection, and if so, at what replication step does this late function manifest itself? Given the fact that other virion-associated proteins such as Gag matrix p17 (11, 12) and viral integrase (13) also appear to facilitate proviral nuclear import, is Vpr's nuclear targeting function essential? If not, what is the relevance of evolutionary conservation of multiple nucleophilic determinants? If so, why has it not been detected in earlier studies? Finally, is Vpr's ability to growth-arrest proliferating cells (14–19) an effect confined to cycling T-cells or does it have functional correlates in nondividing macrophages that are refractive to cell-cycle changes?
In this study we attempt to address these questions by systematically monitoring the relative contribution by HIV-1 Vpr to the early and late viral replication events in primary human macrophage cultures. We monitor the levels of proviral DNA targeted to the cell-nucleus in the presence and absence of Vpr, as well as the ensuing virus production and the pattern of RNA expression at the single cell level using in situ analysis. We also monitor the replication of Vpr-mutants that lack either the protein's ability to localize to the nucleus or its ability to growth-arrest T cells in an effort to gauge the relevance of these phenotypes during macrophage infection.
Materials and Methods
Isolation and Culture of Peripheral Blood–derived Macrophages.
Blood units from HIV seronegative volunteers were obtained from the Hôpital Maisonneuve Rosemont (Montreal, Canada). PBMCs were isolated by Ficoll-Paque centrifugation as recommended by the manufacturer (Pharmacia Biotech AB, Uppsala, Sweden), washed thoroughly to remove contaminating platelets, and cryopreserved. An enriched population of monocyte-derived macrophages were first isolated by adherence to plastic as previously described (5). Further purification was achieved by negative selection where the enriched macrophage population was incubated with a cocktail of monoclonal antibodies against T and B cells followed by subsequent depletion using a secondary antibody conjugated to magnetic beads (Dynal Inc., Lake Success, NY) as previously described (20). Purified macrophages were plated in 6-well plates at a concentration of 2.5 × 106 cells per well and maintained in endotoxin-free RPMI 1640 media (GIBCO BRL, Gaithersburg, MD) containing 10% FCS, l-glutamine, penicillin (100 U/μl), streptomycin (100 U/μl), and gentamycin (10 μg/ml). The resulting adherent macrophages were >93% positive by nonspecific esterase test (Sigma Chemical Co., St. Louis, MO).
Proviral Constructs, Infections, and Replication Kinetics.
The previously well-characterized isogenic HxBRU based clones (21) were rendered macrophage-tropic by transferring the SalI– BamHI fragment from another well-characterized macrophage-tropic construct ADA (NLHXADA-SM), provided by Dr. Lee Ratner (University of Washington School of Medicine, St. Louis, MO) (9). The construction of the HxBRU parental clones that code for the wild-type Vpr or the Vpr-negative ATG− mutant (where the Vpr ATG initiation codon has been mutated to prevent protein translation) have been previously described (21). The R62P and R80A were subcloned in this background using a two-step PCR-based method as previously described (21).
Cos-7 cells were plated at 8 × 105 cells per 100-mm plate, cultured overnight, and transfected with proviral constructs by calcium phosphate method. To generate viral stocks, supernatants were collected 72 h after transfection, concentrated by ultracentrifugation at 35,000 rpm for 2.5 h, filtered through 0.45-μm filters, treated with DNase (25 U/ml) to remove contaminating plasmid DNA, and stored at −80°C. In vitro differentiated macrophages that had been in culture for 7 d were adsorbed with virus for 8 h at 37°C and were subsequently washed thoroughly to remove free virus and were maintained in RPMI 1640 complete medium containing 100 nM Palinavir (BioMega-Boehringer Ingelheim, Laval, Quebec, Canada), a previously well characterized protease inhibitor (22). Half of the medium was changed every 3–4 d with fresh media supplemented with 100 nM Palinavir. Virus production in cultures was determined by measuring supernatant p24 levels by standard ELISA techniques.
In Situ Hybridization.
For in situ hybridization purposes, purified macrophages were maintained after purification for 7 d in Lab-Tech slides (Nunc™, Naperville, IL) and subsequently infected at varying viral inputs under conditions similar to the ones used for one-step replication studies in the presence of 100 nM Palinavir. Hybridization procedures were performed using standard protocols (23, 24) using an antisense probe previously described in detail (25). This probe contains a 92-bp sequence complementary to the Sty-1 (nucleotide 7591) to HindIII (nucleotide 7683) fragment overlapping the HxBc2 envelope sequences found in the ADA chimeric clones (9). Thus, the hybridization procedure identified both full-length and singly spliced mRNAs, both of which contain the target envelope sequences. Based on earlier optimization procedures, RNA pattern was determined at 14 d after infection as all donors tested clearly showed the Vpr-mediated phenotype of augmented replication by this time.
PCR Analysis and Southern Blotting.
