Abstract
The significance of cytokine production by CD4+ regulatory T (T reg) cells after antigen exposure in vivo and its impact on their regulatory activity remains unclear. Pretreatment with donor alloantigen under the cover of anti-CD4 therapy generates alloantigen reactive T reg cells that can prevent rejection of donor-specific skin grafts that are mediated by naive CD45RBhighCD4+ T cells. To examine the kinetics and importance of cytokine gene transcription by such alloantigen-reactive T reg cells, pretreated mice were rechallenged with donor alloantigen in vivo. CD25+CD4+ T cells, but not CD25−CD4+ T cells, showed a fivefold increase in IFN-γ mRNA expression within 24 h of reencountering alloantigen in vivo. This expression kinetic was highly antigen-specific and was of functional significance. Neutralizing IFN-γ at the time of cotransfer of alloantigen reactive T reg cells, together with CD45RBhighCD4+ effector T cells into Rag −/− skin graft recipients, resulted in skin graft necrosis in all recipients; the generation and function of alloantigen-reactive T reg cells was impaired dramatically in IFN-γ–deficient mice. These data support a unique role for IFN-γ in the functional activity of alloantigen-reactive T reg cells during the development of operational tolerance to donor alloantigens in vivo.
T cell subpopulations with regulatory function (regulatory T [T reg] cells) can be identified and were shown to play a key role in controlling immune responsiveness in vivo; however, their precise mechanism of action remains controversial. In particular, how cytokines contribute to immunoregulation by T reg cells, if at all, is far from clear. In vivo, TGF-β and IL-10 play a role in immunoregulation in some (1–4), but not all, settings (5). In vitro, antigen stimulation of T reg cells often results in much higher levels of expression of TGF-β and IL-10 than those produced by naive or effector T cells (6–8). However, the significance of this increased TGF-β and IL-10 expression in vitro is not understood because several groups have shown that neutralization of TGF-β and IL-10 has no effect on immunoregulatory activity (5–7). Moreover, in vitro stimulation of T reg cells produces little or no IL-2 and IFN-γ compared with that produced by effector cells under similar conditions; however, IL-2 and IFN-γ are required for the induction of tolerance to alloantigens in vivo (9, 10). Thus, the kinetics and functional significance of cytokine expression by T reg cells after in vivo antigen stimulation require clarification.
T reg cells can play a major role during the induction and maintenance of operational tolerance to donor alloantigens in vivo (11). We showed previously that pretreatment with donor-specific transfusion in combination with anti-CD4 therapy generated donor-specific CD4+ T reg cells that can suppress rejection of skin grafts mediated by naive CD45RBhighCD4+ T cells (3). In this model, T reg cells are contained predominantly in the CD25+ fraction, because equivalent numbers of CD25−CD4+ T cells were unable to prevent rejection.
The goal of our present study was to follow cytokine expression by alloantigen-reactive T reg cells after in vivo alloantigen stimulation and to evaluate its functional significance. Here we demonstrate that in contrast to findings after ex vivo or in vitro stimulation, CD25+CD4+ T cells transiently up-regulate IFN-γ mRNA expression after alloantigen exposure in vivo. This increase in IFN-γ transcription is antigen specific and is not a result of contamination with antigen-experienced or recently activated T cells. Furthermore, neutralization experiments and studies with IFN-γ–deficient mice reveal an important role of IFN-γ production for the generation and the function of alloantigen reactive T reg cells in vivo.
RESULTS
Cytokine expression of alloantigen reactive regulatory T cells after antigen exposure in vivo
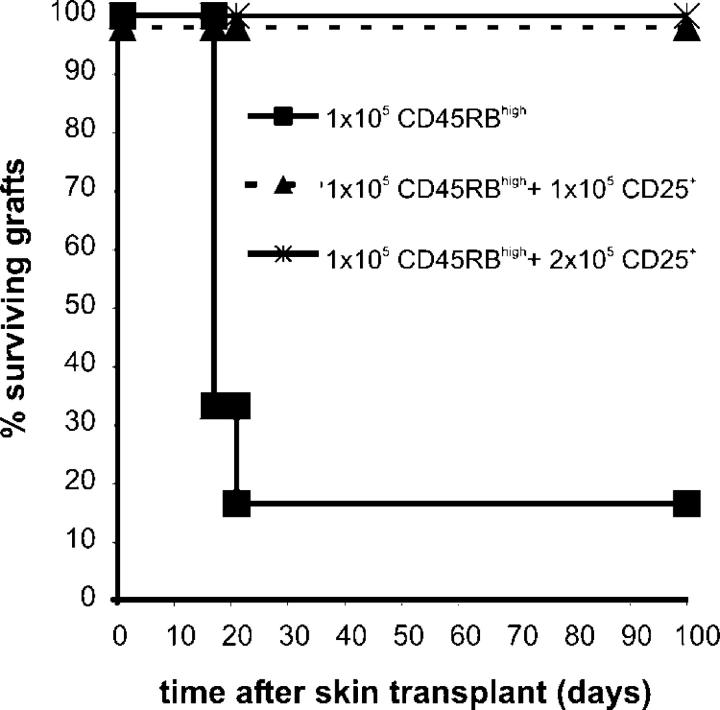
The first goal of this study was to determine the kinetics of cytokine mRNA expression by alloantigen-reactive T reg cells after alloantigen exposure in vivo. Naive CBA (H2k) mice were pretreated with YTS177 anti-CD4 antibody at days −28/27 (200 μg i.v.) and a B10 (H2b) blood transfusion at day −27 (250 μl whole blood i.v.; 177/B10DST). This pretreatment protocol results in long-term survival and induction of operational tolerance to blood donor–specific—B10—but not third-party allografts transplanted on day 0 (12). The induction and maintenance of operational tolerance to donor alloantigens is a result of the development of alloantigen-reactive immunoregulatory CD4+ T cells (3). In this study, 177/B10DST-pretreated mice received an additional B10 blood transfusion 1 d or 3 d before CD25+CD4+ and CD25−CD4+ T cells were purified from the spleen by flow cytometry (day 0). B10 blood transfusion was chosen to provide the antigen challenge instead of a transplant to ensure that antigen-specific CD25+CD4+ T cells were found in the spleen and regional lymph nodes rather than at the graft site, as may be the case after transplantation (13). The presence of CD25+CD4+ T cells with regulatory potential in the spleen 24 h after an additional B10 blood transfusion was demonstrated by their ability to prevent skin graft rejection upon adoptive transfer (Fig. 1).
Figure 1.
Regulation of skin graft rejection by CD25+CD4+ T cells from 177/B10DST-pretreated CBA mice after a rechallenge with B10 blood at day −1. 177/B10DST-pretreated CBA mice were rechallenged with 250 μl B10 blood on day −1. On day 0, spleens were harvested and cells were sorted into CD25+CD4+ T cells. CBARag(−/−) mice that were reconstituted with 105 CD45RBhighCD4+ T cells from naive CBA mice acutely rejected B10 skin grafts (n = 6, mean survival time [MST] = 17 d). Cotransfer of 1 × 105 or 2 × 105 CD25+CD4+ T cells from 177/B10DST-pretreated CBA mice that were re-exposed to donor alloantigen 1 d before cell purification prevented rejection of B10 skin grafts (n = 4, MST >100 d).
