Abstract
The Ca2+ sensor synaptotagmin (Syt) VII regulates the exocytosis of conventional lysosomes in several cell types. In CTLs, the Ca2+-regulated exocytosis of lytic granules/secretory lysosomes is responsible for the perforin/granzyme-mediated lysis of target cells. To investigate the role of Syt VII in CTL effector function, the expression and function of Syt VII were examined in wild-type and Syt VII-deficient mice. In comparison with Syt VII+/+ controls, Syt VII−/− animals were impaired in their ability to clear an infection with the intracellular pathogen Listeria monocytogenes. When isolated CTLs were examined, we found that Syt VII is expressed upon CTL activation and localizes to granzyme A-containing lytic granules. Syt VII-deficient CTLs have no defects in proliferation and cytokine production, and their lytic granules contain normal amounts of perforin and granzyme A and polarize normally at the immunological synapse. However, despite normal conjugate formation with target cells, CTLs from Syt VII−/− mice exhibit reduced effector activity, when compared with controls. Treatment of Syt VII+/+ or Syt VII−/− CTLs with an inhibitor of the perforin-mediated lytic pathway resulted in comparable levels of cytotoxic activity, suggesting that Syt VII regulates perforin-mediated cytolytic CTL responses.
Activated CD8+ CTLs play an important role in host defense, by killing malignantly transformed or virally infected cells (1). This killing event requires only transient contact between the effector CTL and the target cell, and is mediated by the highly polarized secretion of the contents of lytic granules. At the zone of CTL-target cell contact, a highly specialized adhesion structure is formed, called the immunological synapse (2). Immunological synapse formation is triggered by TCR recognition of target cells, and involves polarization of the micro-tubule organizing center, Golgi complex, and lytic granules. The lytic granules of CTLs contain lysosomal markers, in addition to the specialized molecules responsible for cytotoxicity, such as perforin and granzymes. For this reason, CTL lytic granules are also referred to as secretory lysosomes, because exocytosis of these granules and delivery of their lytic contents occur in a regulated fashion, triggered by elevations in intracellular free Ca2+ ([Ca2+ ]i)5 (3, 4).
Elevations in [Ca2+]i trigger the exocytosis of conventional lysosomes, in several different cell types (5). Recent studies showed that lysosomal exocytosis is regulated by synaptotagmin (Syt) VII, a ubiquitously expressed and evolutionarily conserved member of the Syt family of Ca2+ sensors (6, 7). Syt VII is localized on the membrane of lysosomes, and of some secretory granules of specialized cells (6, 8, 9). The expression of dominant-negative constructs or the genetic ablation of Syt VII results in impaired lysosomal exocytosis (6, 10, 11), and in marked alterations in the lysosomal secretion pattern (12). Previous studies using cells defective in lysosomal-regulatory proteins, such as the LYST protein that carries the mutation leading to enlarged lysosomes in Chediak-Higashi/beige syndrome (13), showed that similar secretory defects are found in fibroblast conventional lysosomes (14) and in CTL lytic granules (15). Given that exocytosis of conventional lysosomes is also impaired in fibroblasts from Syt VII-deficient mice (10, 11), we investigated whether a similar functional defect was present in CTLs from these animals. Our results show that Syt VII is expressed in CTLs following activation. In response to an infection with the intracellular bacterium Listeria monocytogenes, Syt VII-deficient mice elicit Ag-specific CD8 T cell responses similar to controls, yet demonstrate an impaired ability to clear the infection.
To further characterize the role of Syt VII, Ag-specific CTLs were examined in vitro. CTLs from Syt VII-deficient mice proliferate normally in response to Ag-specific stimulation, have morphologically normal granules containing normal amounts of secretory/lytic products, and form normal immunological synapses upon target cell contact. Moreover, the efficiency of target cell killing by Syt VII-deficient CTLs, both in vivo and in vitro, is consistently reduced, suggesting that Syt VII is required for the efficient exocytic delivery of lytic products at the immunological synapse.
Materials and Methods
Abs, reagents, and cell lines
The rat mAb recognizing granzyme A (7.1) was a gift from M. Simon (Max Planck Institute, Freiburg, Germany). The affinity-purified rabbit Ab against perforin was purchased from Santa Cruz Biotechnology. Rabbit polyclonal Abs against the NH2 terminal of Syt VII were generated and affinity purified, as previously described (6). The rat mAb against Lamp-1 (ID4B) was from the Developmental Studies Hybridoma Bank. Secondary Alexa Fluor-488-labeled goat anti-rat IgG, Alexa Fluor-546 goat anti-rabbit, and mouse IgG were purchased from Molecular Probes; secondary HRP-conjugated anti-rabbit and anti-rat IgG Abs were from Kirkegaard & Perry Laboratories. Purified mAb anti-CD3ε (145.2C11) was obtained from BD Pharmingen, and mAbs against talin were from Sigma-Aldrich. The OVA257–264 peptide (SIINFEKL) was synthesized and purified by the W. M. Keck Biotechnology Resource Center (Yale University).
P815 and EL4 mouse target cell lines (American Type Culture Collection; a gift from M. Starnbach, Harvard Medical School, Boston, MA) were maintained in RPMI 10/5% FBS with penicillin/streptavidin (pen/strep), Na pyruvate, and minimum essential amino acids at 37°C/5% CO2. Normal rat kidney (NRK) cells were maintained in DMEM/10% with pen/strep.
Mice
Syt VII-deficient mice were generated, as previously described (11), and backcrossed for eight generations into the C57BL/6 background. Seven- to 8-wk-old female B6D2F1 mice were used as a source of mitomycin C-treated allogeneic stimulators (Charles River Laboratories) (16). For some experiments, Syt VII wild-type (WT) and knockout (KO) mice were crossed to OT-1 transgenic mice (provided by F. Carbone, University of Melbourne, Melbourne, Australia) to generate Syt VII+/+ OT-1+ and Syt VII−/− OT-1+ mice specific for the OVA257–264 (SIINFEKL) epitope of OVA (17). OT-1+ mice were screened by retro-orbital bleeding and flow cytometry after staining with CD8- and TCRVα5-specific mAbs, and by tail DNA PCR screening using the OT-1-specific sequence using primers: 5′-GCAGCAAGTGACAACTATCAGTTG-3′ (forward) and 5′-CAGATC TCAACTGGACCACAG-3′ (reverse). Syt VII−/− mice were identified by tail PCR screening using primers spanning the Syt VII C2A region, as follows: 5′-CGATGAGTCTGACCGCAGAAC-3′ (forward) and 5′-CCA CCTTACTACCATCTCTGC-3′ (reverse). All mice were maintained at the Yale Animal Resources Center.
Listeria infections
WT (C57BL/6) and Syt VII-deficient animals (8 –10 wk of age) were primed i.v. with 1 × 104 CFU of rOVA-expressing L. monocytogenes strain JJL-OVA (provided by H. Shen, University of Pennsylvania, Philadelphia, PA). Three weeks later, Ag-primed animals received a second challenge with 2 × 105 CFU of JJL-OVA. Five days postchallenge, animals were sacrificed and single-cell suspensions were prepared from the spleens. The number of SIINFEKL-specific CD8 T cells was determined by flow cytometry. Cells were stained with Abs to CD11a and CD8 (both from BD Pharmingen), followed by PE-conjugated control and SIINFEKL peptide-loaded H-2Kb-IgG dimer (BD Pharmingen). Splenic bacterial burden was determined, as previously described (18). In brief, serial dilutions of splenic homogenates were resuspended in PBS containing 0.05% Triton X-100 and plated in triplicate onto brain-heart infusion agar plates and evaluated 48 h after culture at 37°C.