Total DNA lysates were prepared to monitor the efficiency of reverse transcription at different time points early in the infection as previously described (26). Alternatively, proviral levels found in the nuclear compartment were monitored as previously described (26). PCR analysis to detect HIV-1 proviral products was performed using the following primers in the Gag sequence: sense oligonucleotide 5′-ATA ATC CAC CTA TCC CAG TAG GAG AAA T-3′, and antisense oligonucleotide 5′-TTT GGT CCT TGT CTT ATG TCC AGA ATG C-3′. These primers amplified a fragment of 114 bp corresponding to the sequence 1090 to 1204 in the HxBRU strain. Alternatively, the cellular β2-adrenergic receptor (β2-AR) gene sequences were detected using the following primers: sense 5′-TAG GCC TTC AAA GAA GAC CTC C-3′, and antisense 5′-CGT CTA CTC CAG GGT CTT TCA G-3′. PCR products from this amplification generated a 399-bp fragment that corresponded to the sequence 1465 to 1821 of the human β2-AR gene. Amplification was performed using Taq DNA polymerase (Perkin Elmer, Norwalk, CT) for 30 cycles of 1 min at 94°C, 2 min at 50°C, and 3 min at 72°C. α-[32P]dATP-labeled probes capable of recognizing the PCR products were generated as follows: HIV-1 Gag probe was generated by nick translating with α-[32P]dATP (3,000 Ci/mmol; ICN Radiochemicals, Costa Mesa, CA), the 591-bp ApaI to PstI digestion fragment corresponding to positions 964 to 1555 in the HxBc2 provirus sequence. β2-AR probe was generated by similarly nick translating a fragment corresponding to the sequence 1465 to 1821 from a well characterized β2-AR eukaryotic expressor construct (27).
Results
Correlation of the G2-arrest and Nuclear Localization Phenotypes of Vpr with its Ability to Establish a Productive Infection in Macrophages.
To explore what role, if any, Vpr-mediated G2-arrest and nuclear localization played during viral replication in nondividing target cells such as macrophages, we compared the replication of a virus that coded for a wild-type Vpr or no Vpr at all (ATG− mutant), to that of viruses coding for mutations in the gene that selectively affected either the G2-arrest or the nuclear localization activity of the protein. For this purpose, the macrophage-tropic envelope region from the well-characterized NLHX-ADA-SM construct (9) was transferred into HIV-1 constructs that coded either for the wild-type Vpr or for a mutated version of the protein (21). The two mutations used in this study to gauge how the G2-arrest and nuclear localization phenotypes of Vpr contribute to HIV-1 replication in macrophages were chosen based on previously published reports that clearly separate these functions at the structural level (28, 29).The R62P and R80A proviruses expressed, respectively, a Vpr-mutant that substituted the Arginine residue at position 62 with a Proline and one that substituted the Arginine residue at position 80 with an Alanine. The Proline substitution mutant, R62P, retained its ability to G2-arrest cells but lost its ability to localize to the nucleus as it disrupted a helical domain shown by earlier studies to affect the nuclear localization phenotype specifically (29–32). The R80A mutant was capable of localizing to the nucleus but was impaired in its ability to growth-arrest T cells, also as previously shown (28). These observations are in agreement with a number of earlier functional studies that have characterized central helical and COOH-terminal domains of Vpr (21, 28, 29), and which are summarized in Table 1.
Table 1.
Nuclear Localization and G2-arrest Phenotypes of Various Vpr Genes
| Vpr gene | Wild-type | ATG− | R62P | R80A | ||||
|---|---|---|---|---|---|---|---|---|
| G2-arrest Ability | Positive | Negative | Positive | Negative | ||||
| Subcellular | Nuclear | N/A* | Cytoplasmic | Nuclear | ||||
| localization |
Summary of G2 arrest and nuclear localization phenotypes associated with the wild-type and mutant Vpr-expressing viruses.
The ATG− mutant involved elimination of the Vpr initiation codon and hence did not translate a Vpr product as previously described (21).
Whether or not Vpr is dispensable for viral replication in macrophages has been controversial as there is limited consensus on the extent to which the virus is impaired in the absence of the protein. Reports from independent groups have documented anywhere from 2-fold to close to 100-fold impairment (5, 7, 9). As some investigators have suggested that the viral input may contribute to the in vitro replication kinetics of viruses mutated in another nucleophilic determinant, matrix p17 (33), as well as viruses mutated in Nef, the accessory protein (34, 35), we tested if the input multiplicity can compensate for the Vpr-mediated augmentation of viral replication. To address this issue, we infected purified macrophages kept in culture for 7 d with the wild-type or Vpr-mutant viruses at either a high input titer (50 ng) or one third serial dilutions thereof (16.7 ng, 5.6 ng, 1.85 ng, 619 pg). One-step replication was ensured by maintaining the infected cultures in the presence of the potent protease inhibitor Palinavir (BioMega-Boehringer Ingelheim), which effectively prevented multiple rounds of infection from newly generated viruses in this system (data not shown). We have previously shown that treatment with this inhibitor results in the release of noninfectious particles into the supernatant due to a block at the virion maturation step (22). This culture system allowed us to look at a one-step viral replication without the complication of the progression of viral infection through the culture.