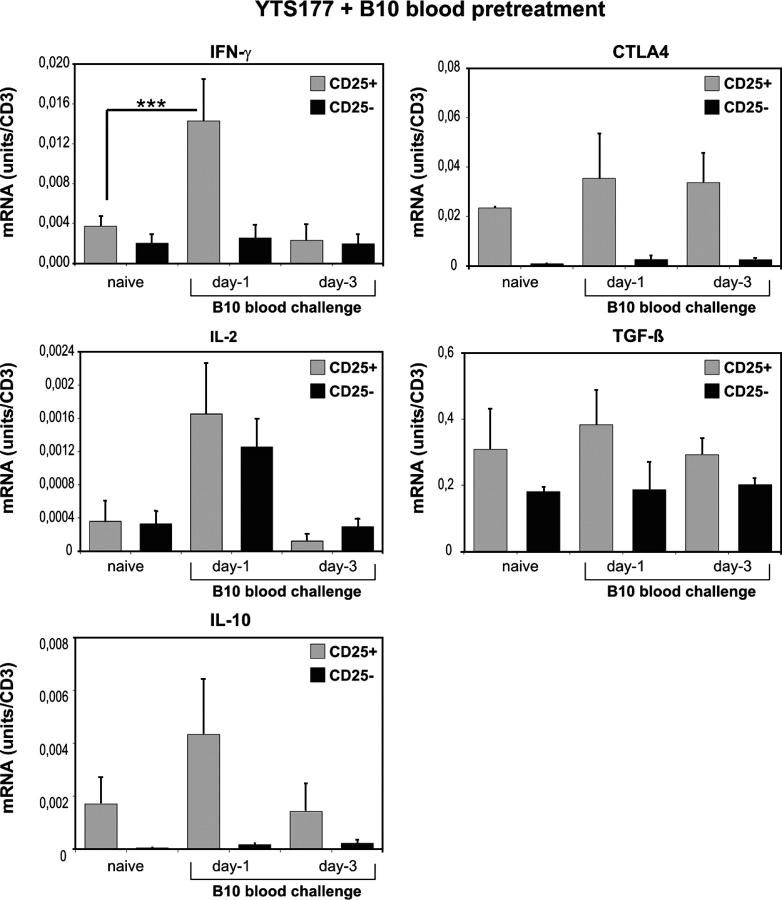
mRNA expression for the following genes, IL-2, IFN-γ, IL-10, TGF-β, and CTLA-4, was quantified in the sorted T cell populations. 177/B10DST pretreatment without restimulation by an additional B10 transfusion did not influence cytokine mRNA expression by CD25+CD4+ or CD25−CD4+ T cells; cytokine expression levels were the same as those in T cells that were purified from the spleen of naive CBA mice (Fig. 2). In contrast, additional exposure to B10 alloantigens resulted in a fivefold increase in IFN-γ mRNA expression by CD25+CD4+ T cells, but not CD25− CD4+ T cells, 24 h after restimulation (P < 0.001). By 72 h after alloantigen challenge, IFN-γ mRNA levels in CD25+ CD4+ T cells that were purified from pretreated mice had decreased to less than those in CD25+CD4+ T cells from naive CBA mice. IL-2 mRNA expression similarly was up-regulated transiently; however, in this case, the increase was detected in CD25+CD4+ and CD25−CD4+ T cells (Fig. 2). In agreement with findings of other groups, CD25+CD4+ T cells displayed a higher basal level expression of IL-10 mRNA and they did up-regulate IL-10 mRNA expression after alloantigen exposure in vivo (Fig. 2). Although CD25+CD4+ T cells displayed a 50-fold greater basal transcription level of CTLA4 compared with CD25−CD4+ T cells, they did not up-regulate CTLA4 mRNA expression after alloantigen exposure in vivo. TGF-β mRNA expression in CD25+CD4+ T cells was not greater than that in CD25−CD4+ T cells; there was no significant increase in TGF-β transcription detectable in CD25+CD4+ T cells from pretreated CBA mice on days 1 or 3 after a B10 blood challenge.
Figure 2.
Cytokine mRNA expression by CD25+CD4+ and CD25−CD4+ T cells from 177/B10DST-pretreated CBA mice after additional exposure to B10 blood at day −1 or −3 before cell isolation. 177/B10DST-pretreated CBA mice were rechallenged with 250 μl B10 blood on days −3 or −1. On day 0, spleens were harvested and cells were sorted into CD25+CD4+ or CD25−CD4+ T cells, and cytokine expression was analyzed. Data are presented as mean ± SD of four independent experiments. ***P < 0.001.
Enhanced IFN-γ expression by alloantigen-reactive regulatory T cells is antigen specific
Next, we investigated whether the increase in IFN-γ mRNA expression by CD25+CD4+ T cells was antigen specific. Naive CBA mice were pretreated with YTS177 and a BALB/c (H2d) blood transfusion under the same conditions as above. Subsequently, pretreated mice received a B10 blood transfusion, and cytokine mRNA expression in CD25+CD4+ and CD25−CD4+ T cells was analyzed 24 h and 72 h later. As shown in Fig. 3, stimulation of CD25+ CD4+ T cells with a third-party antigen did not initiate up-regulation in IFN-γ mRNA.
Figure 3.
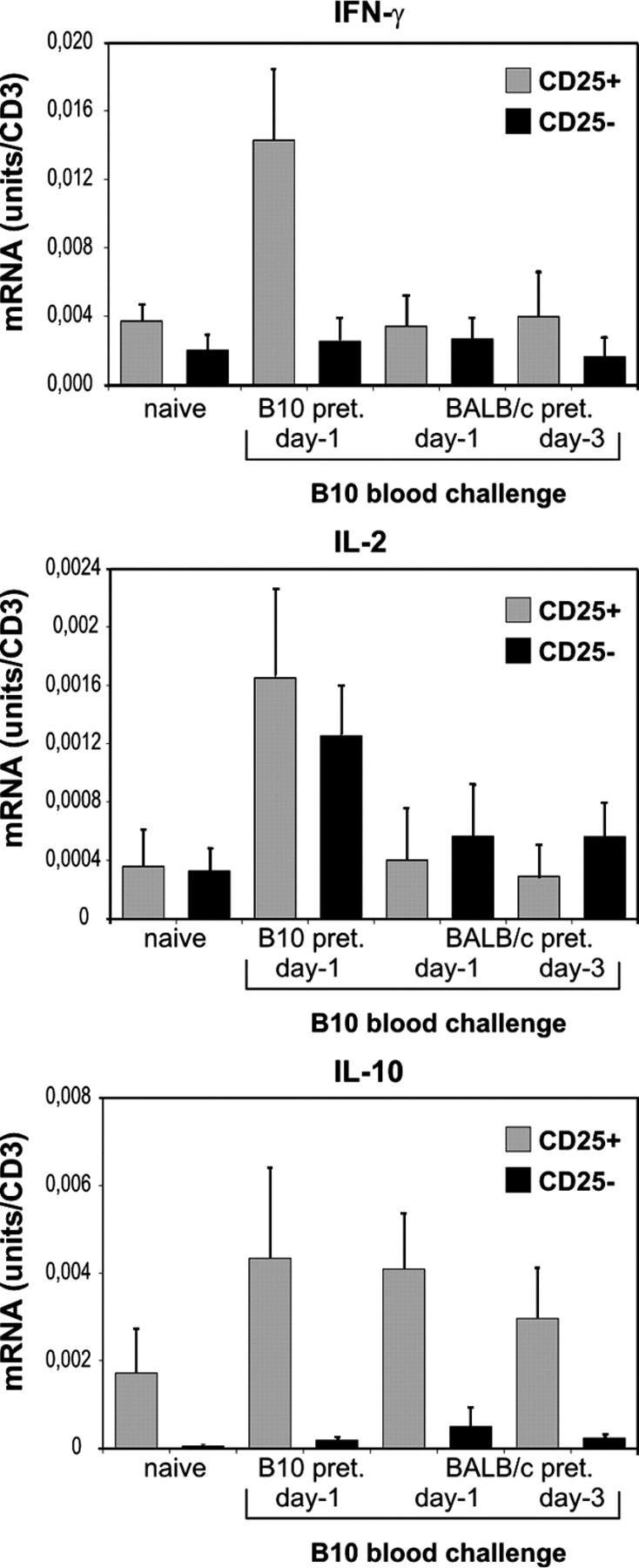
Up-regulation of IFN-γ mRNA expression by CD25+CD4+ T cells after in vivo stimulation is alloantigen specific. CBA mice were pretreated with 200 μg YTS177 on days −28 and −27. On day −27 they also received 250 μl of whole BALB/c blood. Mice were rechallenged with 250 μl B10 blood on days −3 or −1. On day 0, spleens were harvested and cells were sorted into CD25+CD4+ or CD25−CD4+ T cells and cytokine mRNA expression was analyzed. Data are presented as mean ± SD of three independent experiments.