In vitro T cell stimulation
Spleens or mesenteric lymph nodes were removed from Syt VII KO mice and homogenized between frosted glass slides into a single-cell suspensions. RBC were lysed in 1.66% NH4Cl. CTLs were activated by stimulation of splenocytes from B6D2F1 mice with mitomycin C in RPMI 10 medium containing 10% FBS, 10% T-Stim medium (BD Discovery Lab-ware), 5% 1 M methyl α-D-glucopyranoside (Sigma-Aldrich), and pen/strep. After 5 days of stimulation, proliferating CTLs were isolated using lymphocyte separation medium (Fisher Scientific) before assays were performed. OVA-specific CTLs were generated in vitro by stimulation of splenocytes with 0.5–1 μg/ml OVA257–264 peptide (SIINFEKL) in RPMI 10 medium containing 10% FBS and pen/strep. Cells were stimulated for 48 h in the presence of peptide, then washed and cultured in the presence of rIL-2 (20 U/ml) for 24 h before functional assays. CD8+ CTLs were purified using the MACS MS CD8+ separation columns (Miltenyi Biotec), according to the manufacturer’s instructions.
RT-PCR
mRNA was prepared from homogenized whole spleens or day 5-stimulated CTLs in TRIzol. RT-PCR was performed using primers spanning the Syt VII C2A domain as follows: 5′-CGATGAGTCTGACCGCAGAAC-3′ (forward) and 5′-CCACCTTACTACCATCTCTGC-3′(reverse).
Immunoblotting
A total of 2–3 × 106 CTLs was lysed in lysis buffer with protease inhibitors on ice for 20 min. Cell lysates were run on SDS-PAGE gels, and the protein was transferred to nitrocellulose for 1 h. Membranes were blocked in 5% milk/TBS Tween 20 overnight and probed with anti-Syt VII, anti-perforin, or anti-granzyme A Abs for 1 h, before washing three times with TBS/Tween 20 buffer. Membranes were then incubated in secondary HRP-labeled Abs (anti-rabbit, anti-rat) for 1 h, followed by ECL analysis.
Immunofluorescence
Day 5- and 6-stimulated CTLs were used for immunostaining. Cells in suspension were added to poly-L-lysine-coated coverslips at 37°C for 25 min before fixing with 3.7% formaldehyde at 4°C. After washing with PBS, the cells were quenched with 50 mM ammonium chloride for 15 min at room temperature, and coverslips were washed once and permeabilized with 0.1% Triton X-100 for 2 min before blocking in 3 mM glycine/PBS/1% BSA solution for 30 – 45 min. The cells were then stained with mAbs specific for Lamp-1 or granzyme A, and also for Abs against the NH2 terminus of Syt VII. Following staining with conjugated secondary Abs, the DNA of cells was stained for 1 min with 4′,6′-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) and diluted to 5 μg/ml in PBS/2% BSA. Coverslips were mounted in Prolong antifade medium (Molecular Probes) and observed under a Zeiss Axiovert 135 epifluorescent microscope.
Granzyme activity assays
Granzyme A activity was assayed by incubation of 950 μl of benzyloxy-carbonyl-L-lysine thiobenzyl ester (BLT) substrate solution (0.20 mM N-α-BLT (Calbiochem), 0.22 mM 5,5′-dithio-bis-2-nitrobenzoic acid (Sigma-Aldrich), PBS, 0.01% Triton X-100) with 50 μl of culture supernatant, or of total cell lysates in 1% Triton X-100 for 60 min at 37°C. The reaction was stopped by adding 1 ml of cold 1 mM PMSF/PBS, and the absorbance was read at 412 nm. β-hexosaminidase was assayed by incubation of 10 μl of supernatant with 50 μl of B-hex substrate (6 mM 4-methyl-umbelliferyl-N-acetyl-β-D-glucosaminide in 0.1 M citric acid, 0.2 M Na2HPO4 (pH 4.5), Triton X-100) for 15 min at 37°C. A total of 100 μl of Stop buffer (200 mM Na2CO3, 100 mM glycine) was added before reading the fluorescence at excitation 365 nm and emission 450 nm. Percentage of total secretion was calculated as (ODsupernatant/ODlysate) × 100. All assays were performed in triplicate.
Immunological synapse formation
Day 5- and 7-stimulated CTLs were pulsed for 3 h with 10 kDa of dextran-Texas Red in serum-free medium and washed extensively, before chasing for 3 h with RPMI 10. A total of 5 × 105 dextran-Texas Red-pulsed CTLs was mixed with an equal number of P815 cells in 1 ml of RPMI 10. Cells were centrifuged at 100 × g for 5 min at room temperature and resuspended carefully with a Pasteur pipette. The 100-μl aliquots (1 × 105 cells) were then plated on poly(L-lysine)-coated coverslips and placed at 37°C/5% CO2 for 20 – 45 min before fixing in 3% paraformaldehyde for 25 min at 4°C. Cells were subsequently permeabilized for 5 min with 0.1% Triton X-100, washed with PBS, and blocked for 1 h with 1% BSA/PBS. Polyclonal mouse anti-talin Abs (Sigma-Aldrich) were added in the presence of 1% BSA for 1 h and washed extensively in 1% BSA/PBS. Alexa Fluor-488 goat anti-mouse secondary Abs were added in the presence of 1% BSA for 30 min and washed in 1% BSA/PBS, and nuclei were stained with DAPI.
Cytotoxicity assays
Syt VII+/+ or Syt VII−/− CTLs were mixed with 104 P815 (H2d) or EL4 (H2b) target cells at the indicated E:T ratios per well in a final volume of 100 μl in RPMI 10/5% FBS without phenol red. For OT-1 experiments, target cells were coated with or without SIINFEKL peptide (5 μg/ml) for 1 h at 37°C and washed three times with cold medium before mixing with CTLs. After a 4-h incubation at 37°C, cytotoxicity was measured via lactate dehydrogenase activity release from targets, using the CytoTox96 non-radioactive kit, according to the manufacturer’s protocol (Promega). For inhibitor experiments, CTLs were pretreated with 0.25 μM concanamycin A (Alexis Pharmaceuticals) 2 h before being added to targets in the continued presence of the drug. Absorbance was read at 490 nm, and percentage of cytotoxicity was calculated, as follows: (experimental –effector spontaneous – target spontaneous)/(target maximum – target spontaneous) × 100.
FACS analysis
A total of 106 cells was washed with serum-free medium and 1% BSA; stained with CD8 PerCP, CD4 PE, TCRβ FITC, or granzyme A FITC (all from BD Pharmingen) on ice for 20 min in the dark; washed three times with cold PBS; and fixed with 2% paraformaldehyde for 20 min on ice. To evaluate Ag-specific CD8 T cell responses, 106 spleen cells were stained with dimeric Kb (BD Biosciences) loaded with the immunodominant MHC class I-restricted OVA-specific peptide SIINFEKL, as directed by the manufacturer. Samples were then incubated with FITC-labeled anti-mouse IgG to detect dimer staining along with CD11a FITC and CD8 PerCP (all from BD Biosciences) and evaluated on a FACScan flow cytometer.
Splenocyte proliferation assays
A total of 5 × 105 harvested splenocytes was plated into 96-well flat-bottom microtiter plates in 100 μl of RPMI 10 containing soluble anti-CD3 at indicated concentrations. The cultures were incubated for 72 h at 37°C/5% CO2 before proliferation was measured using a nonradioactive 3-(4,5 dimethylthiazol-2yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) (inner salt) assay (CellTiter96; Promega), according to the manufacturer’s instructions. In brief, 20 μl of the substrate solution was added to wells and incubated at 37°C with 5% CO2 for 1– 4 h to allow for full colorimetric change to occur. Absorbance 490 nm was read with a Bio-Rad Spectramax ELISA plate reader. All data are shown as means of quadruplicate wells.