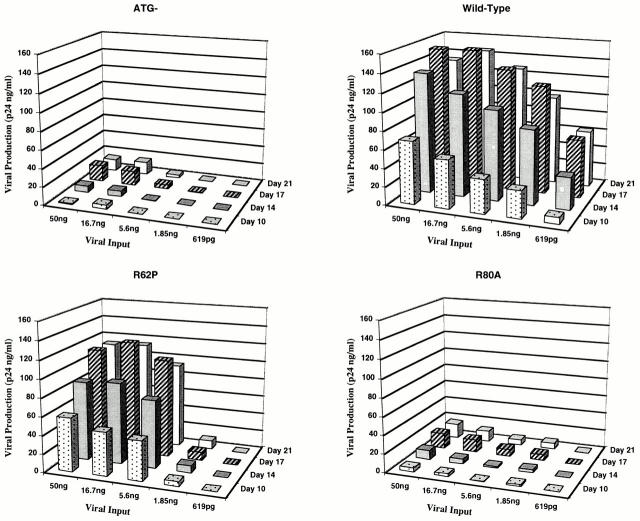
Our data suggests that at low viral inputs both the ability of the protein to localize to the nucleus and its ability to G2-arrest dividing cells appeared to be essential for increased viral replication in macrophages. The wild-type protein capable of both activities established a readily apparent infection even at the lowest multiplicity tested (Fig. 1). However, the R62P mutant, which lacks the nuclear localization activity, and the R80A mutant, which lacks the G2-arrest phenotype (Table 1) are both impaired at low viral inputs (Fig. 1). The ATG− mutant, which lacks both these biological activities, is clearly impaired at the low multiplicities tested (Fig. 1).
Figure 1.
Single cycle replication kinetics of viruses expressing the wild-type and mutant Vpr proteins in primary human macrophages. Peripheral blood monocyte–derived macrophages cultured for 7 d were infected with increasing viral inputs ranging from 619 pg to 50 ng (represented in the horizontal axis). Viral production after infection was monitored at regular intervals by measuring p24 levels in the culture supernatants (represented in the vertical axis as p24 ng/ml). The lateral axis represents days after infection spanning the peak of viral replication. Independent experiments performed with different donors and viral stocks consistently showed similar kinetics.
However, at high viral inputs, the requirement for the nuclear localization is no longer essential for augmented replication, whereas the G2-arrest ability is still required. Note that the R80A mutant that lacks the G2-arrest activity shows impairment of replication at the high viral inputs even though it is capable of localizing to the nucleus, whereas the R62P mutant possessing the G2-arrest activity is rescued even though it fails to localize to the nucleus (Fig. 1).
Ability of the Wild-type and Mutant Vpr Proteins to Target Proviral DNA to the Nuclear Compartment.
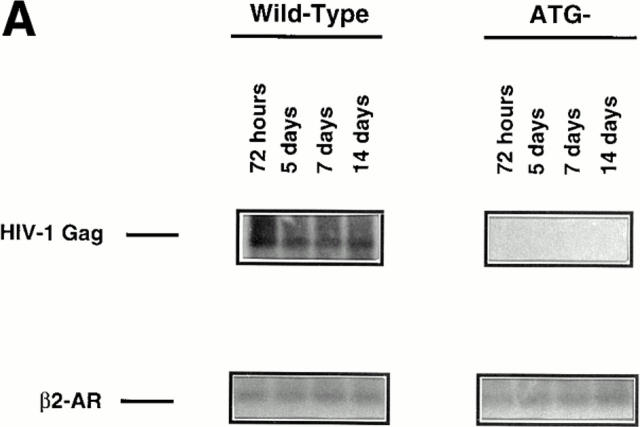
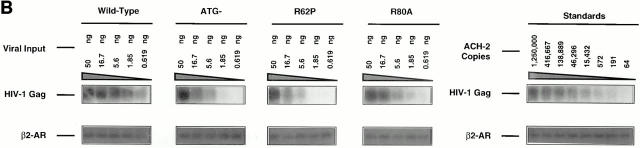
We sought to understand if either the nuclear localization or the G2-arrest activity associated with Vpr affected the protein's ability to target the proviral DNA to the nuclear compartment. To formally address this issue, we used a semiquantitative PCR approach to monitor the total levels of HIV-1 proviral generation and the levels of provirus found in the nuclear compartment at various time points after addition of the initial viral inoculum as previously described (26). Single-step viral replication conditions identical to the ones used to monitor productive infection by p24 were used for this purpose. The total proviral synthesis was approximately the same both in the presence and absence of Vpr, indicating that neither viral entry nor reverse transcription was radically affected by the protein (data not shown), an observation that is in agreement with previous reports (11, 12). However, absence of Vpr drastically reduced the targeting of the proviral DNA to the nuclear compartment and this effect, interestingly, only became apparent at low viral inputs (Fig. 2, A and B, 1.85- and 0.619-ng lanes). It is also clear that the drastic impairment in nuclear targeting is not due to a temporal delay in transport, as nuclear proviral levels remain constant after 72 h (Fig. 2 A). However, at high viral inputs the PCR data clearly demonstrates that the proviral import function proceeds efficiently in spite of the absence of Vpr (ATG−), or its inability to localize to the nucleus (R62P) (Fig. 2 B). This is probably due to complementation of the nuclear import function at high viral inputs by the viral integrase protein (13) and the Gag matrix p17 product (11, 12, 36). Note, however, that at lower viral inputs Vpr was essential for efficient nuclear targeting of the provirus regardless of the presence of these complementary viral nucleophilic factors (Fig. 2 B, compare the 1.85-ng lanes). Also, note that at lower viral inputs, inability of Vpr to localize to the nucleus impairs its ability to target the proviral DNA to the nuclear compartment (compare the 1.85-ng lanes of R62P to that of wild-type).
Figure 2.
Levels of HIV-1 DNA found in the nuclear compartment among viruses expressing the wild-type and mutant Vpr proteins. (A) Kinetics of proviral DNA detection in the nuclear compartment for wild-type and Vpr negative mutants at a nonsaturating viral input (1.85 ng). HIV Gag sequences found targeted to the nucleus reached a plateau by day 3 and remained constant, indicating that multiple rounds of infection in the presence of the protease inhibitor does not occur in this system. (B) HIV-1 proviral detection in the nuclear compartment at day 14 after infection at various viral inputs for the wild-type, ATG−, R62P, and R80A mutants. Similar levels of cellular DNA were analyzed as indicated by the β2-AR detection. Standards for HIV-Gag detection were measured by diluting uninfected macrophages with a known number of chronically infected ACH-2 cells (48).