IFN-γ mRNA up-regulation is not due to contamination with recently activated or antigen-experienced T cells
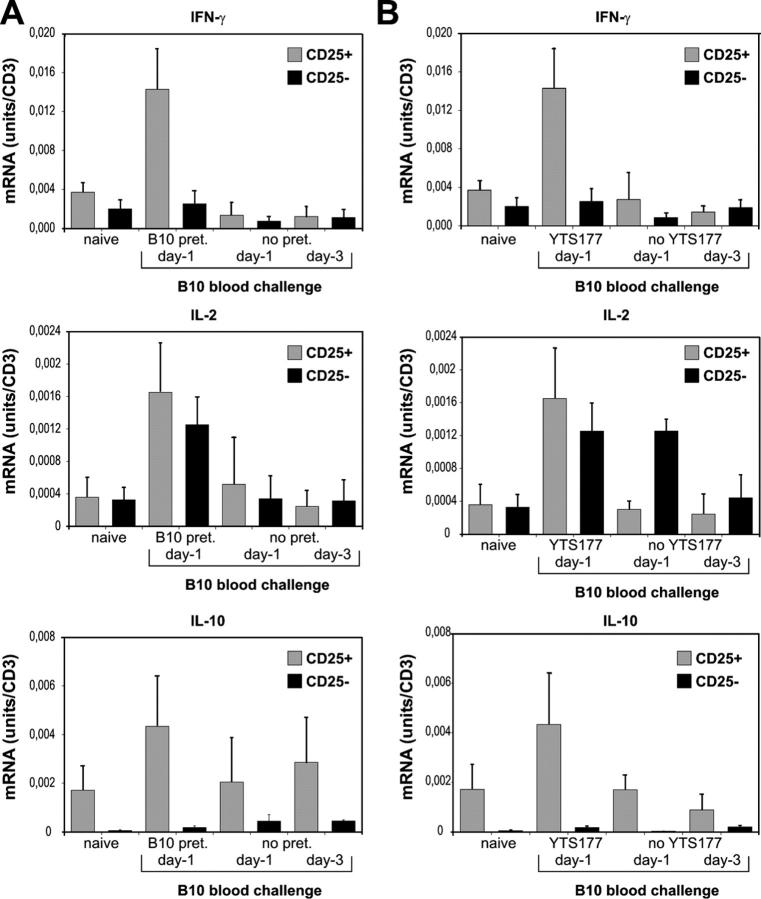
To exclude the possibility that the enhanced IFN-γ transcription that was observed in CD25+CD4+ T cells was due to the presence of recently activated T cells, we quantified cytokine mRNA expression after a single B10 blood transfusion. As shown in Fig. 4, a single blood transfusion (days −3 or −1) without re-exposure to the same alloantigen did not elicit an increase in cytokine mRNA expression in CD25+CD4+ or CD25−CD4+ T cells. To determine if both components of the pretreatment (i.e., anti-CD4 and blood transfusion) were necessary to elicit IFN-γ mRNA up-regulation in CD25+CD4+ T cells after antigen-specific restimulation in vivo, CBA mice were pretreated with a B10 blood transfusion (day −27) only; cytokine transcription by CD25+CD4+ and CD25−CD4+ T cells was analyzed after an additional B10 blood transfusion (day −3 or day −1). Although repeated exposure to alloantigen led to the development of antigen-experienced CD25−CD4+ T cells as demonstrated by enhanced IL-2 transcription within this cell fraction, no increase in IFN-γ mRNA expression in CD25+CD4+ T cells was detectable (Fig. 4). These findings support our previous results where both components—anti-CD4 and donor alloantigen—were needed for the generation of alloantigen-reactive T reg cells and prolonged graft survival (3).
Figure 4.
Enhanced IFN-γ mRNA expression by alloantigen-reactive regulatory T cells after in vivo stimulation is not due to inclusion of recently activated T cells or antigen-experienced T cells. (A) Cytokine mRNA expression of CD25+CD4+ and CD25−CD4+ T cells after administration of B10 blood on day −3 or −1 to naive CBA mice. On day 0, spleens were harvested and cells were sorted into CD25+CD4+ or CD25−CD4+ T cells and cytokine mRNA expression was analyzed. pret, pretreatment. (B) Cytokine mRNA expression by CD25+CD4+ and CD25−CD4+ T cells after administration of B10 blood on days −3 or −1 to CBA mice that were pretreated with B10 blood. CBA mice were pretreated with 250 μl of whole B10 blood on day −27. Mice were rechallenged with 250 μl B10 blood on days −3 or −1. On day 0, spleens were harvested and cells were sorted into CD25+CD4+ or CD25−CD4+ T cells and cytokine mRNA expression analyzed. Data are presented as mean ± SD of three independent experiments.
In vivo neutralization of IFN-γ dramatically reduces regulation by CD25+CD4+ T cells
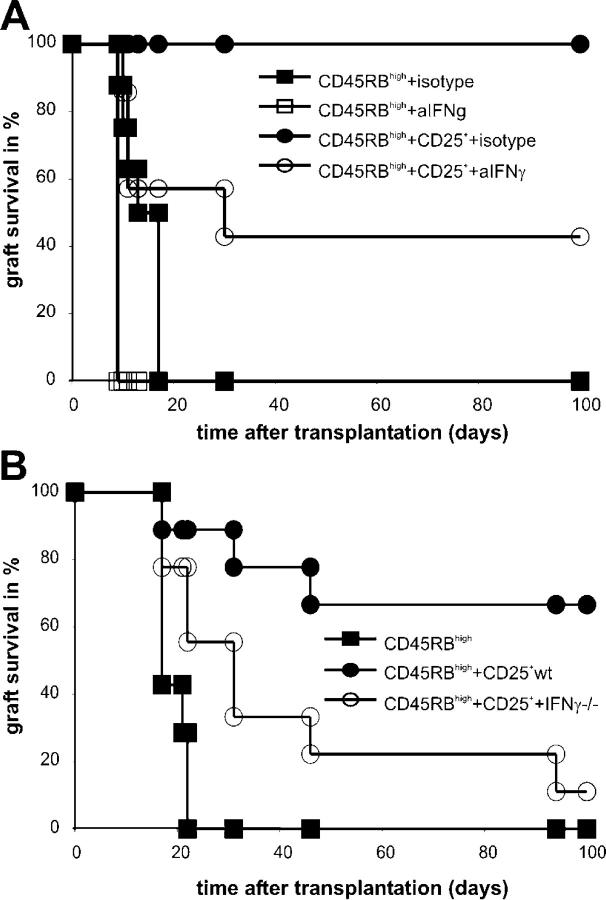
Next we investigated whether neutralizing IFN-γ at the time of cell transfer affected the regulatory capacity of CD25+CD4+ T cells. 5 × 105 CD25+CD4+ T cells from 177/B10DST-pretreated CBA mice were transferred adoptively into CBA(Rag−/−) mice together with 105 CD45RBhighCD4+ T cells from naive CBA mice. At the time of adoptive transfer, a single dose of 200 μg neutralizing anti–mouse IFN-γ or isotype control antibody was administered i.v.; the following day, mice received a B10 skin allograft. Administration of anti-IFN-γ mAb abrogated immunoregulation by CD25+CD4+ T cells from pretreated mice (Table I; Fig. 5). Skin grafts in all mice treated with anti–IFN-γ mAb showed severe signs of necrosis between day 7 and day 11 after transplantation. Four out of seven mice rejected their grafts completely, whereas in the other three mice the signs of graft necrosis resolved (P = 0.0338). Because the definition of skin graft rejection that is used in this study is complete necrosis of the graft, these latter skin grafts—although damaged—could not be considered as rejected. In contrast, all CBA(Rag−/−) recipients that received an isotype control accepted their grafts without any evidence of tissue necrosis at any time point (Table I). Thus, early production of IFN-γ in vivo is required for CD25+CD4+ T cells to prevent effector cells from damaging the graft.
Table I.
Neutralization of IFN-γ dramatically reduced regulation by CD25+CD4+ T cells in vivo
|
Adoptive transfer
|
||||||
|---|---|---|---|---|---|---|
| CD45RBhigh | CD25+ | Anti–IFN-γ mAb | Isotype | Evidence of necrosisbetween days 7 and 11(>50% of graft) | Graft survival time | Graft survival at 100 d |
| d | ||||||
| + | − | − | + | 8/8 | 9, 10, 11, 13, 4 × 17 | 0/8 |
| + | − | + | − | 4/4 | 4 × 9 | 0/4 |
| + | + | − | + | 0/6 | 6× >100 | 6/6 |
| + | + | + | − | 7/7 | 10, 2 × 11, 30, 3× >100 | 3/7a |
P < 0.01.
Figure 5.
Loss of in vivo regulation after IFN-γ neutralization or when cotransferring CD25+CD4+ from YTS177/B10DST-pretreated IFN-γ–deficient mice. (A) Neutralization of IFN-γ dramatically reduced regulation by CD25+CD4+ T cells in vivo. CBARag(−/−) reconstituted with 105 CD45RBhighCD4+ T cells alone treated with a single dose (200 μg) of neutralizing anti–IFN-γ antibody or an isotype control antibody at the time of adoptive cell transfer acutely rejected their B10 skin grafts. Four out of seven mice that were reconstituted with naive 105 CD45RBhighCD4+ T cells and 5 × 105 CD25+CD4+ T cells from 177/B10DST-pretreated CBA mice and treated with 200 μg of the neutralizing anti–IFN-γ antibody at the time of cell cotransfer rejected their B10 skin grafts, whereas all recipients that were treated with 200 μg of an isotype control antibody accepted their grafts. (B) Impaired development of alloantigen-reactive T reg cells in the absence of IFN-γ. C57BL/6 wild-type and C57BL/6IFN-γ(−/−) mice were pretreated with 200 μg YTS177 on days −28 and −27. On day −27 they also received 250 μl of whole BALB/c blood. On day 0, spleens were harvested and cells were sorted into CD25+CD4+ T cells. C57BL/6Rag(−/−) mice that were reconstituted with 105 CD45RBhighCD4+ T cells from naive C57BL/6 mice acutely rejected BALB/c skin grafts. Cotransfer of 5 x105 CD25+CD4+ T cells from 177/BALB/cDST-pretreated C57BL/6 wild-type mice prevented rejection of BALB/c skin grafts in six out of nine recipients. Seven out of nine mice that were reconstituted with naive 105 CD45RBhighCD4+ T cells and 5 × 105 CD25+CD4+ T cells from 177/BALB/cDST-pretreated C57BL/6 IFN-γ(−/−) mice at the time of cell cotransfer rejected their BALBc skin grafts.