Secretion assays
Secretion assays were performed essentially as previously published (19). In brief, round-bottom 96-well plates (Falcon) were coated with 5 μg/ml anti-CD3 Ab (145.2C11) for 30 min at room temperature. Wells were washed three times with 100 μl of RPMI 10 medium before plating 5 × 105 CTLs/50 μl in Ab-coated or uncoated wells. A total of 50 μl of medium was then added to all wells. Total granule release was determined by replacing 10 μl of medium with 10 μl of 1% Triton X-100. Plates were incubated for indicated times at 37°C/5% CO2 before centrifuging for 5 min at 200 × g at 4°C and collecting the supernatants for granzyme A activity assays.
Results
Syt VII is expressed by activated CTLs and present on lytic granules
Syt VII colocalizes with lysosomal markers, and regulates the fusion of conventional lysosomes with the plasma membrane in several cell types (6 –10, 12, 20, 21). These findings suggested that Syt VII might also be involved in the functional regulation of lytic granules of CTLs, which are known to have lysosomal properties and to be secreted in response to elevations in [Ca2+]i (3, 4).
To examine the role of Syt VII in T cells, the expression of Syt VII was examined in stimulated CTLs from C57BL/6 mice. Western blot analysis using an affinity-purified polyclonal Ab against 65-kDa band on lysates the NH2 terminus of Syt VII identified a ∼ of NRK cells (as previously described in Ref. 6) and of spleen-derived CD8+ CTLs (Fig. 1A, left panel). This 65-kDa band corresponds to the expected size of Syt VIIα, the most abundantly expressed isoform of Syt VII in several tissues (22). Because the lysosomal components of CTL lytic granules are primarily expressed upon activation (23), the expression of Syt VII was then examined in unstimulated and stimulated CD8+ T lymphocytes. For this experiment, the T cells were isolated from lymph nodes, to ensure the presence of unstimulated CD8+ CTLs (the spleen normally contains higher percentages of Ag-experienced cells). When unstimulated and allogeneic-stimulated lymph node CD8+ T cells were analyzed, expression of Syt VII was detected only in the stimulated population (Fig. 1A, right panel). Thus, the expression pattern of Syt VII is similar to that of other secretory lysosome components such as granzyme A and perforin, which are up-regulated only after activation (24).
FIGURE 1.
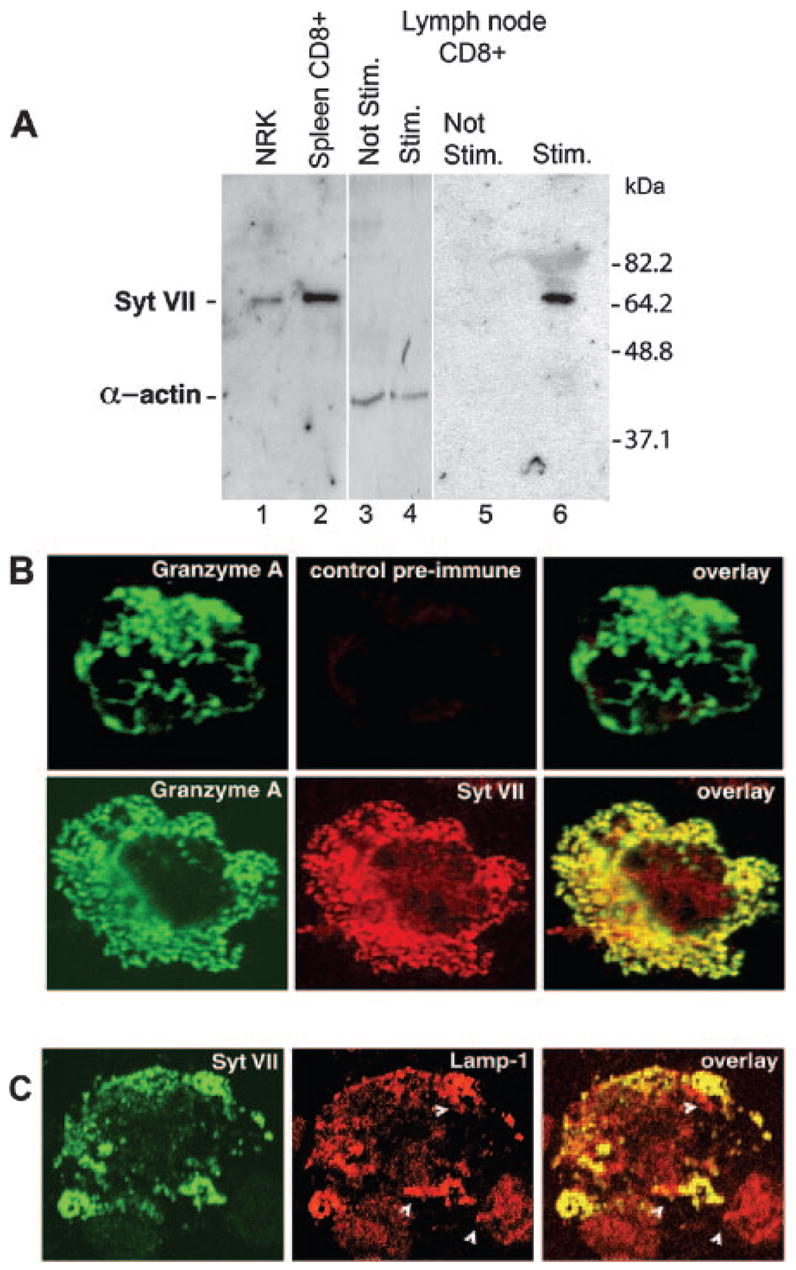
CTLs express Syt VII upon stimulation. A, A ∼66-kDa band corresponding to Syt VII was detected in lysates of NRK cells (lane 1), and on CD8+ T lymphocytes purified from mouse spleens (lane 2), by immunoblot using a N-terminal Syt VII-specific Ab. On lymph node lysates, the band corresponding to Syt VII was detected in activated (lane 6), but not unstimulated alloreactive purified CD8 T cells (lane 5). As a loading control, equivalent lysates of stimulated and nonstimulated purified CD8+ T cells were probed with anti-α-actin Abs (lanes 3 and 4). B, Activated alloreactive CTLs were stained with Abs against granzyme A (green) and either preimmune (red, top row) or anti-Syt VII (red, bottom row). The overlay shows a high degree of colocalization of Syt VII with granzyme A in CTL granules. C, Stimulated CTLs were stained with Abs against Syt VII (green) and Lamp-1 (red). The overlay shows the colocalization of Syt VII and the lysosomal marker Lamp-1. Arrowheads indicate some Syt VII-positive vesicles that are negative for Lamp-1.
The subcellular localization of Syt VII in CTLs was then evaluated by immunofluorescence. To identify the secretory lysosomes/lytic granules, the same cells were also labeled with Abs against granzyme A. As shown in Fig. 1B, an almost complete colocalization of granzyme A-positive granules with Syt VII-positive compartments was seen. No reactivity was observed following similar staining with preimmune serum. The Syt VII-positive compartments also colocalized with the late endosome/lysosome marker Lamp-1 (Fig. 1C), further suggesting that Syt VII localizes to secretory lysosomes/lytic granules of CTLs.