However, an additional contribution to the augmented replication is provided by the protein's G2-arrest ability, as similar nuclear levels of proviral DNA present at higher viral inputs (Fig. 2, compare the 50-ng lanes), still lead to a nonproductive infection in the R80A and ATG− cultures (Fig. 1). This clearly suggests that access to the nucleus by itself is not sufficient to ensure productive infection, and that G2-arrest ability is essential to induce productive infection after achieving efficient proviral nuclear targeting. This conclusion is underscored by the productive infection of R62P mutation once efficient targeting of its proviral DNA is achieved by the use of high viral titers (Fig. 1).
RNA Expression Pattern Among Cultures Infected with Wild-Type and Vpr-Mutant Viruses.
Though analysis of replication kinetics by monitoring supernatant p24 levels allowed us to gauge productive infection, this did not clarify if the highly reduced viral production apparent in some cultures (Fig. 1, ATG− and R80A) is due to a reduction in the number of infected cells, or, alternatively, due to the same number of infected cells producing lower amounts of viruses per cell. For example, it is conceivable that a subpopulation of macrophages are susceptible to HIV-1 infection in the absence of Vpr, whereas others are not. This is a possibility that needs to be considered as it is clear that blood-derived macrophages are a heterogeneous population of cells. Also, it is conceivable that infection itself is established in a small fraction of proliferating cells as previously suggested (37). Hence, in theory, the saturation seen in the p24 assay (Fig. 1) may result from saturation of this subpopulation, after which increasing the viral input results in no further infection as there are no further susceptible cells.
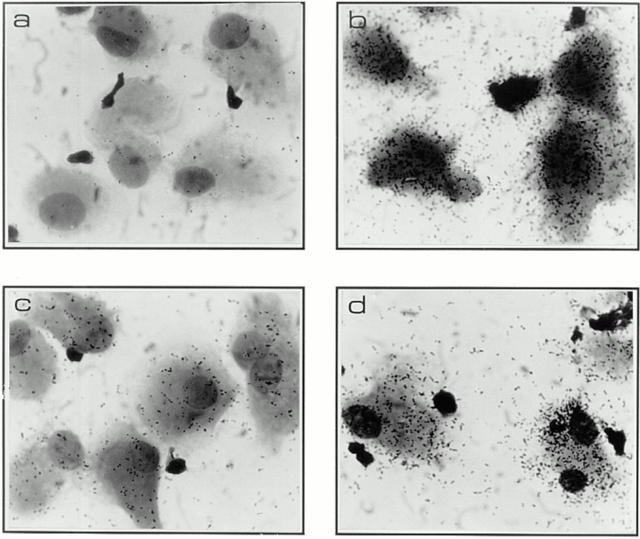
To explore the issue we performed in situ hybridization in lab-tech slides under conditions identical to the ones used to monitor viral replication by p24 and proviral DNA targeting to the nucleus (Fig. 3). Hybridization procedures were performed according to standard protocols (23, 24) using an antisense probe previously described in detail (25). This probe identified both full length and singly spliced mRNAs in HIV-1 infected primary cells (20). We chose to look at two input titers in this experiment, one at the higher saturating level (50 ng) and another at the lower end (1.85 ng).
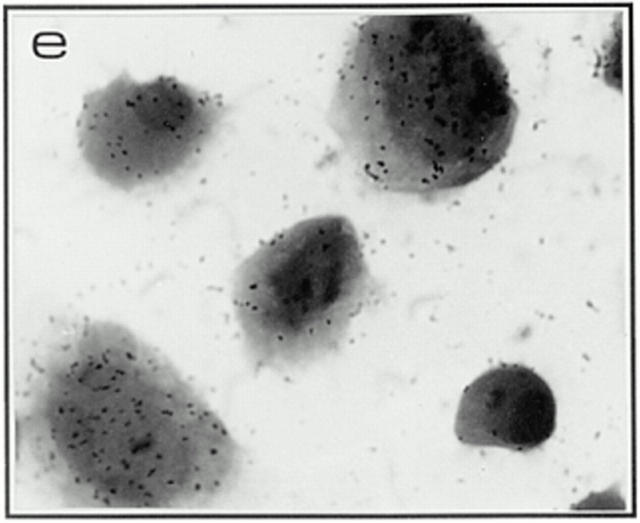
Figure 3.
Productive and silent infection patterns associated with wild-type and Vpr-mutant cultures as assessed by in situ hybridization. Panels depict in situ patterns found in macrophage cultures infected on day 7 after isolation with high viral titers (50 ng) and monitored for RNA expression at 2 wk after infection, when a clear difference in virion production was evident as assessed by supernatant p24 levels (see Fig. 1). Uninfected culture (a), wild-type (b), ATG− (c), R62P (d), and R80A (e).
