Abstract
The triggering receptor expressed on myeloid cells (TREM)-1 is a recently discovered receptor expressed on the surface of neutrophils and a subset of monocytes. Engagement of TREM-1 has been reported to trigger the synthesis of proinflammatory cytokines in the presence of microbial products. Previously, we have identified a soluble form of TREM-1 (sTREM-1) and observed significant levels in serum samples from septic shock patients but not controls. Here, we investigated its putative role in the modulation of inflammation during sepsis. We observed that sTREM-1 was secreted by monocytes activated in vitro by LPS and in the serum of animals involved in an experimental model of septic shock. Both in vitro and in vivo, a synthetic peptide mimicking a short highly conserved domain of sTREM-1 appeared to attenuate cytokine production by human monocytes and protect septic animals from hyper-responsiveness and death. This peptide seemed to be efficient not only in preventing but also in down-modulating the deleterious effects of proinflammatory cytokines. These data suggest that in vivo modulation of TREM-1 by sTREM peptide might be a suitable therapeutic tool for the treatment of sepsis.
Keywords: triggering receptor expressed on myeloid cells-1, inflammation, sepsis, proinflammatory cytokines, mouse model
Introduction
After an infection, innate and cognitive immune responses develop in sequential phases that build up in specificity and complexity, resulting ultimately in the clearance of infectious agents and restoration of homeostasis. The innate immune response serves as the first line of defense and is initiated upon activation of pattern recognition receptors, such as Toll-like receptors (TLRs) (1, 2), by various pathogen-associated microbial patterns (3). Activation of the TLRs triggers the release of large quantities of such cytokines as TNF-α and IL-1β, which, in case of such massive infections as sepsis, can precipitate tissue injury and lethal shock (4, 5). Although antagonists of TNF-α and IL-1β appeared in this context as possibly interesting therapeutic agents of sepsis, they have unfortunately shown limited efficacy in clinical trials (6–8). This could be due to the fact that these cytokines are necessary for the clearance of infections and that their removal would allow for fatal bacterial growth (9–11).
The triggering receptor expressed on myeloid cells (TREM)-1 is a recently discovered cell-surface molecule that has been identified both on human and murine polymorphonuclear neutrophils and mature monocytes (12). It belongs to the immunoglobulin superfamily and activates downstream signaling pathways with the help of an adaptor protein called DAP12 (12–15). Bouchon and coworkers have shown that the expression of TREM-1 was greatly up-regulated on neutrophils and monocytes in the presence of such bacteria as Pseudomonas aeruginosa or Staphylococcus aureus, both in cell culture and in tissue samples from patients with infection (16). In striking contrast, TREM-1 was not up-regulated in samples from patients with noninfectious inflammatory diseases such as psoriasis, ulcerative colitis, or vasculitis caused by immune complexes (16). Moreover, when TREM-1 is bound to its ligand there is a synergistic effect of LPS and an amplified synthesis of the proinflammatory cytokines TNF-α and GM-CSF, together with an inhibition of IL-10 production (17). In a murine model of LPS-induced septic shock, blockade of TREM-1 signaling protected the animals from death, further highlighting the crucial role of this molecule (13, 16).
Here we show that a soluble form of TREM-1 (sTREM-1) is released in the peripheral blood during infectious aggression in mouse. We also confirm monocytes as a major source of sTREM and show that a synthetic peptide mimicking a part of the extracellular domain of TREM-1 can modulate cytokine production by activated monocytes in vitro. We further demonstrate that the same peptide also modulates in vivo the proinflammatory cascade triggered by infection, thus inhibiting hyper-responsiveness and death in an animal model of sepsis.
Materials and Methods
Preparation of Monocytes from Peripheral Blood.
10 ml of peripheral blood samples were collected on EDTA-K from five healthy volunteer donors originating from laboratory staff. After dilution in RPMI (Life Technologies) vol/vol, blood was centrifuged for 30 min at room temperature over a Ficoll gradient (Amersham Biosciences) to isolate PBMCs. The cells recovered above the gradient were washed and counted. To deplete the suspensions of lymphocytes, cells were then plated in 24-well flat-bottom tissue culture plates (Corning) at a concentration of 5 × 106/ml and allowed to adhere during 2 h at 37°C. The resulting lymphocyte suspension was discarded, and the adhering monocytic cells were maintained in a 5% CO2 incubator at 37°C in complete medium (RPMI 1640, 0.1 mM sodium pyruvate, 2 mM penicillin, 50 μg/ml streptomycin; Life Technologies) supplemented with 10% FCS (Invitrogen).
TREM-1 Peptide.
Based on the TREM-1 sequence in GenBank/EMBL/DDBJ (under accession nos. AF287008 and AF241219), a domain highly conserved in mouse and man was found in the extracellular portion of the protein. The corresponding conserved domain (LQVTDSGLYRCVIYHPP) was chemically synthesized as a COOH terminally amidated peptide (Pepscan Systems). The correct peptide was obtained in >99% yield and with measured mass of 1,961 D versus a calculated mass of 1,962 D and was homogeneous after preparative purification, as confirmed by mass spectrometry and analytic reversed phase-high performance liquid chromatography. This peptide was called LP17. A peptide containing the same amino acids as LP17 but in a different sequence order (TDSRCVIGLYHPPLQVY) was similarly synthesized and served as control peptide.
In Vitro Stimulation of Monocytes.
For activation, monocytes were cultured in the presence of Escherichia coli LPS (O111:B4, 1 μg/ml; Sigma-Aldrich). Cell viability was assessed by trypan blue exclusion and by measuring lactate dehydrogenase release. In some experiments, this stimulus was given in combination with TNF-α (5–100 ng/ml; R&D Systems), IL-1β (5–100 ng/ml; R&D Systems), rIFN-γ (up to 100 U/ml; R&D Systems), rIL-10 (500 U/ml; R&D Systems), or up to 100 ng/ml of LP17 or control peptide.