Phenotype and function of T cells from Syt VII-deficient mice
To confirm the lack of Syt VII expression in CTLs of Syt VII-deficient mice, as previously shown for fibroblasts, macrophages, and skeletal muscle (10), we isolated total RNA from whole spleens and purified CTLs of WT and Syt VII KO mice. RT-PCR analysis using primers corresponding to the C2A domain of Syt VII identified a 500-bp band in the WT splenocyte population and in purified CTLs, but not in similar preparations from Syt VII KO mice (Fig. 2A).
FIGURE 2.
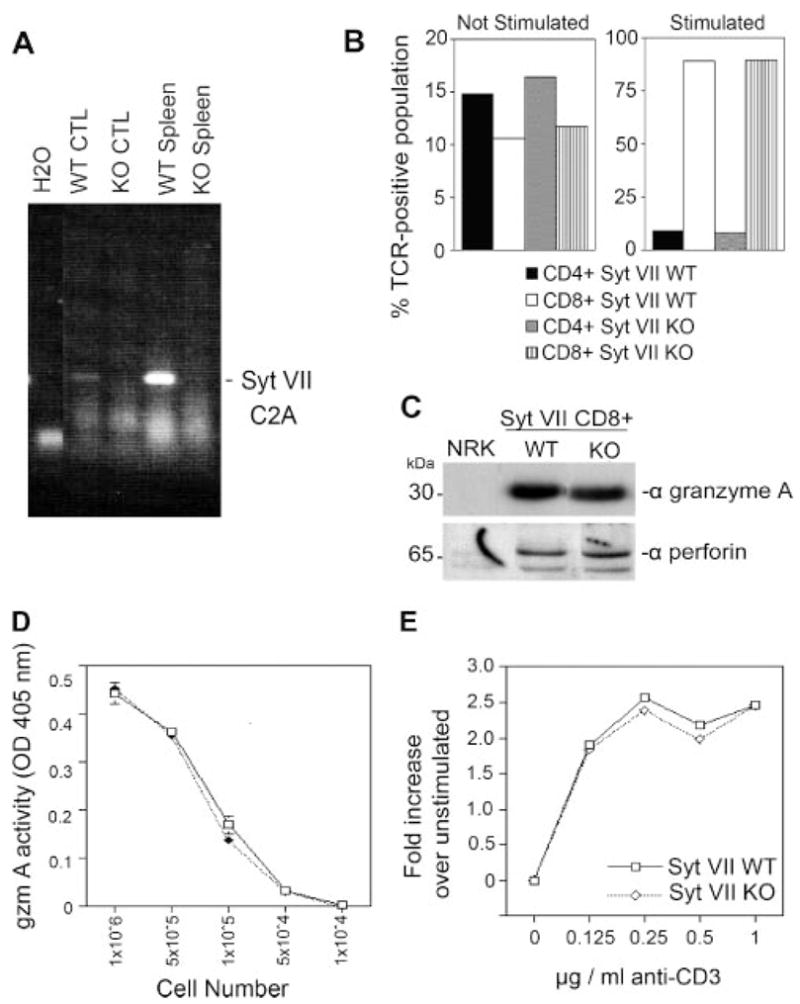
Characterization of CTLs from Syt VII KO mice. A, RT-PCR analysis showing that Syt VII is expressed in the spleen and in purified CTLs of WT, but not Syt VII KO mice. B, Percentage of CD4+ and CD8+ T cells in unstimulated (left) or stimulated splenocytes (right), determined by flow cytometry. C, Western blot analysis showing granzyme A and perforin levels are comparable in lysates of CTLs from Syt VII WT and Syt VII KO mice. D, Total BLT-esterase (granzyme A) activity assay, showing that Syt VII WT and Syt VII KO CTLs contain the same levels of active enzyme 5 days postallogeneic stimulation. E, Proliferation of splenocytes from Syt VII WT (□) and KO (⋄) following allogeneic stimulation was assayed using the CellTiter96 MTS assay. The data represent the mean ± SEM for triplicate samples of one representative experiment.
Compared with their WT littermates, Syt VII-deficient mice are characterized by slightly reduced numbers of splenocytes (5.48 × 107 ± 0.97 × 107 in KO vs 6.49 × 107 ± 1.73 × 107 in WT). Given the observed reduction of total splenocytes, the phenotype and composition of spleen cells in Syt VII-deficient animals were examined. The number of splenic CD4+ and CD8+ T cells was determined by FACS analysis, after harvesting unstimulated cells or after culturing unstimulated cells for 5 days in the presence of allogeneic stimulator cells. No significant differences were detected in the overall composition of Syt VII KO T cell splenocyte populations, in comparison with WT. After stimulation in culture for 5 days, Syt VII WT and KO splenocytes contained an equivalent fraction of CD4+ (9 vs 8%) and CD8+ (89.2 vs 89.8%) T cell populations (Fig. 2B). Taken collectively, these results suggest that the expression of Syt VII is not required for the development of CD4 or CD8 T cells in vivo.
We then examined the CD8+ T cell populations of WT and KO mice for the expression of two important effectors of lytic granule function, perforin and granzyme A. Western blots were performed on lysates from WT and KO CTLs with Abs to these granule products. Abs against granzyme A or perforin detected bands of 30 and 65 kDa, respectively, in both WT and KO CTLs (Fig. 2C). Thus, both of these secretory products showed no change in expression in response to the absence of Syt VII. Similarly, when the total amount of BLT esterase activity (granzyme A) was determined from Triton X-100 CTL lysates, nearly identical levels were observed (Fig. 2D), eliminating the possibility that Syt VII deficiency affects the production or storage of this cytotoxicity effector molecule. Likewise, when the granule morphology was studied by immunofluorescence staining with anti-granzyme A, no apparent differences in granule size or number were seen in CTLs from Syt VII KO mice, when compared with WT CTLs (data not shown). The fraction of CD8+ T cells expressing granzyme A after activation was also similar for WT and KO mice (data not shown), and no differences were detected in the capacity of splenocytes to proliferate following stimulation of allogenic mitomycin C-treated splenocytes (Fig. 2E) or in response to TCR mediated following cross-linking with anti-CD3 Abs (data not shown). Secretion of the cytokines IFN-γ and IL-2 was also comparable between WT and KO splenocytes, following TCR cross-linking stimulation (data not shown).
Syt VII-deficient mice exhibit impaired CD8 effector activity in vivo
To evaluate the effector activity of Syt VII-deficient CTLs in vivo, mice were examined for their ability to resolve an infection with L. monocytogenes. L. monocytogenes is a Gram-positive, facultative intracellular bacterial pathogen. Following infection, the clearance and subsequent immunity to this pathogen are mediated predominantly by Ag-specific CD8 T cells (18). To examine Ag-specific CD8 T cell effector activity, WT and Syt VII KO animals were immunized with PBS or 1 × 104 CFU of OVA-expressing recombinant L. monocytogenes strain JJL-OVA, to prime Ag-specific T cells responses. Three weeks later, animals received a secondary challenge with 2 × 105 CFU of JJL-OVA. Four days following the second challenge, animals were examined for Ag-specific CD8 T cell responses, and for bacterial burden in the spleen. Ag-specific T cell responses to the OVA immunodominant H-2Kb-restricted peptide SIINFEKL were evaluated by flow cytometry. Analysis of CD8 T cells revealed comparable percentages (Fig. 3A) and absolute numbers (Fig. 3B) of Ag-specific CD8+ T cells in both primed WT and Syt VII-deficient animals. As expected, no Ag-specific SIINFEKL CD8 T cell responses were observed in either group when primed with PBS alone (Fig. 3, A and B). These results demonstrate that Syt VII-deficient animals successfully generate Ag-specific CD8 T cell responses that are equivalent to littermate controls. However, despite similar numbers of Ag-specific CD8 T cells, primed Syt VII-deficient animals exhibited significantly higher bacterial burden (Fig. 3C), suggesting an impairment in CD8+ T cell-mediated pathogen clearance.