Loss of in vivo regulation after IFN-γ neutralization is accompanied by reduced iNOS transcription within the graft
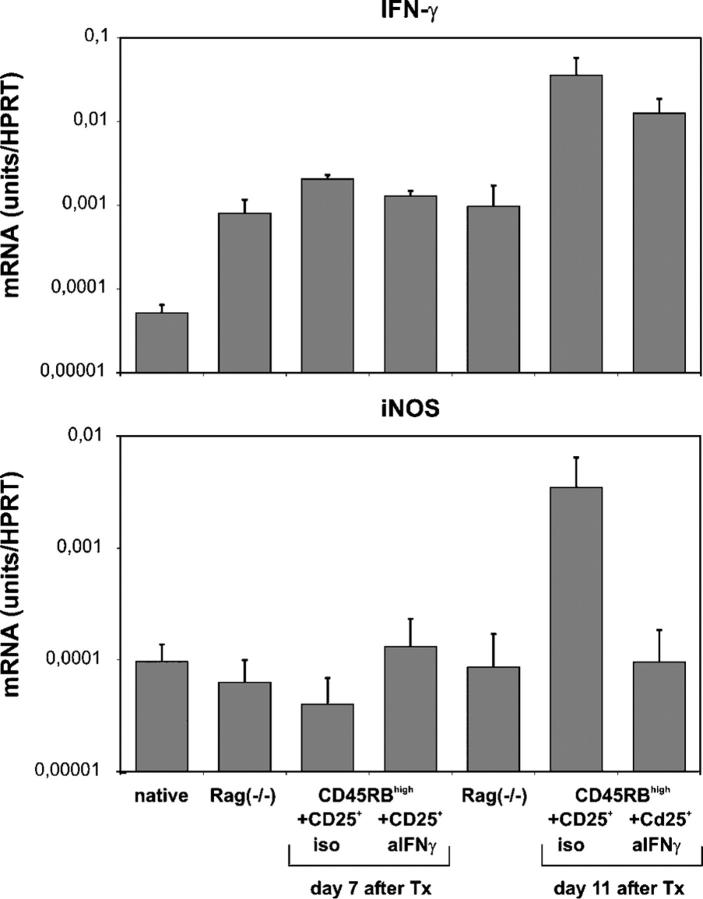
Transcription of inducible nitric oxide synthase (iNOS) is induced when cells are stimulated with IFN-γ (14). To confirm that treatment with anti–IFN-γ mAb resulted in neutralization of IFN-γ in vivo, we analyzed IFN-γ and iNOS mRNA expression in native B10 skin and in B10 skin grafts 7 or 11 d after transplantation onto unreconstituted or reconstituted Rag(−/−) recipients that were treated with an isotype control or anti–IFN-γ mAb (Fig. 6). In the anti–IFN-γ mAb–treated group, only skin grafts from recipients that already had rejected their grafts completely were included in the day 11 analysis. Skin grafts that had not been rejected until day 11 were not tested.
Figure 6.
IFN-γ neutralization is accompanied by reduced iNOS transcription within the graft. IFN-γ and iNOS mRNA expression of native B10 skins, B10 skins harvested 7 and 11 d after transplantation (Tx) onto unreconstituted CBARag(−/−) mice and reconstituted CBARag(−/−) mice with 105 naive CD45RBhighCD4+ T cells and 5 × 105 CD25+CD4+ T cells from 177/B10DST-pretreated CBA mice treated with a single dose of 200 μg of the neutralizing anti–IFN-γ antibody (AN18.17.24) or an isotype control antibody (GL113) at the time of cell cotransfer. Grafts were harvested, and total RNA was isolated and reverse transcribed. Cytokine mRNA expression was analyzed by applying real time RT-PCR. HPRT was used as a housekeeping gene and mRNA concentrations were compared with the sample content of HPRT. Data are presented as mean ± SD of three independent experiments.
Unreconstituted and reconstituted mice displayed elevated IFN-γ mRNA expression in their skin grafts at day 7 after transplantation. However, reconstituted mice showed an additional 50-fold increase in IFN-γ transcription at day 11 that was not detectable in grafts from unreconstituted mice. Thus, the major increase in IFN-γ mRNA expression is dependent on the presence of T cells. A similar increase in IFN-γ transcription was detected in skin grafts from mice that were reconstituted with CD25+CD4+ T cells from 177/B10DST-pretreated CBA mice (unpublished data). Neutralization of IFN-γ did not affect IFN-γ mRNA expression as expected, but iNOS mRNA levels were reduced dramatically in the grafts of mice that were treated with the neutralizing IFN-γ mAb compared with those in skin grafts from isotype mAb–treated mice (Fig. 6). This confirmed the impact of neutralizing the functional activity of IFN-γ in vivo.
Impaired development of alloantigen reactive regulatory T cells in the absence of IFN-γ
To confirm our hypothesis that IFN-γ production by alloantigen-reactive T reg cells is important for their regulatory capacity in vivo, we tested whether in IFN-γ–deficient mice the 177/DST protocol resulted in the generation of alloantigen-reactive T reg cells. Because IFN-γ gene targeted mice are not available on the CBA background, we first confirmed that pretreatment of naive C57BL/6 wild-type mice with 177/BALBDST resulted in the generation of CD25+CD4+ T cells with the ability to control the rejection of BALB skin grafts (unpublished data). C57BL/6 wild-type and C57BL/6IFN-γ(−/−) mice were pretreated with 200 μg YTS177 on days −28 and −27. On day −27 they also received 250 μl of whole BALB/c blood. On day 0, spleens were harvested and CD25+CD4+ T cells were purified by cell sorting.
C57BL/6Rag(−/−) mice reconstituted with 105 CD45RBhigh CD4+ T cells from naive C57BL/6 mice acutely rejected BALB/c skin grafts (medium survival time 19 d; range 17–22 d; Table II; Fig. 5). Cotransfer of 5 × 105 CD25+CD4+ T cells from 177/BALB/cDST-pretreated C57BL/6 wild-type mice prevented rejection of BALB/c skin grafts in six out of nine recipients. All mice that were reconstituted with naive 105 CD45RBhighCD4+ T cells and 5 × 105 CD25+CD4+ T cells from 177BALB/cDST-pretreated C57BL/6 IFN-γ(−/−) mice showed severe signs of necrosis between day 7 and day 11 after skin transplantation, and seven out of nine mice completely rejected their BALB/c skin grafts (P = 0.0138; Table II). These results further indicate that intact IFN-γ production is important for the development of the regulatory capacity of alloantigen-reactive T reg cells in vivo.
Table II.
Impaired development of alloantigen-reactive T reg cells in the absence of IFN-γ
| Adoptive transfer
|
|||||
|---|---|---|---|---|---|
|
CD25+
|
|||||
| CD45RB high | Wild type | IFN-γ knockout | Evidence of necrosisbetween days 7 and 11(>50% of graft) | Graft survival time | Graft survival at 100 d |
| d | |||||
| + | − | − | 7/7 | 4 × 17, 21, 2 × 22 | 0/7 |
| + | + | − | 3/9 | 17, 31, 46, 6× >100 | 6/9 |
| + | − | + | 9/9 | 2 × 17, 2 × 22, 2 × 31, 46, 94, 100 | 1/9a |
P < 0.01.
IFN-γ mRNA expression and protein production by alloantigen-reactive regulatory T cells after in vitro stimulation
Next, we investigated whether stimulation of CD25+CD4+ T cells from pretreated mice with their cognate antigen in vivo or in vitro results in increased IFN-γ transcription and IFN-γ protein production. CD25+CD4+ and CD25−CD4+ T cells from 177/B10DST-pretreated and naive CBA mice were purified from the spleen by flow cytometry. 2 × 105 purified T cells were stimulated with 5 × 105 B10 splenocytes in vitro. At different time points, cells and supernatants were harvested from the cultures for analysis of IFN-γ transcription and protein production by real time RT-PCR and ELISA, respectively.