To activate monocytes through TREM-1, an anti–TREM-1 agonist monoclonal antibody (R&D Systems) was added as follows. Flat-bottom plates were precoated with 10 μg/ml anti–TREM-1 per well. After thorough washing in PBS, the monocyte suspensions were added at a similar concentration as above. Some experiments were performed in the presence of protease inhibitors (PMSF and Protease Cocktail Inhibitor; Invitrogen). Cell-free supernatants were assayed for the production of TNF-α and IL-1β by ELISA according to the recommendations of the manufacturer (BD Biosciences). To address the effect of LP17 on NF-κB activity in monocytes, an ELISA-based assay was performed (BD Mercury Transfactor kit; BD Biosciences). Monocytes were cultured for 24 h in the presence of E. coli LPS (O111:B4, 1 μg/ml), and/or an agonist anti–TREM-1 monoclonal antibody (10 μg/ml), and/or LP17 (100 ng/ml). Whole cell extracts were then prepared, and levels of NF-κB p50 and p65 were determined according to the recommendations of the manufacturer. All experiments were performed in triplicate, and data are expressed as means (SEM).
Identification and Quantitation of sTREM-1 Release.
Primary monocytes suspensions were cultured as described above. The cells were treated with E. coli LPS (O111:B4, 1 μg/ml) for 24 h at 37°C. Cell-conditioned medium was submitted to Western blotting using an anti–TREM-1 monoclonal antibody (R&D Systems) in order to confirm the presence of 27 kD material recognized by anti–TREM-1. Soluble TREM-1 levels were measured by assessing the optical intensity of bands on immunodots by means of a reflectance scanner and the Quantity One Quantitation Software (Bio-Rad Laboratories, Inc.) as reported elsewhere (18). Soluble TREM-1 concentration from each sample was determined by comparing the optical densities of the samples with reference to standard curves generated with purified TREM-1. All measurements were performed in triplicate. The sensitivity of this technique allows the detection of sTREM-1 levels as low as 5 pg/ml.
TREM-1 RT-PCR.
Total mRNA was extracted from primary monocytes cultured in the presence of LPS using a TRIzol reagent (Invitrogen) and reverse transcribed using Superscript RT II (Invitrogen) to generate cDNA. RT-PCR conditions then used for all reactions were 94°C, 30 s/65°C, 30 s/68°C, and 1 min for 30 cycles. Amplification was performed with 2.5 mM MgCl2, 0.2 mM dNTP, 2.0 U Taq polymerase, and 20 pM 5′ and 3′ oligonucleotide primers (Proligos). The sequences of the 5′ and 3′ primer pairs used were the following: TTGTCTCAGAACTCCGAGCTGC and GAGACATCGGCAGTTGACTTGG for TREM-1 (17); GGACGGAGAGATGCCCAAGACC and ACCAGCCAGGAGAATGACAATG for TREM-1 splice variant (TREM-1sv) (19); and GGACGACATGGAGAAGATCTGG and ATAGTAATGTCACGCACGATTTCC for β-actin used as housekeeping amplicon. PCR products were run on agarose gels and visualized by ethidium bromide staining.
LPS-induced Endotoxinemia in Mice.
After approval by the local ethical committee, male Balb/c mice (20–23 g) were randomly grouped and treated with E. coli LPS i.p. in combination with LP17 (in 500 μl normal saline) or control vector before or after LPS challenge. In some experiments, 5 μg of an anti–TREM-1 monoclonal antibody was administered i.p. 1 h after LPS injection. The viability of mice was examined every hour, or animals were killed at regular intervals. Serum samples were collected by cardiac puncture and assayed for TNF-α and IL-1β by ELISA (BD Biosciences) and for sTREM-1 levels by immunodot.
Cecal Ligation and Puncture Polymicrobial Sepsis Model.
Male Balb/c mice (7–9 wk, 20–23 g) were anesthetized by i.p. administration of ketamine and xylazine in 0.2 ml sterile pyrogen-free saline. The cecum was exposed through a 1.0-cm abdominal midline incision and subjected to a ligation of the distal half followed by two punctures with a G21 needle. A small amount of stool was expelled from the punctures to ensure patency. The cecum was replaced into the peritoneal cavity and the abdominal incision closed in two layers. After surgery, all mice were injected s.c. with 0.5 ml of physiologic saline solution for fluid resuscitation and s.c. every 12 h with 1.25 mg (i.e., 50 μg/g) of imipenem. The animals were randomly grouped and treated with normal saline (n = 14), the control peptide (n = 14, 100 μg) or LP17 (100 μg) in a single injection at H0 (n = 18), H+4 (n = 18), or H+24 (n = 18). The last group of mice (n = 18) was treated with repeated injections of LP17 (100 μg) at H+4, H+8, and H+24. All treatments were diluted into 500 μl of normal saline and administered i.p. We next sought to determine the effect of various doses of LP17. For this purpose, mice (n = 15 per group) were treated with a single injection of normal saline or 10, 20, 50, 100, or 200 of LP17 at H0 after the cecal ligation and puncture (CLP) and monitored for survival. Five additional animals per group were killed under anesthesia at 24 h after CLP for the determination of bacterial count and cytokines levels. Peritoneal lavage fluid was obtained using 2 mL RPMI 1640 (Life Technologies), and blood was collected by cardiac puncture. Concentrations of TNF-α and IL-1β in the serum were determined by ELISA (BD Biosciences). For the assessment of bacterial counts, blood and peritoneal lavage fluid were plated in serial log dilutions on tryptic soy supplemented with 5% sheep blood agar plates. After plating, tryptic soy agar plates were incubated at 37°C aerobically for 24 h and anaerobically for 48 h. Results are expressed as CFU per ml of blood and CFU per mouse for the peritoneal lavage.