FIGURE 3.
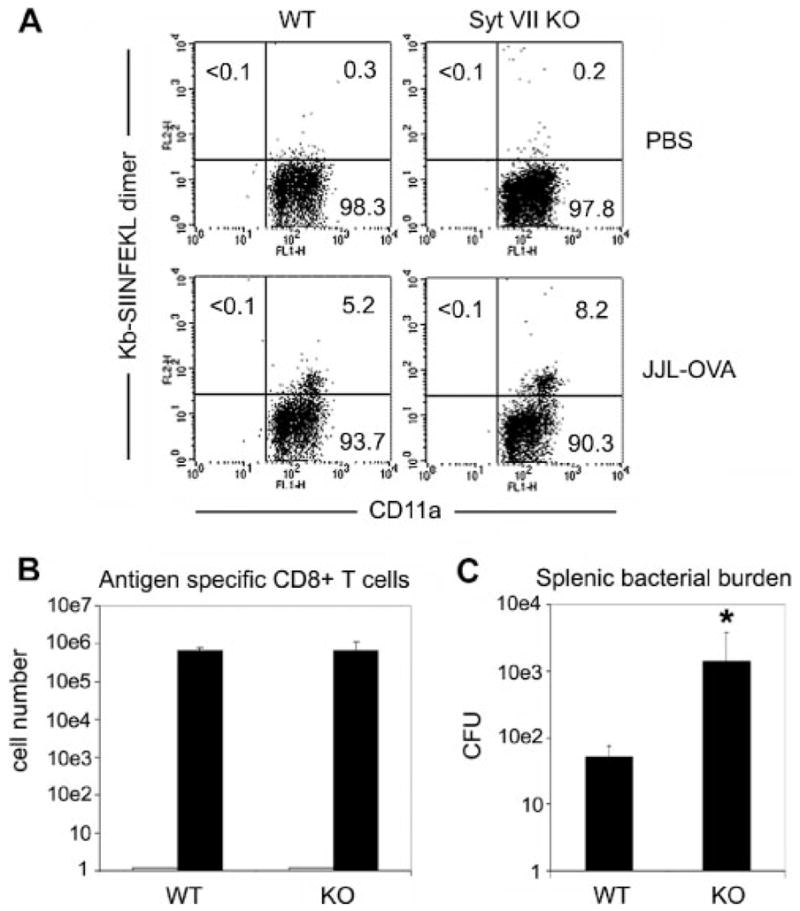
Syt VII KO animals exhibit impaired clearance of the intracellular pathogen L. monocytogenes. Ag-specific T cell responses were primed in WT and Syt VII KO mice with 1 × 104 CFU of OVA-expressing recombinant L. monocytogenes strain, JJL-OVA, or PBS alone. Ag-specific CD8 T cell responses were then evaluated 3 wk later following a secondary challenge with 2 × 105 CFU of JJL-OVA. Five days postsecondary challenge, Ag-specific CD8 T cell responses to the MHC class I-restricted OVA-derived peptide were examined in WT and Syt VII−/− animals. A, Ag-specific CD8+ T cell in PBS (top) and JJL-OVA-primed (bottom) WT and Syt VII KO animals were determined following staining with CD11a FITC, CD8 PerCP, and control peptide- or SIINFEKL-loaded H-2 Kb-IgG dimer (the data shown are for CD8+ -gated cells). B, The average number of SIINFEKL-specific CD8+ T cells in A detected in the spleen 5 days postrechallenge in WT and Syt VII−/− animals primed with PBS (
 ) or 1 × 104 CFU of JJL-OVA (■). C, Splenic bacterial burden in the spleens of WT and Syt VII−/− mice in B were determined with splenic lysates on brain-heart infusion agar plates. The data are presented as the mean ± SEM for individual samples plated in triplicate in one representative experiment. The data in A reflect representative data from three mice per group.*, p >0.05 in paired Student’s t test.
) or 1 × 104 CFU of JJL-OVA (■). C, Splenic bacterial burden in the spleens of WT and Syt VII−/− mice in B were determined with splenic lysates on brain-heart infusion agar plates. The data are presented as the mean ± SEM for individual samples plated in triplicate in one representative experiment. The data in A reflect representative data from three mice per group.*, p >0.05 in paired Student’s t test.
Syt VII-deficient CTLs form normal immunological synapses, but are defective in killing target cells
The data described above indicated that several properties of the CD8+ T cells of Syt VII-deficient mice appeared to be normal, but the animals were not capable of clearing L. monocytogenes infections as efficiently as the WT controls. To gain insight into the mechanism responsible for this defect, we proceeded to examine the interaction of isolated CTLs with target cells in vitro. We initially determined the capacity of Syt VII WT and Syt VII KO CTLs to form immunological synapses, a process that involves a marked polarization of the lytic granules within a ring containing the cytoskeletal protein talin and other adhesion proteins, at the site of contact between CTLs and target cells (25). To be able to visualize the secretory lysosomes/lytic granules by fluorescence microscopy, we loaded the lysosomal compartments of WT and KO CTLs with 10-kDa dextran-Texas Red by a 3-h pulse-chase incubation at 37°C. These dextran-loaded CTLs were then incubated with P815 target cells, and allowed to adhere to coverslips before fixation and staining with Abs against talin. In conjugates formed between Syt VII WT CTLs and their targets, the expected talin enrichment at the synapse site was detected, in the typical ring morphology seen at the site of tight contact between effector and target. The dextran-labeled granules were tightly polarized at the talin ring site, in direct opposition to the target cell membrane (Fig. 4, A–C). In CTLs derived from Syt VII KO mice, a similar granule polarization at the talin-enriched synapse site was detected (Fig. 4, D–F). In both populations of conjugates, the majority of the secretory lysosomes in the CTLs appeared to be polarized at the target cell contact site. A few dextran-positive vesicles that were more dispersed were also observed, in both WT and KO CTL/target cell conjugates (Fig. 4, B and F). Thus, these results suggest that Syt VII-deficient CTLs are not defective in granule polarization, or in the ability to form an immunological synapse when in contact with a target cell.
FIGURE 4.
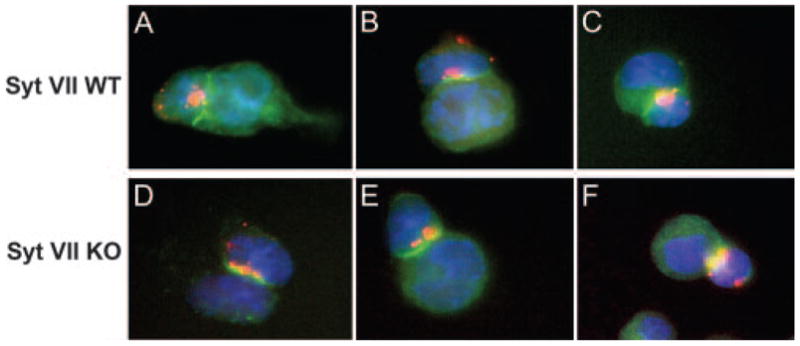
Syt VII KO CTLs form normal immunological synapses. Al-logeneic CTLs from Syt VII WT (A–C) and Syt VII KO (D–F) mice were pulse chased with 10-kDa dextran-Texas Red to label secretory lysosomes, before being incubated with P815 target cells to permit conjugation. Cells were adhered to poly(L-lysine)-coated coverslips and fixed before staining with anti-talin Abs (green, A–F). Three representative pictures of each genotype are shown. CTL nuclei are shown in blue (DAPI DNA stain).