As shown in Fig. 7, CD25+CD4+ T cells from YTS177/B10DST-pretreated CBA mice responded to restimulation by B10 APCs in vitro by rapidly increasing IFN-γ transcription. In contrast, CD25−CD4+ T cells from YTS177/B10DST-pretreated mice showed a much slower response with an increase in IFN-γ mRNA expression detectable only 48 h after stimulation. Both cell types from YTS177/B10DST-pretreated mice responded faster than their counterparts from naive mice. Thus, similar to the findings in vivo, CD25+CD4+ T cells from YTS177/B10DST–pretreated mice respond to alloantigen-specific restimulation by rapidly increasing IFN-γ transcription. Furthermore IFN-γ protein also was detectable in the supernatant of cultures containing CD25+CD4+ T cells from YTS177/B10DST-pretreated mice as early as 12 h after stimulation, whereas in supernatants from CD25−CD4+ T cells, IFN-γ was detectable only after 48 h. These findings confirm that increased IFN-γ mRNA production by CD25+ CD4+ T cells from alloantigen-pretreated mice when they reencounter donor alloantigen translates to increased levels of IFN-γ protein.
Figure 7.
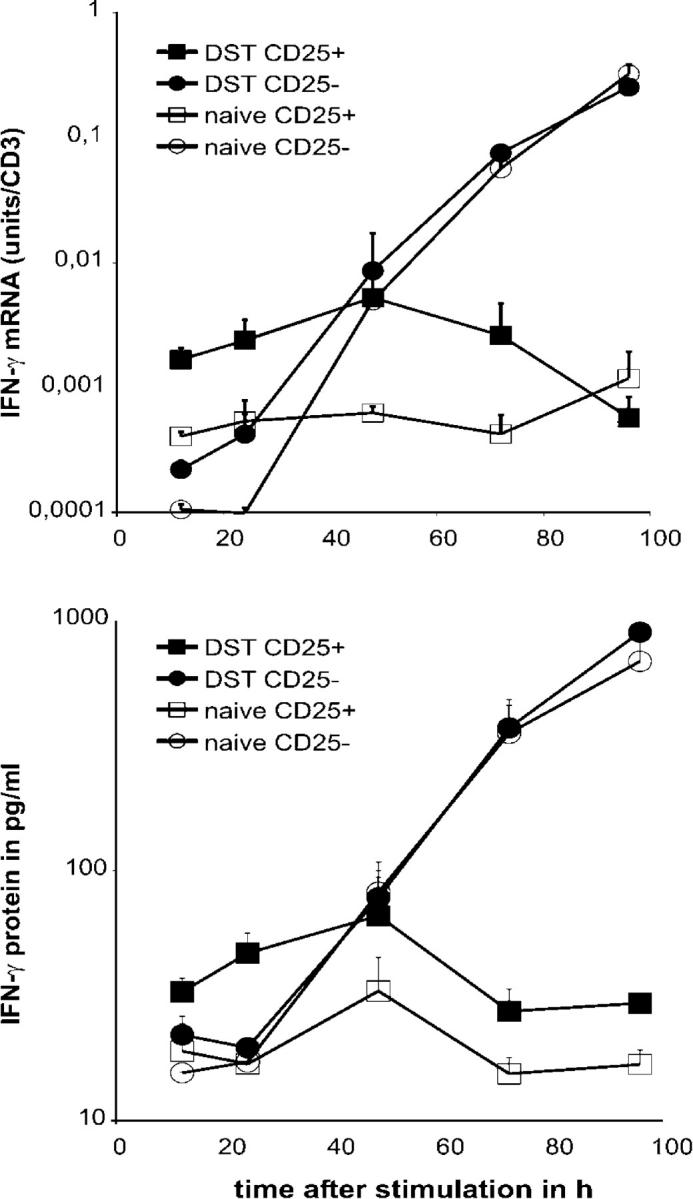
IFN-γ mRNA expression and protein production by alloantigen-reactive regulatory T cells after in vitro stimulation. CD25+CD4+ and CD25−CD4+ T cells from 177/B10DST-pretreated and naive CBA mice were purified from the spleen by flow cytometry. 2 × 105 purified T cells were stimulated with 5 × 105 B10 splenocytes in vitro. At different time points, cells and supernatants were harvested from the cultures for analysis of IFN-γ transcription and protein production by real time RT-PCR and ELISA, respectively.
CD25+CD4+ T cells from YTS177/DST-pretreated mice can inhibit cytokine expression by CD45RBhighCD4+ T cells in vitro
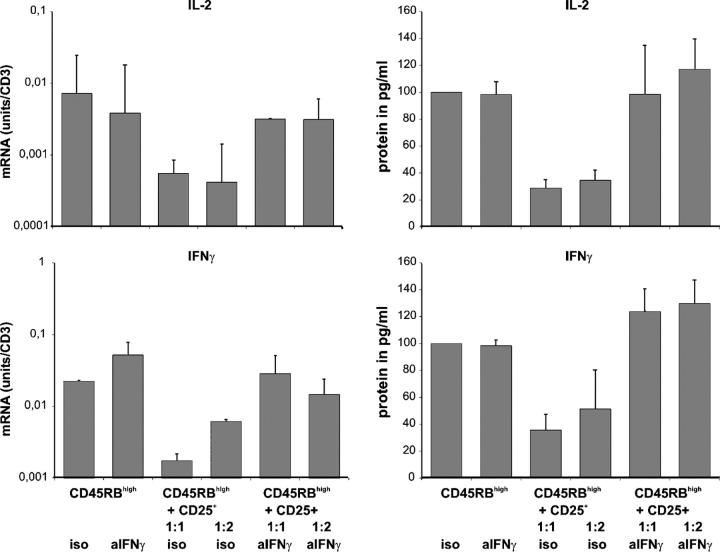
To investigate the importance of IFN-γ production by CD25+CD4+ T cells for their regulatory potential in more detail, we also performed suppression assays in vitro. CD45RBhighCD4+ T cells from naive CBA mice were cultured alone or cocultured with CD25+CD4+ T cells from YTS177/B10DST-pretreated CBA mice in the presence of neutralizing anti–IFN-γ antibody or an isotype control. 5 × 105 B10 splenocytes were added to the cultures as APCs. After 96 h, cells and supernatants were harvested for real time RT-PCR and ELISA analysis of IL-2 and IFN-γ transcription and protein production, respectively. The results are shown in Fig. 8. Stimulation of CD45RBhighCD4+ T cells with allogeneic APCs resulted in an increase in mRNA expression and protein production of IL-2 and IFN-γ. When cocultured with CD25+CD4+ T cells from YTS177/B10DST-pretreated CBA mice, IL-2 and IFN-γ expression was reduced dramatically. Thus, CD25+CD4+ T cells from alloantigen-pretreated mice also are able to inhibit T cell activation in vitro. Addition of a neutralizing anti–IFN-γ antibody completely restored IL-2 and IFN-γ production in the coculture, and thus, undermined the importance of IFN-γ for the regulatory capacity of CD25+CD4+ T cells from YTS177/DST-pretreated CBA mice.
Figure 8.
CD25+CD4+ T cells from YTS177/B10DST-pretreated mice can inhibit cytokine expression by CD45RBhighCD4+ T cells in vitro. CD45RBhighCD4+ T cells from naive CBA mice were cultured alone or cocultured with CD25+CD4+ T cells from YTS177/B10DST-pretreated CBA mice in the presence of neutralizing anti–IFN-γ antibody or an isotype control. 5 × 105 B10 splenocytes were added to the cultures. After 96 h, cells and supernatants were harvested for real time RT-PCR and ELISA analysis of IL-2 and IFN-γ transcription and protein production.
DISCUSSION
The data presented here demonstrate that restimulation of alloantigen-reactive T reg cells by alloantigen in vivo results in a rapid and transient increase in IFN-γ mRNA expression (Fig. 2). This increase in IFN-γ transcription is antigen specific and is not due to contamination with recently activated or antigen-experienced T cells (Figs. 3 and 4). Furthermore, immunoregulation by CD25+CD4+ T cells in vivo is dependent on this early IFN-γ expression (Table I). To our knowledge, this is the first report that analyzes cytokine expression by alloantigen-reactive T reg cells after antigen exposure in vivo.