Statistical Analyses.
Serum sTREM-1 and cytokines levels were expressed as mean (± SD). The protection against LPS lethality by LP17 was assessed by comparison of survival curves using the Log-Rank test. All statistical analyses were completed with Statview software (Abacus Concepts) and a two-tailed P < 0.05 was considered significant.
Results
A Soluble Form of TREM-1 Is Released from Cultured Human Monocytes after Stimulation with E. coli LPS.
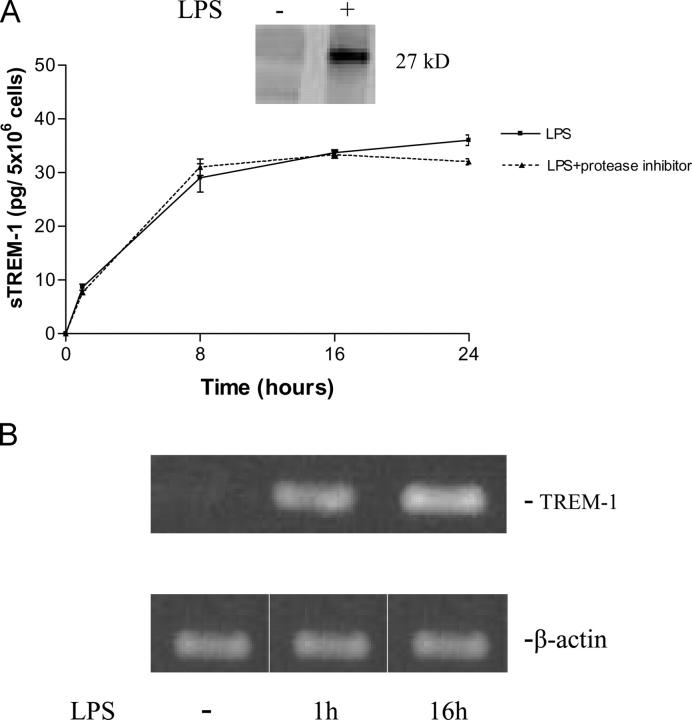
To identify the potential release of sTREM-1 in vitro, we stimulated human monocytes with LPS and analyzed the conditioned culture medium by SDS–PAGE. LPS stimulation induced the appearance of a 27-kD protein in a time-dependent manner (Fig. 1 A). Western blotting analysis revealed that this protein was specifically recognized by a monoclonal antibody directed against the extracellular domain of TREM-1 (Fig. 1 A). Cell viability was unaffected at LPS concentrations that induced the presence of sTREM-1 in conditioned medium, indicating that TREM-1 release was not due to cell death. Similarly, treatment of monocytes with protease inhibitors did not affect TREM-1 release (Fig. 1 A). TREM-1 mRNA levels were increased upon LPS treatment (Fig. 1 B), whereas TREM-1sv mRNA levels remained undetectable. This suggests that TREM-1 release is likely to be linked to an increased transcription of the gene and unrelated to TREM-1sv expression.
Figure 1.
(A) Release of sTREM-1 from cultured monocytes after stimulation with LPS with and without proteases inhibitor. LPS stimulation induced the appearance of a 27-kD protein that was specifically recognized by an anti–TREM-1 mAb (inset). sTREM-1 levels in the conditioned culture medium were measured by reflectance of immunodots. Data are shown as mean ± SD (n = 3). (B) Expression of TREM-1 mRNA in monocytes. Cultured monocytes were stimulated with LPS (1 μg/ml) for 0, 1, and 16 h as indicated. LPS induced TREM-1 mRNA production within 1 h.
Stimulation of monocytes for 16 h with TNF-α (5–100 ng/ml) or IL-1β (5–100 ng/ml) induced very small TREM-1 release in a cytokine dose-dependent manner. Stimulation with IFN-γ did not induce TREM-1 release, even at concentrations of up to 100 U/ml.
LPS-associated Release of Proinflammatory Cytokines Is Attenuated by LP17.
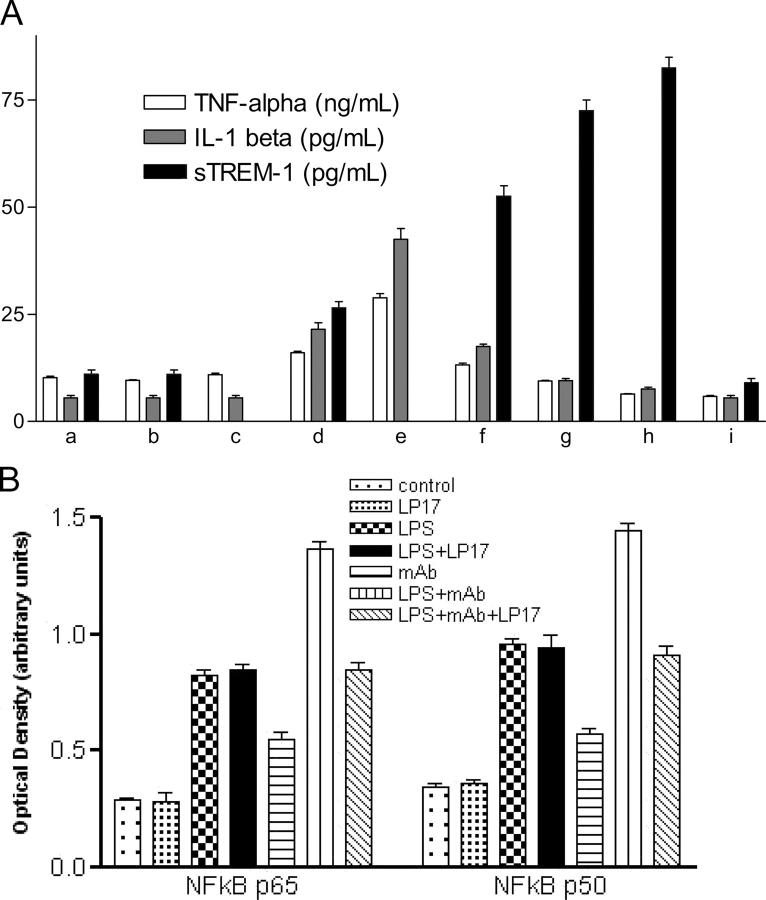
Significant TNF-α and IL-1β production was observed in the supernatant of monocytes cultured with LPS. TNF-α and IL-1β production was even higher for cells cultured with both TREM-1 mAb and LPS compared with those cultured with mAb or LPS alone (Fig. 2 A).