As described above, Syt VII is specifically up-regulated upon CTL activation, and it localizes to secretory lysosomes/lytic granules along with the secreted effector molecules responsible for cytotoxicity. Genetic ablation of Syt VII does not affect the composition of lytic granules, the capacity of CTLs to expand in response to activation, and to form immunological synapses containing polarized granules. However, when CTLs lacking Syt VII were examined in OVA257–264 (SIINFEKL) peptide-specific cytotoxicity assays (after crossing the Syt VII KO mice into the OT1+ background), a marked defect in the ability to lyse peptide-coated target cells was observed (Fig. 5A). Similar observations were made in allogeneic T cell cytotoxicity assays. Following a 4-h incubation with P815 target cells, CTLs from WT mice lysed ∼60% killing of targets, at an E:T ratio of 10. In contrast, under the same conditions, the cytotoxicity levels of Syt VII KO CTLs were 30 – 40% reduced in relation to WT controls (Fig. 5B). This cytotoxicity defect was reproducible in several independent experiments (an ANOVA paired t test performed on the results of seven independent cytotoxicity experiments gave p values of 0.006, 0.079, and 0.010 for E:T ratios of 10, 1, and 0.1, respectively, when the cytotoxicity of Syt VII KO CTLs was compared with WT controls). Thus, CTLs from Syt VII-deficient animals exhibit markedly impaired target cytolysis in response to both Ag-specific and allogenic recognition in vitro.
FIGURE 5.
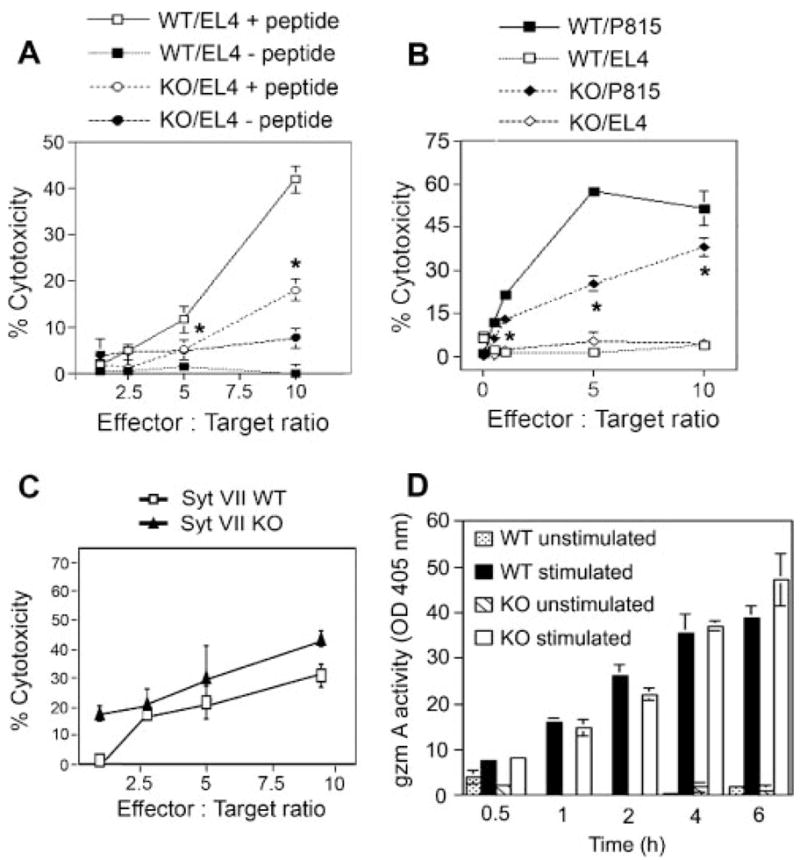
Syt VII KO CTLs demonstrate reduced perforin-mediated cytotoxicity. A, H-2Kd alloreactive CTLs were generated from WT and Syt VII-deficient animals and examined for cytotoxicity at various E:T cell ratios. Allogenic P815 (H-2Kd) (closed symbols) or syngenic EL-4 (H-2Kb) (open symbols) targets were used at 1 × 104 cells/well. EL-4 targets were used as a negative control.*, p <0.05. B, Ag-specific CTL responses by WT and SytVII-deficient OT-1 TCR transgenic animals. CTLs from OT-1+:Syt VII+/+ and OT-1+:Syt VII−/− mice were cultured with EL-4 targets coated with (□/○) or without (■/●) SIINFEKL peptide.*, p <0.05. C, Allogeneic stimulated CTLs from WT and Syt VII-deficient mice were pretreated with 0.25 μM perforin-mediated cytotoxicity inhibitor concanamycin A. Cytotoxicity was determined by assaying released/lactate dehydrogenase (Cytotox96). D, Syt VII KO CTLs secrete granzyme A in levels comparable to that of Syt VII WT CTLs. CTLs were placed in anti-CD3-coated wells (5 μg/ml) for the indicated times, and the supernatants were removed and assayed for BLT-esterase activity. The data in A–D represent the mean ± SEM for triplicate samples of one representative experiment, from a total of seven (A and B), three (C), or five (D) experiments.
The localization of Syt VII on CTL granules suggested that the reduced cytotoxicity of Syt VII KO CTLs resulted from a defect in the granule-mediated killing pathway. To test this hypothesis, the allogeneic target cell lytic assays were repeated after pretreating the effector CTLs with a specific inhibitor of perforin-mediated lysis, concanamycin A. This inhibitor blocks the vacuolar ATPase-dependent acidification that is required for perforin-mediated cytolysis (26). When perforin-mediated cytotoxicity was inhibited (using the lowest concanamycin A dose with inhibitory effect on the cytotoxicity of WT CTLs), Syt VII WT or KO CTLs showed comparable levels of target cell lysis (Fig. 5C), suggesting that Syt VII is specifically involved in regulation of granule-mediated cy-totoxicity. Comparable levels of granzyme secretion were observed when CTLs of WT or Syt VII KO mice were stimulated by TCR cross-linking with anti-CD3 Abs (Fig. 5D), a stimulus that is known to bypass the requirement for additional stimulatory signals normally required during Ag-triggered responses (27). These results demonstrate that CD8+ CTLs of Syt VII-deficient mice, although containing normal levels of secretory products in their lytic granules, are defective in their capacity to kill target cells through the granule-mediated pathway.
Discussion
In this study, we examined cytotoxic CD8+ T cell responses of mice lacking Syt VII, an evolutionarily conserved and ubiquitously expressed member of the Syt family of Ca2+ sensors. Previous studies implicated Syt VII in the regulation of conventional lysosome exocytosis, in fibroblasts and other cell types (28). This suggested that Syt VII might also be required for CTL function, which depends on the Ca2+ -regulated secretion of lysosome-related lytic granules. Our results support this hypothesis, because Syt VII-deficient mice are less capable of clearing L. monocytogenes infections, a process known to depend on Ag-specific CD8+ T cell responses (18), and have a markedly impaired capacity to trigger target cell lysis in vitro.