The fivefold increase in IFN-γ mRNA expression in CD25+CD4+ T cells after restimulation by donor alloantigen was detectable despite only a small fraction of the CD25+CD4+ T cells that were present in the spleen being donor alloantigen specific, suggesting that this is an underestimate of the increase in the alloantigen-reactive cells themselves. IFN-γ mRNA expression by CD25−CD4+ T cells did not change after rechallenge with alloantigen in vivo (Fig. 2). These data differ from previous results—where in contrast to naive T cells—CD25+CD4+ T reg cells produced little or no IFN-γ after stimulation in vitro (6–8). These contradictory observations may be due to a kinetic difference in IFN-γ expression between CD25+CD4+ T reg cells and naive T cells. Although up-regulation of IFN-γ mRNA production by CD25+CD4+ alloantigen-reactive T reg cells was very rapid, transcription was not sustained (Fig. 2). Therefore, it is possible that up-regulation of IFN-γ mRNA expression by naive CD25−CD4+ T cells after alloantigen stimulation in vivo occurs in a delayed fashion (i.e., later than the day 3 time point studied here), but is long lasting. Stimulation of CD25+CD4+ T cells from 177/B10DST-pretreated CBA mice with B10 splenocytes in vitro also resulted in a rapid increase in IFN-γ transcription with subsequent protein production (Fig. 7).
CD25+CD4+ T cells from naive CBA mice had a twofold higher expression of IFN-γ mRNA compared with CD25−CD4+ T cells (Fig. 2) as reported previously (15), and up to 50-fold higher levels of IL-10 mRNA compared with CD25−CD4+ T cells (Fig. 2). This latter observation is in accordance with previous findings from our own and several other laboratories which demonstrated that CD25+CD4+ T reg cells require an environment with intact IL-10 production and signaling to regulate the activity of effector T cells in vivo (1–3). When challenged with alloantigen in vivo, CD25+CD4+ T reg cells did up-regulate IL-10 mRNA expression (Fig. 2); however, a twofold increase in IL-10 mRNA expression also was detected when the mice were challenged with third-party antigen (Fig. 3), although CD25+ CD4+ T cells from pretreated mice cannot inhibit rejection of third-party skin grafts at the same cell number (3).
CD25+CD4+ and CD25−CD4+ T cells from naive mice had a similar basal level of IL-2 mRNA expression, and both populations from pretreated mice responded by increasing IL-2 transcription after restimulation with donor alloantigen in vivo (Fig. 2). Thus, in contrast to in vitro findings (6–8), alloantigen-reactive T reg cells and activated naive T cells up-regulate IL-2 mRNA expression after antigen stimulation in vivo. Whether this is limited to mRNA expression or also applies to IL-2 protein synthesis and secretion needs to be investigated further.
Early expression of IFN-γ by alloantigen-reactive T reg cells was required for regulatory activity in vivo (Table I). Neutralization of IFN-γ signaling resulted in attenuated control of skin allograft rejection by CD25+CD4+ T cells from pretreated mice in vivo, as evidenced by the onset of graft necrosis in all mice (Table I). Furthermore, CD25+CD4+ T cells from IFN-γ–deficient mice were unable to control skin allograft rejection. This is not without precedent because regulatory and antiinflammatory properties of IFN-γ were described previously (16, 17). Endogenous IFN-γ has been shown to act as a protective factor against the onset of experimental autoimmune encephalomyelitis (EAE; references 18–20) and to exert global suppressive effects on T cell trafficking in an asthma model (21). Moreover, transplantation tolerance cannot be induced in IFN-γ knockout mice (10, 22, 23). IFN-γ also is required for T cell depletion and prevention of graft versus host disease by agonistic anti-CD28 antibodies (24). Recently Nishibori et al. reported on an impaired development of CD25+CD4+ T reg cells in signal transducer and activator of transcription (STAT)-1–deficient mice which was associated with an increased susceptibility to autoimmune disease (25). The STAT1 transcription factor plays an important role in IFN-γ–induced signaling, and indicates that IFN-γ–induced STAT1 activation is important for the development and function of CD25+CD4+ T reg cells.
Early administration of IL-12 between days 0 and 5 can suppress EAE through induction of IFN-γ whereas late administration between days 9 and 14 had no effect (26). In this study, the IL-12 treatment induced an early induction of IFN-γ (day 7) in antigen-specific T cells; however, the late IFN-γ and TNF-α response (day 21) was decreased. This was associated with a diminished mononuclear cell infiltration and demyelination. The beneficial effect of early IL-12 administration was clearly dependent on IFN-γ production because IL-12–treated IFN-γ–deficient mice developed severe EAE. Similar findings were made by Tarrant et al. in a model of experimental autoimmune uveitis (16).
Although IFN-γ may elicit inflammatory Th1-driven immune responses in some circumstances, it may exert an immunosuppressive effect in others. This functional dichotomy probably is dependent on the IFN-γ concentration, the microenvironment, and the timing of IFN-γ production. Further evidence from our in vitro studies supports the role of IFN-γ for the regulatory capacity of CD25+CD4+ T cells. We have demonstrated that CD25+CD4+ T cells from 177/B10DST-pretreated CBA mice also are able to inhibit activation of CD45RBhighCD4+ T cells in vitro; this could be restored by the addition of a neutralizing anti–IFN-γ antibody to the coculture (Fig. 8).
The main antiinflammatory properties of IFN-γ are induction of apoptosis and inhibition of proliferation (14, 23, 27). By expressing both types of IFN-γ receptors (IFNR1 and IFNR2), only naive and Th2 cells are sensitive to IFN-γ (28, 29). T cells lose expression of the signaling chain IFNR2 as they develop into Th1 cells, and therefore, become resistant to IFN-γ signaling. Hence, an early transient expression of IFN-γ by alloantigen-reactive T reg cells could prevent the initiation of graft rejection by inhibiting activation and proliferation of naive alloreactive T cells or by controlling alloreactive effector mechanisms. Evidence in support of the latter effect was reported recently by Lin et al. who showed that CD4+ T reg cells did not prevent proliferation or accumulation of alloreactive CD8+ T cells, but rather, inhibited their cytotoxic effector functions by unknown mechanisms (30). Corroborate findings have been made by two other groups (31, 32). In contrast, Lee et al. observed an inhibition of effector cell proliferation by CD25+CD4+ T reg cells in vivo (33). Continued signaling via IFNR2 in CD4+ T cells was shown to lead to impaired Th1-dependent immunity in vivo. CD4+ T cells from IFNR2 transgenic mice still could proliferate upon stimulation in vitro and in vivo, but failed to elicit delayed-type hypersensitivity responses and initiate clearance of Leishmania major (28). Thus, IFN-γ production by alloantigen-reactive T reg cells may influence the ability of effector T cells to initiate graft rejection.
IFN-γ production by T reg cells also may influence the expansion of GR1+ macrophages that can suppress the proliferation of naive T cells by a nitric oxide–dependent mechanism (34). In this study, IFN-γ neutralization was accompanied by a massive reduction in iNOS mRNA expression within skin grafts in which immunoregulation was abrogated (Fig. 6). Furthermore, CD25+CD4+ T reg cells were shown to modulate tryptophan catabolism of dendritic cells, and thereby, prime them for tolerance induction (35). In resting CD25+CD4+ T cells, this effect was mediated via surface expression of CTLA4 which subsequently led to an increase in IFN-γ and indoleamine-2,3-dioxygenase (IDO) production in the cocultured dendritic cells. In contrast, anti-CD3 and LPS stimulation induced IFN-γ production by CD25+CD4+ T cells which led to even greater IDO production and tolerogenic potential of the cocultured dendritic cells. Similar to iNOS mRNA expression, IDO expression also was reduced in skin grafts of recipients of a neutralizing antibody against IFN-γ (unpublished data).
Thus, the protective role of early IFN-γ expression by alloantigen-reactive T reg cells in this model of skin allograft rejection may arise from a direct effect of IFN-γ on naive T cells or indirectly as a result of nitric oxide production by activated residual macrophages or by an increase in IDO production of dendritic cells.
In this study, we associate IFN-γ with the action of T reg cells, and thereby, identify a possible mechanism whereby T reg cells may influence directly the proliferation and effector function of alloreactive T cells in vivo. Furthermore, our findings may explain why IFN-γ acts as a protective factor in certain autoimmune models and is essential in some transplant models of long-term graft acceptance.
MATERIALs AND METHODS
Mice.
CBA.Ca (CBA, H2k), C57BL/10 (B10, H2b), C57BL/6 (BL6, H2b), BALB/c (H2d), and CBA Rag1−/− (H2k) were bred in the SPF facility, Biomedical Services, John Radcliffe Hospital. IFN-γ(−/−) C57BL/6 were provided by U. Klemm, Max Plank Institute of Infection Biology (Berlin, Germany). Sex-matched mice aged 6–12 weeks were used in all experiments. All mice were bred and used in accordance with the Animals (Scientific Procedure) Act 1986 of the United Kingdom.