Figure 2.
(A) Release of cytokines and sTREM-1 from cultured monocytes. For cell activation, primary monocytes were cultured in 24-well flat-bottom tissue culture plates in the presence of LPS (1 μg/ml). In some experiments, this stimulus was provided in combination with LP17 (10–100 ng/ml), control peptide (10–100 ng/ml), or rIL-10 (500 U/ml). To activate monocytes through TREM-1, an agonist anti–TREM-1 mAb (10 μg/ml) was added as indicated. Cell-free supernatants were analyzed for production of TNF-α, IL-1β, and sTREM-1 by ELISA or immunodot. All experiments were performed in triplicate and data are expressed as means (SEM). (a) Media; (b) LP17 10 ng/ml; (c) anti–TREM-1; (d) LPS; (e) LPS + anti–TREM-1; (f) LPS + LP17 10 ng/ml; (g) LPS + LP17 50 ng/ml; (h) LPS + LP17 100 ng/ml; (i) LPS + IL10. (B) Effect of LP17 on NF-κB activation. Monocytes were cultured for 24 h in the presence of E. coli LPS (O111:B4; 1 μg/ml), anti–TREM-1 mAb (10 μg/ml), and/or LP17 (100 ng/ml) as indicated, and the levels of NF-κB p50 and p65 were determined using an ELISA-based assay. Experiments were performed in triplicate, and data are expressed as means of optical densities (SEM).
The inducible release of proinflammatory cytokines was significantly lower after LPS stimulation when the medium was supplemented with LP17 or IL-10. LP17 reduced, in a concentration-dependent manner, the TNF-α and IL-1β production from cells cultured with LPS or with LPS and mAb and simultaneously increased the release of sTREM-1 from cells cultured with LPS. The control peptide displayed no action on cytokines or sTREM-1 release (not depicted). In striking contrast, IL-10 totally inhibited the release of both TREM-1 and inflammatory cytokines (Fig. 2 A). Both LPS and TREM-1 mAb induced a strong activation of monocytic NF-κB p50 and p65, and combined administration of LPS and TREM-1 mAb lead to a synergistic effect. LP17 inhibited the NF-κB activation induced by the engagement of TREM-1 but did not alter the effect of LPS (Fig. 2 B).
Serum sTREM-1 Levels of LPS-treated Mice Are Increased.
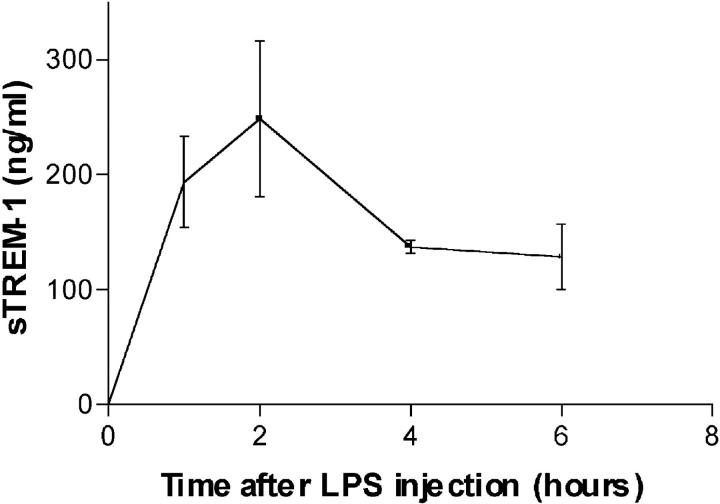
To determine whether sTREM-1 was released systemically during endotoxemia in mice, we measured serum sTREM-1 levels after LPS administration. Serum sTREM-1 was readily detectable 1 h after administration of an LD50 dose of LPS and was maintained at peak plateau levels from 4 to 6 h after LPS treatment (Fig. 3).
Figure 3.
Accumulation of sTREM-1 in serum of LPS-treated mice. Male Balb/c mice (20–23 g) were treated with LPS (LD50, intraperitoneally). Serum was assayed for sTREM-1 by immunodot. Serum sTREM-1 was readily detectable 1 h after LPS administration and was maintained at a plateau level from 4 to 6 h.
LP17 Protects Endotoxemic Mice from Lethality.