Syts are thought to promote exocytosis by facilitating the formation of SNARE complexes (29). The brain-specific isoform Syt I is localized on neuronal synaptic vesicles, where it functions as a Ca2+ sensor for fast, synchronous neurotransmitter release (30 –32). The ubiquitously expressed isoform Syt VII, in contrast, is localized on conventional lysosomes, and also on nonsynaptic secretory granules of PC12 and pancreatic β cells, where it was also shown to regulate Ca2+ -triggered exocytosis (6, 7, 9, 33, 34). Recently, in vitro reconstitution experiments demonstrated that both Syt I and Syt VII, in the absence of any other proteins, are capable of conferring Ca2+ sensitivity to SNARE-mediated membrane fusion (35, 36). Our detection of Syt VII on granzyme A-containing lytic granules of CTLs reinforces the lysosomal nature of these vesicles (37, 38), and raises the possibility that Syt VII may play a role as a sensor for the elevations in cytosolic free Ca2+ concentration that are known to trigger the exocytosis of CTL lytic granules (3, 4). Syt VII expression was also markedly up-regulated during in vitro activation of CTLs isolated from lymph nodes, consistent with what has been reported for other secretory lysosome components (24, 39).
Consistent with a possible role of Syt VII as a Ca2+ sensor regulating SNARE-mediated fusion of lytic granules with the plasma membrane, no abnormalities were detected in the capacity of CTLs from Syt VII-deficient mice to proliferate and secrete cytokines in response to stimulation. Syt VII KO CTL lytic granules also have normal morphology, contain normal amounts of perforin and granzyme A, and show no major abnormalities in their ability to polarize at the sites of immunological synapse formation. It remains to be shown whether Syt VII deficiency affects the ability of lytic granules to dock properly at the plasma membrane, a defect that has been linked to the reduced cytotoxicity of CTLs from ashen mice, which carry a loss of function mutation in Rab27a (40). We find this possibility unlikely, because granzyme secretion triggered by the strong default stimulatory signal provided by TCR cross-linking (27) is not altered in Syt VII KO CTLs. In this respect, Syt VII-deficient CTLs behave similarly to CTLs from gunmetal mice, which also have a reduced capacity for killing target cells, but are still capable of granzyme secretion when stimulated with anti-CD3 Abs (40).
It remains to be determined whether lytic granule exocytosis triggered by TCR cross-linking also requires a Ca2+ sensor molecule such as Syt. Marked differences have been observed in the Ca2+ affinity of different Syt isoforms, and Syt VII has been shown to be capable of sensing significantly lower Ca2+ concentrations, when compared with the neuronal isoform Syt I (35). Syt II, although predominantly expressed in neuronal cells, has been reported to regulate the exocytosis of lysosome-related granules of mast cells (41), and to confer Ca2+ sensitivity to phagocytosis-associated secretion in neutrophils (42). It is conceivable that another Syt isoform, capable of sensing the higher [Ca2+ ]i triggered by TCR cross-linking, mediates granzyme secretion when Syt VII KO CTLs are stimulated with anti-CD3 Abs. Encounters with target cells, in contrast, are likely to generate suboptimal conditions for TCR stimulation, which may generate a requirement for a high-affinity Ca2+ sensor such as Syt VII. This view is reinforced by our observation that an inhibitor of perforin function, concanamycin A, has a strong inhibitory effect on the cytotoxicity of WT CTLs, but not on the residual killing activity of Syt VII-deficient CTLs. Further studies are needed to clarify the exact mechanism mediating the residual cytotoxicity observed in Syt VII-deficient CTLs. Although Fas-mediated killing may be responsible, at this stage we cannot rule out the possibility that the perforin/granzyme pathway is not entirely Syt VII dependent. In any case, our findings indicate that the lytic granule-associated Ca2+ sensor Syt VII is required for full cytotoxicity, specifically under conditions in which exocytosis is triggered by Ag-specific target recognition.
Acknowledgments
We thank Drs. M. Simon (Max-Planck Institute), M. Starnbach (Harvard Medical School), F. Carbone (University of Melbourne), and Hao Shen (University of Pennsylvania) for their gifts of Abs, cell lines, mice, and bacterial strains.
Footnotes
This work was supported by National Institutes of Health Grants RO1GM064625 and RO1A1034867 (to N.W.A.) and by a minority supplement to RO1A1034867 (to K.T.F.).
Abbreviations used in this paper: [Ca2+]i; intracellular free Ca2+; BLT, benzyloxy- carbonyl-L-lysine thiobenzyl ester; DAPI, 4′,6′-diamidino-2-phenylindole; NRK, normal rat kidney; KO, knockout; MTS, 3-(4,5 dimethylthiazol-2yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; pen/strep, penicillin/streptavidin; Syt, synaptotagmin; WT, wild type.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Stinchcombe JC, Griffiths GM. Regulated secretion from hemopoietic cells. J Cell Biol. 1999;147:1– 6. doi: 10.1083/jcb.147.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kupfer A, Singer SJ, Dennert G. On the mechanism of unidirectional killing in mixtures of two cytotoxic T lymphocytes: unidirectional polarization of cytoplasmic organelles and the membrane-associated cytoskeleton in the effector cell. J Exp Med. 1986;163:489 – 498. doi: 10.1084/jem.163.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burkhardt JK, Hester S, Lapham CK, Argon Y. The lytic granules of natural killer cells are dual-function organelles combining secretory and pre-lysosomal compartments. J Cell Biol. 1990;111:2327–2340. doi: 10.1083/jcb.111.6.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters PJ, Borst J, Oorschot V, Fukuda M, Krahenbuhl O, Tschoop J, Slot JW, Geuze HJ. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J Exp Med. 1991;173:1099 –1109. doi: 10.1084/jem.173.5.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez A, Webster P, Ortego J, Andrews NW. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J Cell Biol. 1997;137:93–104. doi: 10.1083/jcb.137.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez I, Chakrabarti S, Hellevik T, Morehead J, Fowler K, Andrews NW. Synaptotagmin VII regulates Ca2+-dependent exocytosis of lysosomes in fibroblasts. J Cell Biol. 2000;148:1141–1149. doi: 10.1083/jcb.148.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy A, Caler E, Andrews N. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 8.Caler EV, Chakrabarti S, Fowler KT, Rao S, Andrews NW. The exocytosis-regulatory protein synaptotagmin VII mediates cell invasion by Trypanosoma cruzi. J Exp Med. 2001;193:1097–1104. doi: 10.1084/jem.193.9.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukuda M, Kanno E, Satoh M, Saegusa C, Yamamoto A. Synaptotagmin VII is targeted to dense-core vesicles and regulates their Ca2+-dependent exocytosis in PC12 cells. J Biol Chem. 2004;279:52677–52684. doi: 10.1074/jbc.M409241200. [DOI] [PubMed] [Google Scholar]
- 10.Chakrabarti S, Kobayashi KS, Flavell RA, Marks CB, Miyake K, Liston DR, Fowler KT, Gorelick FS, Andrews NW. Impaired membrane resealing and autoimmune myositis in synaptotagmin VII-deficient mice. J Cell Biol. 2003;162:543–549. doi: 10.1083/jcb.200305131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy D, Liston DR, Idone VJ, Di A, Nelson DJ, Pujol C, Bliska JB, Chakrabarti S, Andrews NW. A process for controlling intracellular bacterial infections induced by membrane injury. Science. 2004;304:1515–1518. doi: 10.1126/science.1098371. [DOI] [PubMed] [Google Scholar]