Note that the in situ evidence clearly suggests that Vpr upregulates transcription in these nondividing cells, an effect linked to its ability to induce G2-arrest: mutants lacking the Vpr-G2-arrest capability (ATG− and R80A) clearly show drastic reduction in productively infected cells, even though the total number of infected cells were similar (in the range of 90%) at high viral inputs (Fig. 3 c and e, and Table 2). Interestingly, as exemplified by the R62P mutation at low viral inputs, the lack of nuclear targeting takes precedence over the ability to increase transcription as lack of proviral nuclear targeting impedes the establishment of infection (Fig. 2 B, and Table 2). However, the importance of this late transcriptional role is underscored by the lack of productive infection in the R80A mutant where close to wild-type levels of nuclear import occurs (Table 2). Also, the Vpr-negative ATG mutant still fails to show productive infection (Fig. 1 and Table 2) even after effective targeting of its proviral DNA to the nuclear compartment at high viral inputs (Fig. 2 B, 50-ng lane).
Table 2.
Pattern of RNA Expression among HIV-1 Infected Primary Human Macrophage Cultures
| Vpr gene | ATG− | Wild-type | R62P | R80A | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Viral inoculum* | High | Low | High | Low | High | Low | High | Low | ||||||||
| Total number of positive cells‡ | 91.0% | 6.5% | 89.5% | 67.5% | 94.5% | 8.0% | 93.5% | 42.5% | ||||||||
| Productively infected cells§ | 2.5% | 0.0% | 43.5% | 29.0% | 39.0% | 3.0% | 4.5% | 1.5% | ||||||||
RNA expression pattern as assessed by in situ hybridization.
High signifies 50 ng of viral input and low signifies 1.85 ng of viral input as assessed by p24 ELISA.
Positivity is defined as cells expressing >20 grains over background levels.
Productive infection is defined as the proportion of cells expressing 100 grains or more over background levels. Consistent results were also obtained from macrophages derived from a second donor with independent viral stocks. A minimum of 200 cells were quantified using methods we have previously described (20).
Also, both the wild-type and R62P cultures (both of which growth-arrest proliferating cells in the G2 phase) failed to change the DNA profile of these terminally differentiated macrophages, as expected. Both the uninfected and infected cultures showed <1% of the cells in the G2 phase of the cell-cycle (data not shown) even though in the range of 90% of the cells were shown to be infected (Table 2). Hence, the observed correlation between G2-arrest activity and the increased replication in macrophages is likely due to biochemical events rather than actual DNA duplication that characterize the G2-arrest status in proliferating T cells.
Discussion
Current consensus suggests that HIV-1 Vpr mediates two biological activities in target cells. One activity has been described for nondividing and quiescent cells, such as macrophages, where Vpr targets the HIV-1 proviral DNA to the cell's nucleus even in the absence of mitosis (38). The second function has been described in T cells, where Vpr induced growth arrest in the G2-phase of the cell cycle. Most prototypic retroviruses fail to infect nondividing cells, as mitosis and, conceivably, the nuclear membrane disintegration that occurs during cell-division are prerequisites to their gaining access to the cell-nucleus. However, Vpr, and at least two other HIV-1 virion proteins, matrix p17 (11, 12, 36) and integrase (13), have been known to ensure effective targeting of proviral DNA to the nuclear compartment. This leaves open the question why HIV-1 codes for apparently redundant nuclear targeting determinants.
The data presented here suggests that Vpr's contribution to proviral nuclear targeting is an essential rather than an accessory function under conditions that closely mimic in vivo situations, that is, primary cells being infected at low viral inputs. At low viral inputs, lack of Vpr results in the lack of infection, a function not mitigated by the presence of other nucleophilic determinants such as Gag matrix p17 or viral integrase (11–13, 36, 39). This also provides support to the evolutionary conservation of multiple, and apparently redundant, nucleophilic determinants in HIV. It is conceivable that optimal nuclear targeting occurs due to the interdependent nature of these multiple determinants. This may be due to the multivalent nature of the interaction of the preintegration complex with cellular targets. We assume that the lack of even one such valancy could reduce the chance of effective targeting, especially under restrictive conditions such as low viral inputs. These results underscore the importance of using low multiplicity in in vitro studies and provides support to previous suggestions that higher multiplicities of infection may mask certain biological activities in vitro (33).
Mechanistically, data presented here directly link the ability of Vpr to localize to the nucleus with that of its ability to target the provirus to the cellular genome at low multiplicities. This is illustrated by the notable reduction in proviral targeting at low viral inputs associated with mutants that either fail to code for a Vpr product (ATG−), or alternatively, code for a Vpr protein (R62P) incapable of reaching the nucleus (Fig. 2 B). The R80A mutant that is capable of reaching the nucleus, performs the proviral targeting function at levels comparable to its nuclear localization abilities (Table 1). It is unlikely that the failure to detect viral replication in some cases is due to alterations elsewhere in the genome as all viruses used in this study established a readily apparent replication in proliferating T cells (data not shown).
In this study we also provide direct evidence suggesting that HIV-1 Vpr upregulates transcription in infected macrophages. A late function for Vpr has been suggested by an earlier study (7) and our work provides formal proof for a late Vpr function independent of its nuclear targeting ability. Note that at high viral inputs, where a vast majority of cells are infected (in the range of 90%) in both the wild-type and Vpr-negative (ATG−) cultures, the wild-type Vpr expressing viruses generate higher levels of HIV-1 mRNA than the ATG-mutant (Fig. 3). We also demonstrate that the G2-arrest activity of Vpr, first identified in proliferating T cells, correlates with this increased transcriptional activity in macrophages. As macrophages are refractive to actual proliferation, it is conceivable that Vpr induces biochemical changes in macrophages, which in the context of the dividing cells leads to cell cycle arrest. Clearly, further studies are needed to understand the changes brought about by Vpr in the macrophage's cellular milieu.