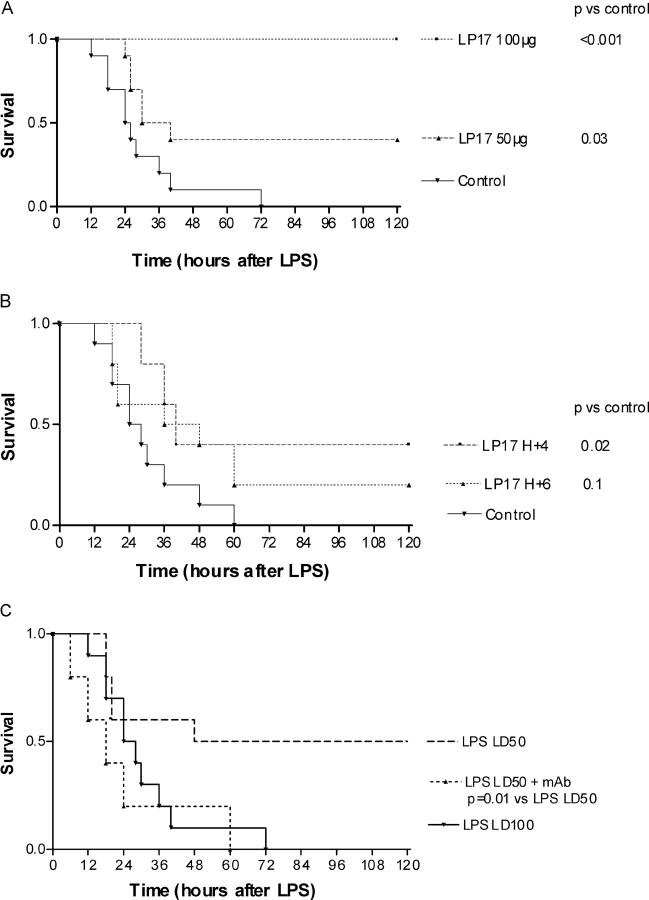
Mice treated by a single dose of LP17 60 min before a lethal dose (LD100) of LPS were prevented from death in a dose-dependent manner (Fig. 4 A). To investigate whether LP17 treatment could be delayed until after the administration of LPS, we injected LP17 beginning 4 or 6 h after LPS injection. This delayed treatment up to 4 h conferred significant protection against an LD100 dose of LPS (Fig. 4 B). No late death occurred over 1 wk, indicating that LP17 did not merely delay the onset of LPS lethality but provided lasting protection. Control mice all developed lethargy, piloerection, and diarrhea before death. By contrast, LP17-treated mice remained well groomed and active, had no diarrhea, and were lively. To clarify the mechanism by which LP17 protected mice from LPS lethality, we determined the serum levels of TNF-α, IL-1β, and sTREM-1 of endotoxemic mice at 2 and 4 h. Compared with controls, pretreatment by 100 μg of LP17 reduced cytokines levels by 30% and increased sTREM-1 levels by twofold (Table I).
Figure 4.
Endotoxinic shock in mice. (A) LP17 pretreatment protects against LPS lethality in mice. Male Balb/c mice (20–23 g) were randomly grouped (10 mice per group) and treated with an LD100 of LPS. LP17 (50 or 100 μg) or control vector was administered 60 min before LPS. (B) Delayed administration of LP17 protects LPS lethality in mice. Male Balb/c mice (20–23 g) were randomly grouped (8 mice per group) and treated with an LD100 of LPS. LP17 (75 μg) or control vector was administered 4 or 6 h after LPS as indicated. (C) Administration of agonist TREM-1 mAb is lethal to mice. Male Balb/c mice (20–23 g) were randomly grouped (eight mice per group) and treated with a combination of an LD50 of LPS + control vector, LD50 of LPS + anti-TREM-1 mAb (5 μg), or LD100 of LPS + control vector as indicated. Control vector and anti–TREM-1 mAb were administered 1 h after LPS injection.
Table I.
Serum Concentrations of TNF-α, IL-1β, and sTREM-1 in Endotoxemic Mice
| TNF-α | IL-1β | sTREM-1 | ||||
|---|---|---|---|---|---|---|
| ng/mL | ng/mL | ng/mL | ||||
| H2 | H4 | H2 | H4 | H2 | H4 | |
| Control | 3.3 ± 1.0 | 0.4 ± 0.1 | 0.3 ± 0.1 | 1.5 ± 0.2 | 249 ± 48 | 139 ± 8 |
| LP17 (100 μg) | 2.4 ± 0.5 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.9 ± 0.2 | 475 ± 37 | 243 ± 28 |
Engagement of TREM-1 Is Lethal to Mice.
To further highlight the role of TREM-1 engagement in LPS-mediated mortality, mice were treated with agonist anti–TREM-1 mAb in combination with the administration of an LD50 dose of LPS. This induced a significant increase in mortality rate from 50 to 100% (Fig. 4 C).
LP17 Protects Mice from CLP-induced Lethality.
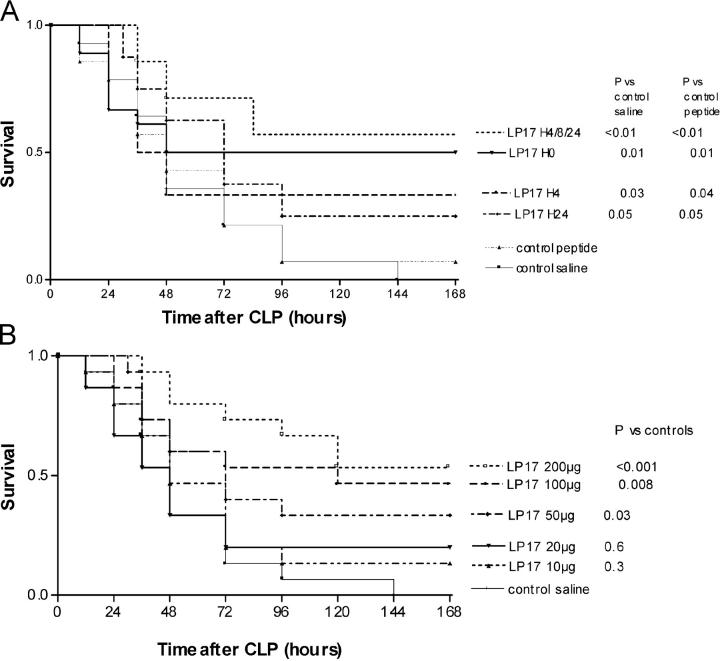
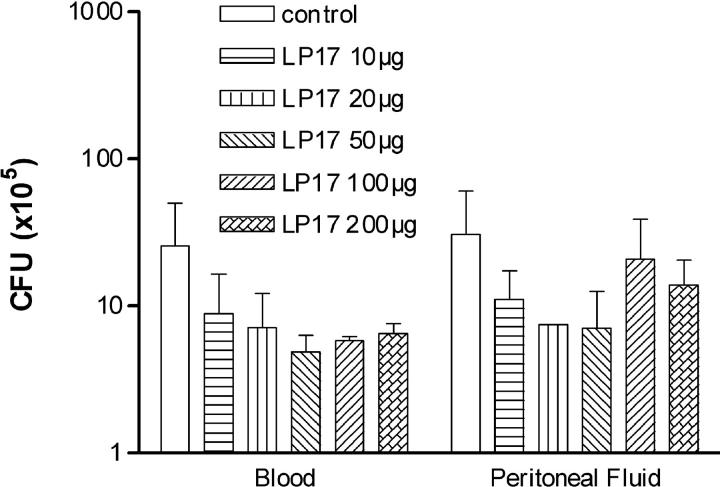
To investigate the role of LP17 in a more relevant model of septic shock, we performed CLP experiments (Fig. 5 A). The control groups comprised mice injected with normal saline or with the control peptide. In this model of polymicrobial sepsis, LP17 still conferred a significant protection against lethality even when administered as late as 24 h after the onset of sepsis. Interestingly, repeated injections of LP17 had the more favorable effect on survival (P < 0.01). There was a dose–response effect of LP17 on survival (Fig. 5 b) and cytokines production (Table II). LP17 had no effect on bacterial clearance (Fig. 6).