Reagents and monoclonal antibodies.
Hybridomas YTS 169.4.2 (anti-CD8) and YTS 177.9 (anti-CD4) were provided by Professor H. Waldmann (Sir William Dunn School of Pathology, Oxford, England, United Kingdom). The neutralizing anti–IFN-γ antibody (clone: AN 18.17.24) was provided by U. Syrbe (German Rheumatology Research Centre, Berlin, Germany). TIB120 (anti-MHC II) was obtained from American Type Culture Collection. RM4-5 (anti-CD4)-cychrome, 16A (anti-CD45RB)-PE, 7D4 (anti-CD25)-biotin, and streptavidin-PE were purchased from BD. The following mAbs were used for in vivo experiments: anti–mouse IFN-γ (clone AN 18.17.24, 200 μg at time of adoptive transfer) and GL113 (rat IgG1, 200 μg at time of adoptive transfer), an isotype control mAb reactive with β-galactosidase (36).
Cell preparation.
Single-cell suspensions from spleen of naive or pretreated CBA mice were prepared as described previously (3). CD25+CD4+, CD25−CD4+, or CD45RBhighCD4+ T cells were obtained by flow cytometry using a FACSVantage or FACSAria (Becton Dickinson). On reanalysis, all populations were >95% pure.
Cell culture.
Culture medium was composed of RPMI 1640 supplemented with 10% FCS (both P.A.A. Laboratories GmBH), 2 mM L-glutamine, 0.5 mM 2-mercaptoethanol (Sigma-Aldrich), and 100 units/ml each penicillin, streptomycin, and kanamycin. All cultures were set in U-shaped 96-well plates (Corning Costar). CD45RBhighCD4+ T cells from naive CBA mice and CD25+CD4+, CD25−CD4+ T cells from naive or pretreated CBA mice were cultured at 2 × 105 cells per well together with 5 × 105 irradiated (3600 rad) allogeneic total splenocytes from B10 mice. For coculture experiments, 2 × 105 CD45RBhighCD4+ T cells from naive CBA mice were cultured with 105 (2:1) or 2 × 105 (1:1) CD25+CD4+ T cells from pretreated CBA mice and 5 × 105 irradiated B10 splenocytes in the presence of 20 μg/ml neutralizing anti–mouse IFN-γ antibody or control isotype.
Cytokine mRNA expression.
Sorted cells or skin grafts were analyzed for the expression of mRNA encoding IL-2, IFN-γ, and IL-10 by quantitative real time RT-PCR (TaqMan; PE Biosystems). RNA isolation and cDNA synthesis were performed as described previously. The cDNA was analyzed for cytokine gene expression by TaqMan PCR as described (37). If not otherwise indicated, CD3 was used as a housekeeping gene and mRNA concentrations were compared with the sample content of CD3. The sequences of the oligonucleotides used are as follows: CD3 sense 5′-ATTGCGGGACAGGATGGAG-3′; CD3 antisense 5′-CTTGGAGATGGCTGTACTGGTCA-3′; CD3 probe 5′-TCGCCAGTCAAGAGCTTCA-GACAAGCA-3′; IL-2 sense 5′-CCCAGGATGCTCACCTTCAA-3′; IL-2 antisense 5′-CATGCCGCAGAGGTCCAA-3′; IL-2 probe 5′-CAATTCTGTGGCCTGCTTGGGCAA-3′; IFN-γ sense 5′-AGCAACAGCAAGGCGAAAAA-3′; IFN-γ antisense 5′-AGCTCATTGAATGCTTGGCG-3′; IFN-γ probe 5′-ATTGCCAAGTTTGAGGTCAACAACCC-ACA-3′; IL-10 sense 5′-GAAGACCCTCAGGATGCGG-3′; IL-10 antisense 5′-CCTGCTCCACTGCCTTGCT-3′; IL-10 probe 5′-CGCTGTCATCGATTTCTCCCCTGTGA-3′; TGF-β sense 5′-GGCTACCATGCCAACTTCTGTCT-3′; TGF-β antisense 5′-CCGGGTTGTGTT-GGTTGTAGA-3′; TGF-β probe 5′-CACACAGTACAGCAAGGTCCTTGCCCT-3′; CTLA4 sense 5′-AACTCATGTACCCACCGCCA-3′; CTLA4 antisense 5′-TCCAAAGGAGGAAGTCAGAATCC-3′; CTLA4 probe 5′-TTATGTCATTGATCCAGAACCATGCCCG-3′; HPRT sense 5′-ATCATTATGCCGAGGATTTGGAA-3′; HPRT antisense 5′-TTGAGCACACAGAGGGCA-3′; HPRT probe 5′-TGGACAGGACTGAAAGACTTGCTCGAGATG-3′; iNOS sense 5′-CTTTGACGCTCGGAACTGTAGC-3′; iNOS antisense 5′-TGAAGTCATGTTTGCCGTCACT-3′; and iNOS probe 5′-GCAACATCAGGTCGGCCAT-TACTGTGTT-3′. Sense and antisense oligonucleotides were purchased from MWG. The probes were labeled with the reporter FAM (6-carboxyfluorescein) at the 5′ end and with the quencher TAMRA (6-carboxy-tetramethyl-rhodamine) at the 3′ end (both from Oswel).
ELISA.
Cell culture supernatants were analyzed for IL-2 and IFN-γ secretion at the time points indicated using commercially available antibody pairs and standards (BD Biosciences).
Adoptive transfer and skin transplantation.
T cell–deficient [Rag(−/−)] CBA mice were reconstituted i.v. with syngeneic fractionated T cells. The day after reconstitution, all mice received a B10 skin graft as described previously (3). Graft rejection was defined as complete destruction of the skin grafts.
Statistical analysis.
Data were analyzed using the statistical software SPSS (SPSS GmbHSoftware) and are reported as mean ± SD. Data for cytokine expression were analyzed using one-way ANOVA with Bonferroni correction. Graft survival data were analyzed using Kaplan Meier Log Rank test. Differences were considered significant when P < 0.05.
Acknowledgments
We would like to thank A. Bushell (Nuffield Department of Surgery) and Christian Meisel (Wellcome Trust Centre of Human Genetics) for the critical review of the manuscript and A. Friedrich (Institute of Medical Immunology, Charité) and S. Chapman (Nuffield Department of Surgery) for technical help.
K.J. Wood holds a Royal Society Wolfson Research Merit Award. This work was supported by The Wellcome Trust.
The authors have no conflicting financial interests.
Abbreviations used: EAE, experimental autoimmune encephalomyelitis; IDO, indoleamine-2,3-dioxygenase; iNOS, inducible nitric oxide synthase; MST, mean survival time; STAT1, signal transducer and activator of transcription–1; T reg, regulatory T.