Our results demonstrating a transcriptional role for Vpr in a relevant target cell provides biological relevance to a number of biochemical observations made earlier. We have previously shown that Vpr can increase reporter gene activity from variety of viral promoters (10). In fact, extracellular Vpr has been shown to induce latently infected cells into productive expression, again suggesting a role for the protein after integration (40, 41). It has also been shown that preventing the late expression of Vpr by using antisense approaches drastically reduces viral replication (6). It is interesting in this regard that a recent report suggests that Vpr can modulate the NFkB pathway by upregulating IκB transcription (42).
Hence, there appears to be a clearly identifiable functional contribution to both early and late viral replication events by HIV-1 Vpr and the results presented here strongly suggest that the notable augmentation in the viral production seen in the presence of Vpr at low viral inputs is probably due to the additive contributions of both the early and late functions of the protein. In in vitro experiments, it may not be possible to demonstrate the early and late functions in exclusion of each other except by using specific mutants or physiologically relevant viral inputs, and this could explain why some early studies vastly underestimated Vpr's contribution to viral replication. It is conceivable that similar mutants favoring one or the other function may be favored in some stages of the disease in vivo. For instance, in vitro studies show that mutating the COOH-terminal region primarily impairs the G2-arrest function of the protein (28, 29), and in this study we correlate this activity with increased viral replication. In this regard, it is interesting to note that such COOH-terminal mutations are associated with low viral loads and lack of disease progression in vivo (43). In fact, Vpr's combined ability to confer optimal infectivity (early function) and its ability to augment viral production after such infection (late function) may provide a considerable selective advantage in vivo to viruses that code for this protein. This notion is strongly supported by the fact that HIV-2 and SIV conserve both Vpx and Vpr-2, two separate proteins amongst which the two functions of HIV-1 Vpr segregate (44).
Some of the regulatory products coded for by HIV-1 have been deemed dispensable or “accessory” based on the fact that viral replication in vitro, albeit attenuated, still occurs even in their absence (45). However, several lines of evidence suggest that accessory genes are not likely to be dispensable in vivo. The ability to code for these genes is maintained in distant lentiviral relatives and specifically within the HIV-1 family of viruses. The open reading frames are invariably retained in all HIV-1 clades and notable conservation of the gene sequences is a common feature. It is particularly difficult to reconcile such conservation of nonessential genes in a highly adaptable system such as HIV-1. In this context, it is interesting to note that some experiments have documented repair or recombinational mechanisms that reestablish the expression of Vpr protein in vivo, suggesting a functional need for this protein during natural infection (46, 47). The potential in vivo relevance of accessory proteins such as Vpr is also suggested by some in vitro experiments in which the inability to code for these proteins appears to affect HIV-1 replication acutely when primary rather than transformed cells are used as targets (for review see reference 45). This observation again suggests experimental conditions more representative of an in vivo milieu may be necessary to truly assess the functional relevance of these genes. Our results suggest that one such accessory protein, HIV-1 Vpr, plays an essential and not a dispensable role in the infection of nondividing cells under conditions that closely mimic in vivo situations, that is, primary cells being infected at low viral inputs.
Acknowledgments
We thank Françoise Boisvert, Jean-Pierre Baril, Marielle Lafontaine, Manon Poirier, and Hôpital Maisonneuve Rosemont for volunteer acquisition; Serge Senechal for FACS® analysis of G2-arrest and macrophage purity tests; and Hugo Dilhuydy for confocal analysis of Vpr nuclear localization. We also thank Dr. Lee Ratner for providing us the macrophage-tropic parental ADA clone, Dr. Bouvier for the β2-AR expressor plasmid, and Dr. Daniel Lammare and BioMega-Boehringer Ingelheim for the generous gift of the protease inhibitors. The authors also acknowledge Nicole Rougeau and Sonia Tremblay for excellent technical support.
Footnotes
1 Abbreviations used in this paper: β2-AR, β2-adrenergic receptor; SIV, simian immunodeficiency virus.
E.A. Cohen is a recipient of a National Health Scientist award from the National Health Research and Development Program (NHRDP) of Canada. This work was supported by grants from the Medical Research Council and Fonds pour La Formation de Chercheurs et L'Aide à La Recherche (FCAR) to E.A. Cohen.