Figure 5.
CLP polymicrobial sepsis model. (A) LP17 partially protects mice from CLP-induced lethality. Male Balb/c mice (20–23 g) were randomly grouped and treated with normal saline (n = 14) or the control peptide (n = 14; 100 μg) or with LP17 (100 μg) in a single infection at H0 (n = 18), H+4 (n = 18), or H+24 (n = 18). The last group of mice (n = 18) was treated with repeated injections of LP17 (100 μg) at H+4, H+8, and H+24. (B) Dose effect of LP17 on survival. Mice (n = 15 per group) were treated with a single injection of normal saline or 10, 20, 50, 100, or 200 μg of LP17 at H0 after the CLP and monitored for survival.
Table II.
Serum Concentrations of TNF-α, IL-1β, and sTREM-1 at 24 h after CLP
| TNF-α pg/mL |
IL-1β pg/mL |
sTREM-1 ng/mL |
|
|---|---|---|---|
| Control peptide | 105 ± 12 | 841 ± 204 | 35 ± 5 |
| Control saline | 118 ± 8 | 792 ± 198 | 35 ± 5 |
| LP17 10 μg | 110 ± 11 | 356 ± 62 | 43 ± 8 |
| LP17 20 μg | 89 ± 10 | 324 ± 58 | 58 ± 8 |
| LP17 50 μg | 24 ± 6 | 57 ± 11 | 93 ± 10 |
| LP17 100 μg | 20 ± 3 | 31 ± 3 | 118 ± 12 |
| LP17 200 μg | 21 ± 7 | 37 ± 8 | 158 ± 13 |
Figure 6.
LP17 has no effect on bacterial counts during CLP. Mice (5 per group) were killed under anesthesia at 24 h after CLP. Bacterial counts in peritoneal lavage fluid and blood were determined and results are expressed as CFU per ml of blood and CFU per mouse for the peritoneal lavage.
Discussion
Sepsis exemplifies a complex clinical syndrome that results from a harmful or damaging host response to severe infection. Sepsis develops when the initial, appropriate host response to systemic infection becomes amplified and then dysregulated (4, 5). Neutrophils and monocyte/macrophages exposed to LPS, for instance, are activated and release such proinflammatory cytokines as TNF-α and IL-1β. Excessive production of these cytokines is widely believed to contribute to the multiorgan failure that is seen in septic patients (20–23).
TREM-1 is a recently identified molecule involved in monocytic activation and inflammatory response (12, 14). It belongs to a family related to NK cell receptors that activate downstream signaling events. The expression of TREM-1 on PMNs and monocytes/macrophages has been shown to be inducible by LPS (16, 17).
Here, we report that a soluble form of TREM-1 was released from cultured human monocytes after stimulation with E. coli LPS. Such a soluble form was also detectable in the serum of endotoxemic mice as early as 1 h after LPS challenge. This is consistent with the implication of TREM-1 in the very early phases of the innate immune response to infection (14, 15, 24). The mechanism by which sTREM-1 is released is not clearly elucidated but seems to be related to an increased transcription of the TREM-1 gene. Nevertheless, although incubation with a protease inhibitor cocktail does not alter the sTREM-1 release, cleavage of the surface TREM-1 from the membrane cannot be totally excluded. Interestingly, stimulation of human monocytes with such proinflammatory cytokines as TNF-α, IL-1β, or IFN-γ induced very small sTREM-1 release unless LPS was added as a costimulus. The expression of an alternative mRNA TREM-1sv has been detected in monocytes that might translate into a soluble receptor (18) upon stimulation with cell wall fraction of Mycobacterium bovis BCG but not LPS (25). This was confirmed in this study as (a) LPS did not increase the level of mRNA TREM-1sv in monocytes and (b) only a 27-kD protein was released by monocytes upon LPS stimulation and not the 17.5-kD variant.
Although its natural ligand has not been identified (13, 14), engagement of TREM-1 on monocytes with an agonist monoclonal antibody resulted in a further enhancement of proinflammatory cytokines production, whereas LP17 induced a decrease of these syntheses in a concentration-dependent manner, and IL-10 completely suppressed it.
Inflammatory cytokines, and especially TNF-α, are considered to be deleterious, yet they also possess beneficial effects in sepsis (5) as shown by the fatal issue of peritonitis in animals with impaired TNF-α responses (9–11). Moreover, in clinical trials the inhibition of TNF-α increased mortality (8). Finally, the role of TNF-α in the clearance of infection has been highlighted by the finding that sepsis is a frequent complication in rheumatoid arthritis patients treated with TNF-α antagonists (26).