B. Sawitzki, C. Kingsley, and V. Oliveira contributed equally to this work.
References
- 1.Asseman, C., S. Mauze, M.W. Leach, R.L. Coffman, and F. Powrie. 1999. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hara, M., C.I. Kingsley, M. Niimi, S. Read, S.E. Turvey, A.R. Bushell, P.J. Morris, F. Powrie, and K.J. Wood. 2001. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J. Immunol. 166:3789–3796. [DOI] [PubMed] [Google Scholar]
- 3.Kingsley, C.I., M. Karim, A.R. Bushell, and K.J. Wood. 2002. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J. Immunol. 168:1080–1086. [DOI] [PubMed] [Google Scholar]
- 4.Powrie, F., J. Carlino, M.W. Leach, S. Mauze, and R.L. Coffman. 1996. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J. Exp. Med. 183:2669–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piccirillo, C.A., J.J. Letterio, A.M. Thornton, R.S. McHugh, M. Mamura, H. Mizuhara, and E.M. Shevach. 2002. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J. Exp. Med. 196:237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levings, M.K., R. Sangregorio, and M.G. Roncarolo. 2001. Human CD25(+)CD(+) T regulatory cells suppress naïve and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 193:1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonuleit, H., E. Schmitt, M. Stassen, A. Tuettenberg, J. Knop, and A.H. Enk. 2001. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 193:1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groux, H.A. O'Garra, M. Bigler, M. Rouleau, S. Antonenko, J.E. de Vries, and M.G. Roncarolo. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 389:737–742. [DOI] [PubMed] [Google Scholar]
- 9.Dai, Z., B. Konieczny, F. Baddoura, and F. Lakkis. 1998. Impaired alloantigen-mediated T cell apoptosis and failure to induce long-term allograft survival in IL-2-deficient mice. J. Immunol. 161:1659–1663. [PubMed] [Google Scholar]
- 10.Konieczny, B.T., Z. Dai, E.T. Elwood, S. Saleem, P.S. Linsley, F.K. Baddoura, C.P. Larsen, T.C. Pearson, and F.G. Lakkis. 1998. IFN-gamma is critical for long-term allograft survival induced by blocking the CD28 and CD40 ligand T cell costimulation pathways. J. Immunol. 160:2059–2064. [PubMed] [Google Scholar]
- 11.Karim, M., A.R. Bushell, and K.J. Wood. 2002. Regulatory T cells in transplantation. Curr. Opin. Immunol. 14:584–591. [DOI] [PubMed] [Google Scholar]
- 12.Saitovitch, D., A. Bushell, D.W. Mabbs, P.J. Morris, and K.J. Wood. 1996. Kinetics of induction of transplantation tolerance with a nondepleting anti-Cd4 monoclonal antibody and donor-specific transfusion before transplantation. A critical period of time is required for development of immunological unresponsiveness. Transplantation. 61:1642–1647. [DOI] [PubMed] [Google Scholar]
- 13.Graca, L., S.P. Cobbold, and H. Waldmann. 2002. Identification of regulatory T cells in tolerated allografts. J. Exp. Med. 195:1641–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shtrichman, R., and C.E. Samuel. 2001. The role of gamma interferon in antimicrobial immunity. Curr. Opin. Microbiol. 4:251–259. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann, J., J. Huehn, M. de la Rosa, F. Maszyna, U. Kretschmer, V. Krenn, M. Brunner, A. Scheffold, and A. Hamann. 2002. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25− regulatory T cells. Proc. Natl. Acad. Sci. USA. 99:13031–13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarrant, T.K., P.B. Silver, J.L. Wahlsten, L.V. Rizzo, C.C. Chan, B. Wiggert, and R.R. Caspi. 1999. Interleukin 12 protects from a T helper type 1-mediated autoimmune disease, experimental autoimmune uveitis, through a mechanism involving interferon gamma, nitric oxide, and apoptosis. J. Exp. Med. 189:219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalton, D.K., L. Haynes, C.Q. Chu, S.L. Swain, and S. Wittmer. 2000. Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J. Exp. Med. 192:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthys, P., K. Vermeire, and A. Billiau. 2001. Mac-1(+) myelopoiesis induced by CFA: a clue to the paradoxical effects of IFN-gamma in autoimmune disease models. Trends Immunol. 22:367–371. [DOI] [PubMed] [Google Scholar]
- 19.Billiau, A., H. Heremans, F. Vandekerckhove, R. Dijkmans, H. Sobis, E. Meulepas, and H. Carton. 1988. Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J. Immunol. 140:1506–1510. [PubMed] [Google Scholar]
- 20.Jones, L.S., L.V. Rizzo, R.K. Agarwal, T.K. Tarrant, C.C. Chan, B. Wiggert, and R.R. Caspi. 1997. IFN-gamma-deficient mice develop experimental autoimmune uveitis in the context of a deviant effector response. J Immunol. 158:5997–6005. [PubMed] [Google Scholar]
- 21.Flaishon, L., I. Topilski, D. Shoseyov, R. Hershkoviz, E. Fireman, Y. Levo, S. Marmor, and I. Shachar. 2002. Cutting edge: anti-inflammatory properties of low levels of IFN-gamma. J. Immunol. 168:3707–3711. [DOI] [PubMed] [Google Scholar]
- 22.Hassan, A.T., Z. Dai, B.T. Konieczny, G.H. Ring, F.K. Baddoura, L.H. Abou-Dahab, A.A. El-Sayed, and F.G. Lakkis. 1999. Regulation of alloantigen-mediated T-cell proliferation by endogenous interferon-gamma: implications for long-term allograft acceptance. Transplantation. 68:124–129. [DOI] [PubMed] [Google Scholar]
- 23.Kishimoto, K., S. Sandner, J. Imitola, M. Sho, Y. Li, P.B. Langmuir, D.M. Rothstein, T.B. Strom, L.A. Turka, and M.H. Sayegh. 2002. Th1 cytokines, programmed cell death, and alloreactive T cell clone size in transplant tolerance. J. Clin. Invest. 109:1471–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu, X.Z., M.H. Albert, P.J. Martin, and C. Anasetti. 2004. CD28 ligation induces transplantation tolerance by IFN-gamma-dependent depletion of T cells that recognize alloantigens. J. Clin. Invest. 113:1624–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishibori, T., Y. Tanabe, L. Su, and M. David. 2004. Impaired development of CD4+ CD25+ regulatory T cells in the absence of STAT1: increased susceptibility to autoimmune disease. J. Exp. Med. 199:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gran, B., N. Chu, G.X. Zhang, S. Yu, Y. Li, X.H. Chen, M. Kamoun, and A. Rostami. 2004. Early administration of IL-12 suppresses EAE through induction of interferon-gamma. J. Neuroimmunol. 156:123–131. [DOI] [PubMed] [Google Scholar]
- 27.Refaeli, Y., L. Van Parijs, S.I. Alexander, and A.K. Abbas. 2002. Interferon gamma is required for activation-induced death of T lymphocytes. J. Exp. Med. 196:999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tau, G.Z., T. von der Weid, B. Lu, S. Cowan, M. Kvatyuk, A. Pernis, G. Cattoretti, N.S. Braunstein, R.L. Coffman, and P.B. Rothman. 2000. Interferon gamma signaling alters the function of T helper type 1 cells. J. Exp. Med. 192:977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernabei, P., E.M. Coccia, L. Rigamonti, M. Bosticardo, G. Forni, S. Pestka, C.D. Krause, A. Battistini, and F. Novelli. 2001. Interferon-gamma receptor 2 expression as the deciding factor in human T, B, and myeloid cell proliferation or death. J. Leukoc. Biol. 70:950–960. [PubMed] [Google Scholar]
- 30.Lin, C.Y., L. Graca, S.P. Cobbold, and H. Waldmann. 2002. Dominant transplantation tolerance impairs CD8+ T cell function but not expansion. Nat. Immunol. 3:1208–1213. [DOI] [PubMed] [Google Scholar]
- 31.Klein, L., K. Khazaie, and H. von Boehmer. 2003. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc. Natl. Acad. Sci. USA. 100:8886–8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin, B., A. Banz, B. Bienvenu, C. Cordier, N. Dautigny, C. Becourt, and B. Lucas. 2004. Suppression of CD4+ T lymphocyte effector functions by CD4+CD25+ cells in vivo. J. Immunol. 172:3391–3398. [DOI] [PubMed] [Google Scholar]
- 33.Lee, M.K.T., D.J. Moore, B.P. Jarrett, M.M. Lian, S. Deng, X. Huang, J.W. Markmann, M. Chiaccio, C.F. Barker, A.J. Caton, and J.F. Markmann. 2004. Promotion of allograft survival by CD4+CD25+ regulatory T cells: evidence for in vivo inhibition of effector cell proliferation. J. Immunol. 172:6539–6544. [DOI] [PubMed] [Google Scholar]
- 34.Atochina, O., T. Daly-Engel, D. Piskorska, E. McGuire, and D.A. Harn. 2001. A schistosome-expressed immunomodulatory glycoconjugate expands peritoneal Gr1(+) macrophages that suppress naïve CD4(+) T cell proliferation via an IFN-gamma and nitric oxide-dependent mechanism. J. Immunol. 167:4293–4302. [DOI] [PubMed] [Google Scholar]
- 35.Fallarino, F., U. Grohmann, K.W. Hwang, C. Orabona, C. Vacca, R. Bianchi, M.L. Belladonna, M.C. Fioretti, M.L. Alegre, and P. Puccetti. 2003. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 4:1206–1212. [DOI] [PubMed] [Google Scholar]
- 36.Gulbenkian, A.R., R.W. Egan, X. Fernandez, H. Jones, W. Kreutner, T. Kung, F. Payvandi, L. Sullivan, J.A. Zurcher, and A.S. Watnick. 1992. Interleukin-5 modulates eosinophil accumulation in allergic guinea pig lung. Am. Rev. Respir. Dis. 146:263–266. [DOI] [PubMed] [Google Scholar]
- 37.Sawitzki, B., F. Amersi, T. Ritter, M. Fisser, X.D. Shen, B. Ke, R. Busuttil, H.D. Volk, and J.W. Kupiec-Weglinski. 2002. Upregulation of bag-1 by ex vivo gene transfer protects rat livers from ischemia/reperfusion injury. Hum. Gene Ther. 13:1495–1504. [DOI] [PubMed] [Google Scholar]