References
- 1.Arrigo SJ, Chen ISY. Rev is necessary for translation but not cytoplasmic accumulation of HIV-1 vif, vpr, and env/vpu 2 RNAs. Genes Dev. 1991;5:808–819. doi: 10.1101/gad.5.5.808. [DOI] [PubMed] [Google Scholar]
- 2.Garrett ED, Tiley LS, Cullen BR. Rev activates expression of the human immunodeficiency virus type 1 vif and vpr gene products. J Virol. 1991;65:1653–1657. doi: 10.1128/jvi.65.3.1653-1657.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tristem M, Marshall C, Karpas A, Petrik J, Hill F. Origin of vpx in lentiviruses. Nature. 1990;347:341–342. doi: 10.1038/347341b0. [DOI] [PubMed] [Google Scholar]
- 4.Tristem M, Marshall C, Karpas A, Hill F. Evolution of the primate lentiviruses: evidence from vpx and vpr. EMBO (Eur Mol Biol Organ) J. 1992;11:3405–3412. doi: 10.1002/j.1460-2075.1992.tb05419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balliet JW, Kolson DL, Eiger G, Kim FM, McGann KA, Srinivasan A, Collman R. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology. 1994;200:623–631. doi: 10.1006/viro.1994.1225. [DOI] [PubMed] [Google Scholar]
- 6.Balotta C, Lusso P, Crowley R, Gallo RC, Franchini G. Antisense phosphorothioate oligodeoxynucleotides targeted to the vpr gene inhibit human immunodeficiency virus type 1 replication in primary human macrophages. J Virol. 1993;67:4409–4414. doi: 10.1128/jvi.67.7.4409-4414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connor RI, Kuan K, Chen, Choe S, Landau N. Vpr is required for efficient replication of human immunodeficiency virus type 1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 8.Hattori N, Michaels F, Fargnoli K, Marcon L, Gallo RC, Franchini G. The human immunodeficiency virus type 2 vpr gene is essential for productive infection of human macrophages. Proc Natl Acad Sci USA. 1990;87:8080–8084. doi: 10.1073/pnas.87.20.8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westervelt P, Henkel T, Trowbridge DB, Orenstein J, Heuser J, Gendelman HE, Ratner L. Dual regulation of silent and productive infection in monocytes by distinct human immunodeficiency virus type 1 determinants. J Virol. 1992;66:3925–3931. doi: 10.1128/jvi.66.6.3925-3931.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen EA, Terwilliger EF, Jalinoos Y, Proulx J, Sodroski JG, Haseltine WA. Identification of HIV-1 vpr product and function. J Acquired Immune Defic Syndr. 1990;3:11–18. [PubMed] [Google Scholar]
- 11.Heinzinger NK, Bukinsky MI, Haggerty SA, Ragland AM, Kewalramani V, Lee MA, Gendelman HE, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallay P, Stitt V, Mundy C, Oettinger M, Trono D. Role of the karyopherin pathway in human immunodeficiency virus type 1 nuclear import. J Virol. 1996;70:1027–1032. doi: 10.1128/jvi.70.2.1027-1032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallay P, Hope T, Chin D, Trono D. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc Natl Acad Sci USA. 1997;94:9825–9830. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He J, Choe S, Walker R, Di Marzio P, Morgan DO, Landau NR. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jowett JB, Planelles V, Poon B, Shah NP, Chen ML, Chen IS. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Planelles V, Jowett JB, Li QX, Xie Y, Hahn B, Chen IS. Vpr-induced cell cycle arrest is conserved among primate lentiviruses. J Virol. 1996;70:2516–2524. doi: 10.1128/jvi.70.4.2516-2524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Re F, Braaten D, Franke EK, Luban J. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J Virol. 1995;69:6859–6864. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogel ME, Wu LI, Emerman M. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J Virol. 1995;69:882–888. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stivahtis GL, Soares MA, Vodicka MA, Hahn BH, Emerman M. Conservation and host specificity of Vpr-mediated cell cycle arrest suggest a fundamental role in primate lentivirus evolution and biology. J Virol. 1997;71:4331–4338. doi: 10.1128/jvi.71.6.4331-4338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beaulieu S, Kessous A, Landry D, Montplaisir S, Bergeron D, Cohen EA. In vitro characterization of purified human thymic dendritic cells infected with human immunodeficiency virus type-1. Virology. 1996;222:214–226. doi: 10.1006/viro.1996.0412. [DOI] [PubMed] [Google Scholar]
- 21.Yao XJ, Subbramanian RA, Rougeau N, Boisvert F, Bergeron D, Cohen EA. Mutagenic analysis of human immunodeficiency virus type 1 Vpr: role of a predicted N-terminal alpha-helical structure in Vpr nuclear localization and virion incorporation. J Virol. 1995;69:7032–7044. doi: 10.1128/jvi.69.11.7032-7044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamarre D, Croteau G, Wardrop E, Bourgon L, Thibeault D, Clouette C, Vaillancourt M, Cohen EA, Pargellis C, Yoakim C, Anderson PC. Antiviral properties of Palinavir, a potent inhibitor of the human immunodeficiency virus type 1 protease. Antimicrob Agents Chemother. 1997;41:965–971. doi: 10.1128/aac.41.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasse A, Brahie L, Stowring L, Blum H. Detection of viral nucleic acids by in situ hybridization. Methods Virol. 1984;7:189–226. [Google Scholar]