The mechanism by which LP17 modulates cytokine production is not yet clear. LP17 comprises the complementary determining region-3 and the “F” β strand of the extracellular domain of TREM-1. The latter contains a tyrosine residue mediating dimerization. Radaev et al. postulated that TREM-1 captures its ligand with its complementary determining region-equivalent loop regions (27). Thus, LP17 could impair the TREM-1 dimerization and/or compete with the natural ligand of TREM-1. Moreover, the increase of sTREM-1 release from monocytes mediated by LP17 could prevent the engagement of membrane TREM-1, sTREM-1 acting as a decoy receptor, as in the TNF-α system (28, 29).
Activation of the transcription factor NF-κB is a critical step in monocyte inflammatory cytokine production after exposure to bacterial stimuli such as LPS (30, 31). Among the various NF-κB/Rel dimers, the p65/p50 heterodimer is the prototypical form of LPS-inducible NF-κB in monocytes (32). LP17 abolishes the p65/p50 NF-κB overactivation induced by the engagement of TREM-1. This might at least partially explain the effects of LP17 on cytokine production and the protection from lethality shown here to occur when the peptide was injected 1 h before LPS-induced septic shock, or even up to 4 h after.
Endotoxemia is simple to achieve experimentally but imperfectly suited to reproduce human sepsis, whereas polymicrobial sepsis induced by CLP is a more complex but better model, including the use of fluid resuscitation and antibiotics. Thus, the latter was also used in this study and confirmed the dose-dependent protection provided by LP17, even when administered as late as 24 h after the onset of sepsis. However, the favorable effect of LP17 was unrelated to an enhanced bacterial clearance.
One difficulty in the use of immunomodulatory therapies is that it is not possible to predict the development of sepsis, and thus patients receiving those treatments frequently already have well-established sepsis (6). Since LP17 appeared to be effective even when injected after the outbreak of sepsis, it could thus constitute a realistic treatment (24, 33).
By contrast, engagement of TREM-1 by an agonist anti–TREM-1 monoclonal antibody mediated a dramatic increase of mortality rate in LPS-challenged mice: this further underscores the detrimental effect of TREM-1 engagement during septic shock.
Experimental septic shock reproduces human sepsis only in part. Indeed, our group recently showed that significant levels of sTREM-1 were released in the serum of critically ill patients with sepsis patients (34), the highest levels being observed in patients who survived. This is consistent with our experimental findings indicating that the more important sTREM-1 release, the more favorable is the outcome, and thus sustains, at least theoretically, the potential value of soluble TREM peptides as postonset sepsis therapy.
TREM-1 appears to be a crucial player in the immediate immune response triggered by infection. In the early phase of infection, neutrophils and monocytes initiate the inflammatory response owing to the engagement of pattern recognition receptors by microbial products (3, 4). At the same time, bacterial products induce the up-regulation and the release of sTREM-1. Upon recognition of an unknown ligand, TREM-1 activates signaling pathways which amplify these inflammatory responses, notably in monocytes/macrophages. The modulation of TREM-1 signaling reduces, although without complete inhibition, cytokine production and protects septic animals from hyper-responsiveness and death. Modulation of TREM-1 engagement with such a peptide as LP17 might be a suitable therapeutic tool for the treatment of sepsis, particularly because it seems to be active even after the onset of sepsis after infectious aggression.
Acknowledgments
We are indebted to Chantal Montemont and Eliane Vauthier for technical assistance.
This work was supported in part by a grant (EA 3143) from the French Ministère de la Recherche et de la Technologie.
The authors have no conflicting financial interests.
Abbreviations used in this paper: CLP, cecal ligation and puncture; TREM, triggering receptor expressed on myeloid cells; TREM-1sv, TREM-1 splice variant; TLR, toll-like receptor.
References
- 1.Aderem, A, and R.J. Ulevitch, R.J. 2000. Toll-like receptors in the induction of the innate immune response. Nature. 406:782–786. [DOI] [PubMed] [Google Scholar]
- 2.Thoma-Uszynski, S., S. Stenger, O. Takeuchi, M.T. Ochoa, P.A. Engele, P.A. Sieling, P.F. Barnes, M. Rollinghoff, P.L. Bolcskei, and M. Wagner. 2001. Induction of direct antimicrobial activity through mammalian Toll-like receptors. Science. 291:1544–1549. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov, R., and C. Janeway Jr. 2000. Innate immunity. N. Engl. J. Med. 343:338–344. [DOI] [PubMed] [Google Scholar]
- 4.Cohen, J. 2002. The immunopathogenesis of sepsis. Nature. 420:885–891. [DOI] [PubMed] [Google Scholar]
- 5.Hotchkiss, R.S., and I.E. Karl. 2003. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348:138–150. [DOI] [PubMed] [Google Scholar]
- 6.Vincent, J.L., Q. Sun, and M.J. Dubois. 2002. Clinical trials of immunomodulatory therapies in severe sepsis and septic shock. Clin. Infect. Dis. 34:1084–1093. [DOI] [PubMed] [Google Scholar]
- 7.Riedemann, N.C., R.F. Guo, and P. Ward. 2003. Novel strategies for the treatment of sepsis. Nat. Med. 9:517–524. [DOI] [PubMed] [Google Scholar]