- 12.Jaiswal JK, Chakrabarti S, Andrews NW, Simon SM. Synaptotagmin VII restricts fusion pore expansion during lysosomal exocytosis. PLoS Biol. 2004;2:1224 –1232. doi: 10.1371/journal.pbio.0020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiflett SL, Kaplan J, Ward DM. Chediak-Higashi syndrome: a rare disorder of lysosomes and lysosome related organelles. Pigm Cell Res. 2002;15:251–257. doi: 10.1034/j.1600-0749.2002.02038.x. [DOI] [PubMed] [Google Scholar]
- 14.Huynh C, Roth D, Ward DM, Kaplan J, Andrews NW. Defective lysosomal exocytosis and plasma membrane repair in Chediak-Higashi/beige cells. Proc Natl Acad Sci USA. 2004;101:16795–16800. doi: 10.1073/pnas.0405905101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baetz K, Isaaz S, Griffiths GM. Loss of cytotoxic T lymphocyte function in Chediak-Higashi syndrome arises from a secretory defect that prevents lytic granule exocytosis. J Immunol. 1995;154:6122– 6131. [PubMed] [Google Scholar]
- 16.Pardo J, Balkow S, Anel A, Simon MM. The differential contribution of granzyme A and granzyme B in cytotoxic T lymphocyte-mediated apoptosis is determined by the quality of target cells. Eur J Immunol. 2002;32:1980 –1985. doi: 10.1002/1521-4141(200207)32:7<1980::AID-IMMU1980>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 17.Carbone FR, Sterry SJ, Butler J, Rodda S, Moore MW. T cell receptor α-chain pairing determines the specificity of residue 262 within the Kb-restricted, ovalbumin257–264 determinant. Int Immunol. 1992;4:861– 867. doi: 10.1093/intimm/4.8.861. [DOI] [PubMed] [Google Scholar]
- 18.Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812– 823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 19.Haddad EK, Wu X, Hammer JA, III, Henkart PA. Defective granule exocytosis in Rab27a-deficient lymphocytes from Ashen mice. J Cell Biol. 2001;152:835– 842. doi: 10.1083/jcb.152.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P, Chicka MC, Bhalla A, Richards DA, Chapman ER. Synaptotagmin VII is targeted to secretory organelles in PC12 cells, where it functions as a high-affinity calcium sensor. Mol Cell Biol. 2005;25:8693– 8702. doi: 10.1128/MCB.25.19.8693-8702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hakansson A, Bentley CC, Shakhnovic EA, Wessels MR. Cytolysin-dependent evasion of lysosomal killing. Proc Natl Acad Sci USA. 2005;102:5192–5197. doi: 10.1073/pnas.0408721102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuda M, Ogata Y, Saegusa C, Kanno E, Mikoshiba K. Alternative splicing isoforms of synaptotagmin VII in the mouse, rat and human. Biochem J. 2002;365:173–180. doi: 10.1042/BJ20011877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pena SV, Krensky AM. Granulysin, a new human cytolytic granule-associated protein with possible involvement in cell-mediated cytotoxicity. Semin Immunol. 1997;9:117–125. doi: 10.1006/smim.1997.0061. [DOI] [PubMed] [Google Scholar]
- 24.Berke G. The binding and lysis of target cells by cytotoxic lymphocytes: molecular and cellular aspects. Annu Rev Immunol. 1994;12:735–773. doi: 10.1146/annurev.iy.12.040194.003511. [DOI] [PubMed] [Google Scholar]
- 25.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 26.Tsujimura K, Takahashi T, Iwase S, Matsudaira Y, Kaneko Y, Yagita H, Obata Y. Two types of anti-TL (thymus leukemia) CTL clones with distinct target specificities: differences in cytotoxic mechanisms and accessory molecule requirements. J Immunol. 1998;160:5253–5261. [PubMed] [Google Scholar]
- 27.Hathcock KS, Segal DM, Hodes RJ. Activation of Lyt-2+(CD8+) and L3T4+ (CD4+) T cell subsets by anti-receptor antibody. J Immunol. 1989;142:2181–2186. [PubMed] [Google Scholar]
- 28.Andrews NW, Chakrabarti S. There’s more to life than neurotrans-mission: the regulation of exocytosis by synaptotagmin VII. Trends Cell Biol. 2005;11:626 – 631. doi: 10.1016/j.tcb.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Chapman ER. Synaptotagmin: a Ca2+ sensor that triggers exocytosis? Nat Rev Mol Cell Biol. 2002;3:498 –508. doi: 10.1038/nrm855. [DOI] [PubMed] [Google Scholar]
- 30.Bai J, Tucker WC, Chapman ER. PIP2 increases the speed-of-response of synaptotagmin and steers its membrane penetration activity toward the plasma membrane. Nat Struct Mol Biol. 2004;11:36 – 44. doi: 10.1038/nsmb709. [DOI] [PubMed] [Google Scholar]
- 31.Koh TW, Bellen HJ. Synaptotagmin I, a Ca2+ sensor for neuro-transmitter release. Trends Neurosci. 2003;26:413– 422. doi: 10.1016/S0166-2236(03)00195-4. [DOI] [PubMed] [Google Scholar]
- 32.Yoshihara M, Adolfsen B, Littleton JT. Is synaptotagmin the calcium sensor? Curr Opin Neurobiol. 2003;13:315–323. doi: 10.1016/s0959-4388(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 33.Wang P, Chicka MC, Bhalla A, Richards DA, Chapman ER. Synaptotagmin VII is targeted to secretory organelles in PC12 cells, where it functions as a high-affinity calcium sensor. Mol Cell Biol. 2005;25:8693– 8702. doi: 10.1128/MCB.25.19.8693-8702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo Z, Turner C, Castle D. Relocation of the t-SNARE SNAP-23 from lamellipodia-like cell surface projections regulates compound exocytosis in mast cells. Cell. 1998;94:537–548. doi: 10.1016/s0092-8674(00)81594-9. [DOI] [PubMed] [Google Scholar]
- 35.Bhalla A, Tucker W, Chapman ER. Synaptotagmin isoforms couple distinct ranges of Ca2+, Ba2+, and Sr2+ concentration to SNARE-mediated membrane fusion. Mol Biol Cell. 2005;16:4755– 4764. doi: 10.1091/mbc.E05-04-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tucker WC, Weber T, Chapman ER. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science. 2004;304:435– 438. doi: 10.1126/science.1097196. [DOI] [PubMed] [Google Scholar]
- 37.Andrews NW. Regulated secretion of conventional lysosomes. Trends Cell Biol. 2000;10:316 –321. doi: 10.1016/s0962-8924(00)01794-3. [DOI] [PubMed] [Google Scholar]
- 38.Griffiths GM. Secretory lysosomes: a special mechanism of regulated secretion in hemopoietic cells. Trends Cell Biol. 1996;6:329 –332. doi: 10.1016/0962-8924(96)20031-5. [DOI] [PubMed] [Google Scholar]
- 39.Pena SV, Krensky AM. Granulysin, a new human cytolytic granule-associated protein with possible involvement in cell-mediated cytotoxicity. Semin Immunol. 1997;9:117–125. doi: 10.1006/smim.1997.0061. [DOI] [PubMed] [Google Scholar]
- 40.Stinchcombe JC, Barral DC, Mules EH, Booth S, Hume AN, Machesky LM, Seabra MC, Griffiths GM. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J Cell Biol. 2001;152:825– 834. doi: 10.1083/jcb.152.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baram D, Adachi R, Medalia O, Tuvim M, Dickey BF, Mekori YA, Sagi-Eisenberg R. Synaptotagmin II negatively regulates Ca2+-triggered exocytosis of lysosomes in mast cells. J Exp Med. 1999;189:1649 –1658. doi: 10.1084/jem.189.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindmark IM, Karlsson A, Serrander L, Francois P, Lew D, Rasmusson B, Stendahl O, Nusse O. Synaptotagmin II could confer Ca2+ sensitivity to phagocytosis in human neutrophils. Biochim Biophys Acta. 2002;1590:159 –166. doi: 10.1016/s0167-4889(02)00209-4. [DOI] [PubMed] [Google Scholar]