- 24.Cox KH, DeLeon DV, Angerer LM, Angerer RC. Detection of mRNAs in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984;101:485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- 25.Berrada F, Ma D, Michaud J, Doucet G, Giroux L, Kessous-Elbaz A. Neuronal expression of human immunodeficiency virus type 1 env proteins in transgenic mice: distribution in the central nervous system and pathological alteration. J Virol. 1995;69:6770–6778. doi: 10.1128/jvi.69.11.6770-6778.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon JH, Malim MH. The human immunodeficiency virus type 1 vif protein modulates the postpenetration stability of viral nucleoprotein complex. J Virol. 1996;70:5297–5305. doi: 10.1128/jvi.70.8.5297-5305.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jockers R, Silva AD, Strosberg AD, Bouvier M, Marullo S. New molecular and structural determinants involved in β2-adrenergic receptor desensitization and sequestration. Delineation using chimeric β3/β2-adrenergic receptors. J Biol Chem. 1996;271:9355–9362. doi: 10.1074/jbc.271.16.9355. [DOI] [PubMed] [Google Scholar]
- 28.Di Marzio P, Choe S, Ebright M, Knoblauch R, Landau NR. Mutational analysis of cell cycle arrest, nuclear localization and virion packaging of human immunodeficiency virus type 1 Vpr. J Virol. 1995;69:7909–7916. doi: 10.1128/jvi.69.12.7909-7916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahalingam S, Ayyavoo V, Patel M, Kieber-Emmons T, Weiner DB. Nuclear import, virion incorporation, and cell cycle arrest/differentiation are mediated by distinct functional domains of human immunodeficiency vpr. J Virol. 1997;71:6339–6347. doi: 10.1128/jvi.71.9.6339-6347.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahalingam S, Collman RG, Patel M, Monken CE, Srinivasan A. Functional analysis of HIV-1 Vpr: identification of determinants essential for subcellular localization. Virology. 1995;212:331–339. doi: 10.1006/viro.1995.1490. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Mukherjee S, Narayan O, Zhao LJ. Characterization of a leucine-zipper-like domain in Vpr protein of human immunodeficiency virus type 1. Gene. 1996;178:7–13. doi: 10.1016/0378-1119(96)00312-5. [DOI] [PubMed] [Google Scholar]
- 32.Zhao L-J, Mukherjee S, Narayan O. Biochemical mechanism of HIV-1 vpr function: specific interaction with a cellular protein. J Biol Chem. 1994;289:15827–15832. [PubMed] [Google Scholar]
- 33.Trono D, Gallay P. In response to Freed et al. Cell. 1997;88:173–174. [Google Scholar]
- 34.Miller MD, Warmerdam MT, Gaston I, Greene WC, Feinberg MB. The human immunodeficiency virus 1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J Exp Med. 1994;179:101–113. doi: 10.1084/jem.179.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spina CA, Kwoh TJ, Chowers MY, Guatelli JC, Richman DD. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallay P, Swingler S, Song J, Bushman F, Trono D. HIV Nuclear import is governed by the phosphotyrosine-mediated binding of matrix to the core domain of integrase. Cell. 1995;83:569–576. doi: 10.1016/0092-8674(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 37.Schuitemaker H, Koostra NA, Koppelman MHGM, Bruisten S, Huisman HG, Tersmette M, Miedema F. Proliferation-dependent HIV-1 infection of monocytes occurs during differentiation into macrophages. J Clin Invest. 1992;89:1154–1160. doi: 10.1172/JCI115697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emerman M. HIV-1, Vpr and the cell cycle. Curr Biol. 1996;6:1096–1103. doi: 10.1016/s0960-9822(02)00676-0. [DOI] [PubMed] [Google Scholar]
- 39.Gallay P, Swingler S, Aiken C, Trono D. HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is a key regulator. Cell. 1995;80:379–388. doi: 10.1016/0092-8674(95)90488-3. [DOI] [PubMed] [Google Scholar]
- 40.Levy DN, Refaeli Y, MacGregor RR, Weiner DB. Serum Vpr regulates productive infection and latency of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1994;91:10873–10877. doi: 10.1073/pnas.91.23.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levy DN, Refaeli Y, Weiner DB. Extracellular Vpr protein increases cellular permissiveness to human immunodeficiency virus replication and reactivates virus from latency. J Virol. 1995;69:1243–1252. doi: 10.1128/jvi.69.2.1243-1252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingam R, Kudchodkar S, Willams WV, Green DR, Weiner DB. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor κB. Nat Med. 1997;3:1117–1123. doi: 10.1038/nm1097-1117. [DOI] [PubMed] [Google Scholar]
- 43.Wang B, Ge YC, Palasanthiran P, Xiang SH, Ziegler J, Dwyer DE, Randle C, Dowton D, Cunningham A, Saksena NK. Gene defects clustered at the C-terminus of the vpr gene of HIV-1 in long-term nonprogressing mother and child pair: in vivo evolution of vpr quasispecies in blood and plasma. Virology. 1996;223:224–232. doi: 10.1006/viro.1996.0471. [DOI] [PubMed] [Google Scholar]
- 44.Fletcher TM, 3rd, Brichacek B, Sharova N, Newman MA, Stivahtis G, Sharp PM, Emerman M, Hahn BH, Stevenson M. Nuclear import and cell cycle arrest functions of the HIV-1 Vpr protein are encoded by two separate genes in HIV-2/SIV(SM) EMBO (Eur Mol Biol Organ) J. 1996;15:6155–6165. [PMC free article] [PubMed] [Google Scholar]
- 45.Subbramanian R, Cohen EA. Molecular biology of HIV accessory genes. J Virol. 1994;68:6831–6835. doi: 10.1128/jvi.68.11.6831-6835.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wooley DP, Smith RA, Czajak S, Desrosiers RC. Direct demonstration of retroviral recombination in rhesus monkey. J Virol. 1997;71:9650–9653. doi: 10.1128/jvi.71.12.9650-9653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lang SM, Weeger M, Stahl-Hennig C, Coulibaly C, Hunsmann G, Muller J, Muller-Hermelink H, Fuchs D, Wachter H, Daniel MM, et al. Importance of vpr for infection of rhesus monkeys with simian immunodeficiency virus. J Virol. 1993;67:902–912. doi: 10.1128/jvi.67.2.902-912.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Folks TM, Clouse KA, Justement J, Rabson A, Duh E, Kehrl JH, Fauci A. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T cell clone. Proc Natl Acad Sci USA. 1989;86:2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]