- 8.Fisher, C.J., Jr., J.M. Agosti, S.M. Opal, S.F. Lowry, R.A. Balk, J.C. Sadoff, E. Abraham, R.M. Schein, and E. Benjamin. 1996. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The soluble TNF receptor sepsis study group. N. Engl. J. Med. 334:1697–1702. [DOI] [PubMed] [Google Scholar]
- 9.Echtenacher, B., W. Falk, D.N. Mannel, and P.H. Krammer. 1990. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J. Immunol. 145:3762–3766. [PubMed] [Google Scholar]
- 10.Echtenacher, B., K. Weigl, N. Lehn, and D.N. Mannel. 2001. Tumor necrosis factor-dependent adhesions as a major protective mechanism early in septic peritonitis in mice. Infect. Immun. 69:3550–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eskandari, M.K., G. Bolgos, C. Miller, D.T. Nguyen, L.E. DeForge, and D.G. Remick. 1992. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J. Immunol. 148:2724–2730. [PubMed] [Google Scholar]
- 12.Bouchon, A., J. Dietrich, and M. Colonna. 2000. Inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 164:4991–4995. [DOI] [PubMed] [Google Scholar]
- 13.Colonna, M., and F. Facchetti. 2003. TREM-1 (triggering receptor expressed on myeloid cells): a new player in acute inflammatory responses. J. Infect. Dis. 187:S397–S401. [DOI] [PubMed] [Google Scholar]
- 14.Colonna, M. 2003. TREMs in the immune system and beyond. Nat. Rev. Immunol. 3:445–453. [DOI] [PubMed] [Google Scholar]
- 15.Nathan, C., and A. Ding. 2001. TREM-1: a new regulator of innate immunity in sepsis syndrome. Nat. Med. 7:530–532. [DOI] [PubMed] [Google Scholar]
- 16.Bouchon, A., F. Facchetti, M.A. Weigand, and M. Colonna. 2001. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 410:1103–1107. [DOI] [PubMed] [Google Scholar]
- 17.Bleharski, J.R., V. Kiessler, C. Buonsanti, P.A. Sieling, S. Stenger, M. Colonna, and R.L. Modlin. 2003. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J. Immunol. 170:3812–3818. [DOI] [PubMed] [Google Scholar]
- 18.Gibot, S., A. Cravoisy, B. Levy, M.C. Béné, G. Faure, and P.E. Bollaert. 2004. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N. Engl. J. Med. 350:451–458. [DOI] [PubMed] [Google Scholar]
- 19.Gingras, M.C., H. Lapillonne, and J.F. Margolin. 2002. TREM-1, MDL-1, and DAP12 expression is associated with a mature stage of myeloid development. Mol. Immunol. 38:817–824. [DOI] [PubMed] [Google Scholar]
- 20.Dinarello, C.A. 1997. Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest. 112:S321–S329. [DOI] [PubMed] [Google Scholar]
- 21.Bone, R.C. R.A Balk, F.B. Cerra, R.P. Dellinger, A.M. Fein, W.A. Knauss, R.M. Schein, and W.J. Sibbald. 1992. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 101:1644–1655. [DOI] [PubMed]
- 22.Warren, H.S. 1997. Strategies for the treatment of sepsis. N. Engl. J. Med. 336:952–953. [DOI] [PubMed] [Google Scholar]
- 23.Stone, R. 1994. Search for sepsis drugs goes on despite past failures. Science. 264:365–367. [DOI] [PubMed] [Google Scholar]
- 24.Cohen, J. 2001. TREM-1 in sepsis. Lancet. 358:776–778. [DOI] [PubMed] [Google Scholar]
- 25.Begum, N.A., K. Ishii, M. Kurita-Taniguchi, M. Tanabe, M. Kobayashi, Y. Moriwaki, M. Matsumoto, Y. Fukumori, I. Azuma, K. Toyoshima, and T. Seya. 2004. Mycobacterium bovis BCG cell wall-specific differentially expressed genes identified by differential display and cDNA substraction in human macrophages. Infect. Immun. 72:937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keane, J., S. Gershon, R.P. Wise, E. Mirabile-Levens, J. Kasznica, W.D. Schwieterman, J.N. Siegel, and M.M. Braun. 2001. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 345:1098–1104. [DOI] [PubMed] [Google Scholar]
- 27.Radaev, S., M. Kattah, B. Rostro, M. Colonna, and P. Sun. 2003. Crystal structure of the human myeloid cell activating receptor TREM-1. Structure. 11:1527–1535. [DOI] [PubMed] [Google Scholar]
- 28.Lantz, M., U. Gullberg, and E. Nilsson. 1990. Characterization in vitro of a human tumor necrosis factor binding protein. A soluble form of tumor necrosis factor receptor. J. Clin. Invest. 86:1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Zee, K.J., T. Kohno, and E. Fischer. 1992. Tumor necrosis soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor α in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 89:4845–4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collart, M.A., P. Baeuerle, and P. Vassalli. 1990. Regulation of tumor necrosis factor alpha transcription in macrophages. Involvement of four NFκB motifs and constitutive and inducible form of NFκB. Mol. Cell. Biol. 10:1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiscott, J.M., J.J. Garoufalis, A. Roulston, I. Kwan, N. Pepin, J. Lacoste, H. Nguyen, G. Bensi, and M. Fenton. 1993. Characterization of a functional NFκB site in the human IL-1β promoter: evidence for a positive autoregulatory loop. Mol. Cell. Biol. 13:6231–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urban, M.B., R. Schreck, and P.A. Baeuerle. 1991. NFκB contacts DNA by a heterodimer of the p50 and p65 subunit. EMBO J. 10:1817–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lolis, E., and R. Bucala. 2003. Therapeutic approaches to innate immunity: severe sepsis and septic shock. Nat. Rev. Drug Discov. 2:635–645. [DOI] [PubMed] [Google Scholar]
- 34.Gibot, S., M.N. Kolopp-Sarda, M.C. Béné, A. Cravoisy, B. Levy, G. Faure, and P.E. Bollaert. 2004. Plasma level of a triggering receptor expressed on myeloid cells-1: its diagnostic accuracy in patients with suspected sepsis. Ann. Intern. Med. 141:9–15. [DOI] [PubMed] [Google Scholar]